Abstract
FtsB and FtsL are two essential integral membrane proteins of the bacterial division complex or “divisome”, both characterized by a single transmembrane helix and a juxta-membrane coiled coil domain. The two domains are important for the association of FtsB and FtsL, a key event for their recruitment to the divisome, that in turn enables recruitment of the late divisomal components to the Z-ring and subsequent completion of the division process. Here we present a biophysical analysis performed in vitro that shows that the transmembrane domains of FtsB and FtsL associate strongly in isolation. Using FRET, we have measured the oligomerization of fluorophore-labeled transmembrane domains of FtsB and FtsL in both detergent and lipid. The data indicates that the transmembrane helices are likely a major contributor to the stability of the FtsB-FtsL complex. Our analyses show that FtsB and FtsL form a 1:1 higher-order oligomeric complex, possibly a tetramer. This finding suggests that the FtsB-FtsL complex is capable of multi-valent binding to FtsQ and other divisome components, a hypothesis that is consistent with the possibility that the FtsB-FtsL complex has a structural role in the stabilization of the Z-ring.
Introduction
In Escherichia coli, the multiprotein cell-division mediating complex, or “divisome”, comprises at least ten essential proteins, most of which are integral membrane proteins. The proteins are recruited at mid-cell over a scaffold formed by the polymeric FtsZ (the Z-ring)1–3, where they assemble according to a defined stepwise pathway4. Two such essential components, FtsB and FtsL, occupy a place mid-way into this hierarchy5,6; they are preceded by the early components (FtsZ, FtsA, ZipA, FtsK and FtsQ); in turn, they are required for the recruitment of the late divisome components (FtsW, FtsI, FtsN), which are important for the reconstruction of the cell wall.
The specific role of FtsB and FtsL is still not well understood. It has been suggested that they have a structural role in the assembly of the divisome6,7 and that they are important for the stabilization of the Z-ring8. It is clear, however, that FtsB and FtsL form a stable sub-complex in vivo prior to their association with the divisome9–12 and that their mutual interaction is central to their role in bacterial division.
The recruitment of FtsB and FtsL depends on their interaction with FtsQ. However, even when FtsQ is depleted these two proteins still associate with each other, and as long as they are artificially targeted to the division septum, they are still able to recruit the downstream proteins6. Evidence for the formation of a stable FtsB-FtsL complex is also provided by the fact that their B. subtilis homologs form a complex when co-expressed in E. coli, even though they are unlikely to interact with the significantly divergent division proteins of the host13. Moreover, FtsB and FtsL are quickly depleted from the cell when the proteins are not co-expressed, suggesting that they are functionally dependent on each other9,10,14–16.
Determining the structural organization of the FtsB-FtsL complex, its stoichiometry, and its stability, is critical to understanding its role in division. Topologically, FtsB and FtsL are similar to each other, being small single-pass integral membrane proteins consisting of an N-terminal transmembrane (TM) helix, a juxta-membrane periplasmic coiled coil and a short C-terminal domain (Figure 1a). Previous studies indicate that both the transmembrane and the coiled coil domains are involved in their mutual association6,7,17. To investigate this we recently studied the structural organization of the individual domains of FtsB in isolation18. Using an in vivo interaction assay, we showed that the TM domain of FtsB self-associates in E. coli membranes. We performed extensive mutagenesis and computed a structural model of FtsB-TM domain that is in excellent agreement with the experimental data, showing a left-handed homo-dimer mediated by an inter-helical hydrogen bond (Figure 1b). We also solved the crystal structure of the coiled coil domain of FtsB in homo-dimeric form, solubilized as a fusion construct with the viral coiled coil protein Gp7. From this evidence, we hypothesized that the FtsB-TM homodimer forms a core for the lateral association of FtsL-TM domain (Figure 1c).
Figure 1. Starting hypothesis: FtsB and FtsL form a higher-order oligomer.
a) FtsB and FtsL are two integral membrane proteins of the bacterial division complex. Their topology consists of a single transmembrane domain (TM) and a juxta-membrane coiled coil domain (CC). b) In previous work we determined that the transmembrane domain of FtsB homo-oligomerizes, and obtained an experimentally-supported computational model of a homo-dimer mediated by a critical hydrogen bond. c) We hypothesized that the TM dimer of FtsB (yellow) forms a core mediated by the critical Gln16 (red circles) for the lateral association of FtsL (blue). Here, we further provide support for this hypothesis by demonstrating that the TM domains of FtsB and FtsL associate stably in isolation forming a 1:1 higher-order oligomer.
Here we present a Förster Resonance Energy Transfer (FRET) based study performed in vitro that shows for the first time that FtsB and FtsL TM domains interact and provides new insight into the structural organization of the FtsB-FtsL complex. The data shows that the TM region of the FtsB-FtsL complex is stably folded and that the TM helices are likely a major determinant for the association of the complex. We also show that FtsL and FtsB forms a 1:1 higher-order oligomeric complex, which is consistent with our previous hypothesis that the FtsB-FtsL complex is likely a tetramer18 (Figure 1c). From these results, we hypothesize that the FtsB-FtsL complex may form a multi-valent binding hub that is important for the stabilization of the Z-ring.
