Abstract
3-Nitrobenzanthrone (3-NBA), a nitropolyaromatic hydrocarbon (NitroPAH) pollutant in diesel exhaust, is a potent mutagen and carcinogen. After metabolic activation, the primary metabolites of 3-NBA react with DNA to form dG and dA adducts. One of the three major adducts identified is N-(2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (dGC8-N-ABA). This bulky adduct likely stalls replicative DNA polymerases but can be traversed by lesion bypass polymerases in vivo. Here, we employed running start assays to show that a site-specifically placed dGC8-N-ABA is bypassed in vitro by Sulfolobus solfataricus DNA polymerase IV (Dpo4), a model Y-family DNA polymerase. However, the nucleotide incorporation rate of Dpo4 was significantly reduced opposite both the lesion and the template position immediately downstream from the lesion site, leading to two strong pause sites. To investigate the kinetic effect of dGC8-N-ABA on polymerization, we utilized pre-steady-state kinetic methods to determine the kinetic parameters for individual nucleotide incorporations upstream, opposite, and downstream from the dGC8-N-ABA lesion. Relative to the replication of the corresponding undamaged DNA template, both nucleotide incorporation efficiency and fidelity of Dpo4 were considerably decreased during dGC8-N-ABA lesion bypass and the subsequent extension step. The lower nucleotide incorporation efficiency caused by the lesion is a result of a significantly reduced dNTP incorporation rate constant and modestly weaker dNTP binding affinity. At both pause sites, nucleotide incorporation followed biphasic kinetics with a fast and a slow phase and their rates varied with nucleotide concentration. In contrast, only the fast phase was observed with undamaged DNA. A kinetic mechanism was proposed for the bypass of dGC8-N-ABA bypass catalyzed by Dpo4.
Keywords: Sulfolobus Solfataricus Dpo4, Pre-steady-state kinetics, 3-Nitrobenzanthrone, Mutation, Y-family DNA polymerase, NitroPAH
1. Introduction
Combustion of every form of fossil fuel produces potent environmental pollutants that are known to affect human health at the molecular level.[1] Once metabolized, many of these pollutants become mutagenic and carcinogenic by damaging cellular genomes in a variety of ways, such as forming bulky DNA adducts, causing oxidative damage, and producing single and double stranded DNA breaks.[1] If the DNA adducts are not recognized and repaired by various cellular DNA repair pathways, they will stall replication machinery and eventually induce apoptosis.[2] To rescue stalled DNA replication, cells switch from a replicative polymerase to a DNA lesion bypass polymerase at a lesion site.[2] Notably, most of these lesion bypass polymerases belong to the Y-family, one of the six families (A, B, C, D, X, and Y) of DNA polymerases.[3] The Y-family enzymes possess relatively flexible and solvent-accessible active sites to accommodate bulky DNA lesions. However, these attributes of the Y-family enzymes also lead to their error-prone manner of DNA synthesis with both undamaged and damaged DNA.[4, 5] Interestingly, the Y-family DNA polymerases have been identified in all three domains of life, e.g. four in humans (DNA polymerases η, κ, ι, and Rev1), three in Schizosaccharomyces pombe (DNA polymerases η, κ, and Rev1), two in Escherichia coli (DNA polymerases IV and V), and one in Sulfolobus solfataricus (DNA polymerases IV). Being the lone Y-family enzyme in S. solfataricus, DNA polymerases IV (Dpo4) likely bypasses various lesions in vivo and thus, has been intensely studied as a model enzyme in vitro. With an undamaged DNA template, Dpo4 catalyzes DNA synthesis with a fidelity of one error per 1,000 –10,000 nucleotide incorporations based on our pre-steady-state kinetic studies from 37 to 56 °C.[6, 7] Dpo4 has been found to bypass various DNA lesions, e.g. abasic sites [8, 9] and N-(deoxyguanosin-8-yl)-1-aminopyrene (dGAP).[10] The latter lesion is a bulky adduct resulting from a dG base reacting with the metabolites of 1-nitropyrene, a product of incomplete diesel and gasoline combustion.[11]
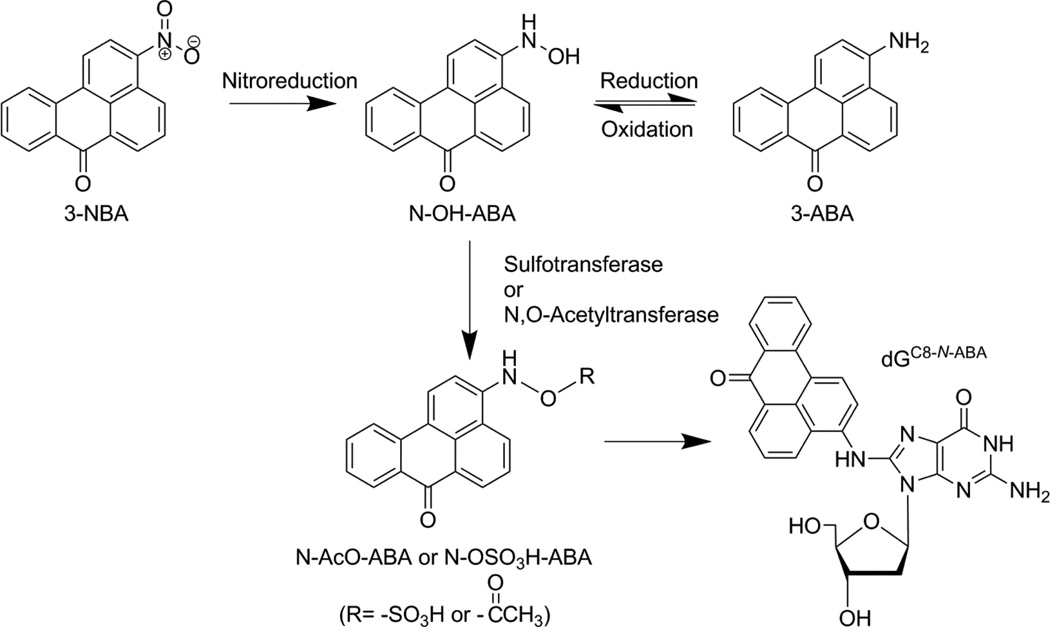
Another interesting product of diesel exhaust, albeit present at a lower concentration than 1-nitropyrene, is 3-nitrobenzanthrone (3-NBA, Scheme 1).[12] 3-NBA, one of the most mutagenic compound ever tested on the Ames Salmonella typhimurium (TA98) assay, is also found in ambient air particulate matter.[12] The mutagenicity of 3-NBA is comparable to 1,8-dinitropyrene, [13] and has been suspected to be carcinogenic to humans.[14–16] This possibility is supported by cellular and animal model data.[17–20] For example, 3-NBA and its metabolites are found to induce micronuclei and DNA adducts in mouse and human cells,[12, 19] and form DNA adducts and tumors in rats.[18–21] Bioactivation of 3-NBA is initiated by an essential nitroreduction step, forming N-hydroxy arylamine (N-OH-ABA) (Scheme 1).[17] This step is catalyzed by cytochrome P450 and cytochrome P450 reductase in rats,[17] and NAD(P)H:Quinone Oxidoreductase in humans.[22] N-OH-ABA is then metabolized to either N-AcO-ABA or N-OSO3H-ABA by N,O-acetyltransferases or sulfotransferases, respectively.[17, 23] Finally, either of these highly reactive derivatives reacts with DNA to form bulky aromatic DNA adducts including N-(deoxyguanosin-8-yl)-3-aminobenzanthrone (dGC8-N-ABA, Scheme 1).
Scheme 1.
Bioactivation of 3-Nitrobenzanthrone. The nitro group of 3-NBA is first activated by a nitroreduction step followed by the formation of an intermediate group (N-AcO-ABA or N-OSO3H-ABA), which is subsequently converted to a nitrenium ion and reacts with adenosine or guanosine to form a DNA adduct, e.g. dGC8-N-ABA.
The DNA adducts derived from 3-NBA, if not repaired, are likely bypassed by lesion bypass polymerases in vivo.[2] In this paper, we kinetically investigated the potential bypass of a site specifically placed dGC8-N-ABA, one of the three major adducts formed by 3-NBA,[24] catalyzed by Dpo4. Our results show that the prototype Y-family polymerase was indeed capable of bypassing dGC8-N-ABA but its polymerase efficiency and fidelity were significantly affected by the bulky lesion.
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. Starting material for synthesis of a DNA oligomer containing dGC8-N-ABA
All starting materials, reagents and solvents were of commercial grade and used as such unless otherwise specified. Anhydrous solvents were purchased from Aldrich (St. Louis, MO). THF was dried by standard methods. NMR (1H and 13C) spectra were recorded on a Bruker (Coventry, UK) AC-400 spectrometer. Samples prepared for NMR analysis were dissolved in either CDCl3 or DMSO-d6. Chemical shifts are reported in δ ppm relative to TMS in the proton spectra and to the deuterated solvent in the carbon spectra. LCMS (electrospray ionization) was recorded on a Micromass Quattro II (Manchester, UK) triple quadrupole mass spectrometer in acetonitrile mobile phase and the cone voltage set to 30 V. Mass spectra of the modified oligonucleotide were acquired on a QStar Elite hybrid QTOF MS mass spectrometer (AB Sciex, USA).
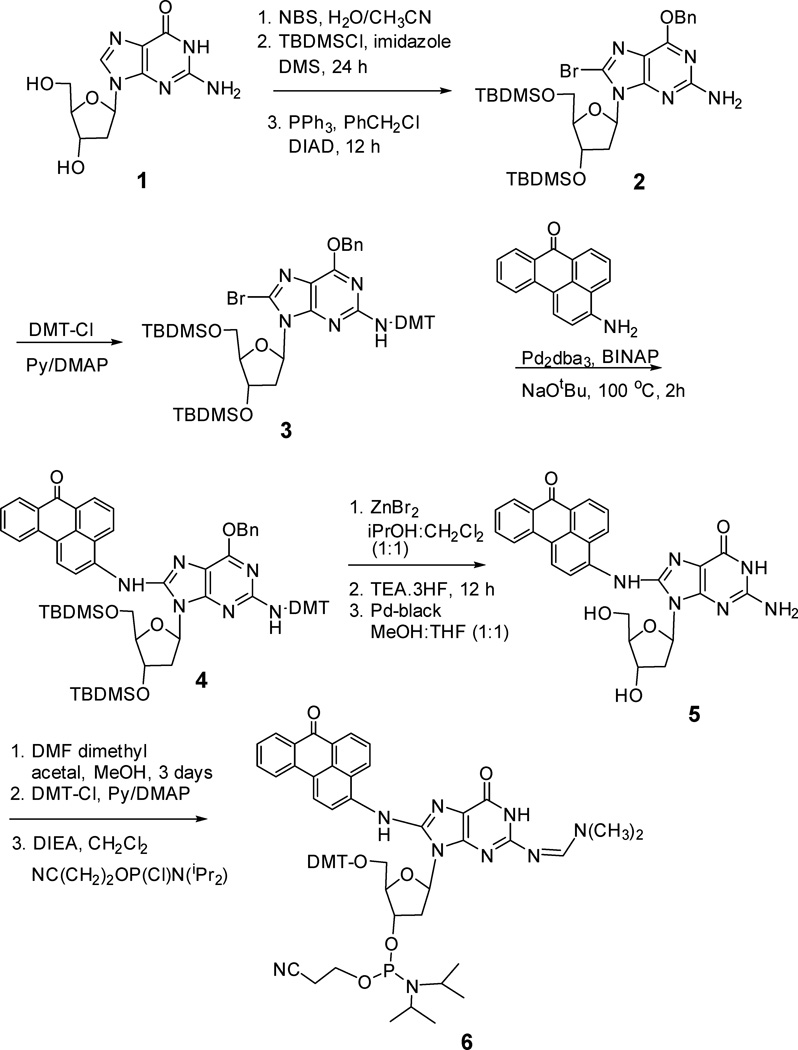
2.1.2. Synthesis and characterization of (O6-Benzyl-3’,5’—bis-O-(tert-butyldimethylsilyl)-N2-dimethoxytrityl-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (4, Scheme 2)
Scheme 2.
Chemical Synthesis of (2-Dimethylformamidine-5'-O-(4,4'-dimethoxytrityl)-3'-O-[N,N'-diisopropylamino(2-cyanoethoxy)phosphinyl]-2'-deoxyguanosin-8-yl)-3-aminobenzanthrone.
