Abstract
Pre- and postnatal developmental studies of the lung have provided compelling evidence demonstrating multiple factors that orchestrate alveolar epithelial cell differentiation. The extent to which reactivation of certain developmental pathways in the adult might influence the course of differentiation of alveolar type 2 cells (AT2) into AT1 cells is not known. In this study, we examined selected members of the forkhead (Fox) family of transcription factors and the Wnt (wingless) family of signaling proteins for expression during human alveolar cell differentiation in vitro and determined their potential responses to sulfated components of extracellular matrix (ECM), like those shed from cell surfaces or found in basement membrane and modeled by heparin. Isolated adult human AT2 cells cultured over a nine day period were used to define the temporal profile of expression of targeted factors during spontaneous differentiation to AT1 cells. FoxA1 protein was up-regulated at early to intermediate time points, where it was strongly elevated by heparin. Gene expression of Wnt7A increased dramatically beginning on day 3 and was enhanced even further on days 7 and 9 by heparin, while protein expression appeared at days 7 and 9. These temporal changes of expression suggest that sulfated ECMs may act to enhance the increase in FoxA1 at the critical juncture when AT2 cells commence the differentiation process to AT1 cells, in addition to enhancing the increase in Wnt7A when the AT1 cell phenotype stabilizes. Collectively, these factors may act to modulate differentiation and stabilize cell numbers in the adult human pulmonary alveolus.
Keywords: β-catenin, differentiation, heparin, sulfated extracellular matrices
INTRODUCTION
The pulmonary alveolus is populated by a simple epithelium of squamous type I cells (AT1) and cuboidal type II (AT2) cells. The latter cells act as epithelial progenitors (Evans et al., 1975), produce surfactant (Mason, 1987), and support host defense (Wright, 1997). Previous work has shown that sulfated extracellular matrices (ECMs), such as heparan sulfate proteoglycans (HSPGs) as modeled by heparin, significantly influence AT2 responses to fibroblast growth factors (FGFs) as they relate to events associated with proliferation (Li et al., 2002b; Newman et al., 2004; Sannes et al., 1998), gene expression (Leiner et al., 2006; Li et al., 2002b), and protein synthesis (Li et al., 2002b). {{28 Li, C.M. 2002;}}. These effects can occur directly at or before receptor binding at the cell surface (Fannon et al., 2000), or indirectly at downstream signaling targets, such as MEK1/2, Erk1/2, SAP/JNK, and Akt/PKB (Newman et al., 2004). Alternatively, heparin itself has been shown to bind to cell surfaces and gain access to the cytoplasm and nucleus, where it could effect multiple biologic targets (Castellot et al., 1985) The rationale for the current investigations built upon earlier work in which the structural domains of the alveolar basement membrane associated with AT2 cells were shown to be quantitatively less sulfated than those of AT1 cells in the adult lung, wherein such differences could directly impact the biology of these cells (Grant et al., 1983; Sannes, 1984; Van Kuppevelt et al., 1984).
From these studies grew the initial paradigm in which the low sulfate ECM environment was predicted to promote the AT2 cell’s capacity to respond to growth factors, while the high sulfate environment of the AT1 cell would retard/inhibit its responsiveness to the same stimuli. It is now understood that the bioactivity of sulfated ECMs is dependent upon their concentration within a microenvironment (Fannon et al., 2000; Kamimura et al., 2001), the nature of the specific sulfate linkages (O-2-, O-6-, -N-, etc.) (Izvolsky et al., 2003; Luo et al., 2006; Yuguchi et al., 2005), and their compositional characteristics (Sannes et al., 1996; Shannon et al., 2003) and shed status (Kainulainen et al., 1998). While these data demonstrate important roles for sulfated ECMs in defining alveolar cell proliferation, their potential role in differentiation and cell fate specification remains unknown. More importantly, the specific factors that control the differentiation of AT2 cells into AT1s in the adult lung have yet to be defined.
A substantial body of work has demonstrated the importance of the forkhead (Fox) family of transcription factors during lung development. Gene-targeted deletion of foxa1 results in transient perturbation of epithelial maturation at precise points in embryonic and postnatal development (Besnard et al., 2005). Deletion of foxa2 precludes formation of the lung bud, resulting in early embryonic death, and its targeted deletion within a subset of lung epithelium using an SP-C promoter construct gave newborn mice severe pulmonary disease similar to respiratory distress syndrome (Wan et al., 2004b). These animals exhibited abnormal, immature alveolar epithelium without lamellar bodies and lacked mature AT1 cells (Wan et al., 2004b). Similarly, animals with reduced expression of foxa1 and foxa2 had inhibited cell proliferation, epithelial differentiation, and branching morphogenesis (Wan et al., 2005). Foxa2 regulates a series of events that control alveolar epithelial cell maturation and which are required for the transition to air breathing at birth (Wan et al., 2004b). These transcription factors, acting in concert with sulfated ECMs, could prove significant in helping drive epithelial cell differentiation in the adult alveolus.