Materials and Methods
Peptide sequences
The predicted TM regions of E. coli FtsB and FtsL (underlined sequence) were synthesized flanked by Gly amino acids on the N terminus to provide a linker between the peptide and the N-terminal fluorophore. Lys residues were added on the C terminus to improve the solubility of the peptide in aqueous media to facilitate purification of the hydrophobic TM peptides19–21. A Trp residue (in bold) was introduced in place of a native His residue in the C-terminus of FtsL-TM domain to facilitate absorbance monitoring at 280nm.
FtsB-TM: GGKLTLLLLAILVWLQYSLWFGKKKK
FtsL-TM: GGGKLPLCLFICIILTAVTVVTT A WHKK
Fmoc Solid Phase Peptide synthesis
Peptides were synthesized at 25 μmole scale on a Protein Technologies Symphony peptide synthesizer, over a low substitution (0.16mmol/g) Fmoc amide resin (Applied Biosystems) using DMF (dimethylformamide) as the solvent, 20% Piperidine with 2% DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) for deprotection and HATU (O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate) for activation (0.40 M in DMF). Unlabeled peptides were N-terminally acetylated using standard procedures. Side chain deprotection and final cleavage from the resin was achieved using a mixture of 95:2.5:2.5 v/v of TFA (trifluoroacetic acid)/EDT(1,2-ethanedithiol)/water at room temperature for 4 hours. The cleaved peptide was precipitated using 1:10 v/v of cleavage mix:cold MTBE (methyl tertiary butyl ether) and dried in a vacuum dessicator.
On-resin N-terminal labeling of peptides
Labeling of the peptides was carried out on the peptide-resin prior to cleavage using variations of a protocol for labeling hydrophobic peptides22. Briefly, excess of a linker, N-ε-Fmoc-aminohexanoic acid (Fmoc-ε-Ahx-OH, AnaSpec) was coupled to the peptide-resin using standard coupling procedures at room temperature overnight. Following thorough washing and deprotection of the Ahx labeled peptide-resin, FITC (Fluorescein isothiocyanate) ‘isomer 1’ (Sigma Aldrich) was coupled with repeated washing and recoupling for ~4 days to achieve efficient labeling. For FtsL, successful coupling was obtained for the 5-FAM (5-carboxyfluorescein) version of the fluorophore in the same manner. Coupling of 7-hydroxycoumarin-3-carboxylic acid (Anaspec) was performed in a similar fashion without prior coupling of the Ahx linker. A detailed labeling procedure is provided in the Supplementary Information.
Purification of peptides
The peptides were purified on a reversed phase HPLC system (Varian ProStar 335) with a Zorbax SB-CN semi-preparative column, 5μm, 9.4 × 250mm (Agilent) for FtsB and a VP 250/10 NUCLEOSIL 100-7 C2 semi-preparative column (Macherey-Nagel) for FtsL, using a linear gradient of water and acetonitrile in the presence of 0.1% TFA. Collected fractions were lyophilized and the masses of the peptides were confirmed by a Bruker REFLEX II MALDI-TOF system using CHCA as the matrix.
Quantification of peptides
Quantification and preparation of peptide stocks in TFE (trifluoroethanol) was carried out by absorbance measurements using a Cary 50 scan UV/Vis spectrophotometer. Accurate quantification and calculation of labeling efficiencies was performed using a detailed procedure23 described in Supplementary Information. The fluorophores were characterized in TFE as described in Figure S1. The calculated labeling efficiencies of the purified FITC-FtsB, coumarin-FtsB, 5-FAM-FtsL and coumarin-FtsL peptides used were >95%. In spite of numerous efforts, the maximum labeling efficiency obtained for FITC-FtsL was <15%. For this reason the 5-FAM version of the fluorophore was used for the FtsL homo-FRET studies.
FRET assay
Peptides were mixed in the desired molar ratios and added to PTFE lined screw cap glass vials containing the calculated amounts of DPC (Dodecylphosphocholine) and POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) (Avanti Polar Lipids) in chloroform. The mixture was vigorously vortexed, and solvents were dried using a stream of nitrogen gas and dessicated overnight in a vacuum desiccator to remove residual organic solvents. Samples were hydrated in 10 mM HEPES, pH 7.5. Lipid samples were vortexed vigorously and equilibrated using three freeze-thaw cycles. Detergent samples were equilibrated by incubating them at room temperature for 4 hours.