O6-Benzyl-8-bromo-3’,5’-O-bis(tert-butyldimethylsilyl)-N2-dimethoxytrityl-2’-deoxyguanosine [25, 26] (3) (5 g, 5.08 mmol), 3-aminobenzanthrone (1.02 g, 4.14 mmol), tris (dibenzylidenacetone)dipalladium (Pd2(dba)3) (380 mg) and rac-2, 2’- bis(diphenylphosphino)-1,1’-binaphthyl (64 mg) were preheated in degassed toluene at 90 °C. After 40 min, sodium tert-butoxide (621 mg, 6.5 mmol) was added and the reaction mixture was stirred for an additional hour at 100 °C. The reaction was cooled and the filtrate was concentrated and purified by chromatography on aluminum oxide using 5–20% ethyl acetate in hexane to provide compound 4 (4.97g, 85%) in Scheme 2. 1H NMR (400 MHz, DMSO-d6) δ −0.27(3H, s), −0.15(3H, s), 0.14 (6H, s), 0.66 (9H, s), 0.95(9H, s), 1.64(1H, br s), 2.22 (1H, br s), 3.80 (7H, br s), 4.14 (3H, m), 4.50 (1H, br s), 5.08 (2H, br s), 6.14 (1H, br s), 6.80 (4H, m), 7.28–7.72 (19H, m),8.24 (2H, m), 8.38 (2H, m), 8.49 (1H, d, J =7.1 Hz), 8.76 (1H, d, J = 7.1 Hz). 13C NMR (100 MHz, DMSO-d6) δ −5.7, −4.7, 1.1, 14.2, 18.0, 18.2, 25.7, 25.8, 25.9, 36.7, 39.9, 39.9, 55.2, 60.4, 63.1, 67.5, 70.2, 72.6, 86.2, 88.5, 112.0, 112.8, 112.9, 112.9, 117.1, 121.0, 122.8, 125.9, 125.9, 126.5, 128.6129.1, 129.1, 133.4, 136.7, 137.1, 137.7, 146.3, 147.2, 156.2, 158.1, 184.1. MS (ESI): m/z: calculated for [M+H]+ C67H75N6O7Si2 1131.5236, observed 1131.5.
2.1.3. DMT deprotection of the dG adduct
To the protected dG adduct 4 (100 mg, 0.088 mmol) dissolved in 2 mL dichloromethane was added 1M zinc bromide solution in isopropanol: dichloromethane (1:1) (2 mL). The reaction was stirred for 15 min, quenched with aqueous sodium bicarbonate, and extracted with dichloromethane. The organic layer was combined, dried and purified by using column chromatography on aluminum oxide using 5–30% ethyl acetate in hexane to afford (O6-benzyl-3’,5’-bis-O-(tert-butyldimethylsilyl)-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (44 mg, 60%). 1H NMR (300 MHz, CDCl3) δ −0.33 (3H, s), −0.19 (3H, s), 0.16 (3H, s), 0.18 (3H, s), 0.60 (9H, s), 0.97 (9H, s), 2.43 (1H, m), 3.08 (1H, m), 3.82 (2H, m), 3.96–3.98 (1H, m), 4.21 (1H, s), 4.59 (1H, s), 4.71 (2H, br s), 5.59 (2H, s), 6.42 (1H, t, J =7.1 Hz), 7.20 (1H, m), 7.34 – 7.36 (4H, m), 7.50 – 7.55 (3H, m), 7.76–7.81 (2H, m), 8.34 – 8.37 (2H, m), 8.50 (3H, m), 8.71 (1H, s), 8.82 (1H, m). 13C NMR (100 MHz, CDCl3) δ −5.65, −4.66, −4.51, 14.2, 18.1, 18.2, 24.7, 36.8, 39.2, 53.5, 60.4, 63.1, 67.8, 72.5, 85.4, 85.4, 88.1, 117.7, 122.8, 125.5, 125.6, 127.8, 127.9, 128.2, 128.3, 130.1, 133.2, 136.5, 136.9, 147.5, 153.6, 157.5, 183.9. MS (ESI): m/z: calculated for [M+H]+ C49H57N6O5Si2 829.3929, observed 829.0.
2.1.4. TBDMS deprotection of the dG Adduct
To the detritylated compound, (O6-benzyl-3’,5’-bis-O-(tert-butyldimethylsilyl)-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (200 mg, 0.242 mmol) in THF (5 mL), triethylamine trihydrogenfluoride (100 µL) was added and stirred overnight at room temperature. Subsequently, triethylamine (88 µL) was added, the solvent was evaporated, and the compound was purified by column chromatography on silica gel using methylene chloride: methanol as an eluent to afford (O6-benzyl-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (122 mg, 84%). 1H NMR (300 MHz, DMSO-d6) δ 2.22 (1H, m). 2.89 (1H, m), 3.68 (1H, m), 3.76 (1H, m), 3.99 (1H, br s), 4.46 (1H, br s), 5.35 (1H, s), 5.44 (2H, br s), 5.53 (1H, m), 6.30 (2H, s),6.50 (1H, m), 7.46-7.30 (5H, s), 7.58 (1H, m), 7.83 (1H, m), 7.90 (1H, m), 7.93 (1H, m), 8.52 (1H, m), 8.60 (1H, m), 8.68 (1H, m), 8.70 (1H, d, J = 8.0 Hz), 9.33 (1H, s). 13C NMR (75 MHz, DMSO-d6) δ 9.0. 47.0, 62.0, 67.1, 71.8, 84.1, 84.5, 88.1, 118.9, 124.0, 126.40, 126.42, 126.7, 126.8, 127.7, 128.0, 128.1, 128.4, 128.4, 128.8, 129.0, 129.9, 130.1, 130.9, 134.3, 135.8, 136.6, 136.8, 137.3, 147.0, 158.7, 183.2. MS (ESI): m/z: calculated for M+ C34H28N6O5 600.2121, observed 600.7.
2.1.5. Benzyl deprotection of the dG adduct
In a round bottom flask (O6-benzyl-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (200 mg, 0.330 mmol) was dissolved in 20 mL THF/MeOH (1:1) under inert (N2) atmosphere. The reaction mixture was purged with N2 for 10 min. Pd-black (15 mg) was added and the reaction mixture was purged with H2 for 2 h. It was left overnight with continued stirring under H2. A solid was separated out and filtered. The filtrate was dried and purified by reversed-phase chromatography on a C18 column using 1–50% acetonitrile in water to give 153 mg of (2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (5, Scheme 2) in 90% yield. 1H NMR (400 MHz, DMSO-d6) δ 2.09–2.16 (1H,m), 2.86 (1H, br s), 3.61–3.72 (2H, m), 3.90(1H, br s),4.39 (1H, s), 5.27 (1H, br s), 5.36 (1H, br s), 6.38 (3H, br s), 7.59 (1H, s), 7.75 (1H, s), 7.84 – 7.91 (2H, m), 8.34 (1H, s), 8.55 (1H, br s), 8.72 (3H, br s), 9.13 (1H, s), 10.63 (1H, s). 13C NMR (75 MHz, DMSO-d6) δ 9.6, 46.1, 62.0, 71.6, 84.0, 88.1, 116.4, 119.8, 123.8, 125.8, 126.2, 127.0, 127.6, 127.8, 128.3, 128.4, 129.7, 130.1, 130.7, 134.2, 136.7, 144.0, 150.5, 153.5, 156.2, 183.2. MS (ESI): m/z: calculated for M+ C27H22N6O5 510.1652, observed 510.8.
2.1.6. DMF protection of (2’-deoxyguanosin-8-yl)-3-aminobenzanthrone
Compound 5 (150 mg, 0.294 mmol) in Scheme 2 was suspended in dry MeOH (10 mL) under argon. N,N-Dimethylformamide dimethyl acetal (1 mL) was added and the mixture was stirred for 3 days. The solid precipitate was filtered to afford the pure N2-DMF protected (2’-deoxyguanosin-8-yl)-3-aminobenzanthrone. The filtrate was dried and purified by reversed-phase chromatography on a C18 column using 1–50% acetonitrile in water to afford an additional crop of the compound (15 mg). Combined yield (157 mg) of the compound was 95 %. 1H NMR (400 MHz, CDCl3) δ 2.20–2.23 (1H, m), 2.87–2.9 (1H, m), 2.93 (3H, s), 3.05 (3H, s), 3.64 (1H, d, J = 12 Hz), 3.75 (1H, d, J = 12 Hz), 3.91 (1H, br s), 4.46 (1H, br s), 5.33 (2H, s), 6.27 (1H, s), 6.44 (1H, s), 7.57–7.60 (1H, m), 7.76–7.78 (1H, m), 7.85 (1H, t, J = 8Hz), 7.92 (1H, t, J = 8 Hz), 8.34 (1H, d, J = 8 Hz), 8.55 (2H, s), 8.70–8.72 (3H, m), 9.24 (1H, s). 13C NMR (75 MHz, CDCl3) δ 38.60, 41.6, 44.64, 65.44, 74.97, 87.4, 91.5, 120.27, 120.28, 123.6, 127.3, 129.42, 129.83, 130.44, 131.25, 131.67, 131.87, 133.25, 133.67, 134.24, 137.84, 140.17, 144.97, 148.57, 152.83, 160.30, 160.72, 161.74, 186.76. HRMS (ESI) m/z calculated for [M+H], C30H28N7O5 566.2152, observed 566.2183.
2.1.7. DMT protection
In a 50 mL round-bottom flask N2-DMF protected (2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (100 mg, 0.177 mmol) was dissolved in dry pyridine (5 mL) under argon., to which DMAP (1 mg) and DMT-Cl (71 mg) were added. The reaction mixture was heated at 50 °C for 2 h. Then solvent was evaporated and purified by column chromatography on silica gel (using 1% Et3N, 3–5% MeOH- 95% CH2Cl2) to afford (2-dimethylformamidine-5’-O-(4,4’-dimethoxytrityl)-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (107 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 3.0 (3H, s), 3.04 (3H, s), 3.10–3.17 (2H, m), 3.38–3.42 (1H, m), 3.48–3.50 (1H, m), 3.56 (3H, s), 3.57 (3H, s), 3.62–3.76 (1H, m), 4.38 (1H, s), 4.59 (1H, s), 6.46 (1H, br s), 6.52–6.54 (4H, m), 7.06 –7.12 (8H, m), 7.18–7.26 (2H, m), 7.42–7.54 (3H, m), 7.71 (1H, t, J = 8 Hz), 8.07 (1H, d, J = 8 Hz), 8.23 (1H, d, J = 8 Hz), 8.33 (1H, d, J = 8 Hz), 8.38 (1H, s), 8.46 (1H, d, J = 8 Hz), 8.63 (1H, s), 8.71 (1H, d, J = 8 Hz). 13C NMR (100 MHz, CDCl3) δ 39.44, 40.29, 55.16, 64.02, 72.02, 86.28, 86.62, 86.92, 107.31, 113.06, 114.12, 116.98, 121.06, 122.95, 125.86, 125.99, 126.18, 126.93, 127.54, 127.85, 128.06, 128.16, 128.25, 128.54, 128.99, 129.74, 130.05, 130.17, 133.71, 135.37, 135.44, 136.64, 128.26, 144.3, 146.87, 149.48, 151.69, 154.76, 155.33, 157.23, 158.20, 158.46, 184.10. HRMS (ESI) m/z calculated for [M+H], C57H46N7O7 868.3459 and observed 868.3507.
2.1.8. (2-Dimethylformamidine-5’-O-(4,4’-dimethoxytrityl)-3’-O-[N,N’-diisopropylamino(2-cyanoethoxy)phosphinyl]-2’-deoxyguanosin-8-yl)-3-aminobenzanthrone (6)
DMF and DMT protected adduct (100 mg, 0.115 mmol) was dissolved in dry CH2Cl2 (10 mL) under argon and DIEA (0.1 mL) was added. 2-Cyanoethoxy diisopropyl chlorophosphoramidite (50 µL) was added and the mixture was stirred for 30 min. Subsequently, the solvent was evaporated and the product was purified by column chromatography on silica gel (using 1% Et3N, 0.5–2% MeOH, 96% CH2Cl2) to afford the phosphoramidite 6 (74 mg, 60%) as a mixture of two diastereomers. 1H NMR (400 MHz, CDCl3) (mixture of two diastereomers) δ 1.07–1.34 (2H, m), 1.99 (1H, br s), 2.42–2.45 (1H,m), 2.59–2.65 (2H, m), 2.69–2.76 (2H, m), 3.09 (3H, br s), 3.15–3.16 (3H, m), 3.29–3.34 (1H, m), 3.39–3.40 (1H, m), 3.44–3.50 (2H, m), 3.56–3.57(6H, br s), 3.57–3.91 (4H, m), 4.44–4.45 (1H, m), 4.63–4.76 (1H, m), 6.40–6.45 (1H, m), 6.50–6.55 (3H, m), 7.07–7.13 (6H, m), 7.23–7.24 (2H, m), 7.49–7.53 (2H, m), 7.72–7.76 (1H,m), 8.07–8.17 (1H,m), 8.28 (1H, br s), 8.35–8.39 (1H, m), 8.48–8.54 (3H,m), 8.73–8.74(1H, m). 13C NMR (100 MHz, CDCl3) (mixture of two diastereomers) δ 20.42, 20.49, 20.66, 20.73, 21.72, 24.80, 35.4, 38.75, 41.47, 43.52, 43.64, 46.28, 53.05, 55.23, 58.13, 58.5, 58.7, 63.26, 63.64, 73.68, 73.85, 74.19, 74.37, 85.9, 86.14, 86.79, 86.82, 113.18, 115.52, 115.77, 117.68, 117.88, 122.96, 120.59, 122.96, 124.97, 126.00, 126.35, 127.09, 127.13, 127.37, 127.96, 128.13, 128.22, 128.25, 128.64, 129.18, 129.77, 130.07, 130.16, 133.64, 135.18, 135.25, 135.37, 136.91, 138.24, 144.3, 145.88, 146.01, 148.84, 148.89, 155.39, 155.45, 156.99, 157.67, 157.79, 158.62, 183.0. 31P NMR (300 MHz, CDCl3): 148.9, 148.8. HRMS (ESI) m/z calculated for [M+H], C60H63N9O8P 1068.4537, observed 1068.4524.
