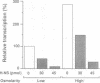
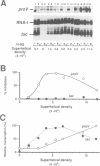
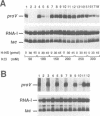
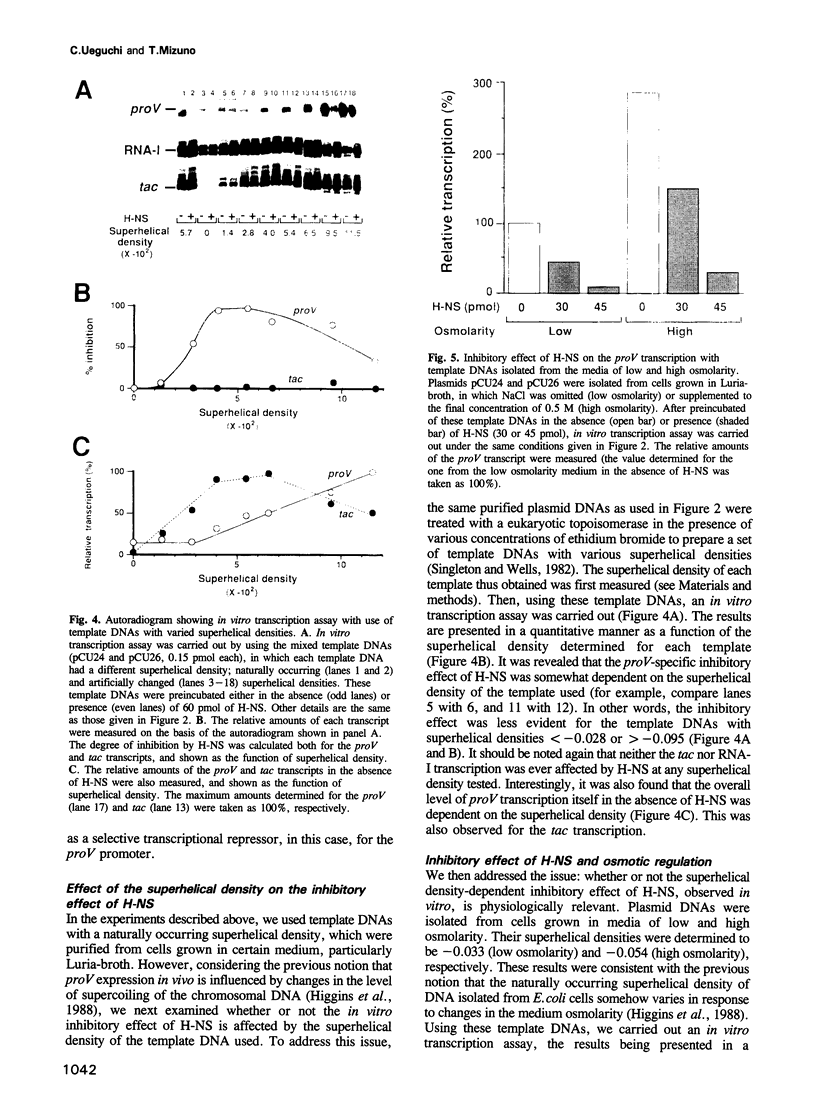
Abstract
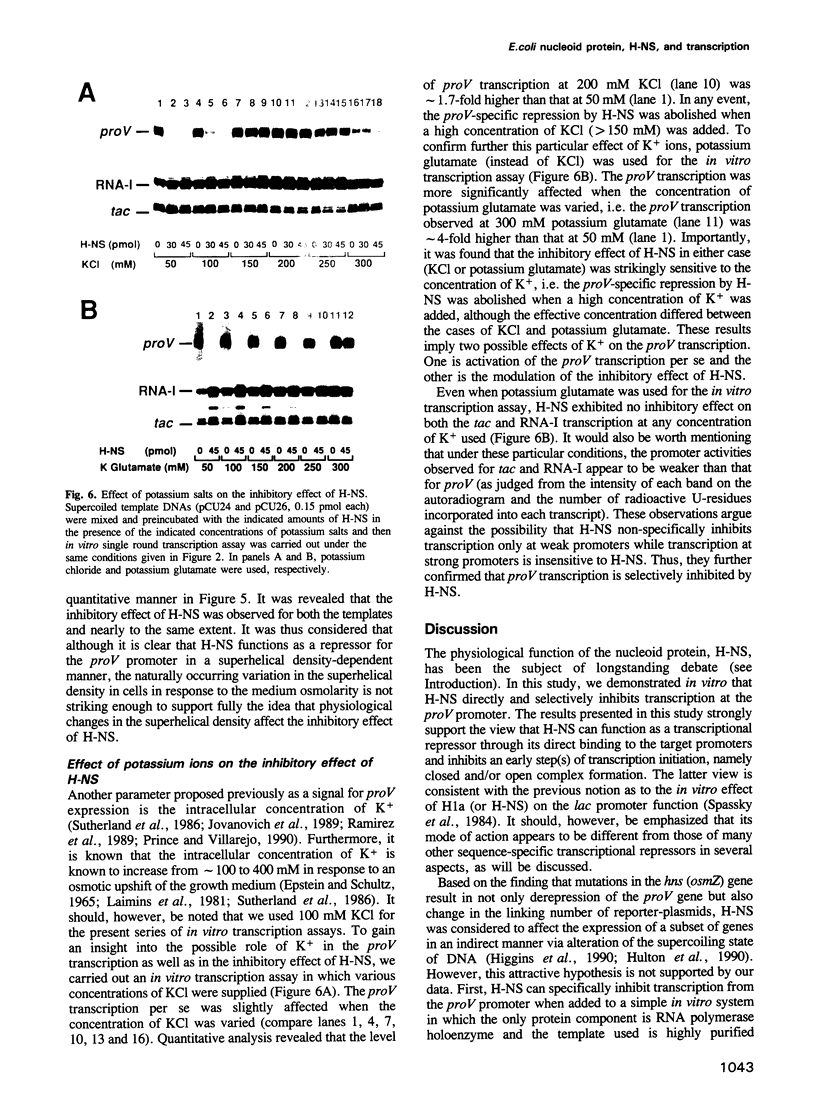
The H-NS protein is a major constituent of the Escherichia coli nucleoid structure and is implicated in the compact organization of the chromosome. Based on recent genetic evidence, this protein appears to influence the transcription of a variety of apparently unlinked genes on the chromosome, although the underlying molecular mechanism is not fully understood. In this study, we carried out a series of in vitro transcription assays including purified H-NS with special reference to the osmotically inducible proV promoter of the proVWX operon (or proU), whose expression is known to be derepressed by lesions of the hns (osmZ) gene. Here, H-NS was revealed to selectively inhibit an early step(s) of proV transcription initiation through its direct binding to the promoter region. It was thus demonstrated that H-NS functions directly as a transcriptional repressor. Under the in vitro conditions used, this in vitro inhibitory effect of H-NS was affected by changes in the superhelical density of template DNAs and more significantly by the concentration of potassium (K+) ions. These results are also discussed with regard to the mechanism underlying regulation of the proV promoter in response to the medium osmolarity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A., May G., Bremer E., Villarejo M. Regulation of envelope protein composition during adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1986 Aug;167(2):433–438. doi: 10.1128/jb.167.2.433-438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin P., Lejeune P., Laurent-Winter C., Danchin A. Mutations in bglY, the structural gene for the DNA-binding protein H1, affect expression of several Escherichia coli genes. Biochimie. 1990 Dec;72(12):889–891. doi: 10.1016/0300-9084(90)90008-5. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J., Booth I. R., Higgins C. F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985 Dec;164(3):1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattananda C. S., Rajkumari K., Gowrishankar J. Multiple mechanisms contribute to osmotic inducibility of proU operon expression in Escherichia coli: demonstration of two osmoresponsive promoters and of a negative regulatory element within the first structural gene. J Bacteriol. 1991 Dec;173(23):7481–7490. doi: 10.1128/jb.173.23.7481-7490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap V. J., Csonka L. N. Osmotic regulation of L-proline transport in Salmonella typhimurium. J Bacteriol. 