A compelling argument could also be made for involvement of the Wnt family of growth factors in the limited reactivation of developmental pathways during alveolar turnover. The Wnt proteins are well known regulators of proliferation, differentiation, adhesion, polarity, and cell fate during lung development and morphogenesis (Borok et al., 2006; Pongracz and Stockley, 2006; Shannon and Hyatt, 2004). Upon Wnt binding, either canonical pathways (which involve activation of the key intermediate, β-catenin) or non-canonical pathways independent of β-catenin (mediated through either c-Jun kinase/AP-1 [JNK/AP-1] or calmodulin kinase II/nuclear factor of activated T cells [CaMKII/NFAT] pathways) are activated. While Wnt2 is not required for the development of apparently normal lungs in mice (Monkley et al., 1996), Wnt5a null mice have late-stage maturational lung defects (Li et al., 2002a) and inactivation of Wnt7b results in defects in lung development (Shu et al., 2002). Similarly, knockdown of Wnt signaling with β-catenin morpholinos results in increased branching and cell proliferation in developing lungs in vitro (Dean et al., 2005). Wnt proteins, like FGFs, signal in a very specific spatiotemporal fashion which appears to be unique for each Wnt family member; it is noteworthy that they are highly influenced by sulfated ECMs. This critical feature could be the key to alveolar epithelial cell differentiation and, hence, their responses to injury. Previous studies have shown that Wnt signaling in early stage embryos is dependent upon HSPGs for progression (Itoh and Sokol, 1994) and that Wnt11 expression by epithelium is dependent upon HSPG expression in the neighboring mesenchyme (Kispert et al., 1996). The glycosaminoglycan components of HSPGs have been shown to modulate extracellular localization and promote signaling of Wnt1 in a sulfation-specific fashion (Baeg et al., 2001; Reichsman et al., 1996). Accordingly, sulfated ECM-Wnt relationships, if operative in the adult pulmonary alveolus, would be predicted to be important determinants of differentiation.
It was the goal of this study to examine the sequence of expression of several factors that might be expected to control or otherwise influence the differentiation of AT2 cells into AT1 cells in culture. From this study, we hoped to gain insights into the control of this process in the adult whole lung which could lead to more targeted studies. To address this important issue, the tendency of isolated AT2 cells to “spontaneously differentiate” into AT1-like cells with time in culture (Dobbs et al., 1985; Manzer et al., 2006; Wang et al., 2006) was exploited. Isolated adult human AT2 cells were cultured on collagen-coated dishes and samples were harvested upon attachment (“day 0”) and at 1, 2, 3, 4, 7, and 9 days. RNA and protein were isolated and levels of selected factors monitored by gene and protein expression assays. In addition, some cells were treated with heparin, a model of both fixed (insoluble) and shed (soluble) sulfated ECMs, to assess whether sulfated components of ECM potentially influence expression levels. Expression levels of Wnt7A, β-catenin, and FoxA1, along with cell-type specific markers, were observed to vary with differentiation and often also in response to the presence of heparin during the time course. These results suggest potential role(s) for these factors and sulfated ECMs in the adult alveolar epithelium during normal lung homeostasis or recovery from pulmonary injury.
MATERIALS AND METHODS
Cell culture
Human alveolar type 2 cells were isolated by elastase digestion according to Dobbs (Dobbs, 1990) from cadaveric organ donor lung tissue obtained from the National Disease Research Interchange (Philadelphia, PA). Preparations of cells from three different human lungs were used in the experiments described herein. Where no statistical data is presented, the data shown are reflective of representative results of the parameters evaluated. All human specimens were handled under Institutional Review Board-approved protocols. The cells were maintained in DMEM medium supplemented with 10% fetal bovine serum and containing additional antibiotics (Amphotericin, Ceftazidime, Tobramycin and Vancomycin at 1.25, 100, 80 and 100 μg/ml, respectively) for the first 2 days or a penicillin, streptomycin, and amphotericin B cocktail with 50 μg/ml gentamicin thereafter. Cells were cultured at 37°C under an atmosphere of 95% air and 5% CO2 in tissue culture dishes pre-coated with type I collagen from calf skin (C-8919, Sigma, St. Louis, MO) or rat tail collagen (BD Biosciences, Franklin Lakes, NJ) at a density of 0.06 μg/mm2. Cells were cultured with or without high molecular weight heparin (Calbiochem, La Jolla, CA) at 500 μg/ml (3μM) for 1, 2, 3, 4, 7, or 9 days after overnight attachment. Cultures were terminated by removing the media, rinsing the dishes once with PBS, and lysing the cells with buffer unique for protein or RNA isolation.
Immunofluorescence
Cells cultured on collagen-coated glass coverslips and fixed in methanol were assessed by immunofluorescence for the localization of pan-cytokeratin to identify epithelial cells and for α-smooth muscle actin to identify contaminating myofibroblasts using commercially available monoclonal antibodies (Cell Signaling Technology, Danvers, MA; and Sigma) and FITC-labeled secondary antibodies (Vector Laboratories, Burlingame, CA). Nuclei were demonstrated with DAPI. Digital photography was performed with a Nikon Optiphot microscope using standard light excitation and filters.