2.2. Kinetic Assays
2.2.1. Buffers
All pre-steady-state kinetic assays were performed in optimized reaction buffer R (50 mM HEPES, pH 7.5, 5 mM MgCl2, 50 mM NaCl, 0.1 mM EDTA, 5 mM DTT, 10% glycerol (v/v), and 0.1 mg/ml bovine serum albumin)5. All electrophoresis mobility shift assays (EMSA) were performed in buffer S (50 mM Tris-Cl, pH 7.5 at 23 °C, 5 mM MgCl2, 50 mM NaCl, 5 mM DTT, 10% glycerol (v/v), and 0.1 mg/ml bovine serum albumin). The EMSAs were conducted using running buffer A (50 mM Tris acetate, pH 7.5, at 25 °C, 0.5 mM EDTA, and 5.5 mM magnesium acetate). All concentrations are final after mixing. Unless otherwise noted, all reactions were performed at 37 °C.
2.2.2. Enzymes and DNA Substrates
Full length Dpo4 was expressed in E. coli and purified as previously described.[6, 7] All primers and templates except 26-mer-dGC8-N-ABA in Table 1 were purchased from the Integrated DNA Technologies, and purified by denaturing polyacrylamide gel electrophoresis (PAGE). The DNA primers were 5′-32P-labeled by incubation with [γ-32P] ATP (Perkin Elmer) and OptiKinase (United States Biochemical) for 3 h at 37 °C. The 5′-32P-labeled primers were annealed to the unlabeled control 26-mer or 26-mer-dGC8-N-ABA at a molar ratio of 1.00:1.35. The solution was first denatured at 95 °C (for undamaged DNA) or 75 °C (for 26-mer-dGC8-N-ABA) for 5 minutes and then slowly cooled to room temperature.
Table 1.
Sequences of DNA oligonucleotides
| Primers (positiona) | Sequences |
|---|---|
| 17-mer (−4) | 5'-AACGACGGCCAGTGAAT-3' |
| 18-mer (−3) | 5'-AACGACGGCCAGTGAATT-3' |
| 19-mer (−2) | 5'-AACGACGGCCAGTGAATTC-3' |
| 20-mer (−1) | 5'-AACGACGGCCAGTGAATTCG-3' |
| 21-mer (0) | 5'-AACGACGGCCAGTGAATTCGC-3' |
| 22-mer (+1) | 5'-AACGACGGCCAGTGAATTCGCG-3' |
| Templates | |
| 26-mer | 3'-TTGCTGCCGGTCACTTAAGCGCGCCC-5' |
| b26-mer-dGC8-N-ABA | 3'-TTGCTGCCGGTCACTTAAGCGCGCCC-5' |
| DNA trap | |
| D-1 (21/41-mer) | 5'-CGCAGCCGTCCAACCAACTCA-3' |
| 3'-GCGTCGGCAGGTTGGTTGAGTAGCAGCTAGGTTACGGCAGG-5' | |
Position of primer 3′-terminus relative to the DNA lesion dGC8-N-ABA.
G designates dGC8-N-ABA.
2.2.3. Running Start Assays
The running start assays were performed as previously described.[8, 9, 27] Briefly, 5′-32P-labeled DNA (100 nM) and Dpo4 (100 nM) were preincubated in buffer R and subsequently mixed rapidly with a solution containing all four dNTPs (200 µM each) at 37 °C via rapid chemical-quench flow apparatus (KinTek). The reactions were quenched by the addition of EDTA to 0.37 M at specific time points. The reaction products were separated by denaturing PAGE (17% polyacrylamide, 8 M urea) and quantitated using a Typhoon Trio (GE Healthcare).
Quantitative analysis of the running start assays was performed by determining the relative lesion bypass efficiencies (dGC8-N-ABA bypass %) as a function of reaction time. For each time point t, dGC8-N-ABA bypass% = (B/E) × 100, where the total dGC8-N-ABA bypass events (B) was calculated from the concentration of all intermediate products with sizes greater than or equal to the 21mer, and the total dGC8-N-ABA “encounter” events (E) equaled the summation of the 20-mer concentration and the total dGC8-N-ABA bypass events (B). To quantitatively define the dGC8-N-ABA bypass efficiency, t50bypass was defined as the time required to bypass 50% of the total dGC8-N-ABA lesions encountered.
2.2.4. Electrophoretic Mobility Shift Assays
Buffer S containing 5′-32P-labeled DNA (5 nM) was titrated with Dpo4 (0 – 220 nM). Binary complexes were then separated from free DNA by native PAGE at a constant voltage of 80 V for 30 min at 25 °C using running buffer A. Complex formation was quantitated by using a Typhoon Trio (GE Healthcare). The plot of the binary complex (Dpo4•DNA) concentrations versus Dpo4 concentrations was fit to Equation 1 to yield Kd, DNA, the equilibrium dissociation constant for the binary complex (Dpo4•DNA) at 25 °C.
| (Eq. 1) |
In Equation 1, E0 is the active Dpo4 concentration while D0 is the DNA concentration.
2.2.5. Substrate Specificity Assays
A previously described single-turnover dNTP incorporation assay was employed to determine the maximum dNTP incorporation rate constant (kp), and equilibrium dissociation constant (Kd, dNTP) of an incoming dNTP.[6–8, 27] These two kinetic parameters yield the dNTP incorporation efficiency (kp/Kd, dNTP). Briefly, Dpo4 (120 nM) and 5′-32P-labeled DNA substrate (30 nM) were preincubated in Buffer R and then mixed rapidly with a solution containing increasing concentrations of dNTP. The reactions were quenched at specific time points by the addition of EDTA to 0.37 M. Reaction samples were analyzed by denaturing PAGE (17% acrylamide, 8 M urea) and quantitated by using a Typhoon Trio (GE Healthcare). The plot of product formation versus time for each dNTP concentration was fit to a single exponential equation (Equation 2),
| (Eq. 2) |
where kobs is the observed reaction rate constant and A is the reaction amplitude. Next, the kobs values were plotted against the dNTP concentrations and the plot was then fit to a hyperbolic equation (Equation 3),
| (Eq. 3) |
where kp is the maximum dNTP incorporation rate constant and Kd, dNTP is the equilibrium dissociation constant for the binding of dNTP to the binary complex (Dpo4•DNA) to form the ternary complex (Dpo4•DNA•dNTP).
2.2.6. Biphasic Kinetic Assays
Dpo4 (120 nM) and 5′-32P-labeled DNA (30 nM) were first preincubated in buffer R and the solution was then mixed rapidly with a solution containing both 5 µM DNA trap D-1 (Table 1) and 1.2 mM correct dNTP in buffer R by using a rapid chemical-quench flow apparatus. The reactions were quenched at specific times by the addition of EDTA to 0.37 M. The samples were resolved and quantitated as described above. The product formation was plotted versus time and the plot was fit to a double-exponential equation (Equation 4),
| (Eq. 4) |
Where Af and As are the reaction amplitudes of the fast and slow phase, respectively, while kf and ks are the rate constants of the fast and slow phase, respectively.
3. Results
3.1. Synthesis of 26-mer-dGC8-N-ABA
Synthesis of C8-dG-ABA was reported by Takamura-Enya and coworkers by Pd-catalyzed cross-coupling of 8-amino-dG with 3-bromobenzanthrone in the presence of XANTPHOS as the ligand.[28] Since conversion of the protected 8-bromo-dG to 8-amino-dG adds two steps and gives low yield of the amine, which drastically reduces the overall yield of the desired adduct, we attempted the reverse coupling of appropriately protected 8-bromo-dG, 3, with 3-aminobenzanthrone (Scheme 2). Using Pd2dba3 as the coupling agent with the ligand BINAP and NaOtBu base at 100 °C for 2 h, we were able to achieve the coupling yield (85%) of adduct 4 (Scheme 2) comparable to the reported reverse approach (80%).[28] Deprotection of 4 and subsequent conversion to the phosphoramidite monomer 6 (Scheme 2) for DNA synthesis also proceeded with a good yield. The protected monomer 6 was used to synthesize a 26-mer-dGC8-N-ABA template (Table 1), by standard protocol in an automated DNA synthesizer, except that the coupling time for this ABA-adducted monomer was increased to 20 min. Deprotection of the oligonucleotides was carried out with concentrated H4OH in the presence of β-mercaptoethanol. The 26-mer-dGC8-N-ABA template was purified by reverse-phase HPLC followed by denaturing PAGE and was analyzed by ESI-MS (Figs. S1 and S2 in Supporting Information). The m/z gave 8142 Da, which is 243 Da higher than the calculated mass of the unmodified 26-mer (7899.1 Da, Table 1) indicating the presence of dGC8-N-ABA in the 26-mer-dGC8-N-ABA template.
