Abstract
Repeated intermittent administration of amphetamines acutely increases appetitive and consummatory aspects of motivated behaviors as well as general activity and exploratory behavior, including voluntary running wheel activity. Subsequently, if the drug is withdrawn, the frequency of these behaviors decrease, which is thought to be indicative of dysphoric symptoms associated with amphetamine withdrawal. Such decreases may be observed after chronic treatment or even after single drug administrations. In the present study, the effect of acute methamphetamine (METH) on running wheel activity, horizontal locomotion, appetitive behavior (food access), and consummatory behavior (food and water intake) was investigated in mice. A multi-configuration behavior apparatus designed to monitor the five behaviors was developed, where combined measures were recorded simultaneously. In the first experiment, naïve male ICR mice showed gradually increasing running wheel activity over three consecutive days after exposure to a running wheel, while mice without a running wheel showed gradually decreasing horizontal locomotion, consistent with running wheel activity being a positively motivated form of natural motor activity. In experiment 2, increased horizontal locomotion and food access, and decreased food intake, were observed for the initial 3 h after acute METH challenge. Subsequently, during the dark phase period decreased running wheel activity and horizontal locomotion were observed. The reductions in running wheel activity and horizontal locomotion may be indicative of reduced dopaminergic function, although it remains to be seen if these changes may be more pronounced after more prolonged METH treatments.
Keywords: Methamphetamine, Running wheel activity, Motivated behavior, Attention deficit hyperactivity disorder
1. Introduction
For stimulant abusers, one of the attractions of drug use is the increased stamina that the drug produces, enabling drug users to perform reward-directed behavior for extended time periods. In experimental animals, repeated intermittent administration of amphetamines (d-amphetamine and methamphetamine (METH)) facilitates natural, incentive motivated aspects of behaviors such as sexual behavior (Fiorino and Phillips, 1999; Holder et al., 2010), appetitive and consummatory aspects of food-directed behaviors (Mendez et al., 2009) and voluntary running wheel activity, a positively motivated form of natural motor activity (Uchihashi et al., 1994; Serwatkiewicz et al., 2000).
These effects on natural reinforcers may be related to increases in the incentive properties of stimuli associated with reinforcers (e.g. incentive sensitization) (Robinson and Berridge, 2003). It has long been known that repeated administration of amphetamines induces a progressive augmentation of motor activity in response to the same drug, termed behavioral sensitization (Ellinwood and Kilbey, 1977; Nishikawa et al., 1983; Robinson and Becker, 1986; Wise and Leeb, 1993; Pierce and Kalivas, 1997). This well-known phenomenon may be part of a broader enhancement of incentive motivation that affects a variety of other circumstances, including non-drug reward-related behavior (Fiorino and Phillips, 1999; Serwatkiewicz et al., 2000; Taylor and Jentsch, 2001; Mead et al., 2004; Mendez et al., 2009). The processes mediating sensitization may involve not just the enhancement of behavior when the drug is initially taken, but also adaptations to drug withdrawal. When the drug is withdrawn, sexual, food-directed and running wheel activity are often decreased for extended periods of time (Uchihashi et al., 1994; Barr et al., 1999; Der-Avakian and Markou, 2010). These decreased responses to natural reinforcers are thought to reflect dysphoric symptoms associated with amphetamine withdrawal including anhedonia, fatigue and locomotor depression (for review, see Kitanaka et al., 2010). Avoidance of the state of dysphoric withdrawal has been suggested to be one factor that motivates amphetamine abusers to continue to use the drug (Kramer et al., 1967; Koob and Le Moal, 1997), in addition to long-term alterations in incentive motivation (Robinson and Berridge, 1993). Therefore, in understanding long-term changes in drug responses relevant to addiction it is important to examine these longer term alterations in drug responses, including whether the first use of the drug actually decreases non-drug reward-related behavior which may be associated with amphetamine withdrawal. Investigation of the effects of a single administration of amphetamine on subsequent naturally motivated behaviors is lacking.
Of amphetamines and related compounds, both d-amphetamine and METH induce similar neurochemical responses in experimental animals, although the doses which induce similar levels of behavioral response are somewhat different (for review see Kitanaka et al., 2008). METH is one of the most powerful drugs of abuse worldwide and the third epidemic of METH abuse among the population is in progress in Japan (Ujike and Sato, 2004). Therefore, we have focused on METH in the present study, and a multi-configuration behavior apparatus designed to monitor five behaviors (running wheel activity, general locomotion, food and water intakes, and access frequency to a food container (“food access”)) was developed, where combined measures were recorded simultaneously. This enabled us to evaluate the expression profile of the natural, motivated aspects of behaviors in mice in an undisturbed home cage environment. Using the automated cages, we conducted two experiments. The first experiment reported here determined that access to exercise on a running wheel in male ICR mice appears to be a positively motivated form of natural motor activity as reported previously (Belke and Wagner, 2005). As presented later, the amount of movement on the wheel increased to a similar extent as general horizontal locomotion observed outside the wheel after a single METH administration. The effects of acute METH administration to mice early during the light phase of the diurnal cycle on running wheel activity, and appetitive behavior was investigated for one day in the second experiment. Since it has been known that even as short as several hours after amphetamine-like drug treatment rodents are reported to enter a dysphoric state, it is possible that even a single dose of METH can decrease running wheel activity, reflective of this opponent process. Our results demonstrated that wheel running decreased after the initial 3-hour post-injection period compared with mice challenged with saline.
2. Results
2.1. Experiment 1: The effects of a running wheel on baseline measures of behavior
Table 1 shows the five behavior categories that were observed in naïve mice for 3 consecutive days measured using a multi-configuration behavior apparatus (Fig. 1). When a running wheel was not present there was obvious adaptation over 3 days of testing: locomotor activity decreased, while food and water consumption increased. When a running wheel was present, mice showed increasing running wheel activity over the 3 days, but no change in overall locomotion. This was accompanied by reduced food access, but increased food intake and water consumption across the three days of testing.
