Summary
Anorexia nervosa (AN) is prevalent in adolescents and young adults, and endocrine changes include hypothalamic amenorrhea, a nutritionally acquired growth hormone resistance with low insulin like growth factor-1 (IGF-1), relative hypercortisolemia, decreases in leptin, insulin, amylin and incretins, and increases in ghrelin, PYY and adiponectin. These changes in turn have deleterious effects on bone, and may affect neurocognition, anxiety, depression and eating disorder psychopathology. Low bone density is particularly concerning; clinical fractures occur and changes in both bone microarchitecture and strength estimates have been reported. Recovery causes improvement of many, but not all, hormonal changes, and deficits in bone accrual may persist despite recovery. Physiologic, primarily transdermal, estrogen replacement increases bone density in adolescents, although catch-up is incomplete. In adults, oral estrogen co-administered with rhIGF-1 in one study, and bisphosphonates in another increased bone density, though not to normal. More studies are necessary to determine the optimal therapeutic approach in AN.
Keywords: estrogen, testosterone, cortisol, growth hormone, IGF-1, ghrelin, leptin, PYY, adiponectin, bone microarchitecture, bone strength, bone density
Introduction
Anorexia nervosa (AN) is a condition of severe undernutrition that is prevalent in adolescent girls and young women, and reported in 0.2–1% of this population 1–5. It is characterized by an altered body image, very low weight associated with an inability to gain or maintain weight, and in females, Diagnostic and Statistical Manual-IV (DSM-IV) included amenorrhea for at least three cycles in the diagnostic criteria 6. The revised DSM-V criteria for AN differ in that weight criteria are less stringent and amenorrhea is no longer required for this diagnosis 7. The condition occurs predominantly in women, and adolescence is a common time for the onset of AN 8, 9. AN is also reported in males, who comprise 10% of all diagnosed AN patients 10, although recent papers suggest a higher prevalence 5. In response to the severe energy restriction, alterations occur in many endocrine axes, most of which are adaptive to stimulate food intake, help maintain euglycemia, and divert available energy for essential body functions. Hypothalamic oligo-amenorrhea in AN causes infertility which typically reverses with stable weight restoration 11, 12. Hormonal alterations contribute to low bone density and increased risk for fractures, a major co-morbidity associated with AN 13. Furthermore, neuropsychiatric co-morbidities such as anxiety and depressive symptoms may be associated with hormonal changes seen in AN 14–17.
Although 50% of adults with AN recover following behavioral, psychiatric and medical therapy 18, about 30% demonstrate only partial recovery, and the remainder are characterized by remissions and relapse or chronic disease 19, 20. A major concern is a high risk for suicide, a common cause of death in AN 21. Among adolescents with AN, relapses occur following inpatient hospital admissions in 30% before medical recovery, however, about 70–75% completely recover over 5–10 years, with a low later risk of relapse 22, 23. About 30% of restrictors will develop binge eating behaviors in the long term 22.
Nutrient Intake and Resting Energy Expenditure in AN
Macronutrient Intake and Resting Energy Expenditure
Adolescents and adults with AN have lower total caloric intake compared with normal-weight controls, and the reduced caloric intake is primarily from marked reductions in fat intake, although decreases are also observed in absolute protein and carbohydrate intake 24, 25. Lower fat intake in AN is associated with lower fat mass 24. Individuals with AN have lower resting energy expenditure than normal-weight controls 24, likely an adaptive mechanism to preserve energy for vital functions. Consistent with findings of lower resting energy expenditure, cold activated brown (or metabolically active) adipose tissue is lower in AN compared with controls 26. One study reports that compared with constitutionally thin individuals and normal-weight controls, individuals with AN have similar total energy expenditure (assessed using doubly labeled water studies), but lower energy intake 27.
Micronutrient Intake
Intake of saturated and unsaturated fat is lower in AN than controls. In contrast, intake of soluble and insoluble fiber is higher in AN, as is intake of oxalates and phytates 24, all of which may potentially reduce absorption of other nutrients. Thus attention to diet composition is important in AN. Vitamin intake from diet and supplements, including of vitamin D, is typically higher in AN than controls, mostly from increased supplement use. In one study of teenagers with AN, 76% of the girls with AN compared with 50% of controls met the recommended dietary intake (RDI) for vitamin D 24. Another study found a low prevalence of vitamin D deficiency in adolescents with AN (2% in AN compared to 24% in controls) 28. Similarly, intake of calcium, magnesium and zinc is higher in AN than controls, and about 59% of AN girls compared with 30% of controls meet the RDI for calcium 24.
Impact of AN on Body Composition and Liver Function
In addition to lower BMI, individuals with AN have lower fat and lean mass, with marked reductions in percent trunk fat, trunk to extremity fat ratio (TEFR) and percent extremity lean mass 29–31. Reductions in trunk fat are related to increased growth hormone (GH) concentrations 31, whereas reductions in percent extremity lean mass are related to higher cortisol 31. Girls with the highest GH and lowest cortisol have the lowest trunk fat. GH and cortisol are gluconeogenic hormones that increase in conditions of energy deprivation and mobilize energy for vital functions. Effects of GH are mediated primarily through increased lipolysis, thus providing required substrate for gluconeogenesis 32. This is consistent with inverse associations of GH with body fat, and particularly with trunk fat. Associations of relative hypercortisolemia in AN with lower extremity lean mass are consistent with the muscle wasting reported in conditions of hypercortisolemia 33, although other factors may contribute. The impact of weight recovery on body composition varies with age group. Both adolescents and adults have increases in fat and lean mass, percent body fat, percent trunk fat and TEFR with weight gain. However, whereas in adolescents, percent trunk fat and TEFR approach that in normal-weight controls 29, in adults with AN, these parameters may exceed that in controls 30.
Endocrine Consequences of AN
Changes occur in multiple endocrine axes, and the severity of changes is related to the degree of undernutrition.
a) Growth hormone (GH)- Insulin like growth factor-1 (IGF-1) axis
Adolescents 34, 35 and adults 36–39 with AN have higher GH levels than controls 35, 37 (Figure 1), and those with the lowest BMI and fat mass have the highest GH 35, 37. However, systemicIGF-1 levels are low in AN 35–39, indicative of a nutritionally acquired resistance to GH. This is likely from a down-regulation of GH receptor expression, corroborated by low levels of GH binding protein, the cleaved extracellular domain of the GH receptor 36, 38. This state of GH resistance in AN is further confirmed by a lack of increase in IGF-1 following administration of supraphysiological doses of recombinant human GH (rhGH) in women with AN 40, 41. Because IGF-1 is an important bone anabolic hormone, GH resistance in AN associated with low IGF-1 levels is an important determinant of impaired bone metabolism.
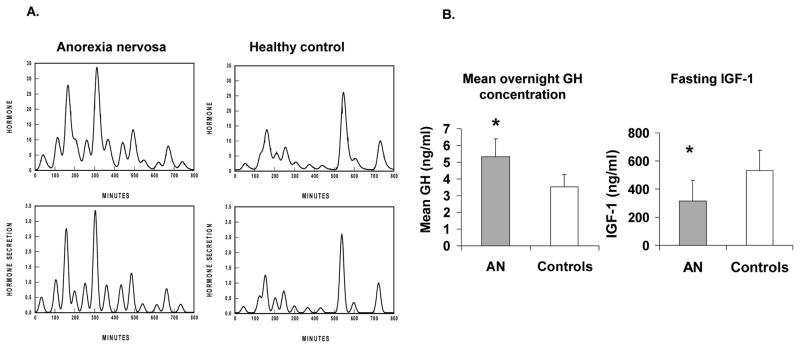
Figure 1. Impact of anorexia nervosa (AN) on growth hormone (GH) and IGF-1.