3.2. Bypass of a Site-Specifically Placed dGC8-N-ABA Lesion by Dpo4
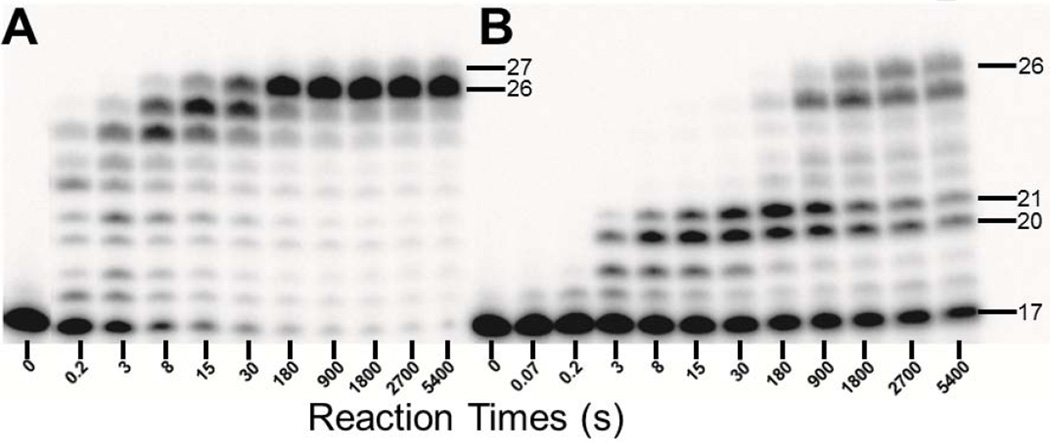
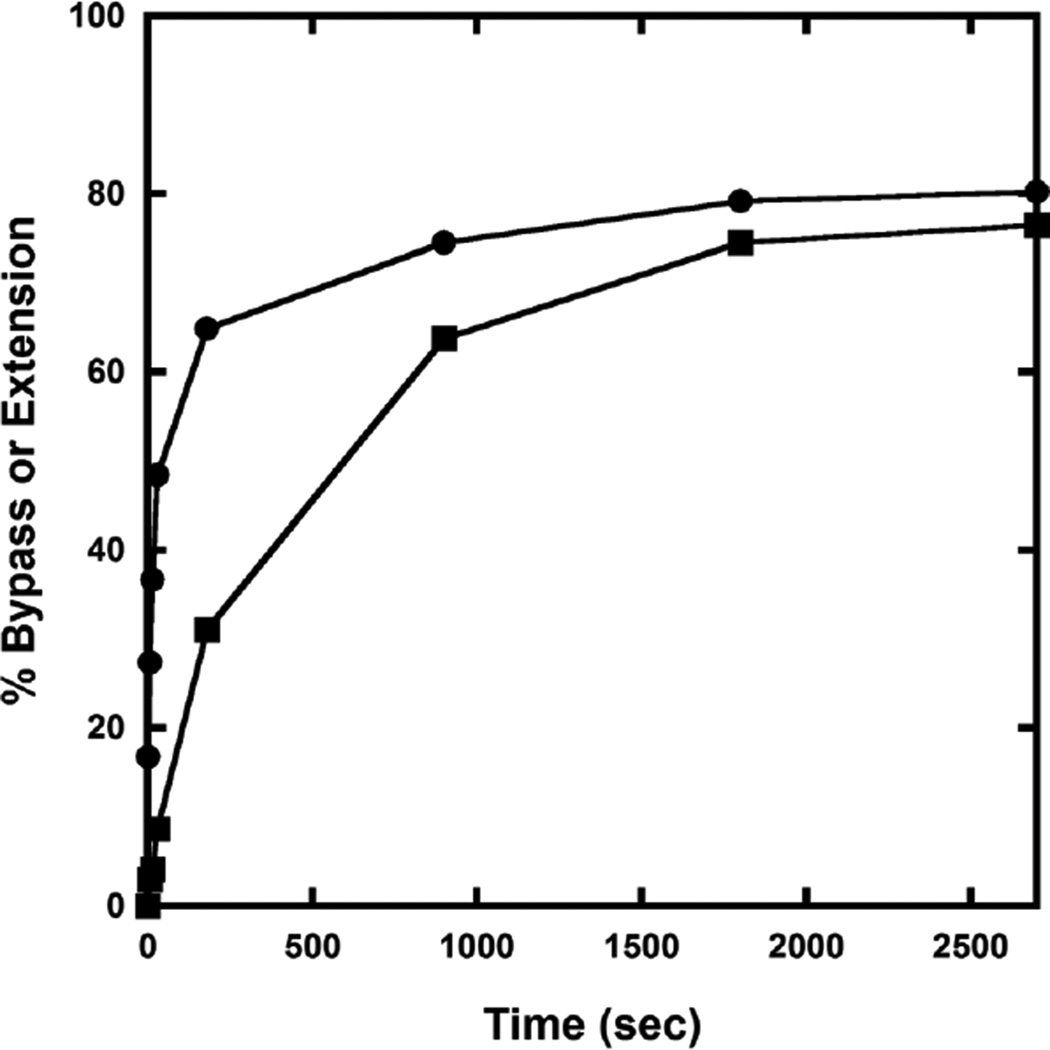
Running start assays were employed to examine the response of Dpo4 to a site specifically placed dGC8-N-ABA lesion in the 26-mer-dGC8-N-ABA template relative to the corresponding undamaged 26-mer template (Table 1). Dpo4 gradually elongated the 17-mer primer and synthesized the full-length product 26-mer in about 8 s with the control 17/26-mer DNA substrate, whereas it took about 900 s to achieve the same result with the damaged 17/26-mer-dGC8-N-ABA DNA substrate (Fig. 1). Thus, a single dGC8-N-ABA lesion slowed Dpo4-catalyzed DNA polymerization by more than 100-fold. To quantitatively study the polymerase pause sites, the t50bypass, defined as the time required to bypass 50% of the total dGC8-N-ABA lesions encountered by Dpo4, was estimated in Fig. 2 (see Experimental Procedures). Consistent with the observations in Fig.1, the t50bypass value was 30 s which is 270-fold greater than the t50 value of 0.11 s for the time to traverse 50% of undamaged dG encountered by Dpo4 at the same template position. Furthermore, the time required to extend 50% of the 21-mer lesion bypass product encountered by Dpo4 (t50extension) was estimated to be 600 s (Fig. 2), which is 320-fold greater than the t50extension value of 1.85 s for the time to traverse 50% of the same position in the undamaged template. As a result, relative to replication of the undamaged DNA substrate, Fig. 1B shows the strong accumulation of intermediate products of 20-mer and 21-mer for replication of the damaged template, indicating that Dpo4 paused strongly during the bypass of the lesion and also at the subsequent extension step. In summary, the 20-fold greater t50extension versus t50bypass (Fig. 2) indicates that Dpo4 pauses significantly more while extending the bypass product than while bypassing the lesion. A similar Dpo4 pausing pattern has been observed previously with another bulky lesion dGAP.[10] Besides the accumulation of the 20-mer and 21-mer products in Fig. 1B, the 25-mer intermediate product also accumulated in the presence of both control and damaged DNA substrates (Fig. 1). The accumulation was caused by “polymerase slippage” via primer realignment against the 26-mer template which contains three consecutive dCs at its 5′-terminus (Table 1).[10] Lastly, a small amount of 27-mer was synthesized by Dpo4 with the control DNA substrate 17/26-mer, resulting from a Dpo4-catalyzed blunt-end addition to the full-length 26-mer product.[29]
Figure 1. Running Start Assay.
A preincubated solution of 100 nM Dpo4 and 100 nM 5′-32P-labeled (A) 17/26-mer, or (B) 17/26-mer-dGC8-N-ABA was rapidly mixed with all four dNTPs (200 µM each) for various times before being quenched by addition of EDTA to 0.37 M. Sizes of important products are indicated, and the 21st position marks the position of the dGC8-N-ABA lesion from the 3′-terminus of the DNA template.
Figure 2.
Determination of t50bypass and t50extension. The percentages of the extension of the intermediate products 20-mer/26-mer-dGC8-N-ABA (●) and 21-mer/26-mer-dGC8-N-ABA (■) out of all polymerase encounter events were determined by quantitation of the gels in Figure 1 (see 2.2.3.) and then plotted against reaction time.
3.3. Moderate Effect of a dGC8-N-ABA Lesion on DNA Binding by Dpo4
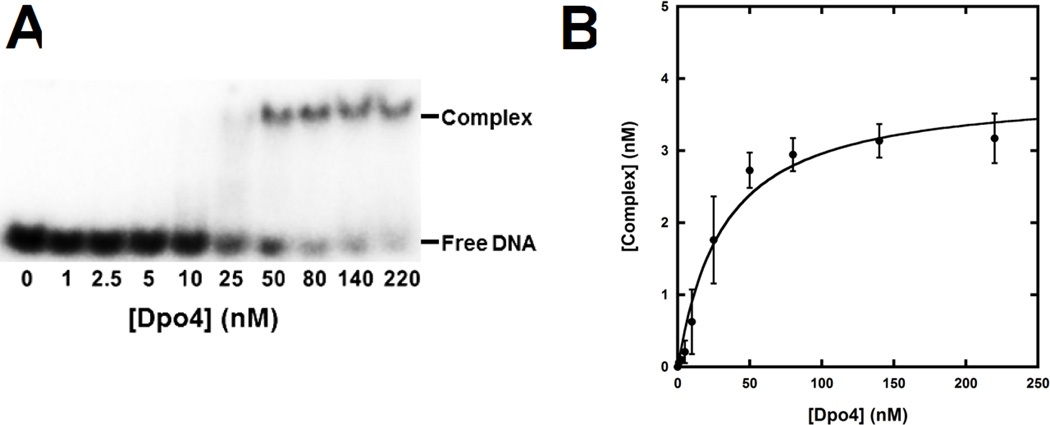
The significant impact of dGC8-N-ABA on the rate of DNA synthesis (Fig. 1B) may be attributed to an adverse effect on DNA binding by Dpo4. To examine this possibility, EMSAs were employed to measure the binding affinities of Dpo4 to several undamaged and damaged DNA substrates. As an example, Fig. 3 shows how the binding affinity between Dpo4 and [32P]-labeled 21/26-mer-dGC8-N-ABA was measured. First, 5′-32P-labeled 21/26-mer-dGC8-N-ABA (5 nM) was titrated with varying concentrations of Dpo4 and the resulting binary complex (Dpo4•21/26-mer-dGC8-N-ABA) was separated from free 21/26-mer-dGC8-N-ABA via native PAGE (Fig. 3A). The titration was repeated four times. After quantitation, the concentration of Dpo4•21/26-mer-dGC8-N-ABA was plotted against the total concentration of Dpo4 and the plot was then fit to Eq. 1 (“Experimental Procedures”) to obtain a Kd, DNA of 29 ± 7 nM (Fig. 3B). Based on measured Kd, DNA values in Table 2, Dpo4 bound to the undamaged DNA substrates with Kd, DNA values ranging from 3.1 to 4.0 nM. In comparison, the single dGC8-N-ABA lesion weakened the binding of the damaged DNA substrates to Dpo4 with affinities in the range of 5.0 to 29 nM. Notably, the affinity ratio (Table 2) is greater with 20/26-mer-dGC8-N-ABA (3-fold) and 21/26-mer-dGC8-N-ABA (7.8-fold). However, we concluded that the impact of the lesion on the DNA binding affinity of Dpo4 contributed but was not sufficient to explain the strong accumulation of the 20-mer and 21-mer in Fig. 1B.
Figure 3. Determination of Kd, DNA.
(A) A gel image shown the formation of the binary complex Dpo4•DNA during titration. Dpo4 (0 – 220 nM) was titrated into a solution containing 5′-32P-labeled 21/26-mer-dGC8-N-ABA (5 nM). The binary complex of Dpo4•DNA was separated from free DNA by native PAGE. The titration was repeated four times. (B) Plot of the binary complex concentration versus the total concentration of Dpo4. Each error bar represents the standard deviation of a complex concentration based on four independent experiments. The data were fit to Equation 1 (see 2.2.4.) which yielded a Kd, DNA of 29 ± 7 nM.
Table 2.
Binding Affinity of Dpo4 to Damaged and Control DNA Substrates at 23 °C
| DNA Substrate | Damaged DNAa (nM) |
Control DNAb,c (nM) |
Affinity Ratiod |
|---|---|---|---|
| 19/26-mer | 7.4 ± 0.8e | 3.1 ± 0.5 | 2.4 |
| 20/26-mer | 12 ± 3 | 4.0 ± 0.2 | 3.0 |
| 21/26-mer | 29 ± 7 | 3.7 ± 0.2 | 7.8 |
| 22/26-mer | 5.0 ± 0.9 | 3.8 ± 0.6 | 1.3 |
Refer to those DNA substrates with the template 26-mer-dGC8-N-ABA
Refer to those undamaged DNA substrates with the control template 26-mer
Values are from Table 2 of reference [10]
Calculated as (Kd, DNA)damaged/(Kd, DNA)control
Error is a data fitting error.
3.4. Kinetics of dNTP Incorporation in the presence of a dGC8-N-ABA lesion
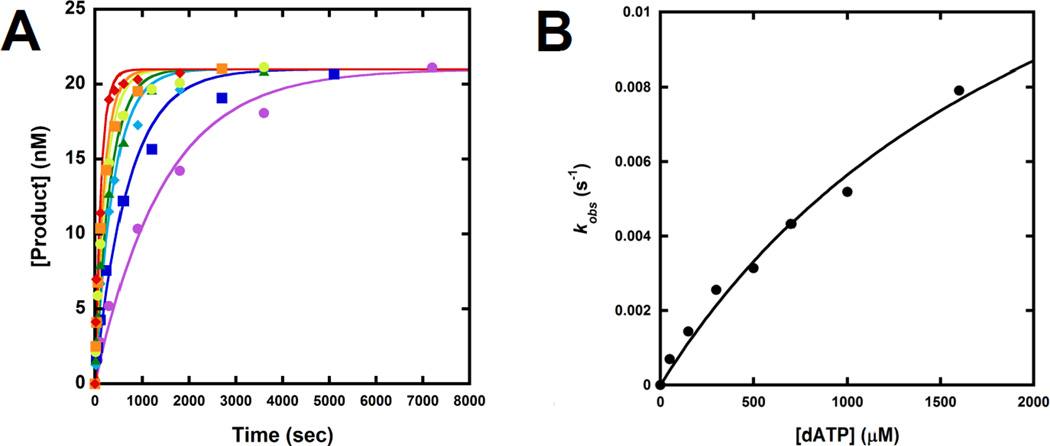
Other than the DNA binding affinity, the dGC8-N-ABA lesion may affect the kinetics of nucleotide incorporation opposite the lesion and/or at adjacent template positions. To test this scenario, we determined the maximum dNTP incorporation rate constant kp, the equilibrium dissociation constant of dNTP binding Kd,dNTP, and the substrate specificity (or efficiency) kp/Kd,dNTP for a single dNTP incorporation into undamaged or damaged DNA substrates with primers 18-mer to 22-mer under single turnover reaction conditions. As a representative example, Fig. 4 shows the measurement of the kp and Kd, dNTP values for dATP incorporation into 18/26-mer-dGC8-N-ABA. First, a preincubated solution of Dpo4 (120 nM) and 5′-32P-labeled 18/26-mer-dGC8-N-ABA (30 nM) was rapidly mixed and reacted with varying concentrations of dATP and quenched at various time points. The plot of product concentrations versus reaction times at each dATP concentration was fit to Eq. 2 (“Experimental Procedures”) to yield the observed rate constant, kobs. The kobs values were then plotted against dATP concentrations and the plot was fit to Eq. 3 (“Experimental Procedures”) to produce a kp of 2.0 ± 0.4 s−1 and a Kd, dATP of 2,200 ± 600 µM (Table 3). With varying sizes of the primer, we similarly measured the kinetic parameters for correct or incorrect nucleotide incorporation opposite dGC8-N-ABA and at the template positions upstream and downstream from the lesion (Table 3). The kp/Kd,dNTP values for correct dNTP incorporations into 18/26-mer-dGC8-N-ABA, 19/26-mer-dGC8-N-ABA, and 22/26-mer-dGC8-N-ABA are either similar to or less than 10-fold lower than the efficiency values with the corresponding control DNA substrates. The efficiency values are 120- and 170-fold lower for correct dNTP incorporation into 20/26-mer-dGC8-N-ABA and 21/26-mer-dGC8-N-ABA than into the control 20/26-mer and 21/26-mer substrates, respectively (Table 3). Thus, relative to undamaged DNA, Dpo4 had little difficulty elongating damaged DNA at non-pause sites (18/26-mer-dGC8-N-ABA, 19/26-mer-dGC8-N-ABA, and 22/26-mer-dGC8-N-ABA) but struggled at the two strong pause sites (20/26-mer-dGC8-N-ABA and 21/26-mer-dGC8-N-ABA), leading to the accumulation of 20-mer and 21-mer observed in Fig. 1B. Furthermore, with damaged DNA substrates containing a single site specifically placed dGC8-N-ABA lesion, we found that Dpo4 incorporated correct over incorrect nucleotides with 10- to 10,000-fold greater efficiency, resulting in polymerase fidelity in the range of 10−1 to 10−6 (Table 3). At the non-pause sites, the polymerase fidelity (10−3 to 10−6) is similar to the fidelity measured with control DNA.[7] In comparison, the polymerase fidelity (10−1 to 10−3) at the pause sites is 100-fold lower on average. Consequently, the probability of correct dNTP incorporation was above 98% at all sites tested in the damaged template, except with 21/26-mer-dGC8-N-ABA, where the probability of correct nucleotide incorporation fell to 84.3% (Table 3).
