Abstract
The prefrontal cortex (PFC) is thought to play an important role in “higher” brain functions such as personality and emotion that may associated with several gender-related mental disorders. In this study, the gender effects of functional connectivity, cortical lateralization and significantly differences in the PFC were investigated by using resting-state functional optical tomography (fOT) measurement. A total of forty subjects including twenty healthy male and twenty healthy female adults were recruited for this study. In the results, the hemoglobin responses are higher in the male group. Additionally, male group exhibited the stronger connectivity in the PFC regions. In the result of lateralization, leftward dominant was observed in the male group but bilateral dominance in the female group. Finally, the 11 channels of the inferior PFC regions (corresponding to the region of Brodmann area 45) are significant different with spectrum analysis. Our findings suggest that the resting-state fOT method can provide high potential to apply to clinical neuroscience for several gender-related mental disorders diagnosis.
OCIS codes: (170.1610) Clinical applications, (170.2655) Functional monitoring and imaging, (170.3880) Medical and biological imaging
1. Introduction
Resting-state functional connectivity, which refers to the spontaneous neural activity, has become one of the important approaches for neuroscience to understanding the functional organization of the human brain [1]. The oscillations of resting-state brain hemodynamic spontaneous fluctuations with low frequency (< 0.1 Hz) can reflect the characteristics of spontaneous neural activity [2–6]. A range of functional magnetic resonance imaging (fMRI) studies pointed out that temporal correlations of blood oxygenation level dependent (BOLD) response between different regions indicate the functional connections with spontaneous activity in brain [7–10]. The result also implies the existed interactions between neuronal populations during the resting-state. Thus, the correlation analysis of spontaneous activity in resting-state brain activity maps can provide insight into the intrinsic functional connectivity of the human brain. Resting-state studies based on fMRI have greatly increased our knowledge to the neuro-connective process of default mode network [11]. Although fMRI provides several strengths such as noninvasive and excellent spatial resolution, however, the limitation of huge size of instruments and confines the participants to restricted positions inside the magnet cannot provide diagnosis with patient-oriented measurement. These characteristics make the imaging modalities as difficult or impossible to apply to many uses, including use with neonates, children, old persons, and claustrophobia patients. Besides, the scanning noise of fMRI cannot provide really undisturbed condition in resting-state measurement.
Recently, measurement of the hemodynamic changes with functional image of near-infrared spectroscopy (NIRS), so-called functional optical tomography (fOT), has become a powerful tool for detecting the neuronal activity of brain with oxygenation dynamic measurement. Functional optical topography method provides several benefits such as less expensive, non-ionizing radiation imaging, real-time measurement, long time monitoring and easy operation [12–15]. Although the spatial resolution of fOT is quite limited, it still takes the own advantages for brain imaging. For example, the high temporal resolution (100 Hz or higher) of fOT methods prevents aliasing of higher frequency cardiac or respiratory activity, which is a significant component of variance in spontaneous BOLD signals of fMRI [16–18]. Therefore, fOT offers a more comprehensive measurement of brain activity than BOLD fMRI [19]. Additionally, fOT imaging offers completely patient-oriented measurement with undisturbed condition in resting-state.
As mentioned before, the functional connectivity has been exhibited to be a potential method for the assessment of the organizational mechanisms of human brain in the fMRI field. To date, fOT method has been widely applied for resting-state functional brain mapping and connectivity studies. Previous studies of resting-state fOT have indicated the feasibility to assess resting-state functional connectivity by using hemodynamic response of brain [2, 20–24]. Although the resting-state in fOT fields with group-level analysis reveals the functional connectivity with inherent characteristic of the different brain systems such as the cognitive, sensorimotor, visual, auditory and language systems. However, the gender-related effects of the results in the group-level analysis with fOT measurement are unclear. The gender differences of structural and functional properties in human brain have reported in lots of previous studies of fMRI [25]. Many clinical studies of fMRI, EEG and fOT indicated the existed gender difference of brain functional connectivity in resting-state [26–28]. Nevertheless, there were few studies of gender-related effects in resting-state functional connectivity via fOT monitoring. Thus, we hope to further investigate the gender-related effects of PFC connectivity in the resting-state by using fOT method in this study. The hemoglobin responses were filtered within 0.01 to 0.08 Hz for statistically evaluation of the PFC region in the resting-state. The functional connectivity and cortical lateralization of gender-related effects are then evaluated by using time series of the total hemoglobin response. The power spectrum analysis was used to estimate the significantly different of PFC regions between male and female groups. The results show that the gender-related effects of resting-state fOT measurement should be considered necessarily for group-level analysis.
2. Methods
2.1 Participants
In this study, 40 right-handed young adults (20 males and 20 females), age between 22 to 25 years (the mean age was 23 ± 2 years old) with no history of neurological diseases, were recruited from National Chiao Tung University, Taiwan. All participants provided written informed consent. The study was in accordance with the latest version of the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) in National Chiao Tung University, Taiwan.