A. Representative overnight GH secretory characteristics in a teenage girl with AN (left) and a normal-weight control (right) showing increased GH secretion in the girl with AN.
B. Mean overnight GH concentrations (left) and fasting IGF-1 levels (right) in adolescent girls with AN and controls. Adolescent girls with AN have lower IGF-1 levels than controls despite higher GH concentrations, suggestive of a nutritionally acquired resistance to GH effects. Adapted from Misra et al. J Clin Endocrinol Metab 88: 5615–5623; 2003. Copyright © by The Endocrine Society 2003.
The increase in GH in AN is consistent with its gluconeogenic role (through increased lipolysis), to maintain euglycemia in a state of low energy availability, and is subsequent to (i) decreased negative feedback from low levels of IGF-1 35, 36, and (ii) increased levels of ghrelin (a GH secretagogue) 34, 42. Importantly, effects of GH on carbohydrate metabolism and lipolysis are not IGF-1 mediated, and these effects are preserved in AN. Hence, supraphysiologic GH administration in adult women with AN leads to a decrease in fat mass, even though IGF-1 levels do not change 40. Weight gain leads to a normalization of GH secretion 36 and GHBP levels 36, 38, consistent with these changes being adaptive to nutritional status.
b) Hypothalamic-pituitary-adrenal (HPA) axis
AN is associated with a state of relative hypercortisolemia in adults and adolescents 15, 39, 43–50 (Figure 2). However, levels rarely exceed twice the upper limit of normal and are not associated with Cushingoid features. In addition to CRH stimulating increased secretion of ACTH and thus cortisol, increased ghrelin secretion in AN 42 may stimulate increased secretion of CRH, ACTH and cortisol 45, 50. In fact, positive associations are reported between ghrelin and cortisol secretory parameters in AN 42. Those with the lowest BMI, fat mass and fasting glucose and insulin levels have the highest cortisol, consistent with the increase in cortisol being an adaptive mechanism to maintain euglycemia in a state of low energy availability 43.
Figure 2. Impact of anorexia nervosa (AN) on cortisol secretion.
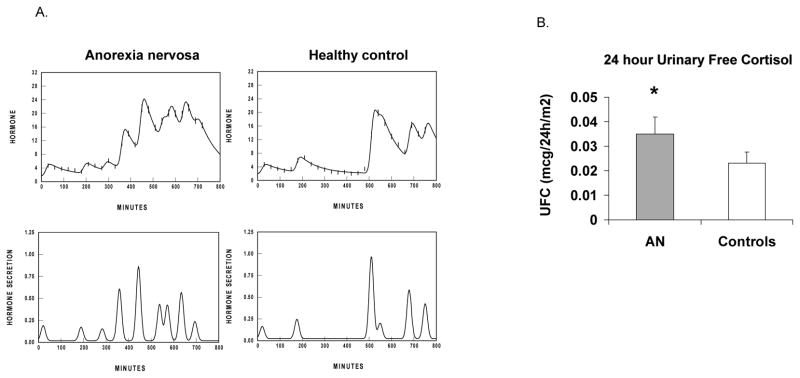
A. Representative overnight cortisol secretory characteristics in a teenage girl with AN (left) and a normal-weight control (right), showing greater cortisol secretion in the girl with AN.
B. 24-h urinary free cortisol levels in adolescent girls with AN and controls. Adolescent girls with AN have higher 24-h urinary free cortisol compared with controls. Adapted from Misra et al. J Clin Endocrinol Metab 89: 4972–4980; 2004. Copyright © by The Endocrine Society 2004.
Higher cortisol levels predict lower percent extremity lean mass 31 and lower bone density in AN15, 43. One study has reported that higher baseline cortisol in girls with AN is predictive of subsequent increases in fat mass, which in turn predicts resumption of menses 51. In contrast, another study suggests that increased secretion of CRH in AN may contribute to the severity of weight loss in AN from the strong anorexigenic drive of CRH 52, and higher cortisol secretion has been demonstrated to predict greater eating disorder psychopathology, independent of BMI 16. No pharmacological approaches are currently recommended to address the relative hypercortisolemia in AN given that these are adaptive changes and do not contribute to a Cushingoid state (in contrast to pathological hypercortisolemia).
c) Hypothalamic-pituitary-thyroid (HPT) axis
Changes observed in the HPT axis in AN are consistent with starvation and those seen with the sick euthyroid syndrome, and do not need to be treated. Levels of total T3 are low, likely an adaptive mechanism to lower resting energy expenditure and conserve energy for vital functions. T3 levels are associated with lower BMI and leptin levels and higher ghrelin and cortisol 42, 43. Free T4 varies from normal to low normal or low, depending on the severity of AN, while TSH levels are typically normal or low normal 39, 42, 43. In adults with AN, the response of TSH to exogenous TRH administration is blunted 53.
d) Insulin, gut peptides and adipokines
Low weight and BMI, and lower glucose levels in AN are associated with lower fasting insulin levels than in controls 54. Lower levels of insulin enable counter-regulatory mechanisms to come into play, including glycogenolysis, lipolysis and gluconeogenesis. Amylin is a hormone secreted by pancreatic beta cells with insulin in a 1:1 ratio, and levels of amylin are low in AN 55, associated with low BMI and percent body fat, as are levels of the incretins, glucagon like peptide-1 56 and glucose-dependent insulinotropic peptide (GIP) 55, 57. Lower insulin and amylin levels help preserve euglycemia, but contribute to lower BMD in AN.
Changes also occur is other gut peptides in AN. It is unknown whether all changes in these peptides are adaptive to starvation and return to normal with sustained weight recovery, or whether some may play a role in the pathogenesis of AN. Levels of ghrelin and peptide YY (PYY) are higher in AN compared with normal-weight controls 34, 42, 58, although studies have also reported unchanged 59 or lower 60 PYY. Ghrelin is an orexigenic hormone (in addition to being a GH secretagogue), and is secreted by the oxyntic cells of the stomach 61. In healthy individuals, ghrelin levels increase immediately before meals, and nadir about 30 minutes after food intake 61. Higher fasting and overnight ghrelin levels have been reported in AN 34, 42, 62, associated inversely with BMI, fat mass, and insulin. Studies have reported differences in ghrelin levels in restrictive vs. binge-purge forms of AN (AN-R vs. AN-BP) vs. bulimia nervosa (BN), with ghrelin levels (compared to controls) being higher in AN-R 59, 60, unchanged in BN 59, and lower in AN-BP 60. Obestatin, a ghrelin gene product that inhibits appetite and gastric motility, is also increased in adults with AN 59, 60, and positively associated with ghrelin 63, 64.
One study has implicated a genetic variation of the ghrelin activator gene ghrelin O-acyltransferase (GOAT) in the etiology of AN 65, and another suggests that the 3056 T-->C single nucleotide polymorphism of the ghrelin gene is related to recovery from AN-R 66. However, another study did not find a higher occurrence of three ghrelin gene polymorphisms in patients with eating disorders 67. It is unclear if and how these polymorphisms contribute to AN.