Figure 4. Kinetics of dATP incorporation onto 18/26-mer-dGC8-N-ABA.
(A) Dpo4 (120 nM) was first preincubated with 5′-32P-labeled 18/26-mer-dGC8-N-ABA (30 nM), and was then rapidly mixed with increasing concentrations of dATP (50 µM, ●; 150 µM, ■; 300 µM, ♦; 500 µM, ▲; 700 µM, ●; 1000 µM, ■; 1600 µM, ♦) for the indicated times before being quenched. Each time course was fitted to Equation 2 to yield a kobs. (B) the kobs values were plotted against corresponding dATP concentrations and the plot was fit to Equation 3 to produce a kp of 2.0 ± 0.4 s−1 and a Kd, dATP of 2,200 ± 600 µM.
Table 3.
Kinetic parameters for dNTP incorporation into damaged DNA substrates containing dGC8-N-ABA
| dNTP |
Kd,dNTP (µM) |
kp (s−1) |
(kp/Kd, dNTP)damaged (µM−1s−1) |
Efficiency Ratioa,b | Fidelityc | Probabilityd |
|---|---|---|---|---|---|---|
| 18/26-mer-dGC8-N-ABA | ||||||
| dCTP | 32 ± 5 | 2.83 ± 0.08 | 0.088 | 1.25 | - | 99.95 |
| dATP | 2200 ± 600 | (2.0 ± 0.4) × 10−2 | 9.1 × 10−6 | 0.85 | 7.3 × 10−6 | 0.01 |
| dGTP | 350 ± 40 | (6.2 ± 0.3) × 10−4 | 1.8 × 10−5 | 1.6 | 1.4 × 10−5 | 0.02 |
| dTTP | - | - | 1.6 × 10−5 | 5.4 | 1.3 × 10−5 | 0.02 |
| 19/26-mer-dGC8-N-ABA | ||||||
| dGTP | 50 ± 9 | 0.48 ± 0.02 | 9.6 × 10−3 | 2.6 | - | 99.82 |
| dATP | 430 ± 60 | (2.9 ± 0.2) × 10−3 | 6.7 × 10−6 | 2.1 | 7.0 × 10−4 | 0.07 |
| dCTP | 220 ± 80 | (1.4 ± 0.2) × 10−3 | 6.4 × 10−6 | 22 | 6.7 × 10−4 | 0.06 |
| dTTP | - | - | 5.5 × 10−6 | 1.6 | 5.7 × 10−4 | 0.05 |
| *20/26-mer-dGC8-N-ABA | ||||||
| dCTP | 510 ± 60 | 0.25 ± 0.02 | 4.9 × 10−4 | 120 | - | 98.93 |
| dATP | 640 ± 170 | (1.9 ± 0.3) × 10−4 | 3.0 × 10−6 | 6.3 | 6.1 × 10−4 | 0.61 |
| dGTP | 1200 ± 400 | (8.1 ± 0.1) × 10−4 | 6.8 × 10−7 | 66 | 1.4 × 10−4 | 0.14 |
| dTTP | 970 ± 390 | (1.6 ± 0.4) × 10−4 | 1.6 × 10−6 | 52 | 3.3 × 10−4 | 0.32 |
| *21/26-mer-dGC8-N-ABA | ||||||
| dGTP | 400 ± 80 | (8.9 ± 0.7) × 10−4 | 2.2 × 10−5 | 170 | - | 84.26 |
| dATP | 450 ± 50 | (4.5 ± 0.2) × 10−4 | 1.0 × 10−6 | 2.4 | 4.3 × 10−2 | 3.83 |
| dCTP | 160 ± 20 | (4.3 ± 0.1) × 10−4 | 2.7 × 10−6 | 12 | 1.1 × 10−1 | 10.34 |
| dTTP | 420 ± 80 | (1.7 ± 0.1) × 10−4 | 4.0 × 10−7 | 4.5 | 1.8 × 10−2 | 1.57 |
| 22/26-mer-dGC8-N-ABA | ||||||
| dCTP | 1400 ± 300 | 4.5 ± 0.7 | 3.2 × 10−4 | 11 | - | 99.55 |
| dATP | 470 ± 70 | (5.4 ± 0.4) × 10−4 | 1.1 × 10−5 | 0.38 | 3.4 × 10−4 | 0.34 |
| dGTP | 1100 ± 200 | (1.4 ± 0.2) × 10−4 | 1.3 × 10−6 | 6.5 | 4.1 × 10−4 | 0.04 |
| dTTP | 770 ± 140 | (1.7 ± 0.2) × 10−4 | 2.2 × 10−6 | 5.0 | 6.9 × 10−4 | 0.07 |
Denote pause sites.
Calculated as (kp/Kd, dNTP)control/(kp/Kd, dNTP)damaged.
Values for (kp/Kd, dNTP)control 19/26-mer to 22/26-mer are from supplemental Table 1 of reference [10]. Kinetic parameters for 18/26-mer are listed in SI Table 1.
Calculated as (kp/Kd, incorrect dNTP)damaged/((kp/Kd, correct dNTP)damaged + (kp/Kd, incorrect dNTP)damaged).
Calculated as ((kp/Kd, dNTP)damaged/(Σ(kp/Kd, dNTP)damaged)) × 100.
3.5. Biphasic Kinetics of dNTP Incorporation at Polymerase Pause Sites
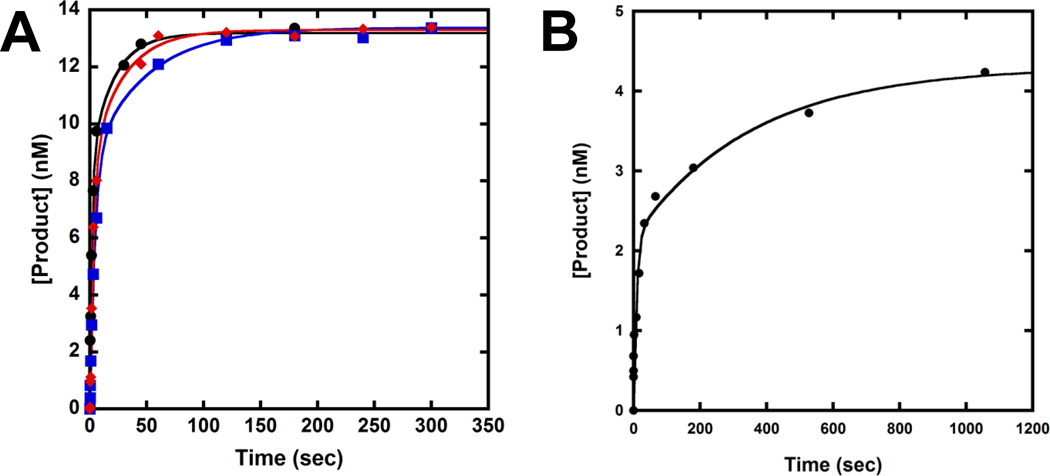
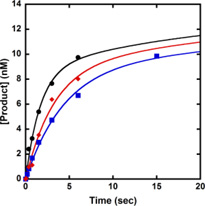
Our previous kinetic studies have shown that correct dNTP incorporation follows biphasic kinetics at each polymerase pause site induced by various DNA lesions.[8, 10, 27, 30] To examine if the kinetic trend is consistent at the two Dpo4 pause sites caused by dGC8-N-ABA, we performed a DNA trap experiment. A preincubated solution of Dpo4 (120 nM) and 5′-32P-labeled 20/26-mer-dGC8-N-ABA or 21/26-mer-dGC8-N-ABA (30 nM) was rapidly mixed with a solution containing correct dNTP (1.2 mM) and unlabeled D-1 DNA (5 µM, Table 1). The reaction was quenched at various times by addition of EDTA to 0.37 M. Previously, we have shown that a 167-fold molar excess of undamaged D-1 over 5′-32P-labeled damaged DNA was sufficient to trap any free Dpo4 before its rebinding to the damaged DNA substrate.[10, 27] As expected, biphasic kinetics of dNTP incorporation was observed with both damaged DNA substrates. Each plot of product concentration versus reaction time (Fig. 5) was fit to a double exponential equation (Eq. 4), yielding the biphasic kinetic parameters listed in Table 4. In comparison, the data fit poorly to a single exponential equation (Data not shown). This can be attributed to the fact that with either 20/26-mer-dGC8-N-ABA or 21/26-mer-dGC8-N-ABA, the first phase rate constant (kf) is substantially greater than the second phase rate constant (ks) while the reaction amplitudes in the first phase (Af) and the second phase (As) are comparable. The total reaction amplitudes (Af + As) were 44% with 20/26-mer-dGC8-N-ABA and 14.3% with 21/26-mer-dGC8-N-ABA, indicating that a substantial amount of damaged DNA was not converted into products during the DNA trap assays. This low overall reaction amplitude was possibly caused by the lesion and/or the reaction conditions. To exclude the latter possibility, similar DNA trap assays were previously performed with the undamaged 20/26-mer and 21/26-mer substrates [10]. Only a single fast phase of nucleotide incorporation was observed with the control substrates [10]. The reaction amplitudes with both 20/26-mer and 21/26-mer (Table 4) are ~ 90% [10], demonstrating that the presence of dGC8-N-ABA significantly lowered overall reaction amplitudes at the polymerase pause sites. Furthermore, to examine if the biphasic kinetics of nucleotide incorporation onto damaged DNA were affected by dNTP concentration, the same DNA trap assay with 20/26-mer-dGC8-N-ABA was performed under 450 or 1,000 µM dCTP (Fig. 5A) and the kinetic data were listed in Table 4. Notably, the biphasic data with 450, 1,000, and 1,200 µM dCTP (Table 4) reveal that the reaction amplitudes of the fast and slow phases were unchanged while their rate constants increased with higher dCTP concentrations.
Figure 5. Biphasic kinetics of correct dNTP incorporation in the presence of a DNA trap.
A preincubated solution of 120 nM Dpo4 and 30 nM 5′-32P-labeled 20/26-mer-dGC8-N-ABA (A) or 21/26-mer- dGC8-N-ABA (B) was mixed rapidly with 5 µM unlabeled 21/41-mer D-1 (DNA trap) and correct dNTP for various times before being quenched by EDTA. The plots of product versus time were fit to Eq. 4 and the data were listed in Table 4. (A) Dpo4•20/26-mer-dGC8-N-ABA was mixed with the DNA trap and 450 (Blue), 1,000 (Red), or 1,200 µM (Black) dCTP. The inset is a magnification of the time points from 0 to 20 seconds, showing the first phase of product formation. (B) Dpo4•21/26-mer-dGC8-N-ABA was mixed with the DNA trap and 1,200 µM dGTP.
Table 4.
Biphasic kinetic parameters for correct dNTP incorporation into a 5'-32P-labeled DNA substrate (30 nM) in the presence of a DNA trap (5 µM) at 37 °C
| DNA substrate | Correct dNTP |
Afa (nM) |
kf (s−1) |
Asa (nM) |
ks (s−1) |
|---|---|---|---|---|---|
| Nucleotide concentration = 1,200 µM | |||||
| b20/26-mer | dCTP | 26 ± 1 (86.7%) | 4.6 ± 0.5 | - | - |
| b21/26-mer | dGTP | 27.0 ± 0.4 (90%) | 2.3 ± 0.1 | - | - |
| 20/26-mer-dGC8-N-ABA | dCTP | 8.6 ± 0.7 (29%) | 0.60 ± 0.07 | 4.5 ± 0.7 (15%) | 0.05 ± 0.01 |
| 21/26-mer-dGC8-N-ABA | dGTP | 2.2 ± 0.4 (7.3%) | 0.12 ± 0.06 | 2.1 ± 0.5 (7%) | 0.0028 ± 0.0025 |
| Nucleotide concentration = 1,000 µM | |||||
| 20/26-mer-dGC8-N-ABA | dCTP | 8.6 ± 1.7 (29%) | 0.32 ± 0.09 | 4.7 ± 1.7 (16%) | 0.04 ± 0.01 |
| Nucleotide concentration = 450 µM | |||||
| 20/26-mer-dGC8-N-ABA | dCTP | 8.7 ± 0.2 (29%) | 0.23 ± 0.03 | 4.7 ± 0.6 (16%) | 0.021 ± 0.005 |
Percentage after each reaction amplitude is calculated as (reaction amplitude/30 nM) × 100%.
