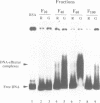
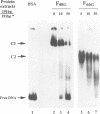
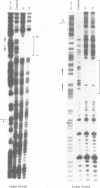
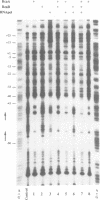
Abstract
The cyanobacterium Calothrix sp. PCC 7601 can adapt its pigment content in response to changes in the incident light wavelength. It synthesizes, as major light-harvesting pigments, either phycocyanin 2 (PC2, encoded by the cpc2 operon) under red light or phycoerythrin (PE, encoded by the cpeBA operon) under green light conditions. The last step of the signal transduction pathway is characterized by a transcriptional control of the expression of these operons. Partially purified protein extracts were used in gel retardation assays and DNase I footprinting experiments to identify the factors that interact with the promoter region of the cpeBA operon. We found that two proteins, RcaA and RcaB, only detected in extracts of cells grown under green light, behave as positive transcriptional factors for the expression of the cpeBA operon. Treatment of the fractions containing RcaA and RcaB with alkaline phosphatase prevents the binding of RcaA but not of RcaB to the cpeBA promoter region. A post-translational modification of RcaA thus modulates its affinity for DNA.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. K., Grossman A. R. Structure and light-regulated expression of phycoerythrin genes in wild-type and phycobilisome assembly mutants of Synechocystis sp. strain PCC 6701. J Bacteriol. 1990 Mar;172(3):1297–1305. doi: 10.1128/jb.172.3.1297-1305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buck M., Cannon W. Specific binding of the transcription factor sigma-54 to promoter DNA. Nature. 1992 Jul 30;358(6385):422–424. doi: 10.1038/358422a0. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Randall D. D. Light as a signal influencing the phosphorylation status of plant proteins. Plant Physiol. 1990 Dec;94(4):1501–1504. doi: 10.1104/pp.94.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano V., Braux A. S., Tandeau de Marsac N., Houmard J. The "anchor polypeptide" of cyanobacterial phycobilisomes. Molecular characterization of the Synechococcus sp. PCC 6301 apce gene. J Biol Chem. 1991 Apr 15;266(11):7239–7247. [PubMed] [Google Scholar]
- Capuano V., Mazel D., Tandeau de Marsac N., Houmard J. Complete nucleotide sequence of the red-light specific set of phycocyanin genes from the cyanobacterium Calothrix PCC 7601. Nucleic Acids Res. 1988 Feb 25;16(4):1626–1626. doi: 10.1093/nar/16.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Damerval T., Guglielmi G., Houmard J., De Marsac N. T. Hormogonium Differentiation in the Cyanobacterium Calothrix: A Photoregulated Developmental Process. Plant Cell. 1991 Feb;3(2):191–201. doi: 10.1105/tpc.3.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakoff S., Scheibe J. Action Spectra for Chromatic Adaptation in Tolypothrix tenuis. Plant Physiol. 1973 Feb;51(2):382–385. doi: 10.1104/pp.51.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs J. M., Bryant D. A. Molecular cloning and transcriptional analysis of the cpeBA operon of the cyanobacterium Pseudanabaena species PCC7409. Mol Microbiol. 1991 Dec;5(12):3073–3085. doi: 10.1111/j.1365-2958.1991.tb01867.x. [DOI] [PubMed] [Google Scholar]
- Elliott T., Geiduschek E. P. Defining a bacteriophage T4 late promoter: absence of a "-35" region. Cell. 1984 Jan;36(1):211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
- Federspiel N. A., Grossman A. R. Characterization of the light-regulated operon encoding the phycoerythrin-associated linker proteins from the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1990 Jul;172(7):4072–4081. doi: 10.1128/jb.172.7.4072-4081.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser M., Sidler W. A., Graham K. W., Bryant D. A., Frank G., Wehrli E., Zuber H. Three C-phycoerythrin-associated linker polypeptides in the phycobilisome of green-light-grown Calothrix sp. PCC 7601 (cyanobacteria). FEBS Lett. 1992 Feb 3;297(1-2):19–23. doi: 10.1016/0014-5793(92)80318-b. [DOI] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989 Nov;3(11):1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Bogorad L. Action Spectra for Phycobiliprotein Synthesis in a Chromatically Adapting Cyanophyte, Fremyella diplosiphon. Plant Physiol. 1977 Dec;60(6):835–839. doi: 10.1104/pp.60.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery D. M., Gannon F., Powell R. A simple method for subcloning DNA fragments from gel slices. Trends Genet. 1990 Jun;6(6):173–173. doi: 10.1016/0168-9525(90)90158-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Kalla S. R., Lind L. K., Lidholm J., Gustafsson P. Transcriptional organization of the phycocyanin subunit gene clusters of the cyanobacterium Anacystis nidulans UTEX 625. J Bacteriol. 1988 Jul;170(7):2961–2970. doi: 10.1128/jb.170.7.2961-2970.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto Y., Sakai S., Tamate H., Gohro T., Inoke T., Tabata S., Tanda H., Kato S., Saka T., Henmi I. [Therapeutic studies on male non-gonorrheal urethritis--use of AT-2266--Sapporo Clinical Research Group for STD]. Hinyokika Kiyo. 1986 Aug;32(8):1203–1212. [PubMed] [Google Scholar]
- Lang J. D., Haselkorn R. Isolation, sequence and transcription of the gene encoding the photosystem II chlorophyll-binding protein, CP-47, in the cyanobacterium Anabaena 7120. Plant Mol Biol. 1989 Oct;13(4):441–457. doi: 10.1007/BF00015556. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Guglielmi G., Houmard J., Sidler W., Bryant D. A., Tandeau de Marsac N. Green light induces transcription of the phycoerythrin operon in the cyanobacterium Calothrix 7601. Nucleic Acids Res. 1986 Nov 11;14(21):8279–8290. doi: 10.1093/nar/14.21.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Houmard J., Castets A. M., Tandeau de Marsac N. Highly repetitive DNA sequences in cyanobacterial genomes. J Bacteriol. 1990 May;172(5):2755–2761. doi: 10.1128/jb.172.5.2755-2761.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. J., Hasekorn R. RNA polymerase subunit homology among cyanobacteria, other eubacteria and archaebacteria. J Bacteriol. 1988 Sep;170(9):4136–4140. doi: 10.1128/jb.170.9.4136-4140.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]