Table 1.
The five behavior categories (running wheel activity, locomotion, food access, food intake and water intake) observed in naïve mice for every 24 h of days 1 and 2 and 23.5 h of day 3. One drop of water intake corresponds to 0.05 ml of water. Values are shown as means ± SEM (n = 9 and 10 for mice without and with a running wheel, respectively).
| Day | Running wheel (revolutions) |
Locomotion (activity counts) |
Food access (access counts) |
Food intake (grams) |
Water intake (drops) |
|---|---|---|---|---|---|
| 1 | 7890 ± 1327 | 55562 ± 4150 | 3176 ± 389 | 3.0 ± 0.4 | 95 ± 15 |
| 2 | 9408 ± 1372** | 48953 ± 4714 | 2709 ± 274** | 4.6 ± 0.4*** | 123 ± 18* |
| 3 | 13572 ± 2342** | 63083 ± 7748 | 2598 ± 307* | 5.1 ± 0.2*** | 148 ± 21*** |
| Total | 30870 ± 4775 | 167598 ± 15138††† | 8483 ± 889†† | 12.8 ± 0.8 | 365 ± 50 |
| 1 | No wheel | 31580 ± 2493 | 4699 ± 356 | 3.7 ± 0.4 | 102 ± 14 |
| 2 | No wheel | 26313 ± 1245* | 3940 ± 237** | 4.4 ± 0.4 | 111 ± 16 |
| 3 | No wheel | 24898 ± 1548* | 4455 ± 320 | 4.5 ± 0.3*** | 113 ± 17 |
| Total | - | 82790 ± 4246 | 13094 ± 746 | 12.6 ± 0.9 | 326 ± 45 |
P < 0.05,
P < 0.01,
P < 0.001, compared with day 1 (post-hoc Bonferroni-Dunn test).
P < 0.01,
P < 0.001, compared with no running wheel (t test).
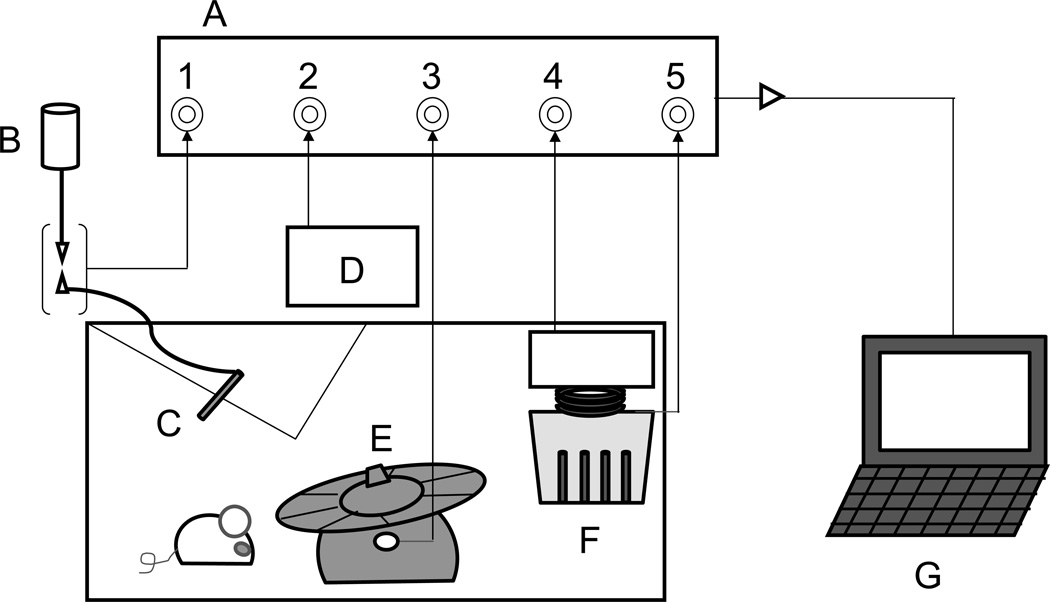
Fig. 1.
Diagram of an automated cage (multi-configuration behavior apparatus). Data for five behavioral measures (i.e. 1, water intake; 2, horizontal locomotion; 3, wheel rotation; 4, food intake; 5, food access) are simultaneously collected into CompACT® interface (A), then transferred to a computer installed the software CompACT® AMS (G). B, Water bottle; C, Sipper tube; D, Supermex® sensor; E, Fast-Trac® equipped mouse igloo; F, Food container.
For the running wheel activity, one-way repeated-measures ANOVA showed a significant main effect of Day (F(2,18) = 11.3, P < 0.001). Post-hoc analyses showed that running wheel activity increased significantly over days (Bonferroni-Dunn test, P < 0.01).
Note that the term “locomotion” used here refers to horizontal locomotor activity as measured by the infrared sensor (see Methods for a full description). This measure is not affected by repetitive behavior that does not involve horizontal movement (e.g. eating, drinking, grooming, and running wheel activity). For the locomotion, two-way repeated-measures ANOVA (Exposure to wheel × Day) showed significant main effects of Exposure to wheel (F(1,51) = 26.4, P < 0.0001) and Day (F(2,51) = 3.4, P < 0.05), and a significant Exposure to running wheel × Day interaction (F(2,51) = 5.0, P < 0.05). Post-hoc pair-wise comparisons showed significant habituation of locomotor activity over days in the mouse group without a running wheel (Bonferroni-Dunn test, P < 0.05), but no change in mice with a running wheel. Mice with a running wheel had greater locomotion than those without a running wheel for all 3 days of testing (Bonferroni-Dunn test, P < 0.05). There was also a significant difference of 3-day total locomotor activities between mouse groups with and without a running wheel (t test, P < 0.001).