2.2 Data acquisition
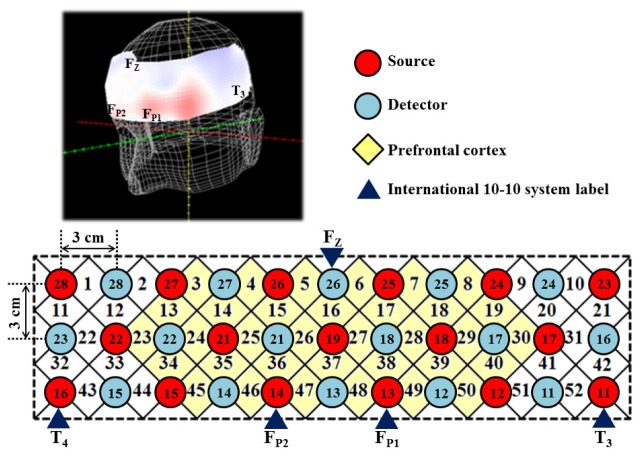
A continuous wave fOT system (ETG-4000, Hitachi Medical Co., Tokyo, Japan) was used to record brain response in this study. The fOT measurements were conducted with multiplexing channels (52 channels), which can allow 2-D tomographic functional maps of brain activity. The near-infrared sources at dual-wavelength 695 and 830 nm were used and the sampling rate was set to 10 Hz. According to the modified Beer-Lambert Law [29], the concentration changes in oxygenated hemoglobin (ΔHbO2) and deoxygenated hemoglobin (ΔHb) could be obtained. Then the total hemoglobin (ΔtHb) can be calculated by the components of ΔHbO2 and ΔHb (the sum of ΔHbO2 and ΔHb). In this system, the ΔHbO2 and ΔHb values include differential path-length factor (DPF) which is wavelength and age dependent [30]. In the multi-channel fOT system, 17 light emitters (the red circles of Fig. 1) and 16 photo-detectors (the blue circles of Fig. 1) are plugged into a probing holder and arranged as 3 × 11 array, resulting in a total of 52 channels detection with 3 cm source-detector separation, which permitted to measure the regions at the approximate depth of 2 to 3 cm from the scalp that corresponded to the surface of cerebral cortex [31–34]. In order to cover the whole PFC, the optode was positioned by according to the international 10-20 system [35]. Figure 1 indicates the schematic arrangement of the fOT optode array. The bottom row of channels was arranged along the T3–FP1–FP2–T4 line of the international 10-20 system, with sources 11, 13, 14 and 16 exactly at the T3, FP1, FP2 and T4 position, respectively. Furthermore, the detector 26 was fitted around FZ.
Fig. 1.
The fOT channels localization. (Top left) the 3-D image of the optode position; (Bottom) The localizations of all 52 channels were positioned according to the international 10-20 system. Red and blue circles indicate near-infrared light emitter and detector positions, respectively. By using the international 10-20 system the detector 26 was positioned at the FZ marker point while the bottom row of sources 11, 13, 14 and 16 were placed on the T3–FP1–FP2–T4 line. Yellow diamonds indicate the measuring region of PFC.
During the experiment, each subject only needed to sit on a comfortable chair in a silent room with the dim lighting and cap with the optode on the head and underwent 10 minutes resting-state session of fOT data collection. During the resting-state session, the subjects were instructed to keep their eyes closed without falling asleep, relax their mind, and remain motionless as much as possible.
2.3 Data analysis
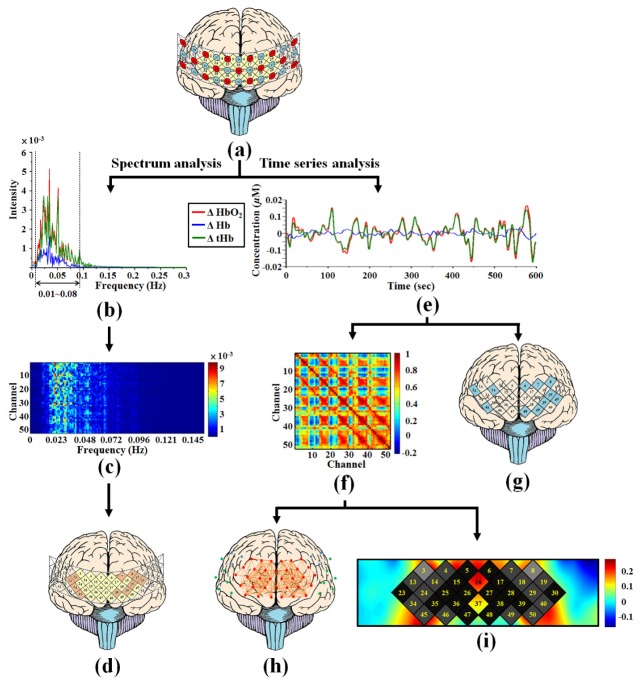
Although the optode arrangement (52 channels) of ETG-4000 can cover the whole PFC, part of temporal cortex and part of motor cortex. In this study, the resting-state brain functional analysis between male and female groups was only focused in PFC region that consist of 34 channels of yellow diamonds in the Fig. 1. Figure 2 shows the flowchart of resting-state fOT data analysis. Here, the gender-specific effects of hemispheric asymmetry of PFC in the resting-state were investigated with time series and spectrum analysis of optical signals at low frequency because the low-frequency band (< 0.1 Hz) were used in the numerous fMRI and fNIRS studies for functional connectivity study in resting-state. In order to obtain the relatively steady signals and reduce the potential effects of unstable signals for further data analysis, the first 2 minutes data was discarded for each participant. This process is employed in previous studies of fNIRS field [21, 23]. The band-passed optical signal analysis avoids the physiological noise of low and high frequency such as respiratory (0.1-0.3 Hz) and cardiac-related fluctuations (0.75-1 Hz) in oxygen supply [2–6]. In our study, the optical signal of each fOT channel was filtered through a band-pass filter within 0.01 to 0.08 Hz to reduce the effects of physiological noise [2, 6, 24]. The butterworth low pass filter (cutoff frequency is 0.08 Hz) and butterworth high pass filter (cutoff frequency is 0.01 Hz) were used for band-pass filtering with MATLAB after raw data of ΔHbO2 and ΔHb were obtained from the system. Then the average data was obtained for group-level analysis to reduce the effects of individual differences. The distribution of the filtered signals of the time series and spectrum data are demonstrated in Fig. 2(b) and 2(e), respectively. In the Fig. 2(b), the power spectrum of a filtered signal shows that the physiological-noise (include respiratory and cardiac frequencies) are completely filtered. These remaining signals after filtering are used to perform subsequent data analysis. Three different analyses of gender-specific effects of hemispheric asymmetry in PFC were performed with processed fOT data that include: 1) functional connectivity, 2) cortical lateralization and 3) significant differences analysis. All of the data were analyzed with group-level brain functional response of PFC area in resting-state.
Fig. 2.
The flowchart of resting-state fOT data analysis. (a) fOT channels localization above the PFC; (b) distribution of power spectrum within low-frequency band 0.01 to 0.08 Hz of ΔHbO2, ΔHb and ΔtHb for a single channel of one subject; (c) spectrogram from each channel of group-level statistics on averaging that was used for (d) significant difference analysis; (e) distribution of time series within low-frequency band 0.01 to 0.08 Hz of ΔHbO2, ΔHb and ΔtHb for a single channel of one subject that was used for correlation and lateralization analysis; (f) schematic diagram of group average correlation matrices for all channels; (g) schematic diagram of cortical lateralization analysis; (h) schematic diagram of functional connectivity in PFC; (i) schematic diagram of correlation coefficient between left vs. right PFC.