Reaction amplitude (A) and rate constant (k) values are from Table 4 of reference [10].
4. Discussion
4.1. Polymerase Pause Sites
During genomic replication, a bulky lesion like dGC8-N-ABA is expected to act as a road-block for high-fidelity, replicative DNA polymerases, which are known to have a tight active site and select against DNA base pairs with non-canonical or distorted geometry.[31] For low-fidelity, Y-family DNA polymerases like Dpo4, their loose and flexible active site tolerates damaged DNA which is geometrically distorted by various lesions as demonstrated by the ternary crystal structures of Dpo4, an incoming nucleotide, and DNA containing various lesions.[32–40] Our running start assays show that Dpo4 was indeed capable of bypassing the site-specifically placed dGC8-N-ABA lesion and synthesized full-length products albeit at a significantly slower rate than copying a corresponding undamaged DNA template (Fig. 1). The slower full-length product formation rate is primarily due to two consecutive strong polymerase pause sites evidenced by the accumulation of the 20-mer and 21-mer intermediate products (Fig. 1B). A similar polymerase pausing pattern was observed in our published kinetic studies of the bypass of dGAP.[10] During polymerization, Dpo4 paused when incorporating a nucleotide opposite the dGC8-N-ABA lesion, and even more so while extending the bypass product. These conclusions are consistent with the 270- and 320-fold longer t50 values for Dpo4 to traverse the 21st and 22nd positions, respectively, from the 3′-terminus of the damaged template 26-mer-dGC8-N-ABA than the corresponding positions on the control template 26-mer.
4.2. Kinetic Basis for Polymerase Pausing as a Result of dGC8-N-ABA
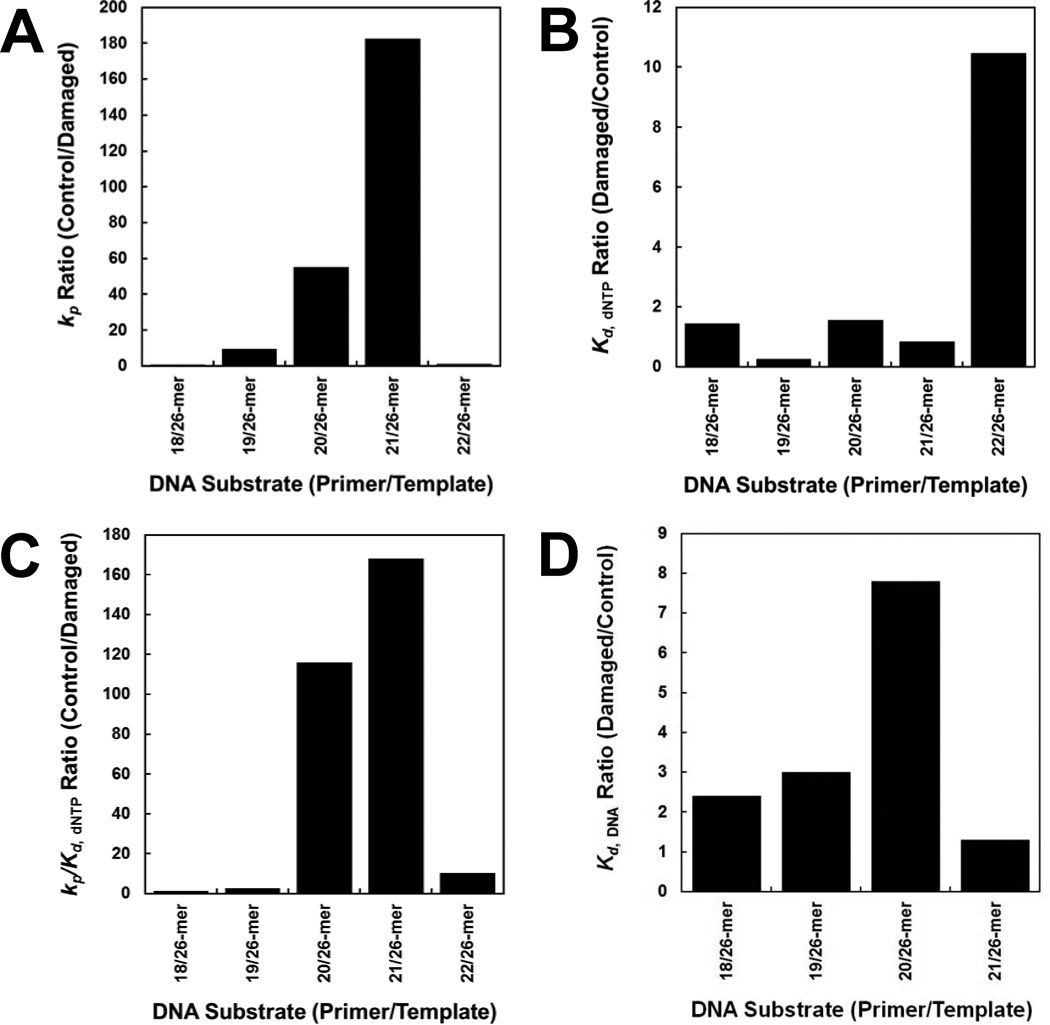
Our DNA-binding assays revealed that the dGC8-N-ABA lesion only reduced the binding affinity of Dpo4 to the damaged DNA substrates by up to 7.8-fold when compared to the corresponding undamaged DNA substrates (Fig. 6D), and Dpo4 binds to the damaged DNA substrates at non-pause sites with 2–6 fold higher affinities than at pause sites (Table 2). Although these affinity differences contribute to the strong pausing of Dpo4 at the two template positions (Fig. 1B) based on the large t50 differences, a reduction in DNA-binding affinity alone is not sufficient to induce such significant pausing by Dpo4. To further investigate the kinetic basis behind the bypass of dGC8-N-ABA catalyzed by Dpo4, individual nucleotide incorporation efficiencies at five template positions were measured with either damaged or undamaged DNA substrates (Table 1). Our kinetic data (Table 3) demonstrated that the dGC8-N-ABA lesion did not alter the kinetic trend that correct nucleotides were incorporated with significantly higher rate constants (kp) and efficiencies (kp/Kd,dNTP) than incorrect nucleotides at all template positions. For the three series reactions during polymerization including 18-mer→19-mer→20-mer, 19-mer→20-mer→21-mer, and 20-mer→21-mer→22-mer, they share the following kinetic pattern based on the kinetic data in Table 3: the first conversion step has higher kp and kp/Kd,dNTP values for correct nucleotide incorporation than the 2nd conversion step, leading to the accumulation of the intermediates 19-mer, 20-mer, and 21-mer, respectively. The reason why the 19-mer accumulated only at early time points is because the fast and efficient conversion of 19-mer→20-mer (0.48 s−1, 9.6 × 10−3 µM−1s−1) was completed at later time points (Figure 1B). In contrast, the strong accumulation of 21-mer product is due to large differences (22–28 fold) in both the rate constants and efficiencies in the conversion of 21-mer→22-mer (0.0089 s−1, 2.2 × 10−5 µM−1s−1) relative to the conversion of 20-mer→21-mer (0.25 s−1, 4.9 × 10−4 µM−1s−1). Similarly, a strong accumulation of 20-mer was contributed by the slower and less efficient conversion of 20-mer→21-mer (0.25 s−1, 4.9 × 10−4 µM−1s−1) than the conversion of 19-mer→20-mer (0.48 s−1, 9.6 × 10−3 µM−1s−1).
Figure 6. Quantitative effects of a dGC8-N-ABA lesion on DNA binding and correct dNTP incorporation catalyzed by Dpo4.
The ratios of the kinetic parameters including kp (A),Kd, dNTP (B), kp/Kd, dNTP (C), and Kd, DNA (D) between damaged and corresponding control DNA substrates were plotted against the primer sizes.
Notably, the 22-mer intermediate product did not accumulate (Fig. 1B), despite that the conversion of 22-mer→23-mer (3.2×10−3 µM−1s−1) has 3-fold lower efficiency than the conversion of 19-mer→20-mer (9.6 × 10−3 µM−1s−1) and the correct nucleotide incorporation efficiency ratio is 11-fold higher at this template position (Fig. 6A). The lack of 22-mer accumulation was due to the kinetic pattern in the reaction series 21-mer→22-mer→23-mer, whereas the 2nd step (4.5 s−1) is much faster than the first step (8.9 × 10−3 s−1). This kinetic pattern is opposite from the pattern deduced from the kinetic series reactions discussed above. Notably, these kinetic patterns for intermediate product formation have been previously revealed in the Dpo4 catalyzed bypass of an abasic site,[8] a cisplatin-d(GpG) adduct,[27] and a dGAP [10].
4.3. Large Effect of dGC8-N-ABA on the Kinetic Parameters of dNTP Incorporation
When comparing the kinetic parameters obtained with control and damaged templates, the correct nucleotide incorporation efficiency ratio, (kp/Kd,dNTP)control/(kp/Kd,dNTP)damaged, increases dramatically from non-pause to pause sites (Fig. 6C). For example, the ratios (Table 3) are less than 10-fold at non-pause sites while the ratios are 120 and 170 with primers 20-mer and 21-mer, respectively. Furthermore, the single dGC8-N-ABA lesion in 20/26-mer-dGC8-N-ABA and 21/26-mer-dGC8-N-ABA weakened their binding to Dpo4 by 3.0- and 7.8-fold, respectively (Fig. 6D and Table 2). Thus, the impact of dGC8-N-ABA on Dpo4•DNA binding is significantly smaller than on nucleotide incorporation at and near the lesion site. Since the overall efficiency of a polymerase at a template position is a function of both its binding affinity to DNA and nucleotide incorporation efficiency during processive polymerization, Dpo4 was 360- and 1,330-fold less efficient while elongating primers 20-mer and 21-mer, respectively, with the damaged template 26-mer-dGC8-N-ABA than with the control template 26-mer. Notably, the kp was 46- and 182-fold slower with 20/26-mer-dGC8-N-ABA and 21/26-mer-dGC8-N-ABA, respectively, than with the corresponding control substrates (Fig. 6A), while the equilibrium dissociation constant ratio (Kd,dNTP)damage/(Kd,dNTP)control was less than 2-fold (Fig. 6B). Thus, the ratio of (kp/Kd,dNTP)control/(kp/Kd,dNTP)damaged at each Dpo4 pause site (Fig. 6C) is dictated by the ratio of (kp)control/(kp)damaged. Oddly, correct dCTP was incorporated with a 10-fold greater Kd,dNTP in the presence of 22/26-mer-dGC8-N-ABA than in the presence of control 22/26-mer while the kp value was not altered by the lesion, leading to a nucleotide incorporation efficiency ratio of 10 at this non-pause site (Fig. 6). It is unclear how an embedded template nucleotide dGC8-N-ABA weakened the ground-state binding of an incoming nucleotide to Dpo4•22/26-mer-dGC8-N-ABA.