Regarding food access, two-way repeated-measures ANOVA (Exposure to running wheel × Day) showed significant main effects of Exposure to running wheel (F(1,51) = 15.4, P < 0.01) and Day (F(2,51) = 5.1, P < 0.05), but no significant Exposure to running wheel × Day interaction (F(2,51) = 1.3, P = 0.29). Post-hoc pair-wise comparisons showed that food access significantly decreased over days in mouse group with a running wheel (Bonferroni-Dunn test, P < 0.01 and P < 0.05, respectively), and that food access decreased on day 2, compared with day 1, in group without a running wheel (Bonferroni-Dunn test, P < 0.01). There was also a significant difference in overall 3-day total food access between groups with and without a running wheel (t test, P < 0.01).
Regarding food intake, two-way repeated-measures ANOVA (Exposure to running wheel × Day) showed a significant main effect of Day (F(2,51) = 16.5, P < 0.0001), but no significant main effect of Exposure to running wheel (F(1,51) = 0.008, P = 0.93) or Exposure to running wheel × Day interaction (F(2,51) = 2.6, P = 0.09). Post-hoc pair-wise comparisons showed that food intake increased over the days in mouse group (Bonferroni-Dunn test, P < 0.001).
Regarding water intake, two-way repeated-measures ANOVA (Exposure to running wheel × Day) showed a significant main effect of Day (F(2,51) = 8.6, P < 0.001), but no significant main effect of Exposure to running wheel (F(1,51) = 0.34, P = 0.57). There was a significant Exposure to running wheel × Day interaction (F(2,51) = 3.6, P < 0.05). Post-hoc pair-wise comparisons showed that water intake significantly increased over days in mouse group with running wheel (Bonferroni-Dunn test, P < 0.05 and P < 0.001, respectively).
2.2. Experiment 2: The effect of acute METH challenge and the presence of a running wheel on baseline measures of behavior
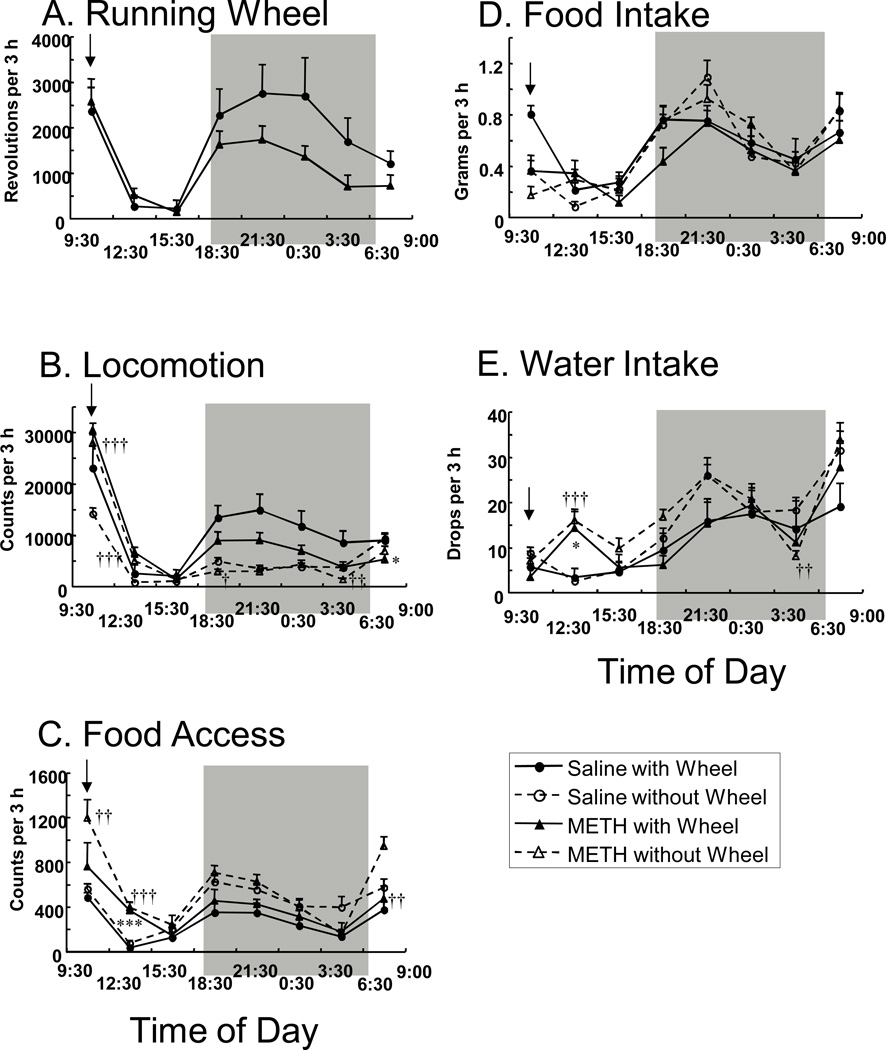
Fig. 2 shows the time course of behavioral changes observed in mice after acute METH (or saline) challenge. Statistical comparisons are shown in Table 2. In summary, there were both acute effects of METH, occurring immediately or shortly after injection, as well as differences that occurred later during the dark period; exposure to the running wheel also produced effects that were only observed during certain time periods. As shown in Fig. 2, after the initial injections (either METH or saline) the initial levels of most behaviors were high but decreased to low levels for the rest of the light period, increasing again at the onset of the dark period and then decreasing again toward the end of the dark period. These changes, observed especially in the mouse group challenged with saline, appeared to be a classic circadian response in rodents and were reflected by a significant main effect of Time for each behavioral category (Table 2). In addition, there were effects of both Exposure to running wheel and METH challenge, but only at certain time-points. Thus, repeated-measures ANOVA indicated significant Exposure to running wheel × Time interactions for locomotion and food intake, and METH Challenge × Time interactions for locomotion and water intake. There were no interactions between these effects; that is, no significant Exposure to running wheel × Challenge interactions or Exposure to running wheel × METH Challenge × Time interactions for any of the behavioral categories (Table 2).
Fig. 2.