2.3.1 Functional connectivity
Basically, the functional connectivity of brain is a statistical concept. It can be considered as temporal correlation between regions that are defined naturally as detection channels of fOT measurement [36–39]. In this study, brain functional connectivity can be explored with time-series optical signals through the following four steps (Fig. 2(a)-2(e)-2(f)-2(g) and 2(h)). First, the network modes that correspond to detection channels were defined as optode (optical-to-detector) array of fOT (Fig. 2(a)). Then the fOT signal of each channel was processed as low-frequency time-series by using band-pass filter within 0.01 to 0.08 Hz (Fig. 2(e)). The low-frequency change of hemoglobin concentration was analyzed by calculating the Pearson’s correlation coefficient rXY:
| (1) |
In Eq. (1), Xi and Yi are the temporal response of group-level oxygenation dynamics (ΔHbO2, ΔHb and ΔtHb) of each detection channels; and are the mean of the group-level oxygenation dynamic response across a 10 minutes of each channels in resting-state; rXY is the correlation coefficient of each channels in male and female groups; n is the number of time-series in resting-state. Here, n is 600 with 1-second time increments. A correlation matrix (Fig. 2(f)) of functional brain network is obtained from correlation coefficients. Correlation analysis was performed for each channels in the left and right region of PFC (Fig. 2(h)) and between left versus right PFC (Fig. 2(i)) to understand the gender-specific effects of hemispheric asymmetry in resting-state functional connectivity.
2.3.2 Cortical lateralization
In previous studies, the cortical lateralization was observed for hemispheric dominance investigation via the laterality index (LI) [40–43]. For fNIRS measurement, the LI indicates the quantification of left and right hemispheres contributions of oxygenation dynamic response [41, 44]. We can define the LI as:
| (2) |
where TL is the mean concentration of ΔtHb of a given left channel of PFC in resting-state; TR is the mean concentration of ΔtHb of a given right channel of PFC in resting-state. Accordingly, a positive value of LI indicates leftward dominance, and a negative value of LI indicates rightward dominance. A near-zero value of LI indicates bilateral dominance that depends on statistical analysis. A two-tailed t-test was performed to determine if LI is a near-zero value or not. Then the one-tailed t-test was performed to determine the leftward or rightward dominance.
2.3.3 Significant differences
The average power spectrum of each channel on PFC area was used to analyze the significantly different channel between male and female groups through the following steps of Figs. 2(a)-2(d). In the Fig. 2(b), the power spectrum of the low-frequency (0.01 to 0.08 Hz) of ΔHbO2, ΔHb, and ΔtHb were obtained from time series signal by using FFT method. Then the spectrogram (Fig. 2(c)) of ΔtHb of each channel was structured from power spectrum curve to determine the significantly different channel (Fig. 2(d)) between male and female groups by using two-sample t-test with p value < 0.001.
3. Results
3.1 Functional connectivity
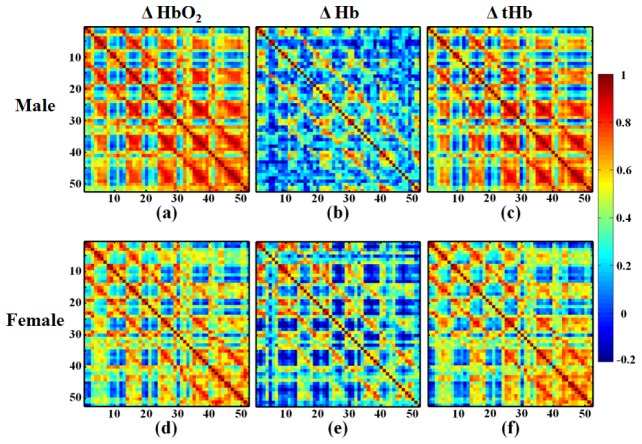
The correlation coefficients between each paired channel of PFC hemodynamic responses were calculated with Pearson’s correlation coefficient that the formula was described in Eq. (1). The male and female groups showed a rather similar distribution of correlation matrices (Fig. 3). The results of correlation matrices represent the strength of resting-state functional connectivity for ΔHbO2, ΔHb and ΔtHb of each paired channel of male and female groups in group-level analysis. In the Fig. 3, the redder boxes represent stronger connection between each PFC region, whereas bluer boxes represent lower connection between each PFC region. Observably, the connection between each pair channel of ΔHbO2, ΔHb and ΔtHb in male group were stronger than in female group. We also noticed that the adjacent and bilateral paired channels represented stronger connection.
Fig. 3.
The correlation matrices derived from male and female groups. (a) and (d) are the correlation matrices of each pair channel of ΔHbO2; (b) and (e) are the correlation matrices of each pair channel of ΔHb; (c) and (f) are the correlation matrices of each pair channel of ΔtHb. Each figure shows a 52 × 52 square matrix, where the x and y axes correspond to the channels listed in optode geometry, and where each unit of matrix indicates the mean strength of the functional connectivity between each pair channel.