For the donor+acceptor (FRET) samples, total peptide amount used was 0.5 nmoles containing 1:1 donor to acceptor molar ratios, and amount of detergent or lipid was varied to span a range of peptide:lipid/detergent molar ratios from 1:10000 to 1:300. Donor only (for ‘no FRET’ control) and acceptor only (for bleed-through correction) samples contained 0.25 nmoles of donor peptides and acceptor peptides, respectively, in the same range of peptide:detergent/lipid molar ratios. A sample with unlabeled FtsB:lipid ratio of 1:10000 was used as a scattering control for the lipid experiments. Triton-x 100 (0.5%) was added to coumarin-FtsB-lipid samples and fluorescence intensity measurements were taken before and after the detergent addition to account for effect of scattering on fluorescence intensity values. Fluorescence readings were taken in a HITACHI F-4500 Fluorescence Spectrophotometer. Samples were excited at 415 nm and emission scans were recorded from 425 to 650 nm at 25°C. Supplementary Figure S2 shows a characteristic FRET curve between donor and acceptor labeled FtsB peptides. FRET efficiency (E%) values were calculated using donor-quenching of coumarin emission λmax at 450nm, using the formula E = [(ID−IDA)/ID], where ID is the fluorescence intensity of the donor only sample, and IDA is the fluorescence intensity of the donor in the presence of the acceptor24,25. Alternatively, 0.5% Triton X-100 was added to the FRET samples and incubated for 10 minutes to disrupt the liposomes and their peptide complexes, and yield a ‘no FRET’ control. In these cases, FRET efficiency was measured as E = [(IT−IDA)/IT], where IT is the donor emission at 450nm after Triton addition and IDA is the donor emission before Triton addition26. For the competition experiment (Figure 4), equimolar amounts of unlabeled FtsL were added to 1:1 donor:acceptor ratios of FtsB in a range of [total FtsB peptide]:[lipid/detergent] values of 1:10000 to 1:300. For the stoichiometric experiment (Figure 5), increasing amounts of FtsL were added to 1:1 donor:acceptor ratios of FtsB in a fixed [total FtsB peptide]:[lipid] ratio of 1:1000. Experiments were performed at least in triplica.
Figure 4. The TM domains of FtsB and FtsL form a higher-order oligomeric complex.
a) Schematic view of a competition experiment in which FRET for a coumarin-FtsB/FITC-FtsB donor/acceptor pair (white helices, D: donor, A: acceptor) is measured in the presence of an equimolar amount of unlabeled FtsL (black helices). The concentration-dependent FRET in DPC detergent (b) and in POPC multi-lamellar vesicles (c) is reported as a function of FtsB peptide concentration (not as FtsB+FtsL total peptide) as black squares. The FtsB self-association data (Figure 2) is repeated here as a reference (gray, dashed lines). The data is consistent with an FtsL-dependent stabilization of an FtsB homo-oligomer (right-most equilibrium in panel a) rather than a competing disruption (left-most equilibrium), suggesting the formation of a higher-order FtsB-FtsL oligomer.
Figure 5. FtsB and FtsL form a 1:1 complex.
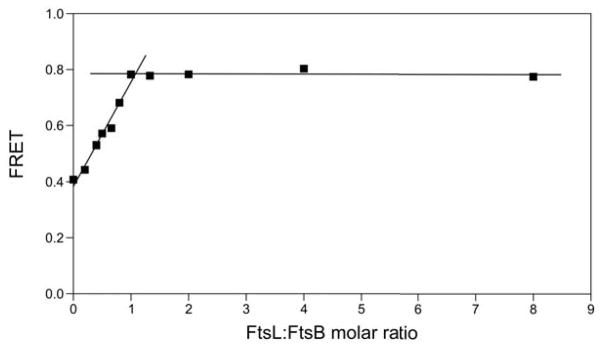
Titration experiment in which unlabeled FtsL is added to donor and acceptor labeled FtsB, in a 1:1000 FtsB peptide to lipid ratio. The data shows a steep increase of FtsB homo-FRET until the stoichiometric ratio with FtsL reaches 1:1, followed by a sharp plateauing of the signal. The data is consistent with formation of a tetrameric (2:2) or hexameric (3:3) or higher complex. The lines represent two linear regressions to the set of molar ratio points from 0 to 1, and to those with ratios from 1 to 8..
Results
The TM domain of FtsB homo-oligomerizes in vitro
Using a biological assay (TOXCAT), we previously established that FtsB-TM self-associates in E. coli membranes, while FtsL-TM appeared to be largely monomeric18. To confirm these observations in vitro, we measured FRET between coumarin/FITC or coumarin/5-FAM labeled peptides as a function of peptide concentration (Figure 2). Fluorescence measurements were taken by maintaining the concentration of the peptides constant while increasing the amount of detergent or lipid to vary the available “hydrophobic volume”, and for this reason peptide concentrations are expressed as peptide:detergent or peptide:lipid molar ratios27. FRET efficiency values were calculated using donor quenching at coumarin emission maxima (450 nm).
Figure 2. FtsB self-associates in vitro in lipid.
Concentration-dependent FRET between a coumarin/FITC donor/acceptor pair, measured in (a) DPC micelles and (b) POPC multi-lamellar vesicles. The peptide concentration is expressed as a function of “hydrophobic volume” as peptide:detergent or peptide:lipid molar ratio. a) FtsB (filled squares) and FtsL (open squares) self-associate very weakly in detergent. b) The self-association of FtsL is not enhanced in lipid, while significant association is observed for FtsB. The finding confirm the self-association of FtsB previously reported using TOXCAT18. The lines correspond to fits to monomer-dimer equilibria.