1985 Jul;163(1):296–304. doi: 10.1128/jb.163.1.296-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger M., La Teana A., Citro G., Venanzi F., Gualerzi C. O., Pon C. L. Escherichia coli DNA-binding protein H-NS is localized in the nucleoid. Res Microbiol. 1991 May;142(4):373–380. doi: 10.1016/0923-2508(91)90106-k. [DOI] [PubMed] [Google Scholar]
- Gowrishankar J. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol. 1985 Oct;164(1):434–445. doi: 10.1128/jb.164.1.434-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J Bacteriol. 1989 Apr;171(4):1923–1931. doi: 10.1128/jb.171.4.1923-1931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hinton J. C., Hulton C. S., Owen-Hughes T., Pavitt G. D., Seirafi A. Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence? Mol Microbiol. 1990 Dec;4(12):2007–2012. doi: 10.1111/j.1365-2958.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Jovanovich S. B., Record M. T., Jr, Burgess R. R. In an Escherichia coli coupled transcription-translation system, expression of the osmoregulated gene proU is stimulated at elevated potassium concentrations and by an extract from cells grown at high osmolality. J Biol Chem. 1989 May 15;264(14):7821–7825. [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Rhoads D. B., Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jan;78(1):464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht J. M., Bremer E. Characterization of mutations affecting the osmoregulated proU promoter of Escherichia coli and identification of 5' sequences required for high-level expression. J Bacteriol. 1991 Jan;173(2):801–809. doi: 10.1128/jb.173.2.801-809.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G., Dersch P., Haardt M., Middendorf A., Bremer E. The osmZ (bglY) gene encodes the DNA-binding protein H-NS (H1a), a component of the Escherichia coli K12 nucleoid. Mol Gen Genet. 1990 Oct;224(1):81–90. doi: 10.1007/BF00259454. [DOI] [PubMed] [Google Scholar]
- May G., Faatz E., Lucht J. M., Haardt M., Bolliger M., Bremer E. Characterization of the osmoregulated Escherichia coli proU promoter and identification of ProV as a membrane-associated protein. Mol Microbiol. 1989 Nov;3(11):1521–1531. doi: 10.1111/j.1365-2958.1989.tb00138.x. [DOI] [PubMed] [Google Scholar]
- May G., Faatz E., Villarejo M., Bremer E. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol Gen Genet. 1986 Nov;205(2):225–233. doi: 10.1007/BF00430432. [DOI] [PubMed] [Google Scholar]