Protein analysis
Western blot analysis of total proteins from AT2 cells from three individuals was performed using standard protocols. Cell lysates in CLB (Cell Lysis Buffer; Cell Signaling Technology) were sonicated (4 X 5 sec) and centrifuged (14,000 rpm, 20 min, at 4°C). The total protein in each supernatant was quantified by a standard Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA). Equal amounts of protein (35 μg) from each sample were electrophoretically separated on NuPAGE 4–12% Bis-Tris gels in MES Running Buffer (Invitrogen, Grand Island, NY) followed by transfer to nitrocellulose membranes. After blocking for 1 hr in 5% milk in TBS-T [20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Tween 20], the blots were probed overnight at 4°C with antibodies in TBS-T/5% milk at 1:1000 dilution. Primary antibodies to activated β-catenin and pan-cytokeratin (Cell Signaling Technology), FoxA1 (Novus Biologicals, Inc., Littleton, CO), Wnt7A, FoxA2, and GATA-6 (R&D Bio-systems, Minneapolis, MN), PAI-1 (Plasminogen activator inhibitor-1; Calbiochem), Nkx2.1/TTF-1 (Lab Vision Products, Thermo Fisher Scientific, Fremont, CA), SP-C and aquaporin5 (Aqp5) (Santa Cruz Biotechnology, Santa Cruz, CA), and ACTA2/alpha-smooth muscle actin (Sigma) were used. Secondary antibodies (Cell Signaling Technology) conjugated to horseradish peroxidase were diluted (1:4000 to 1:8000) in TBS-T with 5% nonfat dry milk. Specific bands were detected by chemiluminescence using SuperSignal West Pico or SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology, Rockford, IL) and visualized by autoradiography. For normalization and quantification, the same blots were hybridized with anti-GAPDH monoclonal antibody (Santa Cruz Biotechnology). Specific bands were scanned at 600 dpi resolution and bitmapped images were quantified in LabWorks (UVP, Inc, Upland, CA). Integrated Optical Densities (IOD) were normalized to GAPDH and graphed in Excel.
Quantitative Real-Time PCR
To obtain RNAs for gene expression studies, AT2/AT1-like cells were lysed in Buffer RLT (RNeasy; Qiagen, Valencia, CA) directly in culture dishes. Total RNAs were purified using the Qiagen RNeasy Mini Kit with on-column DNase digestion, and an equal amount of each sample was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). TaqMan primers and probes for Wnt7A (Hs00171699_m1), and GAPDH (Hs99999905_m1) were obtained from Applied Biosystems and 100 ng of each cDNA sample was amplified in duplicate or triplicate TaqMan qRT-PCR reactions on the MyiQ iCycler (BioRad). For Wnt7A, reactions were normalized for the housekeeping gene GAPDH and analyzed for gene expression using the REST-MCS program (Pfaffl et al., 2002). Fold-change values were generated for each of three alveolar isolations and these were combined and graphed, with error bars representing standard deviations from the average fold change values.
RESULTS
AT2 cell preparation purity
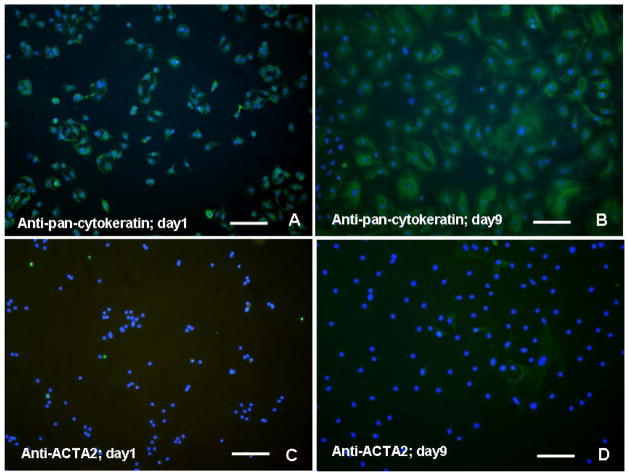
Over 95% of isolated human AT2 cells in culture at day 1 stained positively for pan-cytokeratin (Figs. 1A). By day 9 of culture, over 90% of the cells stained for pan-cytokeratin, although the intensity of signal was slightly diminished due to their spreading (Figs. 1B). Alpha-smooth muscle actin (ACTA2)-positive cells were sparse at day 1 (Figs. 1C). However, by day 9, approximately 4% of the total cell population demonstrated ACTA2, confirming the relatively minor contribution of myofibroblasts to the cell population during later time points (Figs. 1D).
Figure 1.
Immunofluorescent detection of pan-cytokeratin and smooth muscle actin (ACTA2) in isolated human alveolar cell preparations counterstained with DAPI. (A) Over 95% of “day 1” cells appear to be pan-cytokeratin positive, reflective of their epithelial origin; (B) Over 90% of “day 9” cells remained pan-cytokeratin positive, although significantly larger in size than “day 1” cells. (C) Cells positive for the myofibroblast marker ACTA2 at “day 1” in culture are difficult to resolve; (D) Only a few scattered myofibroblasts staining for ACTA2 in “day 9” cells confirm the relative purity of the AT2 isolation. Bar in each = 80 μm.
Expression of AT2 and AT1 markers with heparin treatments
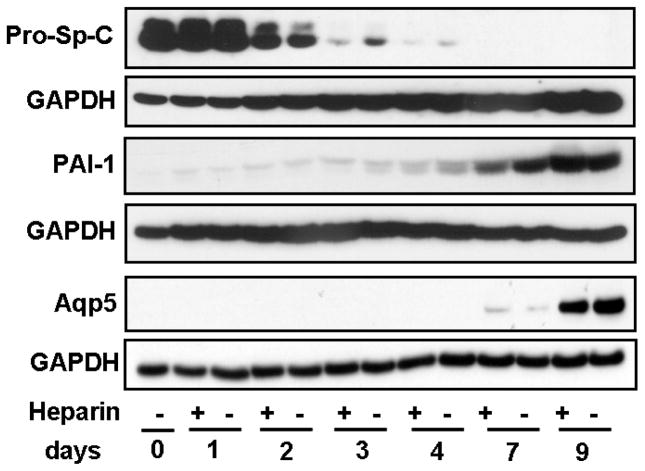
Pro-SP-C was strongly-expressed in isolated cells at days 0 and 1, reflecting the AT2 phenotype (Fig. 2). Pro-SP-C rapidly diminished thereafter, signaling the transition to the AT1 phenotype, and its disappearance was accelerated by heparin treatments (Fig. 2). PAI-1, a marker for the AT1 cell phenotype, was faintly detectable through day 2, began to increase on day 3, and then was highly expressed on days 7 and 9 (Fig. 2). Aqp5, a marker for the AT1 phenotype, rose steadily beginning on day 3 through day 9, indicating progressive differentiation into an AT1-like phenotype (Fig. 2).