A concentration dependent increase in FRET is observed for FtsB-TM in lipid (Figure 2b), confirming that it self-associates. Fit to a monomer-dimer equilibrium, the FRET data in lipid yield an estimated dissociation constant of 9.4×10−4, corresponding to a free energy of association of approximately −4 kcal/mol. A small increase in FRET is also observable in detergent (Figure 2a), but the apparent association energy appears to be very low. Consistent with our previous TOXCAT analysis18, FtsL-TM domain appears to oligomerize very weakly in both detergent and lipid environments.
The TM domains of FtsB and FtsL associate
Having confirmed in vitro that the FtsB-TM homo-oligomerizes, our main interest was to verify whether the TM domains of FtsB and FtsL interact, as hypothesized. Figure 3 shows the FRET data for coumarin-FtsL (donor) and FITC-FtsB (acceptor) peptides, mixed in a 1:1 ratio, and equilibrated with decreasing amounts of DPC detergent (panel a) or POPC lipid (panel b). Increase in FRET efficiency values with increasing peptide concentration demonstrates that FtsB and FtsL TM peptides indeed associate both in detergent and in lipid. In both environments, the FtsB-FtsL heterologous FRET curves grow more rapidly than the FtsB-FtsB homo-FRET, which is displayed in Figure 3 in gray for direct visual comparison. The curves in Figure 3 are fit to a monomer-tetramer equilibrium and provide estimated Kd values of 2.3×10−9 in DPC and 1.3×10−11 in POPC.
Figure 3. The TM domains of FtsB and FtsL form a stable oligomeric complex.
Concentration-dependent FRET (black squares) between a coumarin-FtsL/FITC-FtsB pair, measured in a) DPC micelles and b) POPC multi-lamellar vesicles. The curves represent fits to monomer-tetramer equilibria. The FtsB self-association data (Figure 2) is reported as a reference (gray, dashed lines). The data indicate that FtsB and FtsL TM domains interact in both detergent and lipid environments.
FtsB and FtsL form a higher-order oligomer
After establishing that the TM domains of FtsB and FtsL associate, the next question was whether FtsL competes with and disrupts the FtsB homo-dimer to form an FtsB-FtsL hetero-dimer, or whether FtsL associates with the FtsB homo-dimer to form a tetramer or, potentially, another higher-order complex (scheme in Figure 4a). To investigate this question, we performed a competition experiment in which an equimolar amount of unlabeled FtsL-TM peptide was added to samples containing labeled FtsB-TM donor and acceptor pairs. The data shows that addition of FtsL led to a significant increase in the FtsB-TM FRET both in detergent micelles (Figure 4b) as well as in lipid vesicles (Figure 4c). The apparent dissociation constant of the FtsB homo-oligomer in the presence of an equimolar amount of FtsL decreases by almost two orders of magnitude from 9.4×10−4 to 1.3×10−5, corresponding to an apparent stabilization of an FtsB dimer of 2.5 kcal/mol. Control experiments were conducted by the addition of an equimolar amount of either an unrelated polyleucine based monomeric model TM peptide (pL-3F-dC28), or an equimolar amount of unlabeled FtsB (supplementary Figure S3). The unrelated peptide did not alter the FtsB homo-FRET values. Conversely, addition of unlabeled FtsB decreased FRET, as expected.
FtsB and FtsL form a 1:1 oligomer
To further investigate the stoichiometry of the FtsB-FtsL complex, we performed a titration experiment in which unlabeled FtsL was added in increasing amounts to donor and acceptor labeled FtsB in a fixed [total FtsB peptide]:[lipid] ratio, and FtsB homo-FRET was measured. Figure 5 shows that addition of unlabeled FtsL leads to a linear increase in the FRET efficiency of FtsB homo-oligomer until the FtsL:FtsB molar ratio reaches a value of approximately 1, after which the signal flattens. The data clearly indicate that the oligomer has an equal number of FtsB and FtsL molecules, which is consistent with the hypothesized tetramer (2:2). A tetrameric FtsB-FtsL is also consistent with one of the hypothesized by Villanello et al. on the basis of a bioinformatic analysis of the soluble domains29. The steep linear rise in the FRET efficiency until equal stoichiometry Is reached confirms that the complex is stable at the peptide:lipid ratio in which the experiment was performed (1:1000). The FRET signal compare well with Figure 4c where the FtsB-FRET increases from ~0.4 to ~0.8 FRET efficiency upon addition of equimolar amount of unlabeled FtsL.
Discussion
An important question for understanding the FtsB-FtsL complex is how their two adjacent interaction domains – the TM helix and the juxta-membrane coiled-coil region – contribute and cooperate toward the stability of the complex. We have begun addressing this question by studying the individual domains in isolation. In our previous work we reported that the TM domain of FtsB self-associates18. We used extensive mutagenesis to identify the interaction interface and used computational modeling to interpret the experimental data. The analysis produced a complementary packed homo-dimer mediated by an inter-helical hydrogen bond (Figure 1b). While a biological role for the FtsB homo-dimer is not excluded, we hypothesized that the dimer likely represents the core of an FtsB-FtsL higher oligomer. The current results provide further evidence for this hypothesis.