4.4. Kinetic Mechanism for dGC8-N-ABA Bypass by Dpo4
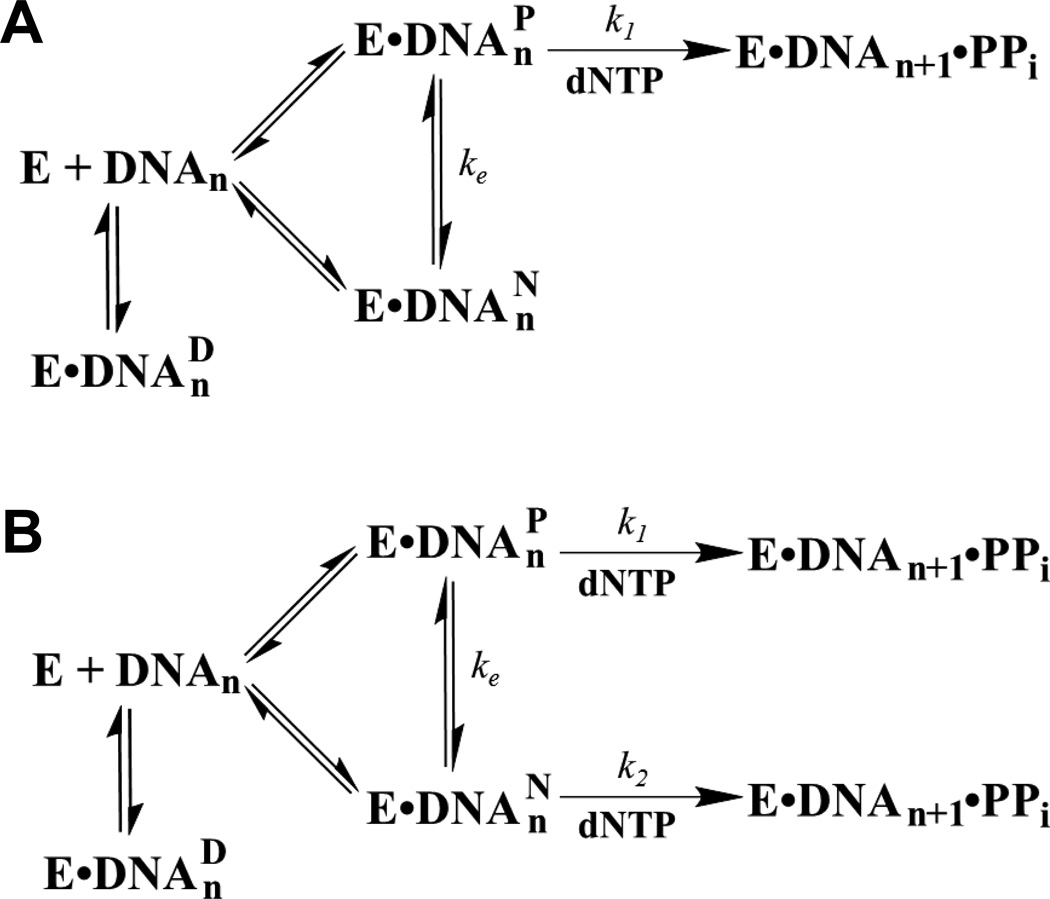
Since the presence of dGC8-N-ABA did not significantly affect the binding of Dpo4 to both 20/26-mer-dGC8-N-ABA and 21/26-mer-dGC8-N-ABA, in comparison to the respective control DNA substrates, it is likely that Dpo4 holds onto the damaged DNA substrates when incorporating a correct incoming dNTP with low kp values. This possibility was confirmed by the DNA trap assays which demonstrated that some of the Dpo4•DNA complex did not dissociate even in the slow reaction phase (Fig. 5). The biphasic kinetics of nucleotide incorporation at each pause site show that a fast phase (Af, kf) preceded a slow phase (As, ks) (Fig. 5 and Table 4). In both cases, the sum of the contribution from the fast (Afkf) and slow (Asks) phases yielded rate constants with 20/26-mer-dGC8-N-ABA (0.18 s−1) and 21/26-mer-dGC8-N-ABA (0.009 s−1) which were relatively close to the respective observed kobs values of 0.18 and 0.007 s−1, estimated using Equation 3, 1.2 mM dCTP in Fig. 5, and corresponding measured Kd,dNTP and kp values (Table 3). In comparison, similar DNA trap assays with the control 20/26-mer and 21/26-mer substrates only exhibited single, fast phase kinetics with greater reaction amplitudes of 87% and 90%, respectively (Table 4). The less than 100% reaction amplitudes with the control substrates can be attributed to experimental errors, inaccurate measurements of oligomer concentrations, incomplete or imperfect annealing of DNA duplexes, or/and Dpo4 binding at the blunt end rather than the staggered end of DNA.[29] Together, the kinetic data in the presence of a DNA trap suggest that the slow phase observed with the damaged DNA substrates was due to the formation of nonproductive complexes between Dpo4 and DNA (E•DNAnN) while the fast phase exhibited by each control or damaged DNA substrate indicates the formation of a productive complex (E•DNAnP), which was turned over rapidly once a nucleotide was bound. Notably, the kf values are smaller with the damaged DNA substrates than with the control DNA substrates, suggesting that even in the fast phase, Dpo4 did not bind to damaged DNA as productively as it interacted with undamaged DNA. Furthermore, since the slow elongation of E•DNAnN occurred in the presence of a large molar excess of unlabeled DNA trap, E•DNAnN was first transformed into E•DNAnP without dissociation with a first order rate of ke, and subsequently elongated (Scheme 3A). Interestingly, the mechanism in Scheme 3A has been implicated into the bypass of an abasic site,[8] a cisplatin-d(GpG) adduct,[27] and a dGAP lesion by Dpo4.[10] If the mechanism in Scheme 3A is correct at each of the two polymerase pause sites in Fig. 1B, the slow phase rate constant (ks) which is dominated by ke, should not be altered when the dNTP concentration is changed. To test this possibility, we performed the same DNA trap assays with 20/26-mer-dGC8-N-ABA in the presence of different dCTP concentrations. We did not perform such DNA trap experiments with 21/26-mer-dGC8-N-ABA because the overall reaction amplitude (4.3 nM) with this DNA substrate (Table 4) is small and the limited accuracy of the kinetic data may not allow us to draw a definite conclusion. With 20/26-mer-dGC8-N-ABA, we performed the DNA trap assays with dCTP concentration of 450, 1,000, and 1,200 µM (Figure 5A). The biphasic kinetic data in Table 4 indicate that the reaction amplitudes (Af and As) do not change with the variation of correct dCTP concentration, which is expected since dNTP concentration should not affect the binding of DNA and Dpo4. In contrast, both the fast and slow phase rate constants are 2- to 3-fold higher in the presence of 1,200 than 450 µM of dCTP while the rate constants with 1,000 µM dCTP are in the middle. These biphasic kinetic data suggest that the kinetic mechanism in Scheme 3A is inaccurate for the bypass of dGC8-N-ABA by Dpo4 and prompted us to propose a new one shown in Scheme 3B. In this mechanism, while the fast phase kf is still governed by the process of nucleotide incorporation onto E•DNAnP as in Scheme 3A, the slow phase rate constant (ks) is a function of both the process of slow nucleotide incorporation onto E•DNAnN (k2) and the interconversion between the non-productive and productive binary complexes (ke). Additionally, our kinetic data in Table 4 also suggest that ke cannot be much larger than k2. Otherwise, Scheme 3B will be simplified as Scheme 3A.
Scheme 3.
Proposed Kinetic Mechanisms (A) and (B) of Lesion Bypass.
Lastly, the sum of Af and As with either 20/26-mer-dGC8-N-ABA (44%) or 21/26-mer-dGC8-N-ABA (14.3%) is significantly smaller than the reaction amplitude (~90%) observed with either 20/26-mer or 21/26-mer (Table 4). Therefore, significant percentages of 20/26-mer-dGC8-N-ABA (90% − 44% = 46%) and 21/26-mer-dGC8-N-ABA (90% − 14% = 76%) bound by Dpo4 were either catalytically incompetent (E•DNAnD) or dissociated during the trap assays if the maximum reaction amplitudes with the two damaged substrates can be as high as their corresponding control substrates (~90%). As the formation of the ternary complexes E•DNAnN•dNTP and E•DNAnP•dNTP, dNTP binds to E•DNAnD and yields E•DNAnD•dNTP. Previously, such ternary complexes (E•DNAnD•dNTP) have been proposed as Dpo4, HIV-1 reverse transcriptase, and T7 DNA polymerase bind to damaged DNA containing dGAP,[10] N2-methylguanine,[41] O6-benzylguanine,[42] and O6-methylguanine.[42] Taken together, the above analysis suggests the kinetic mechanism in Scheme 3B, rather than in Scheme 3A, is accurate for the bypass of dGC8-N-ABA by Dpo4.
Although there are no reported crystal structures of Dpo4 in complex with DNA containing dGC8-N-ABA, several published structures of the Y-family polymerases and DNA containing similarly bulky lesions support the lesion bypass mechanisms (Scheme 3).[35, 36, 43] For example, the binary crystal structures of yeast Polη and DNA containing a damaged templating nucleotide N-2-acetylaminofluorene-dG (AAF-dG) [43] show that the AAF moiety either stacks above the junction base pair, or partially rotates out of the DNA helix but still blocks incoming dCTP. The first conformation of AAF completely blocks an incoming dNTP and thereby represents E•DNAnD. The second conformation of AAF likely represents E•DNAnN, which requires subtle to mild structural changes in order for AAF to be completely rotated out of the DNA helix. If the AAF moiety is completely out of the DNA duplex, the binary complex is considered to be in the form of E•DNAnP, which productively binds an incoming nucleotide and rapidly incorporates it. Interestingly, such a productive conformation (E•DNAnP•dNTP) has been displayed by the ternary structures of Dpo4, dNTP, and DNA containing either benzo[a]pyrene diol epoxide (BPDE)-dG or BPDE-dA,[35, 36] illustrating that the BPDE moiety is flipped out of the DNA helix into a structural gap between the Little Finger and Palm domains of Dpo4 and the distance between the primer 3′-OH and the α–phosphate of the incoming dNTP (3.9 Å) is close to the optimum catalytic distance (3.4 Å).[36, 44] Although these binary and ternary structures with AAF-dG[43] and BPDE[35, 36] are instructive, it remains to see how DNA containing dGC8-N-ABA binds to Dpo4 and forms the three conformations of E•DNAnD, E•DNAnN, and E•DNAnP.
4.5. Biological relevance of the kinetic studies of dGC8-N-ABA
Fig. 1B shows that a single dGC8-N-ABA considerably stalls DNA replication catalyzed by Dpo4 in vitro. Consistently, dGC8-N-ABA is recently shown to be the strongest replication block of the three major 3-NBA derived DNA adducts in an assay with nucleotide excision repair-deficient human XPA cells.[45] Furthermore, the fidelity values in Table 3 indicate that Dpo4 is prone to misincorporate nucleotides at the two pause sites (Fig. 1B). The error-prone manner is especially severe during the extension of the lesion bypass product, where Dpo4 misincoporates dCTP, dATP, and dTTP opposite dC with high frequencies of 10.3, 3.8, and 1.6%, respectively. Notably, dGC8-N-ABA, inducing primarily G-to-A transitions followed by G-to-T transversions, is found to be the most mutagenic 3-NBA adduct with a mutational frequency of 30.6% in the nucleotide excision repair-deficient human XPA cells.[45] The cellular results agree with our in vitro fidelity values although it remains to determine the identity of the polymerase which bypasses dGC8-N-ABA in the XPA cells.
Supplementary Material
Highlights.
3-Nitrobenzanthrone is one of the most mutagenic compounds from diesel exhaust
Pre-steady-state kinetic analysis of DNA damage bypass
A mechanism of lesion bypass by a model Y-family DNA polymerase is proposed
ACKNOWLEDGEMENTS
We are grateful to Dr. You-Jun Fu for his help with mass spectrometry. We thank Mr. Walter Zahurancik, Mr. Austin Raper, and Drs. David J. Taggart and Sheng Cao for reviewing this manuscript. Additionally we thank Mr. Brian A. Maxwell for suggestions.
FUNDING SOURCES
This work was supported by the National Institutes of Health Grant ES09127 to both A.K.B. and Z.S.
Abbreviations
- ABA
Aminobenzanthrone
- dGAP
N-(deoxyguanosin-8-yl)-1-aminopyrene
- dGC8-N-ABA
N-(deoxyguanosin-8-yl)-3-aminobenzanthrone
- dNTP
3’-deoxynucleotide 5′-triphosphate
- Dpo4
Sulfolobus Solfataricus DNA polymerase IV
- EMSA
electrophoresis mobility shift assay
- E•DNAnD
catalytically incompetent or dead-end binary complex
- E•DNAnN
non-productive binary complex
- E•DNAnP
productive binary complex
- E•DNAnD•dNTP
catalytically incompetent ternary complex
- E•DNAnN•dNTP
non-productive ternary complex
- E•DNAnP•dNTP
productive ternary complex
- 3-NBA
3-Nitrobenzanthrone
- NitroPAH
nitro polyaromatic hydrocarbon
- PAGE
polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPORTING INFORMATION AVAILABLE
A table of the kinetic parameters for the 18/26-mer control substrate (Table S1), denaturing PAGE of 32P-labeled control and adducted 26-mer (Fig. S1), and ESI-MS analysis of the adducted 26-mer-dGC8-N-ABA (Fig. S2) are available in the Supporting Information. This information is available free of charge via the Internet at http://www.journals.elsevier.com/dna-repair/.