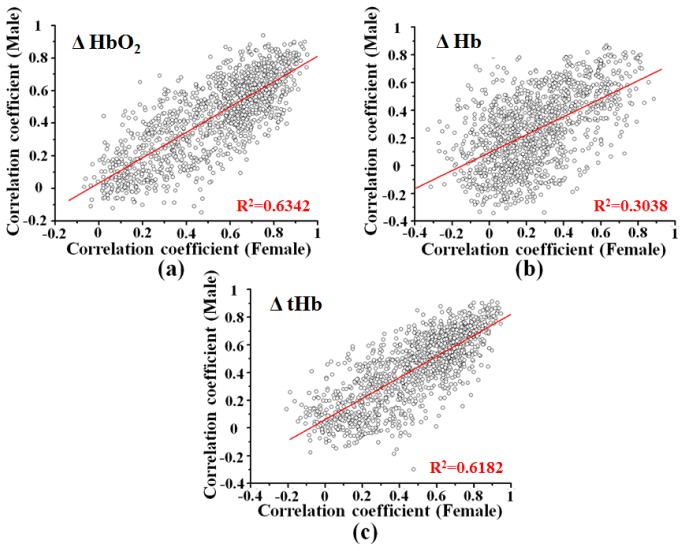
Figure 4 shows the distributions of correlation coefficient of male and female group of the same channel. The results of quantitative correlation analysis of ΔHbO2, ΔHb and ΔtHb indicated strong linear correlation between male and female group with R2 = 0.6342, p < 0.0001; R2 = 0.3038, p < 0.0001; and R2 = 0.6182, p < 0.0001, respectively. The results suggest the fOT-based brain functional connectivity matrices are reliable across different groups. This allowed us to further analyze the brain functional connectivity of gender effects between male and female groups.
Fig. 4.
The distributions of correlation coefficient of male and female group of the same channel. (a), (b), and (c) are the distributions of correlation coefficient of ΔHbO2, ΔHb, and ΔtHb of male and female group of the same channel, respectively. The data were derived from correlation matrices. The red lines mean the result of linear regression. The dots mean the correlation coefficient of male and female group of the same channel.
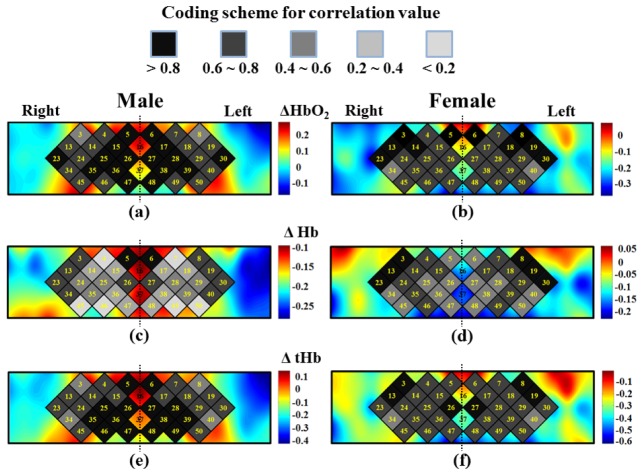
In this study, correlation calculations were also performed between bilateral regions of PFC region (between left vs. right PFC region) for each group. In the results, we can observe that the value of correlation coefficients of ΔHb is lower than ΔHbO2 and ΔtHb especially in male group. In the results of bilateral correlation analysis of ΔHbO2 and ΔtHb (Figs. 5(a), 5(e) and 5(b), 5(f)), the male group shows the stronger connections in the dorsolateral PFC regions (BA 10, BA 46, and BA 9) than inferior PFC regions (BA 44, and BA 45 - Broca's area). Conversely, the female group shows the stronger connections in the inferior PFC regions than dorsolateral PFC regions. Additionally, the mean variations of intensity in PFC of male group are stronger than female group in the resting-state condition. Overall, this result indicates that the bilateral brain functional connectivity is strong in PFC regions of both male and female groups.
Fig. 5.
Correlation analysis of hemodynamic response between left vs. right (Ch 3 vs. Ch 8, Ch4 vs. Ch7 et al.) bilateral PFC regions from male and female groups. (a), (c), and (e) are the correlation analysis of male bilateral PFC regions of ΔHbO2, ΔHb, and ΔtHb, respectively. (b), (d), and (f) are the correlation analysis of female bilateral PFC regions of ΔHbO2, ΔHb, and ΔtHb, respectively. The color bars indicate the mean concentration changes of hemoglobin include ΔHbO2, ΔHb and ΔtHb.
As mentioned before, the correlation matrices of ΔHbO2, ΔHb and ΔtHb were obtained from fOT data to analyze the connection properties between male and female groups. In this study, the ΔtHb was chose to perform the hemispheric functional connectivity analysis. Figure 6 demonstrates the functional connectivity patterns (group-level correlation network) of left-side and right-side PFC regions in resting-state of male (Fig. 6(a)) and female (Fig. 6(b)) groups. The functional connectivity were identified as the values of correction coefficients greater than 0.6. In the results, 203 lines (left-side: 102 lines, right-side: 101 lines) and 139 lines (left-side: 68 lines, right-side: 71 lines) brain functional connectivity of PFC were presented in male and female groups, respectively.
Fig. 6.
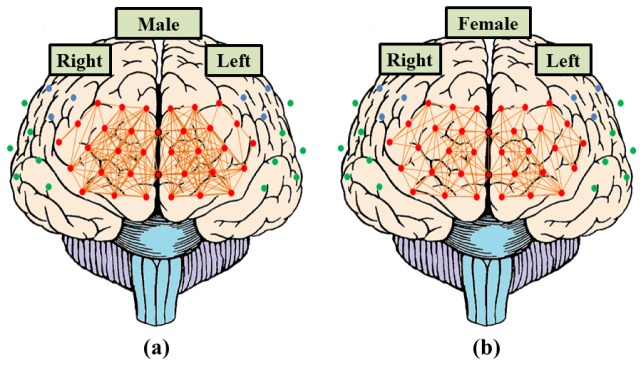
The functional connectivity pattern of left-side and right-side PFC in resting-state of (a) male group and (b) female group. The red dots represent the channels of each PFC region. The orange lines represent the functional connectivity (group-level correlation network) of left-side and right-side PFC region with correlation coefficient rXY larger than 0.6.
3.2 Cortical lateralization
The LI can help to determine the hemispheric dominance in fOT measurement during resting-state condition. Figure 7 shows the dominant channels in the PFC regions of male and female groups. In the male group, we can observe that the leftward dominant is greater than rightward (Fig. 7(a)). Compared with male group, the female group demonstrates approximate bilateral dominance (Fig. 7(b)). Besides, there is no dominance in the medial prefrontal during resting-state condition in both male and female groups.
Fig. 7.