We have confirmed that FtsB self-associates in vitro in lipid bilayers (Figure 2b). The calculated free energy of association is approximately −4 kcal/mol, a value that places FtsB as a moderately stable dimer, compared to other examples from the literature30. The oligomerization of FtsB is only marginal in DPC micelles (Figure 2a): its association curve is superimposable to that of FtsL, which does not associate even in lipid. The higher stability of FtsB in lipid could be in part due to the fact that a bilayer provides an environment closer to a natural membrane31. We note the additional possibility that the inter-helical hydrogen bonding formed by the polar Gln 16 at the FtsB dimer interface (Figure 1b) may contribute differently to the energy of oligomerization in the two environments. Polar side chains can contribute to the interaction of TM helices32,33. The contribution of a polar amino acid to association depends on the net balance between any gain of hydrogen bonding and electrostatic interactions in the bound state, and any loss of favorable interactions between the polar groups of the side chain and water in the unassociated state34. For a TM helix, however, this desolvation cost is presumably lower when, in the monomeric state, the side chain is sequestered away from water into the hydrophobic core of a lipid bilayer32,33. The desolvation cost may be more significant in a detergent environment, either because of the higher propensity of water molecules to penetrate deeply into a micellar environment35,36, or because of the difference in the ability of the long Gln side chain to ‘snorkel’ toward the surface of a micelle than in a lipid.
The main highlight of the present work is that we have obtained for the first time experimental evidence that the TM domains of FtsB and FtsL interact in isolation (Figure 3) and narrowed down the stoichiometry of the complex (Figure 5). The FRET results indicate that FtsB and FtsL associate strongly in both detergent and lipid. The calculated dissociation constants obtained by fitting the coumarin-FtsL/FITC-FtsB FRET data to a theoretical monomer-tetramer equilibrium are 2.3×10−9 and 1.3×10−11 in DPC and POPC respectively, and the corresponding ΔG° values of association are −11.7 and −14.7 kcal/mol, respectively. It is again noteworthy that the association is more stable in lipid than in detergent. The high stability of the transmembrane region of the FtsB-FtsL complex postulates that this domain has an important structural, and potentially functional role.
While it is challenging to determine the precise oligomeric state of a heterologous TM complex by FRET analysis, we provide evidence that the FtsB-FtsL complex is a higher oligomer with a 1:1 stoichimetric ratio. The observed increase of FtsB self-association in the presence of an equimolar amount of FtsL both in lipids and in detergent clearly indicates that FtsL has a strong effect in promoting the formation of a complex that contains more than one FtsB molecule (Figure 4). When the relative amount of FtsL is varied compared to FtsB, we found that the apparent FtsB stabilization increases linearly until a 1:1 FtsB-FtsL molar ratio is reached, at which point the curve sharply flattens (Figure 5).
The observed stabilization of FtsB self-association is due to a specific interaction with FtsL as opposed to non specific FRET due to crowding, as supported by two controls (Figure S3). The addition of an unrelated unlabeled monomeric model TM peptide does not perturb the FtsB self-association equilibrium, indicating that the increase in FtsB FRET is a sequence dependent effect of FtsL. On the other hand, the addition of unlabeled FtsB results in a reduction of FtsB FRET consistent with the expected competition and thus apparent dissociation of the labeled peptide pairs.
The FRET efficiency grows very rapidly as a function of concentration in the competition experiment in Figure 4, compared to the heterologous FtsB/FtsL FRET in Figure 3. It should be noted here that the concentration in Figure 3 is expressed for total peptide (FtsB+FtsL) while in Figure 4 it is expressed for FtsB peptide only, to allow direct comparison with the FtsB homo-FRET. In addition, the FtsB-FtsL experiment is only sensitive to formation of hetero-oligomers, while the competition experiment reports FRET due to FtsB homo-dimer formation as well as hetero-oligomers. It is, however, possible that other factors may also influence the analysis. For example imprecision in the difficult quantification of all labeled and unlabeled species could render the peptide molar ratios inaccurate. Moreover, the calculation of the reported dissociation constants are based on FRET efficiency, which was obtained from donor quenching at its emission maximum. This quantity may contain contributions from self quenching of the donor as well as colocalization effects due to random proximity of donors and acceptors as peptides diffuse randomly in bilayers even in low peptide concentrations. Accounting for these effects could in principle lower the actual FRET efficiency values and yield more precise thermodynamics of association. Furthermore, proximity effects due to different orientation in the opposite bilayer leaflets of multi-lamellar vesicles could also lead to spurious increased FRET efficiency values that have variable contributions for different FRET pairs25,37. Nonetheless, it is reasonable to infer that the contribution of non specific FRET due to all these factors is comparable across experiments performed in similar experimental conditions with the same FRET pair. Therefore, while from a rigorously quantitative stand point this report is an initial thermodynamic analysis, we note that, overall, the magnitude and the consistency of the data across the different experiments provide strong evidence that the TM domains of FtsB and FtsL form a stable 1:1 higher-oligomer complex, a fact that is important for understanding their biological function.