Higher ghrelin levels are consistent with an adaptive mechanism to increase food intake, and two-weeks of twice daily ghrelin infusion in a small group of five women with AN led to reduced gastrointestinal symptoms, and increased hunger and caloric intake 68. More studies are necessary to confirm these promising findings. In addition, ghrelin stimulates GH and ACTH secretion 45 (thus contributing to the counter-regulatory response to maintain euglycemia), and inhibits gonadotropins 69, 70. In girls with AN, higher ghrelin concentrations predict higher GH and cortisol secretion, and lower LH and estradiol 42. Weight gain is associated with a reduction in ghrelin, consistent with an adaptive response to starvation, although levels may remain higher than in normal-weight controls 42. Circulating ghrelin is present in acylated (active) and des-acylated (inactive) forms, and following short-term weight gain, acylated ghrelin and the ratio of acylated/total ghrelin increase, whereas desacylated ghrelin levels decrease 62.
PYY is an anorexigenic hormone secreted by the L (endocrine) cells of the distal gut, whose levels rise 15–30 minutes after food intake and induce postprandial satiety. PYY levels are variably reported to be higher 16, 58, 71, unchanged 59, or lower 60 in AN than in normal-weight controls, and correlate inversely with BMI and fat mass 16, 58, 71. Unlike other hormonal changes that are likely adaptive in AN and work to maintain a state of energy balance, an increase in PYY, an anorexigenic hormone, would not be adaptive and has been hypothesized that it may play an as yet undetermined role in the pathogenesis of this disorder.
Changes occur in adipokines in AN, including levels of leptin, adiponectin and inflammatory cytokines such as IL-6 17, 54, 72, 73. Leptin levels are markedly lower in AN than controls 17, 72, 73. Leptin correlates positively with fat mass, and weight gain is associated with an increase in leptin 72. In contrast, adiponectin levels are variably reported to be unchanged 54, higher 74–76 or lower 77 in AN compared with controls and may depend on measurement of adiponectin isoforms 78. Lower adiponectin in very severe AN 77 may be a consequence of marked reductions in fat mass. All these hormone changes contribute to hypogonadism and low bone density. However, at this time, there are no data to indicate that these changes need to be addressed pharmacologically.
e) Hypothalamic-pituitary-gonadal (HPG) axis
Decreased energy availability in AN is believed to cause hypothalamic amenorrhea 79. Women with AN often have patterns of LH pulsatility that resemble those in prepubertal or early pubertal girls, with very low amplitude LH pulses or a sleep entrained pattern of LH pulsatility 80. Altered gonadotropin secretion in AN has been associated with decreases in fat mass, a reflection of energy stores, and alterations in hormones that are secreted by adipocytes (leptin and adiponectin) or regulated by fat/energy stores (ghrelin, PYY and cortisol) 69, 70, 81–84. Normal leptin levels are permissive for puberty and facilitate normal gondadotropin secretion85.
Altered LH pulsatility manifests as hypothalamic amenorrhea. Some women with AN present with irregular periods rather than complete amenorrhea, and this may reflect changing energy status over time. With weight gain and an increase in fat mass, menstrual function resumes in a large proportion of women with AN, and data suggest that the most important determinant of resumption of menstrual function is an increase in fat mass 51 (Figure 3). Although there is significant overlap of fat content amongst those who do or do not recover weight and menses, one study in adolescents with AN reported that all girls with body fat greater than 24% resumed menses, whereas none of those with body fat less than 18% had menstrual recovery 51. There may be a lag period between increase in body weight and menstrual recovery in AN, and it is prudent to wait a period of at least 6 months following attainment of target weight before considering further investigations. A few studies have suggested that persistent amenorrhea following weight recovery in women with AN may indicate an underlying predisposition for polycystic ovarian syndrome (PCOS) 86, 87.
Figure 3. The proportion of body fat is an important determinant of menstrual function.
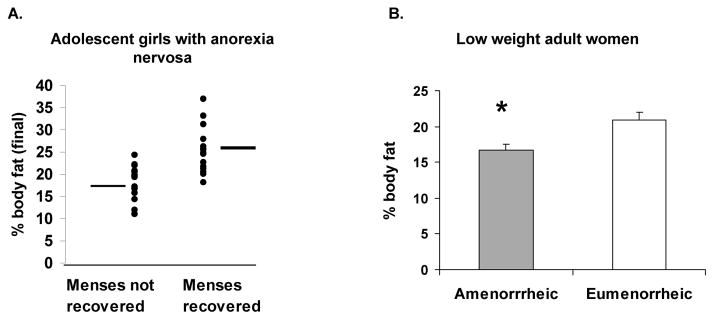
A. Percent body fat in girls with anorexia nervosa (AN) who did or did not resume menses: Girls with AN who recovered menstrual function over a year of follow-up had greater percent body fat at the end of the follow-up period than those who did not. All girls with body fat greater than 24% resumed menses, whereas none of those with body fat less than 18% had menstrual recovery. From Misra et al. Pediatr Res 59: 598–603; 2006. Copyright © by International Pediatric Research Foundation, Inc., 2006.
B. Percent body fat in low weight adult women who were amenorrheic (mean BMI 16.8±0.2 kg/m2) vs. those who were eumenorrheic (mean BMI 17.1±0.2 kg/m2). Despite similarly low BMI status, eumenorrheic women had greater percent body fat than amenorrheic women. Adapted from Miller et al. J Clin Endocrinol Metab 89: 4434–38; 2004. Copyright © by The Endocrine Society 2004.
Hypothalamic oligo-amenorrhea leads to hypoestrogenism and decreased gonadal secretion of testosterone 88, 89. Decreased gonadal steroid secretion has a deleterious effect on bone metabolism, and hypogonadotropic hypogonadism causes infertility that is reversible with recovery from AN 12.
One longitudinal study of the HPG axis in three males with AN reported low morning levels of leptin, gonadotropins and testosterone at the onset of the study, with an increase in these levels with weight gain 90. Another study also demonstrated lower LH, FSH and testosterone, but normal inhibin levels in males with AN than in controls 91. Low testosterone secretion in young men with AN has detrimental effects on bone and body composition 71, 92.
Fertility in Anorexia Nervosa
Women with AN and hypothalamic oligo-amenorrhea are typically infertile until their HPG axis recovers with stable weight restoration 11, 12. The occurrence of AN with bulimia nervosa is associated with a longer time to conceive on treatment, and negative feelings during pregnancy 93. In women with persistent infertility, fertility is inducible with ovulation induction techniques 12, 94. Also, unplanned pregnancies are reported to be more common in AN compared to the general population 93. There are reports of an increased risk for miscarriages, cesarean deliveries, premature births and perinatal lethality in women with a history of AN 11, 95, indicating the need for careful monitoring of these women during pregnancy and their offspring following birth. One study reported that whereas 50 of 140 women gave birth to a total of 86 children, none of 11 males with AN had children 95. While these are small numbers, it is possible that perturbations in the male reproductive axis may have a greater impact on fertility than those in women.
f) Posterior Pituitary Hormones (Including Renal Function)
Some studies have reported altered osmoregulation in AN, attributed to abnormalities in osmoregulation of vasopressin, intrinsic renal defects and the effects of antidepressants 96. Women with AN have lower plasma sodium and osmolality, higher levels of antidiuretic hormone (ADH) and more concentrated urine than controls 97. Following water deprivation, however, ADH secretion is suboptimal with decreased urine concentrating ability 97. Older case reports indicate a risk for nephrocalcinosis, renal calculi, rhabdomyolysis and renal failure in AN; however, there are only a few recent reports of these renal complications 98–103, likely because of earlier diagnosis and improved medical care. Rhabdomyolysis has been associated with both hypokalemia and hypophosphatemia 100, 101. Hypokalemia can also occur from purging behaviors, particularly in bulimia nervosa, and from laxative and diuretic abuse 104, 105. Hypophosphatemia may occur during refeeding 100. Thus, it is important to monitor electrolytes in individuals with AN.