Effect of acute METH (or saline) challenge on mouse behavior. (A) Running wheel activity, (B) horizontal locomotion, (C) food access (access frequency to a food container), (D) food intake and (E) water intake after acute saline or 1.0 mg/kg methamphetamine challenge at 09:30. Data are presented as 3-h bins. Values are shown as the means ± SEM (n = 10 per group). Arrows indicate the time-point of METH (or saline) injection. Gray zones represent the dark period (19:00-07:00). *P < 0.05, ***P < 0.001, compared with saline-injected mice exposed to a running wheel (t-test). ††P < 0.01, †††P < 0.001, compared with saline-injected mice without a running wheel (t-test).
Table 2.
Results of statistics for the effect of acute methamphetamine challenge on mouse behavior represented in Fig. 2. Three-way repeated-measures ANOVA was applied to all behavior categories except the “Running wheel” category, which was analyzed by two-way repeated-measures ANOVA. The F and P values are shown in upper and lower columns, respectively.
| Factor | |||||||
|---|---|---|---|---|---|---|---|
| Behavior category | Exposure to wheel (E) F value P value |
Challenge (C) | Time (T) | E × C | E × T | C × T | E × C × T |
| Running wheel | N/A | 2.590 | 10.367 | N/A | N/A | 1.218 | N/A |
| N/A | 0.1249 | < 0.0001 | N/A | N/A | 0.2976 | N/A | |
| Locomotion | 21.572 | 0.008 | 99.755 | 3.179 | 6.085 | 13.362 | 0.727 |
| < 0.0001 | 0.9288 | < 0.0001 | 0.0830 | < 0.0001 | < 0.0001 | 0.6496 | |
| Food access | 18.755 | 12.174 | 31.311 | 0.129 | 1.907 | 5.913 | 1.812 |
| < 0.0001 | < 0.01 | < 0.0001 | 0.7213 | 0.0689 | < 0.0001 | 0.0853 | |
| Food intake | 0.642 | 2.323 | 20.569 | 2.933 | 2.990 | 1.961 | 0.627 |
| 0.4282 | 0.1362 | < 0.0001 | 0.0954 | < 0.05 | 0.0609 | 0.7334 | |
| Water intake | 6.506 | 1.269 | 22.437 | 0.014 | 1.507 | 2.826 | 0.602 |
| < 0.05 | 0.2674 | < 0.0001 | 0.9077 | 0.1653 | < 0.01 | 0.7544 | |
The effect of METH injection on each behavior was evaluated by t-test when the METH Challenge × Time interaction for each behavior was significant by ANOVA (Table 2). For the running wheel activity, after the initial injections (either METH or saline) the initial levels of running wheel activity were high but decreased to low levels for the rest of the light period, increasing again at the onset of the dark period and then decreasing again toward the end of the dark period (Fig. 2A). However, ANOVA for running wheel activity showed no significant main effect of METH Challenge (Fig. 2A and Table 2). There were significant increases in locomotor activity in mice without a running wheel after METH administration compared with saline-injected mice without a running wheel (time points 9:30–12:30 and12:30–15:30 evaluated by t-test) but after this initial increase lower levels of activity than those observed during the time-point of 9:30–12:30 were observed in both METH and saline treated mice throughout the dark period (Fig. 2B). When a running wheel was present there was a smaller increase in locomotion after METH administration and a consistent reduction in locomotion throughout the dark period (Fig. 2B). There were significant decreases in locomotor activity in mice without a running wheel after METH administration compared with saline-injected mice without a running wheel (time points 18:30–21:30 and 3:30–6:30 evaluated by t-test). By contrast METH did not produce an acute increase in wheel running, although, similar to the effect of METH on locomotion, there was a reduction in wheel running at each time-point during the dark phase, although this effect did not reach statistical significance as evaluated by ANOVA (Table 2). There was a significant decrease in locomotor activity in mice with a running wheel after METH administration compared with saline-injected mice with a running wheel (time points 6:30–9:00 evaluated by t-test). Food access increased substantially after METH challenge in all mice (Fig. 2C). In mice without a running wheel METH increased food access at the first two time points (9:30–12:30 and 12:30–15:30) and again at the onset of the next light cycle (6:30–9:00), as determined by t-test. This effect was slightly depressed in the presence of a running wheel, but was still significant at the 12:30–15:30 time point (Fig. 2C). Water intake was not increased immediately but was significantly increased several hours after METH challenge (12:30–15:30) in both wheel running groups, and then again during the dark period, although these increases were not statistically significant (Fig. 2E). There were significant decreases in water intake in mice without a running wheel after METH administration compared with saline-injected mice without a running wheel (time points 3:30–6:30 evaluated by t-test).
It is clear from Fig. 2 that most effects occurred either immediately after METH injection (or just thereafter in the case of water intake) or some time later during the dark period. Given this pattern a further statistical analysis was performed to focus on these time periods with the data represented in Fig. 2 expressed as Total (09:30–09:00), Initial 3 h (09:30–12:30) and Dark phase period (19:00–07:00), as shown in Table 3.
Table 3.