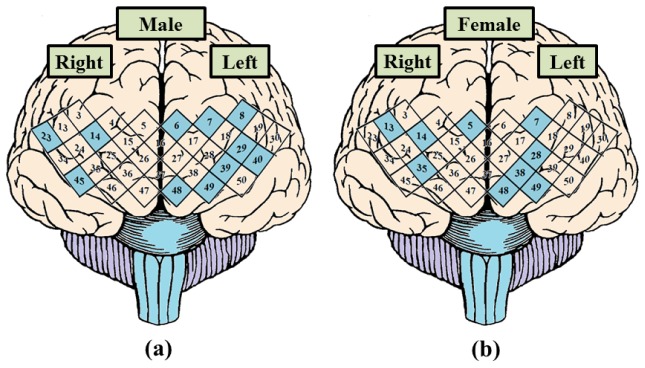
Cortical dominance channel of PFC region by computing LI of all left vs. right channel pairs in resting-state. (a) The result of cortical dominance of male group. (b) The result of cortical dominance of female group. The channels of blue diamond indicate the dominance region of leftward or rightward, and others indicate bilateral dominance.
3.3 Significant differences
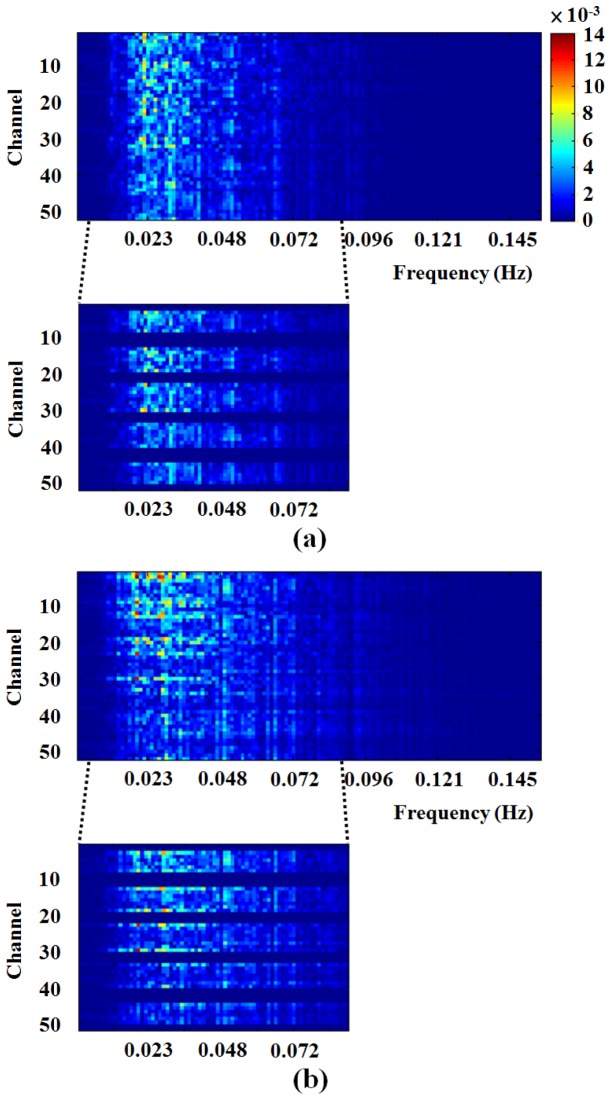
The spectrum analysis was performed to evaluate the significantly different channels between male and female groups. Figure 8 shows the spectrogram of each channel from male (Fig. 8(a)) and female (Fig. 8(b)) groups. Overall, we can observe that the intensity of power spectrum of PFC region (such as Ch. 3 to 8; Ch. 13 to 19; Ch. 23 to 30; Ch. 34 to 40 and Ch. 45 to 50) in female group is greater than male group. The PFC regions of 0.01 to 0.08 Hz were intercepted on the bottom of Fig. 8. In this processing, the values of other regions outside the PFC were filled in value of 0 to highlight the PFC regions. In the detail, the intensity of power spectrum of PFC region in male group is greater than female group around 0.023 Hz. Conversely, the intensity of PFC region in female group is greater than male group around 0.03 Hz. Additionally, the distribution of spectrogram is obvious differences between male and female groups.
Fig. 8.
The spectrogram of ΔtHb of each channel from (a) male and (b) female groups. Color bar indicates the intensity of frequency. The range of frequency 0.01 to 0.08 Hz was used to assess the significantly different channel of PFC between male and female groups. The channels of PFC regions are Ch. 3 to 8; Ch. 13 to 19; Ch. 23 to 30; Ch. 34 to 40 and Ch. 45 to 50 of frequency 0.01 to 0.08 Hz were highlighted on the bottom of figure.
Figure 9 shows the significantly different channels based on two-sample t-test calculated from spectrogram data between male and female groups. The Ch. 7, 8, 19, 29, 30, 38 and 39 of left-side PFC and Ch. 13, 23, 34 and 45 of right-side PFC are significantly different with p < 0.001. It must be noted that the inferior PFC regions (BA 44 and BA 45) of both left-side and right-side are demonstrated significant difference.
Fig. 9.
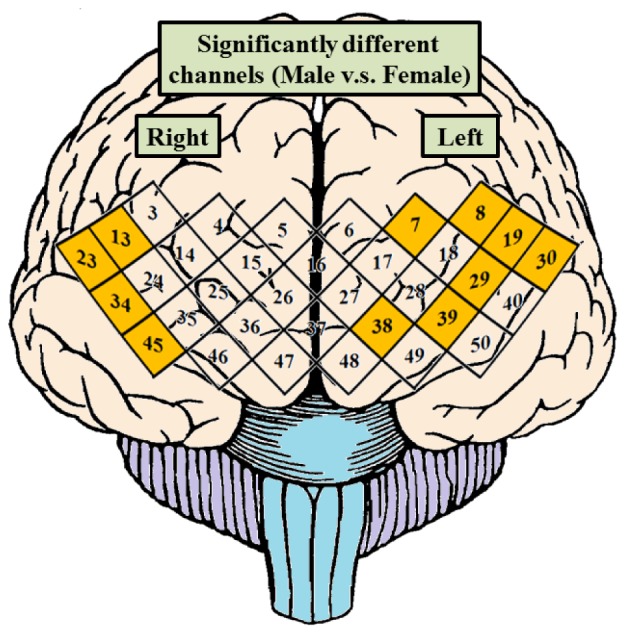
The result of two-sample t-test from power spectrum of each channel of PFC between male and female groups. The Ch. 7, 8, 19, 29, 30, 38, and 39 marked with orange diamonds of left-side PFC and Ch. 13, 23, 34, and 45 marked with orange diamonds of right-side PFC are significantly different with p value < 0.001 between male and female groups.