FIGURE 2.
Time course of markers of alveolar epithelium differentiation from AT2 to AT1 cells. Prosurfactant protein-C (pro-SP-C; marker for AT2 cells), is highly expressed at days 0 and 1, but shows a distinct reduction beginning on day 2, although slightly less with heparin. At days 3 and 4, heparin treatment accelerates the increasing reduction of pro-SP-C. No pro-SP-C expression is evident at days 7 and 9. Plasminogen activator inhibitor-1 (PAI-1; marker for AT1 cells), was faintly detectable at day 1 and steadily increased as the time course progressed to day 9. Another marker for AT1 cells is aquaporin5 (Aqp5), which is detectable at day 7, but was very evident by day 9. GAPDH of the same blots is shown for normalization. Blots shown are representative of the similar results of three separate experiments.
Transcription factor expression
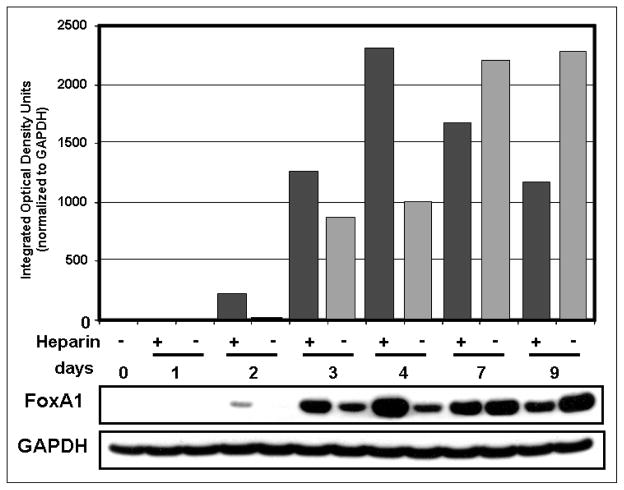
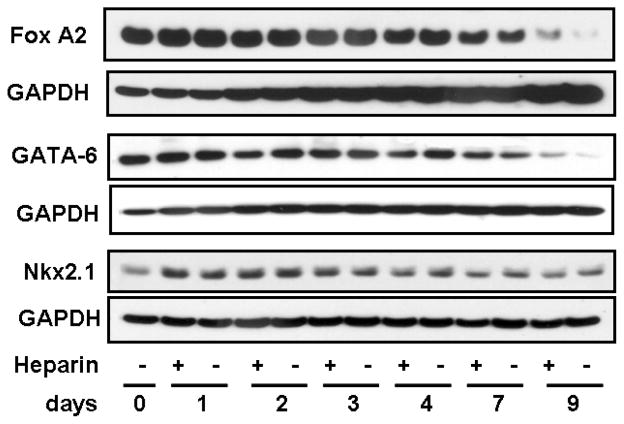
The transcription factor FoxA1 gradually increased from day 2 through day 9 (Fig. 3). Levels of FoxA1 rose significantly with heparin treatment at early time points, while at later time points (especially day 9), heparin treatment actually reduced FoxA1 protein expression (Fig. 3). These conclusions were confirmed by densitometric tracings shown above the gels (Fig. 3). FoxA2 expression gradually decreased after day 4 as the time course progressed to day 9 (Fig. 4). GATA-6 protein was most highly expressed at days 0 and 1 and progressively decreased through day 9 (Fig. 4). Nkx2.1/TTF-1 increased somewhat at day 1, especially with heparin, and gradually decreased through day 9 (Fig. 4).
FIGURE 3.
FoxA1 protein expression is affected by heparin. FoxA1 protein in whole cell lysates of isolated human AT2 cells was analyzed by Western blot. Levels of FoxA1 steadily increased from day 2 in untreated cells, as confirmed in the densitometric tracings (gray bars). FoxA1 was highly increased by heparin treatment (black bars) at days 2, 3, and 4 but was decreased on days 7 and 9 by heparin. GAPDH of the same blot is shown as a loading control. For normalization of FoxA1 densitometry, equal areas of GAPDH signal in each lane were quantified to avoid signal overlap.
FIGURE 4.
Other differentiation-related transcription factors in human alveolar cells are less affected by heparin. FoxA2 protein expression peaked early in the time course, and steadily decreased after day 4 and was relatively insensitive to heparin. GATA-6 protein expression peaked early in the time course and diminished through day 9. TTF-1 expression was low at isolation but strongly increased on day 1 postisolation, was moderately enhanced only at this early time point by heparin, and then tapered off toward day 9. Blots shown are representative of three similar results.