The five behavior categories (running wheel activity, locomotion, food access, food intake and water intake) observed in mice after methamphetamine (or saline) challenge with or without a running wheel during periods as indicated. Total period for behavior observations started at 09:30 and ended the next day at 09:00 (23.5 h) (see Fig. 1B). Initial 3 h period started at 09:30 and ended the next day at 12:30. Dark period started at 19:00 and ended the next day at 07:00. METH, 1.0 mg/kg methamphetamine. Values are shown as means ± SEM (n = 10 per group).
| Period | Challenge | Running wheel (revolutions) |
Locomotion (activity counts) |
Food access (access counts) |
Food intake (grams) |
Water intake (drops) |
|---|---|---|---|---|---|---|
| Total | Saline | 13411 ± 2483 | 84482 ± 12591*** | 2064 ± 244*** | 4.5 ± 0.3 | 89 ± 20* |
| No wheel | 40617 ± 2876 | 3371 ± 198 | 4.2 ± 0.3 | 121 ± 7 | ||
| METH | 9300 ± 598 | 71700 ± 2654*** | 3094 ± 490***,†† | 3.5 ± 0.4 | 102 ± 16* | |
| No wheel | 52170 ± 3529 | 4638 ± 307†† | 4.3 ± 0.4 | 138 ± 4 | ||
| Initial 3 h | Saline | 2356 ± 709 | 22987 ± 4364* | 482 ± 42 | 0.8 ± 0.1*** | 6 ± 2 |
| No wheel | 14099 ± 1109 | 559 ± 44 | 0.4 ± 0.1 | 9 ± 4 | ||
| METH | 2565 ± 310 | 30349 ± 1394*,††† | 759 ± 213†† | 0.4 ± 0.1***,††† | 3 ± 2 | |
| No wheel | 27871 ± 1665††† | 1194 ± 162†† | 0.2 ± 0.1††† | 7 ± 2 | ||
| Dark period | Saline | 9700 ± 1924 | 49801 ± 8640*** | 1102 ± 243*** | 2.5 ± 0.3 | 59 ± 15* |
| No wheel | 17289 ± 1579 | 2067 ± 167 | 2.8 ± 0.1 | 79 ± 5 | ||
| METH | 5534 ± 337† | 29001 ± 1756***,†† | 1405 ± 216*** | 2.2 ± 0.3 | 55 ± 11* | |
| No wheel | 12089 ± 1177†† | 1956 ± 87 | 2.8 ± 0.2 | 76 ± 4 | ||
P < 0.05,
P < 0.001, compared with no running wheel (post-hoc Bonferroni-Dunn test).
P < 0.05,
P < 0.01,
P < 0.001, compared with saline challenge (post-hoc Bonferroni-Dunn test).
One-way ANOVA applied to wheel running data shown in Table 3 showed a significant main effect of METH challenge during the Dark phase period (F(1,18) = 4.5, P < 0.05), but not during the Total and Initial 3 h periods (F(1,18) = 2.6, P = 0.12 and (F(1,18) = 0.04, P = 0.79, respectively).
A two-way ANOVA applied to locomotor data showed significant main effects of Exposure to wheel during the Total, Initial 3 h, and Dark phase periods (F(1,36) = 21.6, P < 0.0001, F(1,36) = 5.2, P < 0.05 and F(1,36) = 29.9, P < 0.0001, respectively) and METH challenge during Initial 3 h and Dark phase periods (F(1,36) = 17.9, P < 0.001 and F(1,36) = 8.3, P < 0.01, respectively) but no significant main effect of METH Challenge during the Total period (F(1,36) = 0.008, P = 0.93). ANOVA also indicated no significant METH Challenge × Exposure to wheel interactions during Total, Initial 3 h and Dark phase periods (F(1,36) = 3.2, P = 0.08, F(1,36) = 1.6, P = 0.21, and F(1,36) = 3.0, P = 0.09, respectively).
ANOVA for food access showed significant main effects of Exposure to running wheel during the Total and the Dark phase periods (F(1,36) = 18.8, P < 0.0001 and F(1,36) = 16.3, P < 0.001, respectively), but not during the Initial 3 h period (F(1,36) = 3.5, P = 0.07), and METH Challenge during the Total and initial 3 h periods (F(1,36) = 12.2, P < 0.01 and F(1,36) = 11.0, P < 0.01, respectively), but not during the Dark phase period (F(1,36) = 0.26, P = 0.61). The ANOVA also indicated no significant METH Challenge × Exposure to wheel interactions during the Total, Initial 3 h or Dark phase periods (F(1,36) = 0.13, P = 0.72, F(1,36) = 1.7, P = 0.20, and F(1,36) = 1.2, P = 0.28, respectively).
ANOVA for food intake indicated significant main effects of Exposure to running wheel during the Initial 3 h period (F(1,36) = 13.4, P < 0.001), but not during the Total and Dark phase periods (F(1,36) = 0.64, P = 0.43 and F(1,36) = 3.2, P = 0.08, respectively) and METH Challenge during the Initial 3 h period (F(1,36) = 13.4, P < 0.001), but not during the Total or Dark phase periods (F(1,36) = 0.23, P = 0.14 and F(1,36) = 0.84, P = 0.37, respectively). The ANOVA found no significant METH Challenge × Exposure to running wheel interactions during the Total, Initial 3 h or Dark phase periods (F(1,36) = 2.9, P = 0.10, F(1,36) = 2.1, P = 0.16, and F(1,36) = 0.41, P = 0.53, respectively).
ANOVA for the water intake data indicated significant main effects of Exposure to running wheel during the Total and Dark phase periods (F(1,36) = 6.5, P < 0.05 and F(1,36) = 4.5, P < 0.05, respectively), but not during the Initial 3 h period (F(1,36) = 2.9, P = 0.10). The ANOVA also indicated no significant main effects of METH Challenge during Total, Initial 3 h or Dark phase periods (F(1,36) = 1.3, P = 0.27, F(1,36) = 0.98, P = 0.33, and F(1,36) = 0.12, P = 0.73, respectively) nor METH Challenge × Exposure to running wheel interactions during the Total, Initial 3 h or Dark phase periods (F(1,36) = 0.014, P = 0.91, F(1,36) = 0.016, P = 0.90, and F(1,36) = 0.011, P = 0.92, respectively).
3. Discussion
The present study demonstrated that acute administration of METH to mice early in the light period (2.5 h after lights on) subsequently decreased voluntary running wheel activity and locomotion for a 12 h-period during the next dark period (9.5 h – 21.5 h after acute METH challenge) (Fig. 2A and Table 3). These effects were far greater, and longer lasting than the initial locomotor stimulant effects of METH. Several interpretations might be posited for these effects. In rats, exercise on a running wheel has been suggested to be rewarding (Iversen, 1993; Ferreira et al., 2006). Thus, these decreases in voluntary wheel running for a 12 h-period during the next dark period might reflect post-METH dysphoria. Consistent with this interpretation, decreased motivated behavior is known to occur during withdrawal from psychostimulant drugs (Uchihashi et al., 1994; Barr et al., 1999; Der-Avakian and Markou, 2010). However, it must be noted that both wheel running and locomotion were decreased, so this may also reflect a broader decrease in activity or arousal during this period, or fatigue.