Pooled nocturnal oxytocin is lower in adult women with AN than in controls and is associated with lower fat mass 106. Oxytocin has anorexigenic effects and is also bone anabolic through effects on osteoblasts 107. Recent studies have also shown that estrogen increases oxytocin secretion by osteoblasts, which then acts on its own receptor to exert an anabolic effect on bone 108. Thus, lower oxytocin levels in AN may contribute to impaired bone metabolism. Importantly, food induced oxytocin secretion in AN-R (and in recovered AN patients) predicts eating disorder psychopathology and hypo-activation of food motivation circuitry 109.
g) Bone mineral metabolism
One of the most concerning consequences of AN that may persist even with weight recovery is low bone mineral density (BMD) 89, 110, 111, associated with altered bone microarchitecture 112, 113 and reduced strength estimates 112, 114. Importantly, women with AN have a higher prevalence of fractures than reported in the general population 13, 115.
Bone mineral density in AN
Adults and adolescents with AN have low areal bone mineral density (aBMD) at the spine and the hip compared with controls, indicating that both trabecular and cortical sites are affected in this disease 89, 110, 111, 116. One study reported that 92% and 38% of adult women with AN have T-scores of <−1 and <−2.5 respectively at one or more skeletal sites 116. In the absence of weight and menstrual recovery, women with AN lose bone mass at an annual rate of 2.6% at the spine and 2.4% at the hip 110 (Figure 4). With weight gain, preferential increases occur in bone density at the total hip, whereas with menstrual recovery, preferential gains are noted at the spine 110 (Figure 4).
Figure 4. Impact of weight and/or menstrual recovery on bone density parameters in adult women with anorexia nervosa not receiving oral contraceptives.
A. Women who both improved weight and resumed menses increased BMD at the PA spine and hip, compared with those who neither improved weight nor resumed menses.
B. Women who resumed menses increased PA spine BMD (but not hip BMD), compared with those who did not improve menstrual function.
C. Women who improved weight increased hip BMD (but not PA spine BMD), compared with those who did not improve weight.
Black bars, PA spine BMD; white bars, hip BMD. *, P < 0.05.
From Miller et al. J Clin Endocrinol Metab 91: 2931–7; 2006. Copyright © by The Endocrine Society 2006.
Similar to adults, low aBMD is characteristic of adolescents with AN. Early studies indicated that as many as 50% of girls with AN may have aBMD Z-scores of <−2 at diagnosis 117. However, recent studies are more encouraging, likely because of greater awareness of this condition and earlier diagnosis and treatment. We have reported that 52% of adolescents with AN have aBMD Z-scores of <−1 at one or more sites, with the spine (a site of trabecular bone) being commonly affected 111. In addition, in contrast to healthy adolescents who demonstrate the continued increase in bone accrual that is necessary for attainment of optimal peak bone mass, bone accrual plateaus in adolescents with AN 89, 118. This raises concerns for attainment of peak bone mass, a critical determinant of future bone health and fracture risk. With weight and menses recovery, increases occur in bone accrual 118. However, rates remain lower than in normal-weight controls, and catch-up does not occur. In fact, aBMD Z-scores often continue to decrease even following weight gain118. In addition, low bone density is observed in adolescent boys with AN 71, 119. In contrast to girls, boys have greater involvement of sites of predominantly cortical bone (such as the hip), with 65% and 50% of boys with AN having Z-scores of <−1 at the femoral neck and spine compared with 18% and 24% of normal-weight boys 71.
Bone microarchitecture and strength estimates
Adult women with AN have decreased cortical thickness and cortical volumetric bone mineral density (vBMD) at the distal radius, as assessed by high resolution peripheral quantitative computed tomography (HRpQCT) 120. Trabecular parameters are also impacted, with reductions in trabecular number and thickness and increased trabecular separation113, 120. Strength estimates assessed using micro finite element analysis (μFEA) are lower in women with AN than controls 121. Similarly, adolescents with AN have altered bone microarchitecture with lower cortical and trabecular thickness, lower cortical area, increased trabecular area, and lower total and trabecular vBMD compared to controls 112. Cortical porosity is higher in AN, and stiffness and failure load (strength estimates) lower 112.
Determinants of impaired bone metabolism
Important determinants of low bone density in AN include lower BMI and lean body mass 110, 111, 116, consistent with known effects of mechanical loading on bone. Particularly, lower lean mass predicts lower bone density at the hip and whole body, and size parameters of bone microarchitecture, such as cortical thickness and total area.
A major contributor to low bone density and impaired bone microarchitecture is hypogonadism. Later age at menarche and a longer duration of amenorrhea are associated with lower bone density and impaired microarchitecture, and lower estradiol and testosterone predict lower bone density in AN 71, 88, 111, 116. Many studies have demonstrated that oral estrogen administration as monotherapy is not effective in increasing bone density in adults and adolescents with AN 122, 123, attributed to the IGF-1 lowering effects of oral estrogen 124, 125. Importantly, physiologic estrogen replacement using transdermal estradiol with cyclic progesterone increases spine and hip aBMD Z-scores in adolescents with AN 126 (Figure 5). However, complete catch-up does not occur, likely because residual alterations persist in other hormones that may impact bone. In a randomized placebo-controlled study, transdermal testosterone replacement did not improve bone density in adult women with AN 127. Data are not available regarding the impact of testosterone replacement on bone density in males with AN. Oral DHEA with ethinyl estradiol was demonstrated in one study to prevent further decreases in bone density 128.
Figure 5. Impact of physiologic estrogen replacement on bone density in adolescent girls with anorexia nervosa (AN). Girls with AN randomized to physiologic estrogen administration (ANE+) had significant increases in bone density at the lumbar spine over 6, 12 and 18 months compared with those randomized to placebo (AN-E−), to approximate bone accrual rates observed in controls (C) (adjusted for baseline age and weight).

From Misra et al. J Bone Mineral Metab. 26; 2430–2438; 2011. Copyright © by The American Society for Bone and Mineral Research, 2011.
A key determinant of impaired bone metabolism in AN is GH resistance with low IGF-1 35, 40, 89. In AN, IGF-1 levels correlate positively with markers of bone turnover, aBMD 35, 89, and bone structural parameters 113, and administration of rhIGF-1 in replacement doses of 30–40 mcg/k twice daily increases levels of bone formation markers in both adolescents and adults with AN 129, 130. One 9-month randomized controlled study of oral estrogen with rhIGF-1 in adults with AN demonstrated an increase in aBMD compared to no treatment, estrogen or rhIGF-1 alone 131, and studies of estrogen and rhIGF-1 administration in adolescents with AN are ongoing.
Other determinants of low BMD include high levels of cortisol15, 43 and PYY 58, 132 and low levels of leptin, insulin and amylin 54, 55. PYY inhibits osteoblastic activity, and PYY transgenic mice have decreased bone formation 133. In AN, high PYY levels correlate with lower BMD in adults, and with lower levels of bone turnover markers in adolescents 58, 132. Leptin, insulin and amylin all have bone anabolic effects, and lower levels of these hormones in AN correlate with lower BMD 54, 55, 113. Finally, adiponectin is deleterious to bone, and inverse associations exist between adiponectin and spine bone mineral apparent density in AN 54.
