Abstract
OBJECTIVE
Besides its classical role in calcium and bone homeostasis, vitamin D is considered a potent immunomodulator that can affect the pathogenesis of several autoimmune diseases. Our aim is to evaluate the effect of vitamin D correction to a patient with new onset Graves’ disease (GD) with an underlying vitamin D deficiency.
METHOD
We describe the effect of vitamin D3 on untreated Graves’ disease with vitamin D deficiency.
RESULTS
A healthy Saudi woman in her 40s sought consultation with a three-month history of palpitation. She denied any history of heat intolerance, weight loss, menstrual irregularity or sweating. She has a history of chronic muscle aches and pains. Physical examination revealed a mild diffusely enlarged and non-tender thyroid gland with no bruit. She had no signs of Graves’ ophthalmopathy. In laboratory examinations, the initial thyroid function test, which was done in an outside hospital, revealed a TSH, 0.01 mIU/L; FT4, 22.5 pmol/L and FT3, 6.5 pmol/L. Vitamin D 25-OH level was done in our hospital and showed a result of 26.0 nmol/L with a TSH, 0.013 mIU/L; FT4, 16.7 pmol/L; and FT3, 3.8 pmol/L. TSH receptor antibody was positive. TC-99 m thyroid scintigraphy demonstrated an enlarged thyroid gland with increased radiotracer trapping and heterogeneous distribution. The patient was given only oral cholecalciferol 4000 IU per day since November 2012 (prescribed by an outside hospital) then from May 2013 onwards she was given 50,000 IU per month. Follow-up laboratory exams revealed improved vitamin D levels as well as TSH and FT4. She eventually improved both clinically and biochemically with a satisfactory outcome.
CONCLUSION
Vitamin D deficiency may exacerbate the onset and/or development of GD and correction of the deficiency may be able to reverse it. However, further prospective clinical studies will be needed to define the role of vitamin D treatment in GD.
Keywords: Autoimmune diseases, Graves’ disease, vitamin D deficiency, Vitamin D3
Introduction
The role of vitamin D is well known in calcium metabolism and skeletal homeostasis. More recently, vitamin D has been shown to be a modulator in both innate and adaptive immunity.1 There is a well-established link between vitamin D deficiency and various autoimmune diseases, including type 1 diabetes mellitus (T1DM), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and multiple sclerosis (MS). Furthermore, it has been found that the supplementation of vitamin D can prevent the onset and/or development of different kinds of autoimmune disorders in human beings and animal models.2 In addition, it has been shown that the prevalence of vitamin D deficiency is common in patients with Graves’ disease (GD),3 and is associated with higher thyroid volume.4 In our case report, we evaluated the effect of vitamin D correction to a patient with new onset GD with an underlying vitamin D deficiency.
Case Presentation
A healthy Saudi woman in her 40s sought consultation with a 3 months history of palpitation. She denied any history of heat intolerance, weight loss, menstrual irregularity, diarrhea, or sweating. She has a history of chronic muscle aches and pains. There was no personal or family history of thyroid disease and no specific medication history. Physical examination revealed a mild diffusely enlarged and non-tender thyroid gland with no bruit. There were no palpable cervical lymph nodes. She had no signs of Graves’ ophthalmopathy or pretibial myxedema. The rest of the examination was unremarkable.
In laboratory examinations, the initial thyroid function test, which was done in an outside hospital, revealed a TSH, 0.01 mIU/L; FT4, 22.5 pmol/L; and FT3, 6.5 pmol/L. Vitamin D 25-OH level was done in our hospital and showed a result of 26.0 nmol/L with a TSH, 0.013 mIU/L; FT4, 16.7 pmol/L; and FT3, 3.8 pmol/L. Anti-thyroid antibodies showed a Tg, 17.1 IU/mL; TPO, 0.19 IU/mL with a positive TSH receptor antibody. TC-99 m thyroid scintigraphy demonstrated an enlarged thyroid gland with increased radiotracer trapping and heterogeneous distribution (Fig. 1).
Figure 1.
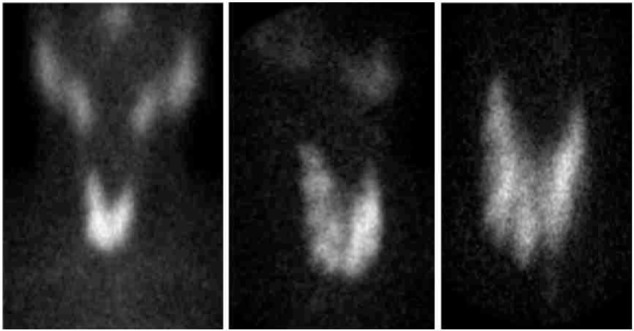
TC-99m thyroid scintigraphy showing enlarged thyroid gland with increased radiotracer trapping with heterogeneous distribution.