Rodents repeatedly administered amphetamines exhibit decreased general spontaneous locomotion during drug withdrawal (Herman et al., 1971; Lynch and Leonard, 1978; Robinson and Camp, 1987; Paulson et al., 1991; Schindler et al., 1994; Russig et al., 2005; White and White, 2006) as well as other dysphoric symptoms including anhedonia and fatigue. Thus, a stimulatory effect of acute METH followed by severe fatigue may have caused the decreased running wheel activity as well. The present results are consistent with a previous study that found increased horizontal locomotion for the initial 3 h after a single METH injection (1.0 mg/kg, i.p.), followed by a tendency to exhibit decreased horizontal locomotion 9.5 h – 21.5 h after METH administration (Kitanaka et al., 2003). In the present study, increased horizontal locomotion was observed in the initial 3 h time period after acute METH challenge. This was observed in mice whether or not a running wheel was present. This increase in locomotor activity was followed by decreases in horizontal locomotion during the subsequent dark phase 9.5 h – 21.5 h after METH injection (Tables 3 and 4). During the initial 3 h after acute METH challenge, there was no significant difference in running wheel activity (Table 3), confirming the independent basis of these two behaviors. However, during the dark phase reductions were observed in both wheel running and horizontal locomotion. METH concentrations in the brain appear to be associated with the magnitudes of the horizontal locomotion, but not wheel running activity, based on the pharmacokinetics of METH: the apparent disappearance half-life of METH from brain is 56.6 min in mice after i.p. administration with 0.64 mg/kg METH (Brien et al., 1978). By contrast, the later effects of METH, during the dark period, would appear to result from some type of opponent process, or acute withdrawal. METH (or amphetamine) withdrawal actually decreases the dopaminergic functioning and such a decrease is closely related to anhedonia/dysphoria (Kitanaka et al., 2008).
Table 4.
Results of statistics for the effect of acute methamphetamine challenge on mouse behavior represented in Table 3. Two-way ANOVA was applied to all behavior categories except the “Running wheel” category, which was analyzed by one-way ANOVA. The F and P values are shown in upper and lower columns, respectively.
| Factor-Total (09:30-09:00) | Initial 3 h (09:30–12:30) | Dark period (19:00-07:00) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavior category | Exposure to wheel (E) F value P value |
Challenge (C) | E × C | E | C | E × C | E | C | E × C |
| Running wheel | N/A | 2.590 | N/A | N/A | 0.073 | N/A | N/A | 4.549 | N/A |
| N/A | 0.1249 | N/A | N/A | 0.7907 | N/A | N/A | < 0.05 | N/A | |
| Locomotion | 21.572 | 0.008 | 3.179 | 5.169 | 17.870 | 1.643 | 29.931 | 8.283 | 2.982 |
| < 0.0001 | 0.9288 | 0.0830 | < 0.05 | < 0.001 | 0.2081 | < 0.001 | < 0.01 | 0.0928 | |
| Food access | 18.755 | 12.174 | 0.129 | 3.471 | 11.022 | 1.693 | 16.269 | 0.263 | 1.211 |
| < 0.0001 | < 0.01 | 0.7213 | 0.0706 | < 0.01 | 0.2015 | < 0.001 | 0.6113 | 0.2784 | |
| Food intake | 0.642 | 2.323 | 2.933 | 13.384 | 13.384 | 2.108 | 3.201 | 0.836 | 0.405 |
| 0.4282 | 0.1362 | 0.0954 | < 0.001 | < 0.001 | 0.1552 | 0.0820 | 0.3666 | 0.5287 | |
| Water intake | 6.506 | 1.269 | 0.014 | 2.905 | 0.984 | 0.016 | 4.527 | 0.123 | 0.011 |
| < 0.05 | 0.2674 | 0.9077 | 0.0969 | 0.3278 | 0.8995 | < 0.05 | 0.7275 | 0.9183 | |
Increased food access but decreased food intake were observed for the initial 3 h time period after acute METH challenge whether a running wheel was present or not (Tables 3 and 4). Thus, there was a strong dissociation between food-directed appetitive and consummatory behavior. This increase in food access might be considered to be consistent with increases in horizontal locomotion, as part of general increase in exploratory (e.g. appetitive) behavior after METH administration. As shown in Fig. 2E, water intake significantly increased in mice challenged with METH at a time point of 12:30–15:30 compared with mice challenged with saline. The increase in the water intake appeared in mice with or without a running wheel, suggesting that the METH-induced excess activity during the initial 3 h of period, or increased temperature, might increase water intake 3 h after the drug injection although the mechanism of the increase is uncertain. In contrast to this acute phenomenon, a significant decrease in the water intake in mice (without a running wheel) challenged with METH during a time period of 3:30–6:30 (Fig. 2E) is likely to be associated with hypoactivity (Fig. 2B).