Management of low bone density and impaired bone accrual
The most important strategy to improve bone density and bone accrual in adolescents with AN is recovery of weight and menstrual function. However, catch-up does not occur even with recovery, and because bone accrual rates remain lower than in controls, bone density Z-scores continue to decrease 118. This lack of catch-up may reflect persistent abnormalities in hormones such as cortisol, or frequent relapses. It is important to supplement vitamin D to maintain 25(OH) vitamin D levels above 30 ng/ml. Because the teenage years are such a narrow window of time during which to optimize bone accrual, one may consider physiologic transdermal estrogen replacement (with cyclic progesterone) 126 in teenagers with AN who have completed growth and have a significant fracture history, or very low bone density Z-scores (Z-scores <−2) 134 with deterioration over time. However, the impact of estrogen replacement on fracture risk in AN remains unclear. Testosterone replacement is not effective in increasing bone density in women with AN 127. Bisphosphonates increase bone density in adults 127, but do not increase spine bone density measures in adolescents with AN 135. If considered as a therapeutic strategy, these drugs should be given cautiously in women of reproductive age, given concerns regarding their long half-life.
Impact of Endocrine Changes on Eating Disorder Psychopathology, Anxiety and Depression in AN
Lower levels of gonadal hormones, oxytocin and leptin, and higher cortisol and PYY have been implicated in eating disorder psychopathology, and symptoms of anxiety and depression in AN 16, 17, 109, 136. Administration of transdermal estradiol reduces trait anxiety in girls with AN without affecting eating attitudes or body shape perception 137. In addition, estrogen replacement prevents the increase in aberrant eating attitudes and body dissatisfaction noted with weight gain in AN.
Conclusion
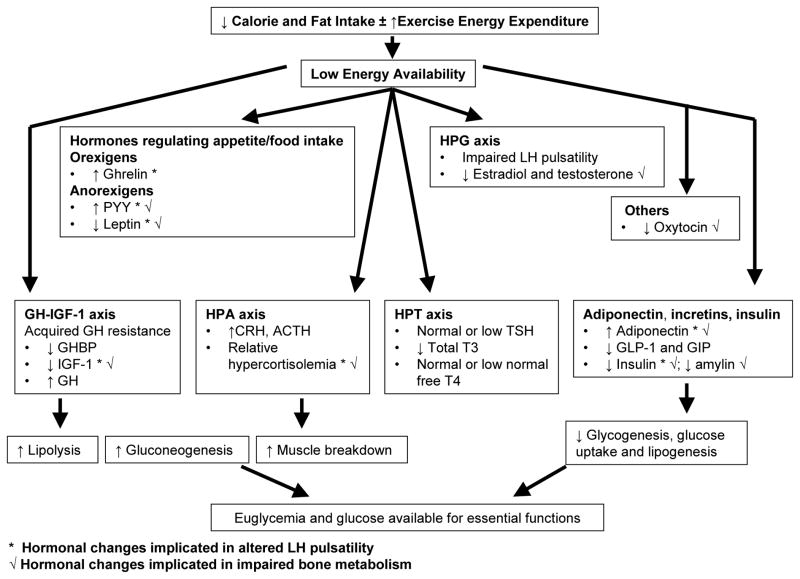
AN is thus associated with mostly adaptive changes in multiple endocrine axes to optimize energy intake and availability for vital functions (Figure 6). However, these changes contribute to low bone density and possibly to neurocognitive changes and psychopathology in AN. Studies are ongoing to assess the impact of various therapeutic strategies on bone accrual, density, and structure n AN, and to better understand the links between various hormonal changes and food motivation pathways that involve reward and satiety.
Figure 6. Endocrine changes in anorexia nervosa that maintain euglycemia and preserve energy for vital functions, and also contribute to impaired bone metabolism.
CRH: corticotrophin releasing hormone; ACTH: adrenocorticotropic hormone
GH-IGF-1 axis: growth hormone- insulin like growth factor-1 axis
GHBP: GH binding protein
GLP-1: glucagon like peptide-1; GIP: glucose-dependent insulinotropic peptide
HPG axis: hypothalamic-pituitary-gonadal axis
HPA axis: hypothalamic-pituitary-adrenal axis
HPT axis: hypothalamic-pituitary-thyroid axis
LH: luteinizing hormone
PYY: peptide YY
T3: tri-iodothyronine; T4: tetra-iodothyronine; TSH: thyroid stimulating hormone
Search Strategy.
We searched PUBMED (1990–2013) using the search terms “anorexia nervosa” or “eating disorders” in combination with the terms “hypogonadism”, “estrogen”, “testosterone”, “growth hormone”, “IGF-1”, “cortisol”, “thyroid”, “ghrelin”, “leptin”, “peptide YY”, “adiponectin”, “amylin”, “GLP-1”, “GIP”, “bone density”, “bone structure or microarchitecture”, “finite element analysis”, or “fractures”. We largely selected publications in the past 15 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. A few review articles and book chapters are cited to provide readers with more details and references than can be accommodated in this Review.”
Acknowledgments
Funding Sources: NIH Grant Numbers 1 R01 HD060827, 1 K24 HD071843-01A, 5 UL1 RR025758 and 2 RO1 DK062249.
Footnotes
The authors have no conflicts of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Jaite C, Hoffmann F, Glaeske G, Bachmann CJ. Prevalence, comorbidities and outpatient treatment of anorexia and bulimia nervosa in German children and adolescents. Eat Weight Disord. 2013;18(2):157–65. doi: 10.1007/s40519-013-0020-4. [DOI] [PubMed] [Google Scholar]
- 2.Wade TD, Bergin JL, Tiggemann M, Bulik CM, Fairburn CG. Prevalence and long-term course of lifetime eating disorders in an adult Australian twin cohort. Aust N Z J Psychiatry. 2006;40(2):121–8. doi: 10.1080/j.1440-1614.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 3.Pelaez Fernandez MA, Labrador FJ, Raich RM. Prevalence of eating disorders among adolescent and young adult scholastic population in the region of Madrid (Spain) J Psychosom Res. 2007;62(6):681–90. doi: 10.1016/j.jpsychores.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry. 2006;19(4):389–94. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- 5.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diagnostic and statistical manual of mental disorders. 3. Washington, D.C: American Psychiatric Association; 1987. [Google Scholar]
- 7.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5. 2013. DSM-5. [Google Scholar]
- 8.Halmi KA, Casper RC, Eckert ED, Goldberg SC, Davis JM. Unique features associated with age of onset of anorexia nervosa. Psychiatry Res. 1979;1(2):209–15. doi: 10.1016/0165-1781(79)90063-5. [DOI] [PubMed] [Google Scholar]
- 9.Favaro A, Caregaro L, Tenconi E, Bosello R, Santonastaso P. Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry. 2009;70(12):1715–21. doi: 10.4088/JCP.09m05176blu. [DOI] [PubMed] [Google Scholar]
- 10.Raevuori A, Hoek HW, Susser E, Kaprio J, Rissanen A, Keski-Rahkonen A. Epidemiology of anorexia nervosa in men: a nationwide study of Finnish twins. PLoS One. 2009;4(2):e4402. doi: 10.1371/journal.pone.0004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik CM, Sullivan PF, Fear JL, Pickering A, Dawn A, McCullin M. Fertility and reproduction in women with anorexia nervosa: a controlled study. J Clin Psychiatry. 1999;60(2):130–5. doi: 10.4088/jcp.v60n0212. quiz 5–7. [DOI] [PubMed] [Google Scholar]
- 12.Perkins RB, Hall JE, Martin KA. Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum Reprod. 2001;16(10):2198–205. doi: 10.1093/humrep/16.10.2198. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders--a nationwide register study. Int J Eat Disord. 2002;32(3):301–8. doi: 10.1002/eat.10101. [DOI] [PubMed] [Google Scholar]
- 14.Miller KK, Wexler TL, Zha AM, Lawson EA, Meenaghan EM, Misra M, et al. Androgen deficiency: association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry. 2007;68(6):959–65. doi: 10.4088/jcp.v68n0621. [DOI] [PubMed] [Google Scholar]
- 15.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94(12):4710–6. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, et al. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol. 2011;164(2):253–61. doi: 10.1530/EJE-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (Oxf) 2012;76(4):520–5. doi: 10.1111/j.1365-2265.2011.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. 2001;31(5):881–90. doi: 10.1017/s003329170100407x. [DOI] [PubMed] [Google Scholar]
- 19.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284–93. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 20.Fichter MM, Quadflieg N, Hedlund S. Twelve-year course and outcome predictors of anorexia nervosa. Int J Eat Disord. 2006;39(2):87–100. doi: 10.1002/eat.20215. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos FC, Karamanis G, Brandt L, Ekbom A, Ekselius L. Childbearing and mortality among women with anorexia nervosa. Int J Eat Disord. 2013;46(2):164–70. doi: 10.1002/eat.22051. [DOI] [PubMed] [Google Scholar]