4. Discussion
The PFC performs several important functions in the brain. Unlike most previous fNIRS studies of brain network in resting-state, the present study used fOT to investigate the time-series and spectrum analysis of gender effect in the PFC during resting-state. In our study, although the quantitative correlation analysis shows strong linear connection of hemodynamic responses between male and female group (Fig. 4), the correlation matrices (Fig. 3) show that the male group possess higher connection of each paired channel than the female group in all hemodynamic responses. Besides, more noteworthy is that the fOT data of ΔHbO2, ΔHb and ΔtHb are higher in male group than in female group with the resting-state condition. Gender differences in brain function of PFC such as memories, sense emotions social sensitivity et al. have been reported by previous studies [25]. Although the behavioral function of resting-state network is still unclearly, it has been suggested that the resting-state brain network plays a role of associated with environmental stimuli as well as organizing processes such as reviewing past knowledge or preparing future actions [45]. Thus, our results imply that the PFC connection in resting-state might be associated with emotion recognition and cognitive differences between male and female group. The hemispheric functional connectivity analysis also shows the greater number of dorsolateral PFC functional connectivity in male group than in female group (Fig. 6). But there are no significant differences between leftward and rightward PFC in both male and female groups.
According to the result of Fig. 5, the male group presents stronger connections in the dorsolateral PFC regions (BA 10, BA 46, and BA 9) that are associated with brain function of memory, particularly working memory, and memory control and organization. Conversely, the female group shows the stronger connections in the inferior PFC regions (BA 44, BA 45). It is interesting to note that the BA 44 and 45 of left PFC are called Broca’s area that possesses strong correlation of speech-language. Thus, our results might help to explain females perform better in verbal memory, verbal fluency tasks, and speed of articulation [46, 47]. Contrarily, males perform batter in quantitative problem solving, and mental rotation, or tasks involving the underlying cognitive processes of maintaining and manipulating a visual image in working memory [48]. The observance of leftward dominance of inferior PFC in male group and bilateral dominance in female group during resting-state may help to explain the finding from previous fMRI study [4] that indicated the phonological processing aroused activation in the left inferior PFC in male but the bilateral PFC was activated in females. In the other words, it may support that why the females perform better in the function of speech-language.
Finally, the spectrum analysis of ΔtHb was used to investigate the significant differences of PFC between male and female groups in resting-state condition. The result indicates that the inferior (Ch. 8, 19, 29, 30 of left-side PFC and Ch. 13, 23, 34, 45 of right-side PFC) and part of dorsolateral PFC (Ch. 7, 38, 39 of right-side PFC) regions are significantly different between male and female groups. Observably, the result of significant differences analysis corresponds to the results of cortical lateralization. They all indicate that the inferior PFC especially in Broca’s area presents the significant differences between male and female groups. Besides, the peaks of frequency in the PFC region are most around at 0.023 Hz in the male group and around at 0.03 Hz in female group. These frequencies we named dominant frequency. In the EEG fields, the brain has delta, theta, alpha, beta and gamma wave whose frequency ranges correlate to brain states from awake to sleep [28]. Is it possible that there are also different frequencies distributes in the brain of resting-state? In this study, the different dominant frequencies are demonstrated in the gender-related effect. Although the meaning of the dominant frequency in the resting-state is unclear, we suggest that it maybe could provide useful information to characterize the brain connectivity changes with disease and should be considered necessarily for future study.
Functional connectivity in the resting-state is an important approach to investigate the intrinsic activity of the human brain. Recent studies of resting-state fOT have emerged as a hot topic and revealed that resting-state brain network is an inherent characteristic of the resting brain [49]. Brain functional connectivity is a complex mechanism especially in the PFC region that includes many complex brain functions, which associated with several neurodegenerative diseases. The gender effects should be necessarily considered for the functional connectivity studies with fOT measurement. Our result suggest that the gender effects of hemispheric asymmetry in spontaneous low-frequency hemodynamic fluctuation may provide useful information to understand the gender difference and may improve the accuracy of the assessment during the resting-state condition in future fOT studies.
5. Conclusion
Our result demonstrated that functional connectivity during resting-state condition of gender effects in the PFC region could be successfully measure by fOT method with high temporal resolution. Time-series analysis indicates the gender effects of functional connectivity and cortical lateralization and spectrum analysis indicates the significant differences in resting-state in PFC between male and female groups. The inferior PFC is significantly different between male and female groups with both time-series and spectrum analysis that can provide useful information for the future resting-state functional connectivity study with fOT-based measurement. Although there are still some limitations and unresolved issues [50], fOT provide high potential to be the ideal choice for resting-state functional studies in the fields of developmental and clinical neuroscience that can apply to several gender-related neurodegenerative diseases diagnosis.
Acknowledgments
This work was supported in part by the Taiwan National Science Council under Grant Nos. NSC 101-2628-E-009-026-MY3, NSC 102-2321-B-009-002, NSC 102-2622-E-009-007-CC3, NSC 102-2627-E-010-001, NSC 102-3011-P-010-003, and a grant from Ministry of Education, Aim for the Top University Plan in National Chiao-Tung University 101W9866.