The results of the present study demonstrated that a single METH administration was sufficient to reduce voluntary wheel and locomotor activities about 9 h later when the animals entered the dark phase of the light/dark cycle. As suggested above, the changes in behavior observed long after the METH injection, during the dark period, could be interpreted as resulting from an opponent process, such as that associated with post-METH dysphoria. Alternatively, it may be more relevant to the long-lasting reductions in motor activity that have been noted to occur in response to several amphetamine-like drugs in animal models (Herman et al., 1971; Lynch and Leonard, 1978; Robinson and Camp, 1987; Paulson et al., 1991; Schindler et al., 1994; Russig et al., 2005; White and White, 2006) and are associated with the therapeutic effects of amphetamine-like drugs in the treatment of attention deficit hyperactivity disorder (ADHD) (Swanson et al., 2011). In the present experiments a behavior analogous to an ADHD symptom (e. g. hyperactivity) was attenuated by METH, a drug with a chemical structure closely related to d-amphetamine, which is existing treatment for ADHD. Since the sort of opponent processes that trigger dysphoric states usually require high doses and extensive treatment regimens (Herman et al., 1971; Lynch and Leonard, 1978; Robinson and Camp, 1987; Paulson et al., 1991; Schindler et al., 1994; Russig et al., 2005; White and White, 2006), and long-lasting reductions in locomotion can be observed after single treatments (Loos et al., 2010), it would seem likely that the current results are more likely to reflect this type of process. This would suggest that the model used here may be an effective approach for examining the therapeutic potential of putative medications for the treatment of ADHD, although a more extensive examination of compounds, doses and treatment regimens will be necessary to fully examine this hypothesis.
4. Materials and Methods
4.1. Subjects
Male ICR mice (11–13 weeks old; Japan SLC, Shizuoka, Japan) were housed in groups of eight (cage size: 37 × 22 × 15 cm; with fresh wood chips), in a temperature- (22 ± 2°C) and humidity-controlled environment (50 ± 10%), under a 12 h light/dark cycle (lights on at 07:00) with food and water available ad libitum. Animal handling and care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (7th edition, Institute of Laboratory Animal Resources-National Research Council, National Academy Press, 1996), and all experiments were reviewed and approved by our Institutional Animal Research Committee. The mice were only used once (body weight on experimental day: 35–48 g, n = 59 total) after at least one-week habituation in the facility.
4.2. Reagents
METH HCl was purchased from Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan) and was dissolved in sterile saline and administered intraperitoneally (i.p.; injection volume: 0.1 ml/10 g). The dose of METH refers to the weight of salt. In our previous study, we demonstrated that total amount of nocturnal activity decreased significantly in mice challenged with METH in the morning (10:00AM) (Kitanaka et al., 2003). The current study has been designed based on the previous work and the reason for choosing the time of injection (09:30AM).
4.3. Multi-configuration behavior apparatus
A multi-configuration behavior apparatus designed to monitor a range of mouse behaviors, including running wheel activity, horizontal locomotion (locomotion), access frequency to a food container (food access), food consumption (food intake) and water consumption (water intake), was developed by Muromachi Kikai Co., Ltd. (Tokyo, Japan), that allows the behaviors to be recorded simultaneously in the home cage (Fig. 1). This enabled us to evaluate the expression profile of natural, motivated behaviors in mice under an undisturbed home cage environment. The behavior apparatus was made of a transparent acrylic cage (cage size, 31 × 25 × 17 cm), with a stainless steel grid top. The floor was covered with Paper Clean™ bedding (Japan SLC). The apparatus was enclosed in a quiet ventilated chamber (53 × 45 × 45 cm) under a 12-h light/dark schedule (lights on at 07:00 in the ventilated chamber, 130 lux when the lights were on). Data were collected in 10 min bins using CompACT AMS software version 3.0 (Muromachi Kikai Co., Ltd.). For some of the subjects a running wheel was added as indicated in Tables and Figures. Running wheel activity was monitored using Fast-Trac equipped mouse igloo (Bio-Serv, Frenchtown, NJ, USA) with modifications: the igloo was equipped with a rotation sensor that electronically detects running wheel revolutions. Locomotion was measured with an infrared pyroelectric sensor Supermex® (Muromachi Kikai Co., Ltd.) that detects thermal radiation from animals, equipped in the ceiling of the ventilated chamber (Kitanaka et al., 2003). Thus, it is noted that the term “locomotion” used in the results of the current study means the general horizontal locomotor activity totally generated by a movement observed when a mouse ambulates or runs freely in the automated cage as well as the movement when the animal has a free access to a food container or to a sipper tube for the daily diet. Supporting this distinction, the present study found differences in locomotion between groups during the period immediately after injections (Fig. 2B) but did not find group differences in wheel running during the same period (Fig. 2A). Food access and food intake were measured simultaneously using an ingestion monitor (model FIC-001, Muromachi Kikai Co., Ltd), which contains a sensor for both food access and food intake. For food access, one count was recorded once a mouse touched the food container. For food intake, the total amount of food (less than 1 g within 10 sec) taken by a mouse was recorded. The feed was standard food pellets (MF for mouse, rat and hamster; Oriental Yeast Co., Ltd., Tokyo, Japan) available ad libitum. The bottom of the food container was raised 5 cm above the floor so that mice would not touch the food container incidentally during other activities. Water intake was measured using a drinking sensor (model MDS-1B, Muromachi Kikai Co., Ltd.). Water was available ad libitum. Within the apparatus a drip chamber with an electric sensor was equipped between a water bottle and a receptacle with a stainless steel sipper tube. As water was consumed from the sipper tube, water passed (dripped) through the sensor so that the amount consumed was recorded as drops of water intake; one drop corresponding to 0.05 ml of water.
4.4. Test protocol
4.4.1. Experiment 1: The effects of a running wheel on baseline measures of behavior
On day 1, mice (n = 19) were weighed and randomly divided into two groups (n = 9 and 10 for groups without and with a running wheel, respectively). The animals were placed individually in transparent acrylic cages located in ventilated chambers (the Multi-configuration behavior apparatus, as described above) and their behavior (locomotion, wheel running, food access, food consumption and water consumption) was automatically recorded for three days, beginning at 09:30 on day 1.
4.4.2. Experiment 2: The effect of acute METH challenge and the presence of a running wheel on baseline measures of behavior
On the day of the experiment the mice (n = 40) were weighed and randomly divided into four groups (n = 10 per group): METH/running wheel, METH/no running wheel, saline/running wheel, and saline/no running wheel. Mice were injected with saline or 1.0 mg/kg METH and subsequently placed individually in a multi-configuration behavior apparatus with, or without, a running wheel and behavior was automatically recorded for 23.5 h, beginning at 09:30 and ending at 09:00 the next day. The mice did not have experience with the running wheel before the drug/saline injection.