Wnt gene and protein expression
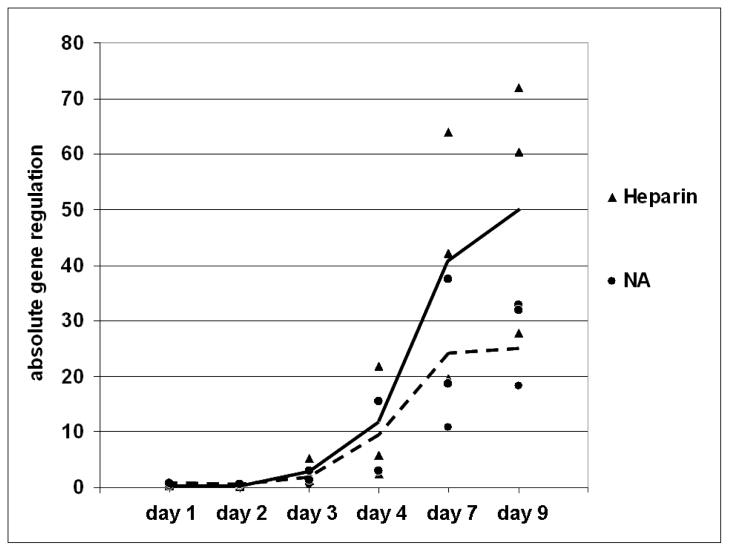
Commercial Wnt signaling pathway gene arrays (GE Arrays, ORN-043, Super Array Biosciences, Frederick, MD) revealed differences in gene expression in rat alveolar cells due to aging over 96 hours and significantly (>1.5-fold) differentially-expressed genes were identified (data not shown). Wnt7A was found to be the most dynamically-expressed Wnt in aging, and therefore differentiating, rat AT2 cells and was selected for examination in human AT2 cells. Expression of Wnt7A was assessed in three different preparations of isolated human alveolar cells as they progressed in culture over nine days and was found to be highly elevated from day 3 through day 9. Of particular note is that treatment of cells with heparin consistently and greatly increased Wnt7A gene expression at days 7 and 9 (Fig. 5). As variance between individuals was relatively small, the results of the fold change in gene expression of three different isolates were combined (Fig. 5).
FIGURE 5.
Wnt7A expression in isolated human alveolar type 2 cells varies with time and heparin treatments. Quantitative real time polymerase chain reaction (qRT-PCR) using TaqMan primers and probes was performed on cDNA equivalent to 100 ng of total RNA isolated from human AT2 cells cultured with or without 500 μg/mL heparin for 0–9 days. The absolute wnt7A gene regulation in cells from three individuals treated in separate experiments with (▲) or without (●) heparin is depicted at each time point. The solid line (—) connects the means of the expression at each time point in heparin-treated cells from the three individuals while the broken line (---) connects the means of expression at each time point in untreated cells. Wnt7A clearly increased rapidly from day 2 through day 9 and was enhanced by heparin on days 7 and 9.
Wnt7A protein was below the limits of detection by Western blot until day 7, and was increased at day 9 (Fig. 6). In contrast to the increased gene expression seen with heparin treatments at days 7 and 9, there appeared to be a slight reduction in protein expression with heparin addition (Fig. 6). Previous studies have noted that secreted Wnt proteins tend to adhere to the cell membrane (Bradley et al., 1990; Smolich et al., 1993), so that our gene expression data (Fig. 6) may be more realistic than quantitation of Wnt7A protein contained in our mild lysates. The timing of the increased expression of Wnt7A protein paralleled an increase at days 7 and 9 in dephosphorylated (active) β-catenin protein expression, above its relatively steady level of expression for at least the first 4 days post-isolation (Fig. 6), confirming increased activation of Wnt signaling pathway(s) concurrent with AT1 differentiation.
FIGURE 6.
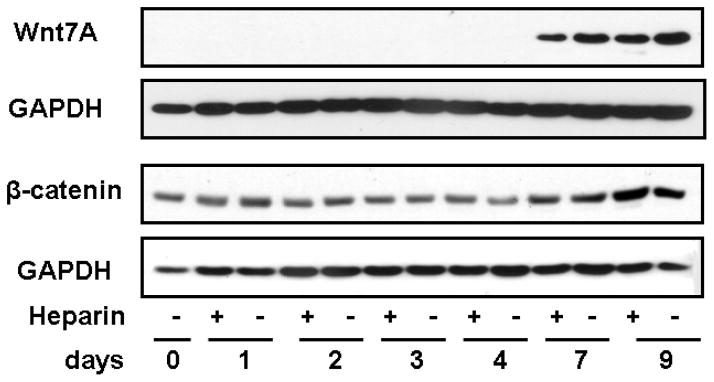
Wnt7A and β-catenin protein expression in whole cell lysates of isolated human alveolar cells, analyzed by Western blot. Wnt7A protein expression was not detected by Western blot until day 7 and increased on day 9. β-catenin protein expression remained relatively steady from day 0 through day 4 and then was elevated at days 7 and 9, confirming activation of Wnt signaling. Each of these proteins is shown with the GAPDH band from the same blot for normalization; the temporal pattern was the same in each of three individuals, and representative blots are shown.
DISCUSSION
Numerous studies on alveolar development in the lung have provided critical data on factors that control or modify spatial-temporal events involved with organ morphogenesis and cell proliferation, differentiation, and cell fate (Dobbs et al., 1985; Manzer et al., 2006; Wang et al., 2006). It was the goal of this study to examine in vitro several potential modulators of alveolar epithelial cell differentiation and their response to the model for sulfated ECMs, heparin, during the time course of isolated human AT2 cells’ phenotypic change into AT1-like cells. The results demonstrate for the first time the reactivation of differentiation pathways with expression of FoxA1 and the emergence of Wnt7A as a likely modulator of differentiation in isolated adult alveolar epithelial cells. Distinct changes in the levels of expression of these proteins and their enhancement by heparin might be expected to reflect changes in cell function and phenotype related to the process of AT1 cell replacement and differentiation in vivo.