Further work is necessary to establish the precise oligomerization state of the FtsB-FtsL complex, using methods that are directly sensitive to the total mass of the complex, such as Analytical Ultracentrifugation38,39. The present work, however, represents a significant step toward understanding the structural organization of the FtsB-FtsL complex and its role in the assembly and function of the divisome. It provides further evidence for our previously reported hypothesis that a tetrameric TM complex is likely formed by the lateral association of FtsL-TM helix onto a FtsB-TM dimer18, and demonstrates that the TM region of FtsB-FtsL is stable and likely a major contributor to the stability of the overall complex (Figure 1c).
The fact that FtsB and FtsL form a higher-oligomeric complex may be significant for the functional role of the proteins. FtsB and FtsL have been hypothesized to be structural proteins that contribute to the stabilization of the Z-ring6–8. There is indeed substantial evidence that FtsB and FtsL bind to with multiple partners: they associate with FtsQ, an interaction that is mediate by the periplasmic domains of the proteins6,29,40–42; the N-terminal tail of FtsL is important for the recruitment of FtsW40; moreover, two-hybrid assay have identified several potential interactions with other proteins43,44. This leads to the hypothesis that a tetrameric and thus multi-valent FtsB-FtsL complex may simultaneously bind multiple partners and act as an interaction hub that tethers together several complexes of the divisome. This hypothetical role is schematically illustrated in Figure 6.
Figure 6. A higher-oligomeric FtsB-FtsL complex may be a multi-valent tethering structural element of the divisome.

A schematic depiction of a tetrameric FtsB-FtsL complex (black and white circles marked B and L) seen from above the plane of the membrane. The FtsB-FtsL complex is bound to FtsQ with the periplasmic tail of FtsB6,42 and to FtsW with the cytoplamic tail of FtsL40. Other hypothetical divisome components are depicted as dotted circles. The scheme illustrates how a tetrameric FtsB-FtsL complex could potentially bind to multiple divisome elements at the same time, therefore acting as a tethering structural element that contributes to holding together multiple sub-complexes within the Z-ring.
Supplementary Material
Acknowledgments
Funding sources: The work was supported by startup funds from the University of Wisconsin-Madison and in part by NIH R01GM099752 to AS.
We are grateful to Dr. Melissa Boersma at the Peptide Synthesis facility of the UW-Madison Biotech Center for assistance and very useful discussion in the synthesis and labeling of the peptides. We are also grateful to Dr. Kalina Hristova and Kristen Duthie for valuable inputs for labeling of peptides and the FRET assay and N. Rangarajan for assistance with characterization of the fluorophores and use of the fluorimeter. We thank Dr. Martha M. Vestling at the Mass Spectrometry facility at the Department of Chemistry and Dr. Darrel McCaslin of the Biophysical Instrumentation Facility of the Department of Biochemistry for assistance and guidance in data collection. Finally, we thank Nawaraj Subedi, Pragya Sidhwani, Jennifer Peotter and Chin Tan for assistance during sample preparation, and Loren LaPointe and Ben Mueller for useful discussion and critical reading of the manuscript.
Abbreviations
- TM
transmembrane
- FtsB-TM
transmembembrane domain of FtsB
- FtsL-TM
transmembembrane domain of FtsL
- FRET
Förster Resonance Energy Transfer
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DPC
dodecylphosphocholine
- FITC
fluorescein isothiocyanate
- 5-FAM
5-carboxyfluorescein
- TFE
trifluoroethanol
Footnotes
Supplementary Methods covering N-terminal labeling of peptides and quantification. Characterization of FITC in TFE (Figure S1). Fluorescence spectra of coumarin (donor) and FITC (acceptor) labeled peptides (Figure S2). Effect of unlabeled control peptides on FtsB homo-FRET (Figure S3). Supporting materials may be accessed free of charge online at http://pubs.acs.org.