4.5. Statistics
The values are presented as mean ± the standard error of the mean (SEM). Statistical analysis was performed using mixed factor analysis of variance (ANOVA) with or without repeated measures followed by Bonferroni-Dunn test or t-test (Statview 5.0 for Apple Macintosh, SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at P < 0.05.
Acknowledgments
The authors are grateful to Ms. A. Yoshioka of the Department of Pharmacology, Hyogo College of Medicine, for preparing the animal study proposal. This research was supported, in part, by a Grant-in-Aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 21790254 to NK), a Grant-in-Aid for Researchers, Hyogo College of Medicine (2011 to NK), and intramural funding from the National Institute on Drug Abuse, USA (to GRU and FSH).
References
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol. Biochem. Behav. 1999;64:597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav. Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Brien JF, Kitney JC, Peachey JE, Rogers BJ. Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res. Commun. Chem. Pathol. Pharmacol. 1978;22:313–328. [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav. Pharmacol. 2010;21:359–368. doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood EH, Kilbey MM. Chronic stimulant intoxication models of psychosis. In: Hanin I, Usdin E, editors. Animal Models in Psychiatry and Neurology. New York: Pergamon Press; 1977. pp. 61–74. [Google Scholar]
- Ferreira A, Lamarque S, Boyer P, Perez-Diaz F, Jouvent R, Cohen-Salmon C. Spontaneous appetence for wheel-running: a model of dependency on physical activity in rat. Eur. Psychiatry. 2006;21:580–588. doi: 10.1016/j.eurpsy.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d-amphetamine-induced behavioral sensitization. Psychopharmacology (Berl.) 1999;142:200–208. doi: 10.1007/s002130050880. [DOI] [PubMed] [Google Scholar]
- Herman ZS, Trzeciak H, Chrusciel TL, Kmiteciak-Kolada K, Drybanski A, Sokola A. The influence of prolonged amphetamine treatment and amphetamine withdrawal on brain biogenic amine content and behaviour in the rat. Psychopharmacologia (Berl.) 1971;21:74–81. doi: 10.1007/BF00403998. [DOI] [PubMed] [Google Scholar]
- Holder MK, Hadjimarkou MM, Zup SL, Blutstein T, Benham RS, McCarthy MM, Mong JA. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus. Psychoneuroendocrinology. 2010;35:197–208. doi: 10.1016/j.psyneuen.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav. 1993;60:219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. Neurochemical consequences of dysphoric state during amphetamine withdrawal in animal models: a review. Neurochem. Res. 2008;33:204–219. doi: 10.1007/s11064-007-9409-7. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Takemura M. Sequential expression of impaired psychomotor and sensorimotor activities in rodents during amphetamine withdrawal. In: Davies RS, editor. Handbook of Neuropsychiatry Research. Chapter 4. New York: Nova Science Publishers; 2010. pp. 97–112. [Google Scholar]
- Kitanaka N, Kitanaka J, Takemura M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur. J. Pharmacol. 2003;474:63–70. doi: 10.1016/s0014-2999(03)02015-6. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse: pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer ANM, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsibility to amphetamine. Behav. Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Lynch MA, Leonard BE. Effect of chronic amphetamine administration on the behaviour of rats in the open field apparatus: reversal of post-withdrawal depression by two antidepressants. J. Pharm. Pharmacol. 1978;30:798–799. doi: 10.1111/j.2042-7158.1978.tb13398.x. [DOI] [PubMed] [Google Scholar]
- Mead AN, Crombag HS, Rocha BA. Sensitization of psychomotor stimulation and conditioned reward in mice: differential modulation by contextual learning. Neuropsychopharmacology. 2004;29:249–258. doi: 10.1038/sj.npp.1300294. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behav. Brain Res. 2009;201:74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Mataga N, Takashima M, Toru M. Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur. J. Pharmacol. 1983;88:195–203. doi: 10.1016/0014-2999(83)90006-7. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl.) 1991;103:480–494. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The natural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DE. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol. Biochem. Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Russig H, Murphy CA, Feldon J. Behavioural consequences of withdrawal from three different administration schedules of amphetamine. Behav. Brain Res. 2005;165:26–35. doi: 10.1016/j.bbr.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Persico AM, Uhl GR, Goldberg SR. Behavioral assessment of high-dose amphetamine withdrawal: importance of training and testing conditions. Pharmacol. Biochem. Behav. 1994;49:41–46. doi: 10.1016/0091-3057(94)90454-5. [DOI] [PubMed] [Google Scholar]
- Serwatkiewicz C, Limebeer C, Eikelboom R. Sensitization of amphetamine-induced wheel running suppression in rats: dose and context factors. Psychopharmacology (Berl.) 2000;151:219–225. doi: 10.1007/s002130000446. [DOI] [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognitions in individuals with attention-deficit hyperactivity disorder: a decade of process. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biol. Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Uchihashi Y, Kuribara H, Yasuda H, Umezu T, Tadokoro S. Long-continuous observation of the effects of methamphetamine on wheel-running and drinking in mice. Prog. Neuro-psychopharmaclol. Biol. Psychiatry. 1994;18:397–407. doi: 10.1016/0278-5846(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann. N.Y. Acad. Sci. 2004;1025:279–287. doi: 10.1196/annals.1316.035. [DOI] [PubMed] [Google Scholar]
- White W, White IM. An activity indicator of acute withdrawal depends on amphetamine dose in rats. Physiol. Behav. 2006;87:368–376. doi: 10.1016/j.physbeh.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leeb K. Psychomotor-stimulant sensitization: a unitary phenomenon? Behav. Pharmacol. 1993;4:339–349. [PubMed] [Google Scholar]