References and links
- 1.Rosazza C., Minati L., “Resting-state brain networks: literature review and clinical applications,” Neurol. Sci. 32(5), 773–785 (2011). 10.1007/s10072-011-0636-y [DOI] [PubMed] [Google Scholar]
- 2.Niu H., Wang J., Zhao T., Shu N., He Y., “Revealing topological organization of human brain functional networks with resting-state functional near infrared spectroscopy,” PLoS ONE 7(9), e45771 (2012). 10.1371/journal.pone.0045771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glerean E., Salmi J., Lahnakoski J. M., Jääskeläinen I. P., Sams M., “Functional magnetic resonance imaging phase synchronization as a measure of dynamic functional connectivity,” Brain Connect. 2(2), 91–101 (2012). 10.1089/brain.2011.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian L., Wang J., Yan C., He Y., “Hemisphere- and gender-related differences in small-world brain networks: A resting-state functional MRI study,” Neuroimage 54(1), 191–202 (2011). 10.1016/j.neuroimage.2010.07.066 [DOI] [PubMed] [Google Scholar]
- 5.Mesquita R. C., Franceschini M. A., Boas D. A., “Resting state functional connectivity of the whole head with near-infrared spectroscopy,” Biomed. Opt. Express 1(1), 324–336 (2010). 10.1364/BOE.1.000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordes D., Haughton V. M., Arfanakis K., Carew J. D., Turski P. A., Moritz C. H., Quigley M. A., Meyerand M. E., “Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data,” AJNR Am. J. Neuroradiol. 22(7), 1326–1333 (2001). [PMC free article] [PubMed] [Google Scholar]
- 7.Song X. W., Dong Z. Y., Long X. Y., Li S. F., Zuo X. N., Zhu C. Z., He Y., Yan C. G., Zang Y. F., “REST: A Toolkit for resting-state functional magnetic resonance imaging data processing,” PLoS ONE 6(9), e25031 (2011). 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox M. D., Greicius M., “Clinical applications of resting state functional connectivity,” Front Syst. Neurosci. 4, 19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransson P., Skiöld B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Åden U., “Resting-state networks in the infant brain,” Proc. Natl. Acad. Sci. U.S.A. 104(39), 15531–15536 (2007). 10.1073/pnas.0704380104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H., Long X. Y., Yang Y., Yan H., Zhu C. Z., Zhou X. P., Zang Y. F., Gong Q. Y., “Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI,” Neuroimage 36(1), 144–152 (2007). 10.1016/j.neuroimage.2007.01.054 [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel M. P., Hulshoff Pol H. E., “Exploring the brain network: A review on resting-state fMRI functional connectivity,” Eur. Neuropsychopharmacol. 20(8), 519–534 (2010). 10.1016/j.euroneuro.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Boas G., “Noninvasive imaging of the brain,” Opt. Photonics News 15, 52–55 (2004). [Google Scholar]
- 13.Sasai S., Homae F., Watanabe H., Taga G., “Frequency-specific functional connectivity in the brain during resting state revealed by NIRS,” Neuroimage 56(1), 252–257 (2011). 10.1016/j.neuroimage.2010.12.075 [DOI] [PubMed] [Google Scholar]
- 14.Sasai S., Homae F., Watanabe H., Sasaki A. T., Tanabe H. C., Sadato N., Taga G., “A NIRS-fMRI study of resting state network,” Neuroimage 63(1), 179–193 (2012). 10.1016/j.neuroimage.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 15.Medvedev A. V., “Does the resting state connectivity have hemispheric asymmetry? A near-infrared spectroscopy study,” Neuroimage 85(Pt 1), 400–407 (2014). 10.1016/j.neuroimage.2013.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Y., Hocke L. M., Licata S. C., Frederick B., “Low-frequency oscillations measured in the periphery with near-infrared spectroscopy are strongly correlated with blood oxygen level-dependent functional magnetic resonance imaging signals,” J. Biomed. Opt. 17(10), 106004 (2012). 10.1117/1.JBO.17.10.106004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birn R. M., Diamond J. B., Smith M. A., Bandettini P. A., “Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI,” Neuroimage 31(4), 1536–1548 (2006). 10.1016/j.neuroimage.2006.02.048 [DOI] [PubMed] [Google Scholar]
- 18.Lund T. E., Madsen K. H., Sidaros K., Luo W. L., Nichols T. E., “Non-white noise in fMRI: Does modelling have an impact?” Neuroimage 29(1), 54–66 (2006). 10.1016/j.neuroimage.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Bunce S. C., Izzetoglu M., Izzetoglu K., Onaral B., Pourrezaei K., “Functional near-infrared spectroscopy,” IEEE Eng. Med. Biol. Mag. 25(4), 54–62 (2006). 10.1109/MEMB.2006.1657788 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Duan L., Zhang Y. J., Lu C. M., Liu H., Zhu C. Z., “Test-retest assessment of independent component analysis-derived resting-state functional connectivity based on functional near-infrared spectroscopy,” Neuroimage 55(2), 607–615 (2011). 10.1016/j.neuroimage.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Zhang Y. J., Lu C. M., Ma S. Y., Zang Y. F., Zhu C. Z., “Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements,” Neuroimage 51(3), 1150–1161 (2010). 10.1016/j.neuroimage.2010.02.080 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y. J., Lu C. M., Biswal B. B., Zang Y. F., Peng D. L., Zhu C. Z., “Detecting resting-state functional connectivity in the language system using functional near-infrared spectroscopy,” J. Biomed. Opt. 15(4), 047003 (2010). 10.1117/1.3462973 [DOI] [PubMed] [Google Scholar]
- 23.Lu C. M., Zhang Y. J., Biswal B. B., Zang Y. F., Peng D. L., Zhu C. Z., “Use of fNIRS to assess resting state functional connectivity,” J. Neurosci. Methods 186(2), 242–249 (2010). 10.1016/j.jneumeth.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 24.White B. R., Snyder A. Z., Cohen A. L., Petersen S. E., Raichle M. E., Schlaggar B. L., Culver J. P., “Resting-state functional connectivity in the human brain revealed with diffuse optical tomography,” Neuroimage 47(1), 148–156 (2009). 10.1016/j.neuroimage.2009.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaidi Z. F., “Gender differences in human brain: A review,” Open Anat. J. 2, 37–55 (2010). 10.2174/1877609401002010037 [DOI] [Google Scholar]