- Overdier D. G., Csonka L. N. A transcriptional silencer downstream of the promoter in the osmotically controlled proU operon of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3140–3144. doi: 10.1073/pnas.89.7.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overdier D. G., Olson E. R., Erickson B. D., Ederer M. M., Csonka L. N. Nucleotide sequence of the transcriptional control region of the osmotically regulated proU operon of Salmonella typhimurium and identification of the 5' endpoint of the proU mRNA. J Bacteriol. 1989 Sep;171(9):4694–4706. doi: 10.1128/jb.171.9.4694-4706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Hughes T. A., Pavitt G. D., Santos D. S., Sidebotham J. M., Hulton C. S., Hinton J. C., Higgins C. F. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992 Oct 16;71(2):255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- Park S. F., Stirling D. A., Hulton C. S., Booth I. R., Higgins C. F., Stewart G. S. A novel, non-invasive promoter probe vector: cloning of the osmoregulated proU promoter of Escherichia coli K12. Mol Microbiol. 1989 Aug;3(8):1011–1023. doi: 10.1111/j.1365-2958.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Histone-like proteins and bacterial chromosome structure. J Biol Chem. 1988 Sep 15;263(26):12793–12796. [PubMed] [Google Scholar]
- Plaskon R. R., Wartell R. M. Sequence distributions associated with DNA curvature are found upstream of strong E. coli promoters. Nucleic Acids Res. 1987 Jan 26;15(2):785–796. doi: 10.1093/nar/15.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pon C. L., Calogero R. A., Gualerzi C. O. Identification, cloning, nucleotide sequence and chromosomal map location of hns, the structural gene for Escherichia coli DNA-binding protein H-NS. Mol Gen Genet. 1988 May;212(2):199–202. doi: 10.1007/BF00334684. [DOI] [PubMed] [Google Scholar]
- Prince W. S., Villarejo M. R. Osmotic control of proU transcription is mediated through direct action of potassium glutamate on the transcription complex. J Biol Chem. 1990 Oct 15;265(29):17673–17679. [PubMed] [Google Scholar]
- Ramirez R. M., Prince W. S., Bremer E., Villarejo M. In vitro reconstitution of osmoregulated expression of proU of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1153–1157. doi: 10.1073/pnas.86.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R. M., Villarejo M. Osmotic signal transduction to proU is independent of DNA supercoiling in Escherichia coli. J Bacteriol. 1991 Jan;173(2):879–885. doi: 10.1128/jb.173.2.879-885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimsky S., Spassky A. Sequence determinants for H1 binding on Escherichia coli lac and gal promoters. Biochemistry. 1990 Apr 17;29(15):3765–3771. doi: 10.1021/bi00467a024. [DOI] [PubMed] [Google Scholar]