The patient was given only oral cholecalciferol 4000 IU per day since November 2012 (took it from an outside hospital) then from May 2013 onwards she was given 50,000 IU per month. The serial thyroid function tests, vitamin D levels, and titer autoantibodies are summarized in Table 1.
Table 1.
Metabolic profile series.
| JULY 2012 | NOV 2012 | OCT 2013 | NORMAL | |
|---|---|---|---|---|
| TSH (mIU/L) | 0.01 | 0.013 | 3.60 | 0.27–4.2 |
| FT4 (pmol/L) | 22.5 | 16.7 | 15.7 | 12–22 |
| FT3 (pmol/L) | 6.5 | 3.8 | ND | 3.1–6.8 |
| 25(OH)Vitamin D total (nmol/L) | ND | 26.0 | 69.1 | 50–250 |
| Anti-Tg Ab (IU/mL) | ND | 17.3 | ND | 0–4.11 |
| Anti-TPO (IU/mL) | ND | 0.19 | ND | 0–5.61 |
| TSH receptor Ab | ND | positive | ND | |
| Ca (mmol/L) | ND | ND | 2.35 | 2.09–2.54 |
Follow-up laboratory exams revealed improved vitamin D levels as well as TSH and FT4. She eventually improved both clinically and biochemically with a satisfactory outcome. She is going for another TC-99m thyroid scintigraphy for follow up. Written informed consent was obtained from the patient for the publication of this case and accompanying images.
Discussion
GD is an autoimmune disease characterized by hyperthyroidism secondary to circulating autoantibodies. It has become apparent that multiple factors contribute to the etiology of GD, including genetic and environmental factors. The pathophysiology of GD involves the infiltration of T cells in the thyroid gland. These activated T cells in turn increase the secretion of thyroid-specific autoantibodies from B cells.
The prevalence of vitamin D deficiency was reported to be common in patients with GD.3 Whether vitamin D deficiency has a causal relationship with GD remains a controversial issue. The role of vitamin D in GD has been investigated in several studies. Misharin et al.5 observed that vitamin D deficiency was found to modulate Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. In this study, BALB/c mice on a vitamin D deficient diet were more likely to develop persistent hyperthyroidism than other mice receiving adequate vitamin D supply. In another study, combination treatment with methimazole and vitamin D3 (1,25 (OH)2D) in patients with GD has more rapid euthyroidism achievement compared with patients receiving methimazole alone.6 In addition, vitamin D supplementation has been shown to inhibit inflammatory responses in human thyroid and T cells.7 Interestingly, vitamin D deficiency is found to be associated with higher thyroid volume in patients with newly onset GD.4 It has been recently discovered that vitamin D-receptor gene and vitamin D-binding protein gene polymorphisms are associated with GD.8,9
Conclusion
Our present case supports the current literature and strongly suggests that vitamin D deficiency may exacerbate the onset and/or development of GD and correction of which may be able to reverse it. However, further prospective clinical studies will be needed to define the role of vitamin D treatment in GD.
Footnotes
Author Contributions
Conceived and designed the experiments: ONH, NA. Analyzed the data: ONH, NA. Wrote the first draft of the manuscript: ONH, NA. Contributed to the writing of the manuscript: ONH, NA. Agree with manuscript results and conclusions: ONH, NA. Jointly developed the structure and arguments for the paper: ONH, NA. Made critical revisions and approved final version: ONH, NA. Both authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita H, Noguchi S, Takatsu K, et al. High prevalence of vitamin D deficiency in Japanese female patients with Graves’ disease. Endocr J. 2001;58:63–9. doi: 10.1507/endocrj.48.63. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda T, Okamoto Y, Hamada N, et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine. 2012;42(3):739–41. doi: 10.1007/s12020-012-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misharin A, Hewison M, Chen CR, et al. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009;150(2):1051–60. doi: 10.1210/en.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami-Tani T, Fukawa E, Tanaka H, Abe Y, Makino I. Effect of 1 alphahydroxyvitamin D3 on serum levels of thyroid hormones in hyperthyroid patients with untreated Graves’ disease. Metabolism. 1997;46:1184–88. doi: 10.1016/s0026-0495(97)90214-6. [DOI] [PubMed] [Google Scholar]
- 7.Borgogni E, Sarchielli E, Sottili M, et al. Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology. 2008;149(7):3626–34. doi: 10.1210/en.2008-0078. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Xu C, Gu M. Vitamin D receptor (VDR) gene polymorphisms and Graves’ disease: a meta-analysis. Clin Endocrinol (Oxf) 2009;70(6):938–45. doi: 10.1111/j.1365-2265.2008.03413.x. [DOI] [PubMed] [Google Scholar]
- 9.Kurylowicz A, Ramos-Lopez E, Bednarczuk T, Badenhoop K. Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves’ disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes. 2006;114(6):329–35. doi: 10.1055/s-2006-924256. [DOI] [PubMed] [Google Scholar]