- 26.Tomasi D., Volkow N. D., “Gender differences in brain functional connectivity density,” Hum. Brain Mapp. 33(4), 849–860 (2012). 10.1002/hbm.21252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong G., He Y., Evans A. C., “Brain connectivity: Gender makes a difference,” Neuroscientist 17(5), 575–591 (2011). 10.1177/1073858410386492 [DOI] [PubMed] [Google Scholar]
- 28.Jaušovec N., Jaušovec K., “Resting brain activity: Differences between genders,” Neuropsychologia 48(13), 3918–3925 (2010). 10.1016/j.neuropsychologia.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 29.Kocsis L., Herman P., Eke A., “The modified Beer-Lambert law revisited,” Phys. Med. Biol. 51(5), N91–N98 (2006). 10.1088/0031-9155/51/5/N02 [DOI] [PubMed] [Google Scholar]
- 30.Koh P. H., Glaser D. E., Flandin G., Kiebel S., Butterworth B., Maki A., Delpy D. T., Elwell C. E., “Functional optical signal analysis: a software tool for near-infrared spectroscopy data processing incorporating statistical parametric mapping,” J. Biomed. Opt. 12(6), 064010 (2007). 10.1117/1.2804092 [DOI] [PubMed] [Google Scholar]
- 31.Chuang C.-C., Chen C.-M., Hsieh Y.-S., Liu T.-C., Sun C.-W., “Brain structure and spatial sensitivity profile assessing by near-infrared spectroscopy modeling based on 3D MRI data,” J. Biophotonics 6(3), 267–274 (2013). 10.1002/jbio.201200025 [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Fox S., Papademetriou M., Darboe M. K., Everdell N. L., Wegmuller R., Prentice A. M., Moore S. E., Elwell C. E., “Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa,” Sci. Rep. 4, 4740 (2014). 10.1038/srep04740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coyle S. M., Ward T. E., Markham C. M., “Brain-computer interface using a simplified functional near-infrared spectroscopy system,” J. Neural Eng. 4(3), 219–226 (2007). 10.1088/1741-2560/4/3/007 [DOI] [PubMed] [Google Scholar]
- 34.Hoshi Y., Tamura M., “Dynamic multichannel near-infrared optical imaging of human brain activity,” J. Appl. Physiol. 75(4), 1842–1846 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Okamoto M., Dan H., Sakamoto K., Takeo K., Shimizu K., Kohno S., Oda I., Isobe S., Suzuki T., Kohyama K., Dan I., “Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping,” Neuroimage 21(1), 99–111 (2004). 10.1016/j.neuroimage.2003.08.026 [DOI] [PubMed] [Google Scholar]
- 36.Bullmore E., Sporns O., “Complex brain networks: graph theoretical analysis of structural and functional systems,” Nat. Rev. Neurosci. 10(3), 186–198 (2009). 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- 37.Fingelkurts A. A., Fingelkurts A. A., Kähkönen S., “Functional connectivity in the brain-is it an elusive concept?” Neurosci. Biobehav. Rev. 28(8), 827–836 (2005). 10.1016/j.neubiorev.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 38.Horwitz B., “The elusive concept of brain connectivity,” Neuroimage 19(2), 466–470 (2003). 10.1016/S1053-8119(03)00112-5 [DOI] [PubMed] [Google Scholar]
- 39.Friston K. J., “Functional and effective connectivity in neuroimaging: A synthesis,” Hum. Brain Mapp. 2(1-2), 56–78 (1994). 10.1002/hbm.460020107 [DOI] [Google Scholar]
- 40.Tomasi D., Volkow N. D., “Laterality patterns of brain functional connectivity: gender effects,” Cereb. Cortex 22(6), 1455–1462 (2012). 10.1093/cercor/bhr230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimoda N., Takeda K., Imai I., Kaneko J., Kato H., “Cerebral laterality differences in handedness: A mental rotation study with NIRS,” Neurosci. Lett. 430(1), 43–47 (2008). 10.1016/j.neulet.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 42.Takeda K., Gomi Y., Imai I., Shimoda N., Hiwatari M., Kato H., “Shift of motor activation areas during recovery from hemiparesis after cerebral infarction: A longitudinal study with near-infrared spectroscopy,” Neurosci. Res. 59(2), 136–144 (2007). 10.1016/j.neures.2007.06.1466 [DOI] [PubMed] [Google Scholar]
- 43.Kennan R. P., Kim D., Maki A., Koizumi H., Constable R. T., “Non-invasive assessment of language lateralization by transcranial near infrared optical topography and functional MRI,” Hum. Brain Mapp. 16(3), 183–189 (2002). 10.1002/hbm.10039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson N. F., Dodrill C., Farrell D., Holmes M. D., Miller J. W., “Determination of language dominance with near-infrared spectroscopy: comparison with the intracarotid amobarbital procedure,” Seizure 13(6), 399–402 (2004). 10.1016/j.seizure.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 45.Koch W., Teipel S., Mueller S., Buerger K., Bokde A. L. W., Hampel H., Coates U., Reiser M., Meindl T., “Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter?” Neuroimage 51(1), 280–287 (2010). 10.1016/j.neuroimage.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 46.Wallentin M., “Putative sex differences in verbal abilities and language cortex: A critical review,” Brain Lang. 108(3), 175–183 (2009). 10.1016/j.bandl.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 47.Weiss E. M., Ragland J. D., Brensinger C. M., Bilker W. B., Deisenhammer E. A., Delazer M., “Sex differences in clustering and switching in verbal fluency tasks,” J. Int. Neuropsychol. Soc. 12(4), 502–509 (2006). 10.1017/S1355617706060656 [DOI] [PubMed] [Google Scholar]
- 48.Andreano J. M., Cahill L., “Sex influences on the neurobiology of learning and memory,” Learn. Mem. 16(4), 248–266 (2009). 10.1101/lm.918309 [DOI] [PubMed] [Google Scholar]
- 49.Niu H., He Y., “Resting-State Functional Brain Connectivity: Lessons from Functional Near-Infrared Spectroscopy,” Neuroscientist 20(2), 173–188 (2014). 10.1177/1073858413502707 [DOI] [PubMed] [Google Scholar]
- 50.Hoshi Y., “Functional near-infrared spectroscopy: current status and future prospects,” J. Biomed. Opt. 12(6), 062106 (2007). 10.1117/1.2804911 [DOI] [PubMed] [Google Scholar]