- Schmid M. B. More than just "histone-like" proteins. Cell. 1990 Nov 2;63(3):451–453. doi: 10.1016/0092-8674(90)90438-k. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. The facile generation of covalently closed, circular DNAs with defined negative superhelical densities. Anal Biochem. 1982 May 15;122(2):253–257. doi: 10.1016/0003-2697(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Spassky A., Rimsky S., Garreau H., Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984 Jul 11;12(13):5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. A., Hulton C. S., Waddell L., Park S. F., Stewart G. S., Booth I. R., Higgins C. F. Molecular characterization of the proU loci of Salmonella typhimurium and Escherichia coli encoding osmoregulated glycine betaine transport systems. Mol Microbiol. 1989 Aug;3(8):1025–1038. doi: 10.1111/j.1365-2958.1989.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Sutherland L., Cairney J., Elmore M. J., Booth I. R., Higgins C. F. Osmotic regulation of transcription: induction of the proU betaine transport gene is dependent on accumulation of intracellular potassium. J Bacteriol. 1986 Nov;168(2):805–814. doi: 10.1128/jb.168.2.805-814.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Muramatsu S., Yamada H., Mizuno T. Systematic characterization of curved DNA segments randomly cloned from Escherichia coli and their functional significance. Mol Gen Genet. 1991 May;226(3):367–376. doi: 10.1007/BF00260648. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima R., Fujita N., Ishihama A. DNA supercoiling and temperature shift affect the promoter activity of the Escherichia coli rpoH gene encoding the heat-shock sigma subunit of RNA polymerase. Mol Gen Genet. 1989 Jan;215(2):185–189. doi: 10.1007/BF00339716. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Bakayev V. V., Bakayeva T. G., Georgiev G. P. Histone-like proteins in the purified Escherichia coli deoxyribonucleoprotein. Nucleic Acids Res. 1977 Aug;4(8):2725–2745. doi: 10.1093/nar/4.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yamada H., Muramatsu S., Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem. 1990 Sep;108(3):420–425. doi: 10.1093/oxfordjournals.jbchem.a123216. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yoshida T., Tanaka K., Sasakawa C., Mizuno T. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet. 1991 Nov;230(1-2):332–336. doi: 10.1007/BF00290685. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]