References
- 1.Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- 2.Mingorance J, Rivas G, Vélez M, Gómez-Puertas P, Vicente M. Strong FtsZ is with the force: mechanisms to constrict bacteria. Trends Microbiol. 2010;18:348–356. doi: 10.1016/j.tim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Erickson HP, Anderson DE, Osawa M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol Mol Biol Rev MMBR. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goehring NW, Beckwith J. Diverse Paths to Midcell: Assembly of the Bacterial Cell Division Machinery. Curr Biol. 2005;15:R514–R526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Goehring NW, Gonzalez MD, Beckwith J. Premature targeting of cell division proteins to midcell reveals hierarchies of protein interactions involved in divisome assembly. Mol Microbiol. 2006;61:33–45. doi: 10.1111/j.1365-2958.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez MD, Beckwith J. Divisome under construction: distinct domains of the small membrane protein FtsB are necessary for interaction with multiple cell division proteins. J Bacteriol. 2009;191:2815–2825. doi: 10.1128/JB.01597-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robichon C, Karimova G, Beckwith J, Ladant D. Role of Leucine Zipper Motifs in Association of the Escherichia coli Cell Division Proteins FtsL and FtsB ▽. J Bacteriol. 2011;193:4988–4992. doi: 10.1128/JB.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissler B, Margolin W. Evidence for functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. Mol Microbiol. 2005;58:596–612. doi: 10.1111/j.1365-2958.2005.04858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buddelmeijer N, Judson N, Boyd D, Mekalanos JJ, Beckwith J. YgbQ, a cell division protein in Escherichia coli and Vibrio cholerae, localizes in codependent fashion with FtsL to the division site. Proc Natl Acad Sci. 2002;99:6316–6321. doi: 10.1073/pnas.092128499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel RA, Errington J. Intrinsic instability of the essential cell division protein FtsL of Bacillus subtilis and a role for DivIB protein in FtsL turnover. Mol Microbiol. 2000;36:278–289. doi: 10.1046/j.1365-2958.2000.01857.x. [DOI] [PubMed] [Google Scholar]
- 11.Ghigo JM, Weiss DS, Chen JC, Yarrow JC, Beckwith J. Localization of FtsL to the Escherichia coli septal ring. Mol Microbiol. 1999;31:725–737. doi: 10.1046/j.1365-2958.1999.01213.x. [DOI] [PubMed] [Google Scholar]
- 12.Katis VL, Wake RG, Harry EJ. Septal Localization of the Membrane-Bound Division Proteins of Bacillus subtilis DivIB and DivIC Is Codependent Only at High Temperatures and Requires FtsZ. J Bacteriol. 2000;182:3607–3611. doi: 10.1128/jb.182.12.3607-3611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robichon C, King GF, Goehring NW, Beckwith J. Artificial Septal Targeting of Bacillus subtilis Cell Division Proteins in Escherichia coli: an Interspecies Approach to the Study of Protein-Protein Interactions in Multiprotein Complexes. J Bacteriol. 2008;190:6048–6059. doi: 10.1128/JB.00462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel RA, Harry EJ, Katis VL, Wake RG, Errington J. Characterization of the essential cell division gene ftsL (yllD) of Bacillus subtilis and its role in the assembly of the division apparatus. Mol Microbiol. 1998;29:593–604. doi: 10.1046/j.1365-2958.1998.00954.x. [DOI] [PubMed] [Google Scholar]
- 15.Daniel RA, Noirot-Gros MF, Noirot P, Errington J. Multiple Interactions between the Transmembrane Division Proteins of Bacillus subtilis and the Role of FtsL Instability in Divisome Assembly. J Bacteriol. 2006;188:7396–7404. doi: 10.1128/JB.01031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramkamp M, Weston L, Daniel RA, Errington J. Regulated intramembrane proteolysis of FtsL protein and the control of cell division in Bacillus subtilis. Mol Microbiol. 2006;62:580–591. doi: 10.1111/j.1365-2958.2006.05402.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowland SL, Wadsworth KD, Robson SA, Robichon C, Beckwith J, King GF. Evidence from Artificial Septal Targeting and Site-Directed Mutagenesis that Residues in the Extracytoplasmic ? Domain of DivIB Mediate Its Interaction with the Divisomal Transpeptidase PBP 2B. J Bacteriol. 2010;192:6116–6125. doi: 10.1128/JB.00783-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaPointe LM, Taylor KC, Subramaniam S, Khadria A, Rayment I, Senes A. Structural organization of FtsB, a transmembrane protein of the bacterial divisome. Biochemistry (Mosc) 2013;52:2574–2585. doi: 10.1021/bi400222r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rath A, Deber CM. Design of transmembrane peptides: Coping with sticky situations. Methods Mol Biol Clifton NJ. 2013 doi: 10.1007/978-1-62703-583-5_11. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Liu LP, Deber CM. Guidelines for membrane protein engineering derived from de novo designed model peptides. Pept Sci. 1998;47:41–62. doi: 10.1002/(SICI)1097-0282(1998)47:1<41::AID-BIP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Melnyk RA, Partridge AW, Deber CM. Retention of Native-like Oligomerization States in Transmembrane Segment Peptides: Application to the Escherichia coli Aspartate Receptor†. Biochemistry (Mosc) 2001;40:11106–11113. doi: 10.1021/bi010642e. [DOI] [PubMed] [Google Scholar]