The forkhead transcription factors FoxA1 and FoxA2 are intriguing prospective players in the modulation of alveolar differentiation in the adult. Targeted disruption of Foxa1 in mice altered the timing of maturation of respiratory epithelium, such that delays were observed in dilation of peripheral lung saccules at E16.5 and alveolarization at PN5 (Besnard et al., 2005). In each case, however, these delays were met with compensatory growth that resulted in subsequent normalization of the tissue within a short period (Besnard et al., 2005). Similarly, Clara cell secretory protein (CCSP), pro-surfactant protein (SP)-C, and SP-B protein expression were decreased in Foxa1−/− mice between E16.5 and E18.5, but were at normal levels at birth (Besnard et al., 2005). These data support a role for Foxa1 in alveolar epithelial cell maturation at very precise time points during development.
Deletion in early embryogenesis of the essential transcription factor Foxa2, when conditionally targeted to peripheral pulmonary cells only, results in immature AT2 cells and the absence of AT1 cells at birth (Wan et al., 2004b). However, when Foxa2 is deleted just before birth, neonate lungs show no overt morphologic abnormalities but then accumulate alveolarization and septation pathologies (Wan et al., 2004a). As Foxa1 and Foxa2 share patterns of expression in respiratory epithelium and bind similar consensus DNA binding sites, it may not be surprising that they serve similar functions in lung morphogenesis. Foxa2 can compensate for the targeted deletion of Foxa1, and Foxa1 mRNA was enhanced in animals in which Foxa2 was conditionally deleted (Wan et al., 2005). This study demonstrated that Foxa1 and Foxa2 have overlapping as well as distinct roles in the control of gene expression. It is likely that they interact and cooperate with a variety of other transcription factors to drive patterns of expression during development (Minoo et al., 2007). Both Foxa1 and Foxa2 are expressed in the normal adult lung (Besnard et al., 2004). Foxa2 expression may play an active role in regulation of the repair and redifferentiation of bronchiolar epithelium following injury (Park et al., 2006), but little is known about the function of Foxa1 in the adult.
The present data are consistent with a prominent role for FoxA1 in alveolar cell turnover in the adult. FoxA1 expression was clearly upregulated during the temporal progression toward the AT1 cell without heparin. But FoxA1 expression was significantly enhanced by heparin treatment at early time points, suggesting a potentially important role for increased sulfated ECMs in their soluble, shed forms or insoluble, fixed states, as found in the alveolar basement membranes (Sannes, 1984). Foxa1 has been shown to be upregulated by TTF-1 binding to two sites in the Foxa1 promoter (Peterson et al., 1997). In our studies, TTF-1 protein increased soon after AT2 isolation and was slightly enhanced on day 1 by heparin treatment. Heparin’s effects on TTF-1 could be part of the signal through which heparin treatments increase FoxA1. FoxA2 was much more consistent over time and less affected by heparin. Nevertheless, it is possible that FoxA1 and FoxA2 act in concert – with varying sensitivities to a sulfated environment –perhaps ensuring progression of differentiation regardless of the pericellular environment. But the facts that FoxA1 varies so directly with the progression of differentiation and that it is enhanced by modulators present in the immediate pericellular environment (sulfated ECMs) make it an excellent candidate for influencing, if not initiating, the differentiation process.
Although Wnt7a is not known to play any specific roles in lung development, it has been shown to have antitumor effects in non-small cell lung carcinoma (NSCLC) (Winn et al., 2005; Winn et al., 2006). In these studies, Wnt7a-transfection reversed transformation and decreased anchorage-independent proliferation in NSCLC cell lines. Furthermore, Wnt7a protein induced epithelial differentiation through induction of cadherin and Sprouty 4 and through activation of peroxisome proliferator-activated receptor γ (PPARγ) by ERK-5 (Winn et al., 2005). In the present study, Wnt7A gene expression began a rapid increase beginning at day 3 and continuing through day 9, with a clear enhancement with heparin treatment (Fig. 8), as the cells assumed more of an AT1-like phenotype. What initiates this rapid increase is not apparent, but a recent study demonstrated that TGFβ1, which has been shown to up-regulate Wnt7a in bone marrow stromal cells (Zhou et al., 2004), increases during in vitro trans-differentiation of rat AT2 to AT1 cells from days 1–5 (Bhaskaran et al., 2007). Addition of excess TGFβ1 prior to proliferation inhibited AT2 proliferation and later differentiation, while inhibition of TGFβ1 signaling after proliferation prevented differentiation (Bhaskaran et al., 2007) – demonstrating the important role TGFβ1 at key points in this process. Preliminary data from our lab has indicated that TGFβ1 protein and Smad 2/3 signaling increases in human AT2 cells during differentiation in culture parallel to the enhanced expression of Wnt7A as in Figure 8. It is established that TGFβ can suppress surfactant protein gene expression in fetal lung AT2 cells (McDevitt et al., 2007) and antagonize keratinocyte growth factor-induced AT2 cell proliferation (Zhang et al., 2004). Interestingly, sulfated ECMs have been shown to inhibit proliferation (Li et al., 2002b; Newman et al., 2004; Sannes et al., 1998) and AT2 cells in culture add progressively more sulfate to their biosynthesized ECMs with time (Sannes et al., 1997). Wnt7A, TGFβ1, and sulfated ECMs are likely to act in concert to significantly affect the inhibition/retardation of proliferation and ensure stabilization of phenotype with progression toward the AT1-like cell.