References
- 1.Oh SM, Kim HR, Park YJ, Lee SY, Chung KH. Organic extracts of urban air pollution particulate matter (PM2.5)-induced genotoxicity and oxidative stress in human lung bronchial epithelial cells (BEAS-2B cells) Mutat. Res. 2009;723:142–151. doi: 10.1016/j.mrgentox.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Kawanishi M, Kanno T, Nishida H, Takamura-Enya T, Yagi T. Translesion DNA synthesis across various DNA adducts produced by 3-nitrobenzanthrone in Escherichia coli. Mutat. Res. 2013;754:32–38. doi: 10.1016/j.mrgentox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker GC, Wang Z, Woodgate R. The Y-family of DNA polymerases. Mol Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 5.Fowler JD, Suo Z. Biochemical, structural,m and physiological characterization of terminal deoxynucleotidyl transferase. Chem Rev. 2006;106:2092–2110. doi: 10.1021/cr040445w. [DOI] [PubMed] [Google Scholar]
- 6.Fiala KA, Suo Z. Mechanism of DNA Polymerization Catalyzed by Sulfolobus solfataricus P2 DNA Polymerase IV. Biochemistry. 2004;43:2116–2125. doi: 10.1021/bi035746z. [DOI] [PubMed] [Google Scholar]
- 7.Fiala KA, Suo Z. Pre-Steady-State Kinetic Studies of the Fidelity of Sulfolobus solfataricus P2 DNA Polymerase IV. Biochemistry. 2004;43:2106–2115. doi: 10.1021/bi0357457. [DOI] [PubMed] [Google Scholar]
- 8.Fiala KA, Hypes CD, Suo Z. Mechanism of abasic lesion bypass catalyzed by a Y-family DNA polymerase. J Biol Chem. 2007;282:8188–8198. doi: 10.1074/jbc.M610718200. [DOI] [PubMed] [Google Scholar]
- 9.Fiala KA, Suo Z. Sloppy bypass of an abasic lesion catalyzed by a Y-family DNA polymerase. J Biol Chem. 2007;282:8199–8206. doi: 10.1074/jbc.M610719200. [DOI] [PubMed] [Google Scholar]
- 10.Sherrer SM, Brown JA, Pack LR, Jasti VP, Fowler JD, Basu AK, Suo Z. Mechanistic Studies of the Bypass of a Bulky Single-base Lesion Catalyzed by a Y-family DNA Polymerase. J Biol Chem. 2009;284:6379–6388. doi: 10.1074/jbc.M808161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallager J HU, George M, Hendee L, Phillips DH, Lewtas J. Formation of DNA adducts in rat lung following chronic inhalation of diesel emissions, carbon black and titanium dioxide particles. Carcinogenesis. 1994;15:1291–1299. doi: 10.1093/carcin/15.7.1291. [DOI] [PubMed] [Google Scholar]
- 12.Enya T SH, Watanabe T, Hirayama T, Hisamatsu Y. 3-Nitrobenzanthrone a powerful bacterial mutagen and suspected human carcinogen found in diesel exhausts and airborne particulates. Environ. Sci. Technol. 1997;31:2772–2776. [Google Scholar]
- 13.Enya T SH, Watanabe T, Hirayama T, Hisamatsu Y. 3-Nitrobenzanthrone a powerful bacterial mutagen and suspected human carcinogen found in diesel exhausts and airborne particulates. Environ. Sci. Technol. 1997;31:2772–2776. [Google Scholar]
- 14.U.S. EPA. Health Assessment Document for Diesel Engine Exhaust. 2002 [Google Scholar]
- 15.Farmer PB. Diesel fuel and exhaust emissions: is there a human carcinogenic risk? Lancet. 1997;350:1118. doi: 10.1016/S0140-6736(05)63786-5. [DOI] [PubMed] [Google Scholar]
- 16.IARC monographs on the evaluation of carcinogenic risks to humans. Diesel and gasoline engine exhausts and some nitroarenes. International Agency for Research on Cancer. IARC Monogr Eval Carcinog Risks Hum. 1989;46:1–458. [PMC free article] [PubMed] [Google Scholar]
- 17.Arlt VM, Glatt H, Muckel E, Pabel U, Sorg BL, Seidel A, Frank H, Schmeiser HH, Phillips DH. Activation of 3-nitrobenzanthrone and its metabolites by human acetyltransferases, sulfotransferases and cytochrome P450 expressed in Chinese hamster V79 cells. Int J Cancer. 2003;105:583–592. doi: 10.1002/ijc.11143. [DOI] [PubMed] [Google Scholar]
- 18.Arlt VM, Sorg BL, Osborne M, Hewer A, Seidel A, Schmeiser HH, Phillips DH. DNA adduct formation by the ubiquitous environmental pollutant 3-nitrobenzanthrone and its metabolites in rats. Biochem Biophys Res Commun. 2003;300:107–114. doi: 10.1016/s0006-291x(02)02789-4. [DOI] [PubMed] [Google Scholar]
- 19.Bieler CA, Arlt VM, Wiessler M, Schmeiser HH. DNA adduct formation by the environmental contaminant 3-nitrobenzanthrone in V79 cells expressing human cytochrome P450 enzymes. Cancer Lett. 2003;200:9–18. doi: 10.1016/s0304-3835(03)00418-x. [DOI] [PubMed] [Google Scholar]
- 20.Nagy E, Zeisig M, Kawamura K, Hisamatsu Y, Sugeta A, Adachi S, Moller L. DNA adduct and tumor formations in rats after intratracheal administration of the urban air pollutant 3-nitrobenzanthrone. Carcinogenesis. 2005;26:1821–1828. doi: 10.1093/carcin/bgi141. [DOI] [PubMed] [Google Scholar]
- 21.Bieler CA, Cornelius MG, Klein R, Arlt VM, Wiessler M, Phillips DH, Schmeiser HH. DNA adduct formation by the environmental contaminant 3-nitrobenzanthrone after intratracheal instillation in rats. Int J Cancer. 2005;116:833–838. doi: 10.1002/ijc.21095. [DOI] [PubMed] [Google Scholar]
- 22.Arlt VM, Stiborova M, Henderson CJ, Osborne MR, Bieler CA, Frei E, Martinek V, Sopko B, Wolf CR, Schmeiser HH, Phillips DH. Environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols. Cancer Res. 2005;65:2644–2652. doi: 10.1158/0008-5472.CAN-04-3544. [DOI] [PubMed] [Google Scholar]
- 23.Oda Y, Zhang Y, Buchinger S, Reifferscheid G, Yang M. Roles of human sulfotransferases in genotoxicity of carcinogens using genetically engineered umu test strains. Environ Mol Mutagen. 2012;53:152–164. doi: 10.1002/em.20696. [DOI] [PubMed] [Google Scholar]
- 24.Arlt VM, Schmeiser HH, Osborne MR, Kawanishi M, Kanno T, Yagi T, Phillips DH, Takamura-Enya T. Identification of three major DNA adducts formed by the carcinogenic air pollutant 3-nitrobenzanthrone in rat lung at the C8 and N2 position of guanine and at the N6 position of adenine. Int J Cancer. 2006;118:2139–2146. doi: 10.1002/ijc.21622. [DOI] [PubMed] [Google Scholar]
- 25.Colis LC, Chakraborti D, Hilario P, McCarty C, Basu AK. Synthesis of oligonucleotides containing 2'-deoxyguanosine adducts of nitropyrenes, Nucleosides. nucleotides & nucleic acids. 2009;28:67–77. doi: 10.1080/15257770902736426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillet LC, Scharer OD. Preparation of C8-amine and acetylamine adducts of 2'-deoxyguanosine suitably protected for DNA synthesis. Org Lett. 2002;4:4205–4208. doi: 10.1021/ol026474f. [DOI] [PubMed] [Google Scholar]
- 27.Brown JA, Newmister SA, Fiala KA, Suo Z. Mechanism of double-base lesion bypass catalyzed by a Y-family DNA polymerase. Nucleic Acids Res. 2008;36:3867–3878. doi: 10.1093/nar/gkn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamura-Enya T, Ishikawa S, Mochizuki M, Wakabayashi K. A practical approach for the chemical synthesis of 2 '-deoxyguanosine-C8 adducts with mutagenic/carcinogenic amino- or nitro-arenes. Tetrahedron Lett. 2003;44:5969–5973. [Google Scholar]
- 29.Fiala KA, Brown JA, Ling H, Kshetry AK, Zhang J, Taylor JS, Yang W, Suo Z. Mechanism of Template-independent Nucleotide Incorporation Catalyzed by a Template-dependent DNA Polymerase. J Mol Biol. 2007;365:590–602. doi: 10.1016/j.jmb.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherrer SM, Sanman LE, Xia CX, Bolin ER, Malik CK, Efthimiopoulos G, Basu AK, Suo Z. Kinetic analysis of the bypass of a bulky DNA lesion catalyzed by human Y-family DNA polymerases. Chem Res Toxicol. 2012;25:730–740. doi: 10.1021/tx200531y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kool ET. Active site tightness and substrate fit in DNA replication. Annu Rev Biochem. 2002;71:191–219. doi: 10.1146/annurev.biochem.71.110601.135453. [DOI] [PubMed] [Google Scholar]
- 32.Zang H, Irimia A, Choi JY, Angel KC, Loukachevitch LV, Egli M, Guengerich FP. Efficient and High Fidelity Incorporation of dCTP Opposite 7,8-Dihydro-8-oxodeoxyguanosine by Sulfolobus solfataricus DNA Polymerase Dpo4. J Biol Chem. 2006;281:2358–2372. doi: 10.1074/jbc.M510889200. [DOI] [PubMed] [Google Scholar]
- 33.Ling H, Boudsocq F, Plosky BS, Woodgate R, Yang W. Replication of a cis-syn thymine dimer at atomic resolution. Nature. 2003;424:1083–1087. doi: 10.1038/nature01919. [DOI] [PubMed] [Google Scholar]
- 34.Ling H, Boudsocq F, Woodgate R, Yang W. Snapshots of replication through an abasic lesion; structural basis for base substitutions and frameshifts. Mol Cell. 2004;13:751–762. doi: 10.1016/s1097-2765(04)00101-7. [DOI] [PubMed] [Google Scholar]
- 35.Bauer J, Xing G, Yagi H, Sayer JM, Jerina DM, Ling H. A structural gap in Dpo4 supports mutagenic bypass of a major benzo[a]pyrene dG adduct in DNA through template misalignment. Proc Natl Acad Sci U S A. 2007;104:14905–14910. doi: 10.1073/pnas.0700717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling H, Sayer JM, Plosky BS, Yagi H, Boudsocq F, Woodgate R, Jerina DM, Yang W. Crystal structure of a benzo[a]pyrene diol epoxide adduct in a ternary complex with a DNA polymerase. Proc Natl Acad Sci U S A. 2004;101:2265–2269. doi: 10.1073/pnas.0308332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zang H, Goodenough AK, Choi JY, Irimia A, Loukachevitch LV, Kozekov ID, Angel KC, Rizzo CJ, Egli M, Guengerich FP. DNA adduct bypass polymerization by Sulfolobus solfataricus DNA polymerase Dpo4: analysis and crystal structures of multiple base pair substitution and frameshift products with the adduct 1,N2-ethenoguanine. J Biol Chem. 2005;280:29750–29764. doi: 10.1074/jbc.M504756200. [DOI] [PubMed] [Google Scholar]
- 38.Zang H, Chowdhury G, Angel KC, Harris TM, Guengerich FP. Translesion synthesis across polycyclic aromatic hydrocarbon diol epoxide adducts of deoxyadenosine by Sulfolobus solfataricus DNA polymerase Dpo4. Chem Res Toxicol. 2006;19:859–867. doi: 10.1021/tx060056s. [DOI] [PubMed] [Google Scholar]
- 39.Eoff RL, Irimia A, Egli M, Guengerich FP. Sulfolobus solfataricus DNA polymerase Dpo4 is partially inhibited by "wobble" pairing between O6-methylguanine and cytosine, but accurate bypass is preferred. J Biol Chem. 2007;282:1456–1467. doi: 10.1074/jbc.M609661200. [DOI] [PubMed] [Google Scholar]
- 40.Eoff RL, Angel KC, Egli M, Guengerich FP. Molecular basis of selectivity of nucleoside triphosphate incorporation opposite O6-benzylguanine by sulfolobus solfataricus DNA polymerase Dpo4: steady-state and pre-steady-state kinetics and x-ray crystallography of correct and incorrect pairing. J Biol Chem. 2007;282:13573–13584. doi: 10.1074/jbc.M700656200. [DOI] [PubMed] [Google Scholar]
- 41.Choi JY, Guengerich FP. Analysis of the effect of bulk at N2-alkylguanine DNA adducts on catalytic efficiency and fidelity of the processive DNA polymerases bacteriophage T7 exonuclease- and HIV-1 reverse transcriptase. J Biol Chem. 2004;279:19217–19229. doi: 10.1074/jbc.M313759200. [DOI] [PubMed] [Google Scholar]
- 42.Woodside AM, Guengerich FP. Misincorporation and stalling at O(6)-methylguanine and O(6)-benzylguanine: evidence for inactive polymerase complexes. Biochemistry. 2002;41:1039–1050. doi: 10.1021/bi011496f. [DOI] [PubMed] [Google Scholar]
- 43.Schorr S, Schneider S, Lammens K, Hopfner KP, Carell T. Mechanism of replication blocking and bypass of Y-family polymerase {eta} by bulky acetylaminofluorene DNA adducts. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20720–20725. doi: 10.1073/pnas.1008894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Magnesium-induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawanishi M, Fujikawa Y, Ishii H, Nishida H, Higashigaki Y, Kanno T, Matsuda T, Takamura-Enya T, Yagi T. Adduct formation and repair, and translesion DNA synthesis across the adducts in human cells exposed to 3-nitrobenzanthrone. Mutat Res. 2013;753:93–100. doi: 10.1016/j.mrgentox.2013.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.