- 22.Stahl PJ, Cruz JC, Li Y, Michael Yu S, Hristova K. On-the-resin N-terminal modification of long synthetic peptides. Anal Biochem. 2012;424:137–139. doi: 10.1016/j.ab.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khadria A, Senes A. Measurement of Transmembrane Peptide Interactions in Liposomes Using Förster Resonance Energy Transfer (FRET) Methods Mol Biol Clifton NJ. 2013;1063:19–36. doi: 10.1007/978-1-62703-583-5_2. [DOI] [PubMed] [Google Scholar]
- 24.Li E, You M, Hristova K. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Förster Resonance Energy Transfer Suggest Weak Interactions between Fibroblast Growth Factor Receptor 3 (FGFR3) Transmembrane Domains in the Absence of Extracellular Domains and Ligands†. Biochemistry (Mosc) 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- 25.You M, Li E, Wimley WC, Hristova K. Förster resonance energy transfer in liposomes: Measurements of transmembrane helix dimerization in the native bilayer environment. Anal Biochem. 2005;340:154–164. doi: 10.1016/j.ab.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schick S, Chen L, Li E, Lin J, Köper I, Hristova K. Assembly of the M2 Tetramer Is Strongly Modulated by Lipid Chain Length. Biophys J. 2010;99:1810–1817. doi: 10.1016/j.bpj.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming KG, Ren CC, Doura AK, Eisley ME, Kobus FJ, Stanley AM. Thermodynamics of glycophorin A transmembrane helix dimerization in C14 betaine micelles. Biophys Chem. 2004;108:43–49. doi: 10.1016/j.bpc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Pace CJ, Huang Q, Wang F, Palla KS, Fuller AA, Gao J. A FlAsH–Tetracysteine Assay for Quantifying the Association and Orientation of Transmembrane α-Helices. ChemBioChem. 2011;12:1018–1022. doi: 10.1002/cbic.201000736. [DOI] [PubMed] [Google Scholar]
- 29.Villanelo F, Ordenes A, Brunet J, Lagos R, Monasterio O. A model for the Escherichia coli FtsB/FtsL/FtsQ cell division complex. BMC Struct Biol. 2011;11:28. doi: 10.1186/1472-6807-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKenzie KR, Fleming KG. Association energetics of membrane spanning α-helices. Curr Opin Struct Biol. 2008;18:412–419. doi: 10.1016/j.sbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Zhou F, Cocco MJ, Russ WP, Brunger AT, Engelman DM. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat Struct Mol Biol. 2000;7:154–160. doi: 10.1038/72430. [DOI] [PubMed] [Google Scholar]
- 33.Choma C, Gratkowski H, Lear JD, DeGrado WF. Asparagine-mediated self-association of a model transmembrane helix. Nat Struct Mol Biol. 2000;7:161–166. doi: 10.1038/72440. [DOI] [PubMed] [Google Scholar]
- 34.Hendsch ZS, Tidor B. Electrostatic interactions in the GCN4 leucine zipper: Substantial contributions arise from intramolecular interactions enhanced on binding. Protein Sci. 1999;8:1381–1392. doi: 10.1110/ps.8.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renthal R, Brancaleon L, Pena I, Silva F, Chen LY. Interaction of a two-transmembrane-helix peptide with lipid bilayers and dodecyl sulfate micelles. Biophys Chem. 2011;159:321–327. doi: 10.1016/j.bpc.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulumello DV, Deber CM. Positions of Polar Amino Acids Alter Interactions between Transmembrane Segments and Detergents. Biochemistry (Mosc) 2011;50:3928–3935. doi: 10.1021/bi200238g. [DOI] [PubMed] [Google Scholar]
- 37.Posokhov YO, Merzlyakov M, Hristova K, Ladokhin AS. A SIMPLE “PROXIMITY” CORRECTION FOR FRET EFFICIENCY DETERMINATION IN MEMBRANES USING LIFETIME MEASUREMENTS. Anal Biochem. 2008;380:134–136. doi: 10.1016/j.ab.2008.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doura AK, Fleming KG. Complex interactions at the helix-helix interface stabilize the glycophorin A transmembrane dimer. J Mol Biol. 2004;343:1487–1497. doi: 10.1016/j.jmb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Burgess NK, Stanley AM, Fleming KG. Determination of Membrane Protein Molecular Weights and Association Equilibrium Constants Using Sedimentation Equilibrium and Sedimentation Velocity. In: Correia John J, William Detrich HI., editors. Methods in Cell Biology. Academic Press; 2008. pp. 181–211. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez MD, Akbay EA, Boyd D, Beckwith J. Multiple interaction domains in FtsL, a protein component of the widely conserved bacterial FtsLBQ cell division complex. J Bacteriol. 2010;192:2757–2768. doi: 10.1128/JB.01609-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masson S, Kern T, Le Gouëllec A, Giustini C, Simorre JP, Callow P, Vernet T, Gabel F, Zapun A. Central domain of DivIB caps the C-terminal regions of the FtsL/DivIC coiled-coil rod. J Biol Chem. 2009;284:27687–27700. doi: 10.1074/jbc.M109.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Berg van Saparoea HB, Glas M, Vernooij IGWH, Bitter W, den Blaauwen T, Luirink J. Fine-mapping the Contact Sites of the Escherichia coli Cell Division Proteins FtsB and FtsL on the FtsQ Protein. J Biol Chem. 2013;288:24340–24350. doi: 10.1074/jbc.M113.485888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Ulisse V, Fagioli M, Ghelardini P, Paolozzi L. Three functional subdomains of the Escherichia coli FtsQ protein are involved in its interaction with the other division proteins. Microbiol Read Engl. 2007;153:124–138. doi: 10.1099/mic.0.2006/000265-0. [DOI] [PubMed] [Google Scholar]
- 44.Karimova G, Dautin N, Ladant D. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J Bacteriol. 2005;187:2233–2243. doi: 10.1128/JB.187.7.2233-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