GATA-6 and TTF-1/Nkx2.1 regulate differentiation of alveolar epithelial cells (Wert et al., 2002; Yang et al., 2002) and act synergistically to regulate lung specific gene expression and development (Zhang et al., 2007). The data presented here demonstrate strong expression of GATA6 and TTF-1 at early time points in culture while the cells are still presumed to be AT2 cells, but over the 9-day time course, both factors diminish in expression. As these proteins are known regulators of SP-C production (Liu et al., 2002), their lack of or reduced expression in the day 9 post-isolation alveolar cell should not be surprising.
These data offer potentially important perspectives on the in vitro differentiation of AT2 cells to AT1-like cells. Of particular note was the level of gene and protein expression - and heparin-induced regulation - of Wnt7A, which may not only play a role in differentiation and cell fate of AT1 cells but may also affect their ability to proliferate under homeostatic conditions. The temporal expression patterns of FoxA1 during early and intermediate time points were not as surprising, but the change in expression of FoxA1 with heparin treatments may be significant. Up-regulation at early times by heparin may mimic the effects on a newly-generated replacement alveolar cell of the heavily sulfated basal lamina associated with AT1 cells in vivo (Grant et al., 1983; Sannes, 1984; Van Kuppevelt et al., 1984) and/or the shedding of sulfated ectodomains of surface proteoglycans during injury (Kainulainen et al., 1998). Following the asymmetric division of the limited stem-like AT2 cell to replace a damaged AT1 cell, the first ECM encountered by the new (daughter) cell would be heavily sulfated. This sulfated ECM could trigger enhancement of FoxA1 which, we believe, may initiate progression toward the AT1 phenotype. Enhancement of Wnt7A by the sulfated ECM could then stabilize the AT1 phenotype and prevent further proliferation. Collectively, these factors are candidates for playing roles in the transition to AT1-like cells – possibly driving differentiation and stabilizing phenotype while reducing proliferation. It may be useful to consider the changes in expression observed – their parallels and temporal overlaps – in the context of epithelial plasticity, wherein alterations in signaling via growth stimulating or retarding events can produce different outcomes (Borok et al., 1998; Wang et al., 2007). It is possible that the factors described here, among others, help modulate this important process in an orchestrated sequence in the adult both in response to injury and in normal alveolar turnover, while the precise mechanisms involved remain to be elucidated.
Acknowledgments
Supported by: PHS grant HL44497 (PLS), Core Grant DK065988, (SHR), and the State of North Carolina
The authors gratefully acknowledge the staff of the University of North Carolina Cystic Fibrosis/Pulmonary Research and Treatment Center Tissue Procurement and Cell Culture Core for the provision of human lung tissue.
LITERATURE CITED
- Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of wingless. Development. 2001;128:87–94. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Kaestner KH, Whitsett JA. Stage-specific regulation of respiratory epithelial cell differentiation by Foxa1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L750–9. doi: 10.1152/ajplung.00151.2005. [DOI] [PubMed] [Google Scholar]
- Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the smad pathway. J Biol Chem. 2007;282:3968–3976. doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1alpha expression with alveolar epithelial cell phenotype in vitro. Am J Physiol. 1998;275:L155–64. doi: 10.1152/ajplung.1998.275.1.L155. [DOI] [PubMed] [Google Scholar]
- Borok Z, Li C, Liebler J, Aghamohammadi N, Londhe VA, Minoo P. Developmental pathways and specification of intrapulmonary stem cells. Pediatr Res. 2006;59:84R–93R. doi: 10.1203/01.pdr.0000203563.37626.77. [DOI] [PubMed] [Google Scholar]
- Bradley RS, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990;9:1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol. 1990;258:L134–47. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta. 1985;846:155–166. doi: 10.1016/0167-4889(85)90121-1. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Fannon M, Forsten KE, Nugent MA. Potentiation and inhibition of bFGF binding by heparin: A model for regulation of cellular response. Biochemistry. 2000;39:1434–1445. doi: 10.1021/bi991895z. [DOI] [PubMed] [Google Scholar]
- Grant MM, Cutts NR, Brody JS. Alterations in lung basement membrane during fetal growth and type 2 cell development. Dev Biol. 1983;97:173–183. doi: 10.1016/0012-1606(83)90074-x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Sokol SY. Heparan sulfate proteoglycans are required for mesoderm formation in xenopus embryos. Development. 1994;120:2703–2711. doi: 10.1242/dev.120.9.2703. [DOI] [PubMed] [Google Scholar]
- Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, Cardoso WV. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol. 2003;285:L838–46. doi: 10.1152/ajplung.00081.2003. [DOI] [PubMed] [Google Scholar]
- Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Fujise M, Villa F, Izumi S, Habuchi H, Kimata K, Nakato H. Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene. structure, expression, and function in the formation of the tracheal system. J Biol Chem. 2001;276:17014–17021. doi: 10.1074/jbc.M011354200. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Leiner KA, Newman D, Li CM, Walsh E, Khosla J, Sannes PL. Heparin and fibroblast growth factors affect surfactant protein gene expression in type II cells. Am J Respir Cell Mol Biol. 2006;35:611–618. doi: 10.1165/rcmb.2006-0159OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002a;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li CM, Newman D, Khosla J, Sannes PL. Heparin inhibits DNA synthesis and gene expression in alveolar type II cells. Am J Respir Cell Mol Biol. 2002b;27:345–352. doi: 10.1165/rcmb.2002-0002OC. [DOI] [PubMed] [Google Scholar]