- 22.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22(4):339–60. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Herpertz-Dahlmann B, Muller B, Herpertz S, Heussen N, Hebebrand J, Remschmidt H. Prospective 10-year follow-up in adolescent anorexia nervosa--course, outcome, psychiatric comorbidity, and psychosocial adaptation. J Child Psychol Psychiatry. 2001;42(5):603–12. [PubMed] [Google Scholar]
- 24.Misra M, Tsai P, Anderson EJ, Hubbard JL, Gallagher K, Soyka LA, et al. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr. 2006;84(4):698–706. doi: 10.1093/ajcn/84.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadigan CM, Anderson EJ, Miller KK, Hubbard JL, Herzog DB, Klibanski A, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord. 2000;28(3):284–92. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97 (4):E584–90. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossu C, Galusca B, Normand S, Germain N, Collet P, Frere D, et al. Energy expenditure adjusted for body composition differentiates constitutional thinness from both normal subjects and anorexia nervosa. Am J Physiol Endocrinol Metab. 2007;292(1):E132–7. doi: 10.1152/ajpendo.00241.2006. [DOI] [PubMed] [Google Scholar]
- 28.Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int. 2008;19(3):289–94. doi: 10.1007/s00198-007-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra M, Soyka LA, Miller KK, Grinspoon S, Levitsky LL, Klibanski A. Regional body composition in adolescents with anorexia nervosa and changes with weight recovery. Am J Clin Nutr. 2003;77(6):1361–7. doi: 10.1093/ajcn/77.6.1361. [DOI] [PubMed] [Google Scholar]
- 30.Grinspoon S, Thomas L, Miller K, Pitts S, Herzog D, Klibanski A. Changes in regional fat redistribution and the effects of estrogen during spontaneous weight gain in women with anorexia nervosa. Am J Clin Nutr. 2001;73(5):865–9. doi: 10.1093/ajcn/73.5.865. [DOI] [PubMed] [Google Scholar]
- 31.Misra M, Miller KK, Almazan C, Worley M, Herzog DB, Klibanski A. Hormonal determinants of regional body composition in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2005;90(5):2580–7. doi: 10.1210/jc.2004-2041. [DOI] [PubMed] [Google Scholar]
- 32.Ottosson M, Lonnroth P, Bjorntorp P, Eden S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J Clin Endocrinol Metab. 2000;85(2):799–803. doi: 10.1210/jcem.85.2.6358. [DOI] [PubMed] [Google Scholar]
- 33.Simmons PS, Miles JM, Gerich JE, Haymond MW. Increased proteolysis. An effect of increases in plasma cortisol within the physiologic range. J Clin Invest. 1984;73(2):412–20. doi: 10.1172/JCI111227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misra M, Miller KK, Herzog DB, Ramaswamy K, Aggarwal A, Almazan C, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89(4):1605–12. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 35.Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88(12):5615–23. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 36.Argente J, Caballo N, Barrios V, Munoz MT, Pozo J, Chowen JA, et al. Multiple endocrine abnormalities of the growth hormone and insulin-like growth factor axis in patients with anorexia nervosa: effect of short- and long-term weight recuperation. J Clin Endocrinol Metab. 1997;82(7):2084–92. doi: 10.1210/jcem.82.7.4090. [DOI] [PubMed] [Google Scholar]
- 37.Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, et al. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. J Clin Endocrinol Metab. 1999;84(6):2056–63. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- 38.Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75(3):762–7. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 39.Estour B, Germain N, Diconne E, Frere D, Cottet-Emard JM, Carrot G, et al. Hormonal profile heterogeneity and short-term physical risk in restrictive anorexia nervosa. J Clin Endocrinol Metab. 2010;95(5):2203–10. doi: 10.1210/jc.2009-2608. [DOI] [PubMed] [Google Scholar]
- 40.Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, et al. Effects of recombinant human growth hormone in anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(11):4889–97. doi: 10.1210/jc.2010-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):369–74. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(2):E347–56. doi: 10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 43.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89(10):4972–80. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 44.Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol. 2001;145(2):165–71. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 45.Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86(3):1169–74. doi: 10.1210/jcem.86.3.7314. [DOI] [PubMed] [Google Scholar]
- 46.Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab. 1989;68(3):548–54. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 47.Duclos M, Corcuff JB, Roger P, Tabarin A. The dexamethasone-suppressed corticotrophin-releasing hormone stimulation test in anorexia nervosa. Clin Endocrinol (Oxf) 1999;51(6):725–31. doi: 10.1046/j.1365-2265.1999.00872.x. [DOI] [PubMed] [Google Scholar]
- 48.Estour B, Pugeat M, Lang F, Lejeune H, Broutin F, Pellet J, et al. Rapid escape of cortisol from suppression in response to i.v. dexamethasone in anorexia nervosa. Clin Endocrinol (Oxf) 1990;33(1):45–52. doi: 10.1111/j.1365-2265.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 49.Lawson EA, Misra M, Meenaghan E, Rosenblum L, Donoho DA, Herzog D, et al. Adrenal glucocorticoid and androgen precursor dissociation in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(4):1367–71. doi: 10.1210/jc.2008-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mozid AM, Tringali G, Forsling ML, Hendricks MS, Ajodha S, Edwards R, et al. Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm Metab Res. 2003;35(8):455–9. doi: 10.1055/s-2003-41801. [DOI] [PubMed] [Google Scholar]
- 51.Misra M, Prabhakaran R, Miller KK, Tsai P, Lin A, Lee N, et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res. 2006;59(4 Pt 1):598–603. doi: 10.1203/01.pdr.0000203097.64918.63. [DOI] [PubMed] [Google Scholar]
- 52.Connan F, Lightman SL, Landau S, Wheeler M, Treasure J, Campbell IC. An investigation of hypothalamic-pituitary-adrenal axis hyperactivity in anorexia nervosa: the role of CRH and AVP. J Psychiatr Res. 2007;41(1–2):131–43. doi: 10.1016/j.jpsychires.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Leslie RD, Isaacs AJ, Gomez J, Raggatt PR, Bayliss R. Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. Br Med J. 1978;2(6136):526–8. doi: 10.1136/bmj.2.6136.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92(6):2046–52. doi: 10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 55.Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone. 2010;46(3):796–800. doi: 10.1016/j.bone.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Horm Metab Res. 2002;34(2):77–80. doi: 10.1055/s-2002-20519. [DOI] [PubMed] [Google Scholar]
- 57.Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 58.Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91(3):1027–33. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 59.Sedlackova D, Kopeckova J, Papezova H, Hainer V, Kvasnickova H, Hill M, et al. Comparison of a high-carbohydrate and high-protein breakfast effect on plasma ghrelin, obestatin, NPY and PYY levels in women with anorexia and bulimia nervosa. Nutr Metab (Lond) 2012;9(1):52. doi: 10.1186/1743-7075-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Germain N, Galusca B, Grouselle D, Frere D, Billard S, Epelbaum J, et al. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J Clin Endocrinol Metab. 2010;95(6):3057–62. doi: 10.1210/jc.2009-2196. [DOI] [PubMed] [Google Scholar]
- 61.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 62.Koyama KI, Yasuhara D, Nakahara T, Harada T, Uehara M, Ushikai M, et al. Changes in acyl ghrelin, des-acyl ghrelin, and ratio of acyl ghrelin to total ghrelin with short-term refeeding in female inpatients with restricting-type anorexia nervosa. Horm Metab Res. 2010;42(8):595–8. doi: 10.1055/s-0030-1252017. [DOI] [PubMed] [Google Scholar]
- 63.Nakahara T, Harada T, Yasuhara D, Shimada N, Amitani H, Sakoguchi T, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64 (3):252–5. doi: 10.1016/j.biopsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Harada T, Nakahara T, Yasuhara D, Kojima S, Sagiyama K, Amitani H, et al. Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biol Psychiatry. 2008;63(2):245–7. doi: 10.1016/j.biopsych.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Muller TD, Tschop MH, Jarick I, Ehrlich S, Scherag S, Herpertz-Dahlmann B, et al. Genetic variation of the ghrelin activator gene ghrelin O-acyltransferase (GOAT) is associated with anorexia nervosa. J Psychiatr Res. 2011;45(5):706–11. doi: 10.1016/j.jpsychires.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Ando T, Komaki G, Nishimura H, Naruo T, Okabe K, Kawai K, et al. A ghrelin gene variant may predict crossover rate from restricting-type anorexia nervosa to other phenotypes of eating disorders: a retrospective survival analysis. Psychiatr Genet. 2010;20(4):153–9. doi: 10.1097/YPG.0b013e32833a1f0e. [DOI] [PubMed] [Google Scholar]
- 67.Kindler J, Bailer U, de Zwaan M, Fuchs K, Leisch F, Grun B, et al. No association of the neuropeptide Y (Leu7Pro) and ghrelin gene (Arg51Gln, Leu72Met, Gln90Leu) single nucleotide polymorphisms with eating disorders. Nord J Psychiatry. 2011;65(3):203–7. doi: 10.3109/08039488.2010.525258. [DOI] [PubMed] [Google Scholar]
- 68.Hotta M, Ohwada R, Akamizu T, Shibasaki T, Takano K, Kangawa K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: a pilot study. Endocr J. 2009;56(9):1119–28. doi: 10.1507/endocrj.k09e-168. [DOI] [PubMed] [Google Scholar]
- 69.Kluge M, Uhr M, Bleninger P, Yassouridis A, Steiger A. Ghrelin suppresses secretion of FSH in males. Clin Endocrinol (Oxf) 2009;70(6):920–3. doi: 10.1111/j.1365-2265.2008.03440.x. [DOI] [PubMed] [Google Scholar]
- 70.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92(8):3202–5. doi: 10.1210/jc.2007-0593. [DOI] [PubMed] [Google Scholar]
- 71.Misra M, Katzman DK, Cord J, Manning SJ, Mendes N, Herzog DB, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008;93(8):3029–36. doi: 10.1210/jc.2008-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab. 2005;289(3):E373–81. doi: 10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 73.Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, et al. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81:3861–3. doi: 10.1210/jcem.81.11.8923829. [DOI] [PubMed] [Google Scholar]
- 74.Housova J, Anderlova K, Krizova J, Haluzikova D, Kremen J, Kumstyrova T, et al. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2005;90(3):1366–70. doi: 10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 75.Modan-Moses D, Stein D, Pariente C, Yaroslavsky A, Ram A, Faigin M, et al. Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. J Clin Endocrinol Metab. 2007;92(5):1843–7. doi: 10.1210/jc.2006-1683. [DOI] [PubMed] [Google Scholar]
- 76.Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G, et al. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88(4):1748–52. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- 77.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89(4):1833–7. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 78.Amitani H, Asakawa A, Ogiso K, Nakahara T, Ushikai M, Haruta I, et al. The role of adiponectin multimers in anorexia nervosa. Nutrition. 2013;29(1):203–6. doi: 10.1016/j.nut.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 79.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363(4):365–71. doi: 10.1056/NEJMcp0912024. [DOI] [PubMed] [Google Scholar]
- 80.Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, et al. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med. 1974;291(17):861–5. doi: 10.1056/NEJM197410242911701. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez-Fernandez R, Aguilar E, Tena-Sempere M, Pinilla L. Effects of polypeptide YY(3-36) upon luteinizing hormone-releasing hormone and gonadotropin secretion in prepubertal rats: in vivo and in vitro studies. Endocrinology. 2005;146(3):1403–10. doi: 10.1210/en.2004-0858. [DOI] [PubMed] [Google Scholar]
- 82.Padmanabhan V, Keech C, Convey EM. Cortisol inhibits and adrenocorticotropin has no effect on luteinizing hormone-releasing hormone-induced release of luteinizing hormone from bovine pituitary cells in vitro. Endocrinology. 1983;112(5):1782–7. doi: 10.1210/endo-112-5-1782. [DOI] [PubMed] [Google Scholar]
- 83.Ackerman KE, Patel KT, Guereca G, Pierce L, Herzog DB, Misra M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin Endocrinol (Oxf) 2013;78(1):114–9. doi: 10.1111/j.1365-2265.2012.04458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ackerman KE, Slusarz K, Guereca G, Pierce L, Slattery M, Mendes N, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab. 2012;302(7):E800–6. doi: 10.1152/ajpendo.00598.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant Human Leptin in Women with Hypothalamic Amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 86.Pinhas-Hamiel O, Pilpel N, Carel C, Singer S. Clinical and laboratory characteristics of adolescents with both polycystic ovary disease and anorexia nervosa. Fertil Steril. 2006;85(6):1849–51. doi: 10.1016/j.fertnstert.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 87.Sum M, Warren MP. Hypothalamic amenorrhea in young women with underlying polycystic ovary syndrome. Fertil Steril. 2009;92(6):2106–8. doi: 10.1016/j.fertnstert.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 88.Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2007;92(4):1334–9. doi: 10.1210/jc.2006-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87(9):4177–85. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 90.Wabitsch M, Ballauff A, Holl R, Blum WF, Heinze E, Remschmidt H, et al. Serum leptin, gonadotropin, and testosterone concentrations in male patients with anorexia nervosa during weight gain. J Clin Endocrinol Metab. 2001;86(7):2982–8. doi: 10.1210/jcem.86.7.7685. [DOI] [PubMed] [Google Scholar]
- 91.Galusca B, Leca V, Germain N, Frere D, Khalfallah Y, Lang F, et al. Normal inhibin B levels suggest partial preservation of gonadal function in adult male patients with anorexia nervosa. J Sex Med. 2012;9(5):1442–7. doi: 10.1111/j.1743-6109.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 92.Misra M, Katzman DK, Cord J, Manning SJ, Mickley D, Herzog DB, et al. Percentage extremity fat, but not percentage trunk fat, is lower in adolescent boys with anorexia nervosa than in healthy adolescents. Am J Clin Nutr. 2008;88(6):1478–84. doi: 10.3945/ajcn.2008.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Easter A, Treasure J, Micali N. Fertility and prenatal attitudes towards pregnancy in women with eating disorders: results from the Avon Longitudinal Study of Parents and Children. BJOG. 2011;118(12):1491–8. doi: 10.1111/j.1471-0528.2011.03077.x. [DOI] [PubMed] [Google Scholar]
- 94.Healy DL, Kovacs GT, Pepperell RJ, Burger HG. A normal cumulative conception rate after human pituitary gonadotropin. Fertil Steril. 1980;34(4):341–5. doi: 10.1016/s0015-0282(16)45021-1. [DOI] [PubMed] [Google Scholar]