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277:4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- Luo Y, Ye S, Kan M, McKeehan WL. Control of fibroblast growth factor (FGF) 7- and FGF1-induced mitogenesis and downstream signaling by distinct heparin octasaccharide motifs. J Biol Chem. 2006;281:21052–21061. doi: 10.1074/jbc.M601559200. [DOI] [PubMed] [Google Scholar]
- Manzer R, Wang J, Nishina K, McConville G, Mason RJ. Alveolar epithelial cells secrete chemokines in response to IL-1beta and lipopolysaccharide but not to ozone. Am J Respir Cell Mol Biol. 2006;34:158–166. doi: 10.1165/rcmb.2005-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RJ. Surfactant synthesis, secretion, and function in alveoli and small airways. review of the physiologic basis for pharmacologic intervention. Respiration. 1987;51(Suppl 1):3–9. doi: 10.1159/000195267. [DOI] [PubMed] [Google Scholar]
- McDevitt TM, Gonzales LW, Savani RC, Ballard PL. Role of endogenous TGF-beta in glucocorticoid-induced lung type II cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L249–57. doi: 10.1152/ajplung.00088.2006. [DOI] [PubMed] [Google Scholar]
- Minoo P, Hu L, Xing Y, Zhu NL, Chen H, Li M, Borok Z, Li C. Physical and functional interactions between the homeodomain NKX2.1 & the winged Helix/Forkhead FOXA1 in lung epithelial cells. Mol Cell Biol. 2007 doi: 10.1128/MCB.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Newman DR, Li CM, Simmons R, Khosla J, Sannes PL. Heparin affects signaling pathways stimulated by fibroblast growth factor-1 and -2 in type II cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L191–200. doi: 10.1152/ajplung.00284.2003. [DOI] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Clevidence DE, Ye H, Costa RH. Hepatocyte nuclear factor-3 alpha promoter regulation involves recognition by cell-specific factors, thyroid transcription factor-1, and autoactivation. Cell Growth Differ. 1997;8:69–82. [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res. 2006;7:15. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol. 1996;135:819–827. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannes PL. Differences in basement membrane-associated microdomains of type I and type II pneumocytes in the rat and rabbit lung. J Histochem Cytochem. 1984;32:827–833. doi: 10.1177/32.8.6747274. [DOI] [PubMed] [Google Scholar]
- Sannes PL, Khosla J, Cheng PW. Sulfation of extracellular matrices modifies responses of alveolar type II cells to fibroblast growth factors. Am J Physiol. 1996;271:L688–97. doi: 10.1152/ajplung.1996.271.5.L688. [DOI] [PubMed] [Google Scholar]
- Sannes PL, Khosla J, Li CM, Pagan I. Sulfation of extracellular matrices modifies growth factor effects on type II cells on laminin substrata. Am J Physiol. 1998;275:L701–8. doi: 10.1152/ajplung.1998.275.4.L701. [DOI] [PubMed] [Google Scholar]
- Sannes PL, Khosla J, Peters BP. Biosynthesis of sulfated extracellular matrices by alveolar type II cells increases with time in culture. Am J Physiol. 1997;273:L840–7. doi: 10.1152/ajplung.1997.273.4.L840. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Shannon JM, McCormick-Shannon K, Burhans MS, Shangguan X, Srivastava K, Hyatt BA. Chondroitin sulfate proteoglycans are required for lung growth and morphogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1323–36. doi: 10.1152/ajplung.00226.2003. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Smolich BD, McMahon JA, McMahon AP, Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kuppevelt TH, Domen JG, Cremers FP, Kuyper CM. Staining of proteoglycans in mouse lung alveoli. I. ultrastructural localization of anionic sites. Histochem J. 1984;16:657–669. doi: 10.1007/BF01003393. [DOI] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004a;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A. 2004b;101:14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36:661–668. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang S, Manzer R, McConville G, Mason RJ. Ozone induces oxidative stress in rat alveolar type II and type I-like cells. Free Radic Biol Med. 2006;40:1914–1928. doi: 10.1016/j.freeradbiomed.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Wert SE, Dey CR, Blair PA, Kimura S, Whitsett JA. Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Dev Biol. 2002;242:75–87. doi: 10.1006/dbio.2001.0540. [DOI] [PubMed] [Google Scholar]
- Winn RA, Marek L, Han SY, et al. Restoration of wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Jr, Heasley LE, Nemenoff RA. Antitumorigenic effect of wnt 7a and fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Yuguchi Y, Kominato R, Ban T, Urakawa H, Kajiwara K, Takano R, Kamei K, Hara S. Structural observation of complexes of FGF-2 and regioselectively desulfated heparin in aqueous solutions. Int J Biol Macromol. 2005;35:19–25. doi: 10.1016/j.ijbiomac.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang F, Nielsen LD, Lucas JJ, Mason RJ. Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am J Respir Cell Mol Biol. 2004;31:679–686. doi: 10.1165/rcmb.2004-0182OC. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. GATA and nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–198. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
- Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]