- 95.Brinch M, Isager T, Tolstrup K. Anorexia nervosa and motherhood: reproduction pattern and mothering behavior of 50 women. Acta Psychiatr Scand. 1988;77(5):611–7. doi: 10.1111/j.1600-0447.1988.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 96.Kanbur N, Katzman DK. Impaired osmoregulation in anorexia nervosa: review of the literature. Pediatr Endocrinol Rev. 2011;8(3):218–21. [PubMed] [Google Scholar]
- 97.Evrard F, da Cunha MP, Lambert M, Devuyst O. Impaired osmoregulation in anorexia nervosa: a case-control study. Nephrol Dial Transplant. 2004;19(12):3034–9. doi: 10.1093/ndt/gfh507. [DOI] [PubMed] [Google Scholar]
- 98.Roberts MA, Thorpe CR, Macgregor DP, Paoletti N, Ierino FL. Severe renal failure and nephrocalcinosis in anorexia nervosa. Med J Aust. 2005;182(12):635–6. [PubMed] [Google Scholar]
- 99.Walder A, Baumann P. Increased creatinine kinase and rhabdomyolysis in anorexia nervosa. Int J Eat Disord. 2008;41(8):766–7. doi: 10.1002/eat.20548. [DOI] [PubMed] [Google Scholar]
- 100.Wada S, Nagase T, Koike Y, Kugai N, Nagata N. A case of anorexia nervosa with acute renal failure induced by rhabdomyolysis; possible involvement of hypophosphatemia or phosphate depletion. Intern Med. 1992;31(4):478–82. doi: 10.2169/internalmedicine.31.478. [DOI] [PubMed] [Google Scholar]
- 101.Dive A, Donckier J, Lejeune D, Buysschaert M. Hypokalemic rhabdomyolysis in anorexia nervosa. Acta Neurol Scand. 1991;83(6):419. doi: 10.1111/j.1600-0404.1991.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 102.Abe K, Mezaki T, Hirono N, Udaka F, Kameyama M. A case of anorexia nervosa with acute renal failure resulting from rhabdomyolysis. Acta Neurol Scand. 1990;81(1):82–3. doi: 10.1111/j.1600-0404.1990.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 103.Jonat LM, Birmingham CL. Kidney stones in anorexia nervosa: a case report and review of the literature. Eat Weight Disord. 2003;8(4):332–5. doi: 10.1007/BF03325036. [DOI] [PubMed] [Google Scholar]
- 104.Pines A, Kaplinsky N, Olchovsky D, Frankl O, Goldfarb D, Iaina A. Anorexia nervosa, laxative abuse, hypopotassemia and distal renal tubular acidosis. Isr J Med Sci. 1985;21(1):50–2. [PubMed] [Google Scholar]
- 105.Liang CC, Yeh HC. Hypokalemic nephropathy in anorexia nervosa. CMAJ. 2011;183(11):E761. doi: 10.1503/cmaj.101790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011;72(11):1546–51. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, et al. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci U S A. 2009;106(17):7149–54. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colaianni G, Sun L, Di Benedetto A, Tamma R, Zhu LL, Cao J, et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J Biol Chem. 2012;287(34):29159–67. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE, et al. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. J Clin Endocrinol Metab. 2012;97(10):E1898–908. doi: 10.1210/jc.2012-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91(8):2931–7. doi: 10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114(6):1574–83. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 112.Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent Girls With Anorexia Nervosa Have Impaired Cortical and Trabecular Microarchitecture and Lower Estimated Bone Strength at the Distal Radius. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, et al. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46 (2):458–63. doi: 10.1016/j.bone.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walsh CJ, Phan CM, Misra M, Bredella MA, Miller KK, Fazeli PK, et al. Women with anorexia nervosa: finite element and trabecular structure analysis by using flat-panel volume CT. Radiology. 2010;257(1):167–74. doi: 10.1148/radiol.10100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lucas AR, Melton LJ, 3rd, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74(10):972–7. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 116.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133(10):790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86(3):440–7. [PubMed] [Google Scholar]
- 118.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93(4):1231–7. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castro J, Toro J, Lazaro L, Pons F, Halperin I. Bone mineral density in male adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2002;41(5):613–8. doi: 10.1097/00004583-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 120.Milos G, Spindler A, Ruegsegger P, Seifert B, Muhlebach S, Uebelhart D, et al. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16(7):783–90. doi: 10.1007/s00198-004-1759-2. [DOI] [PubMed] [Google Scholar]
- 121.Walsh CJ, Phan CM, Misra M, Bredella MA, Miller KK, Fazeli P, et al. Finite Element and Trabecular Structure Analysis in Anorexia Nervosa via Flat-Panel Volume CT. Radiology. 2010;257(1):167–74. doi: 10.1148/radiol.10100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klibanski A, Biller B, Schoenfeld D, Herzog D, Saxe V. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80(3):898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 123.Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double-blind, placebo-controlled study. J Adolesc Health. 2006;39(6):819–27. doi: 10.1016/j.jadohealth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 124.Ho K, Weissberger A. Impact of short-term estrogen administration on growth hormone secretion and action: distinct route-dependent effects on connective and bone tissue metabolism. J Bone Miner Res. 1992;7(7):821–7. doi: 10.1002/jbmr.5650070711. [DOI] [PubMed] [Google Scholar]
- 125.Weissberger AJ, Ho KK, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab. 1991;72 (2):374–81. doi: 10.1210/jcem-72-2-374. [DOI] [PubMed] [Google Scholar]
- 126.Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430–8. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96 (7):2081–8. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism. 2012;61(7):1010–20. doi: 10.1016/j.metabol.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81(11):3864–70. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 130.Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, et al. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45(3):493–8. doi: 10.1016/j.bone.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab. 2002;87(6):2883–91. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 132.Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, et al. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43 (1):135–9. doi: 10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wong IP, Driessler F, Khor EC, Shi YC, Hormer B, Nguyen AD, et al. Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One. 2012;7 (7):e40038. doi: 10.1371/journal.pone.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone. 2008;43(6):1115–21. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 135.Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, et al. Alendronate for the treatment of osteopenia in anorexia nervosa: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(6):3179–85. doi: 10.1210/jc.2004-1659. [DOI] [PubMed] [Google Scholar]
- 136.Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, et al. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Res. 2004;132(3):197–207. doi: 10.1016/j.pscychresns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 137.Misra M, Katzman DK, Estella NM, Eddy K, Weigel T, Goldstein MA, et al. Impact of Physiologic Estrogen Replacement on Anxiety Symptoms, Body Shape Perception and Eating Attitudes in Adolescent Girls with Anorexia Nervosa: Data from a Randomized Controlled Trial. J Clin Psychiatry. 2013 doi: 10.4088/JCP.13m08365. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]