Abstract
Objective
There is enormous clinical potential in exploiting the spatial and temporal resolution of optical techniques to modulate pathophysiological neuronal activity, especially intractable focal epilepsy. We have recently utilized a new ruthenium-based caged compound, ruthenium-bipyridine-triphenylphosphine–γ-aminobutyric acid (RuBi-GABA), which releases GABA when exposed to blue light, to rapidly terminate paroxysmal activity in vitro and in vivo.
Methods
The convulsant 4-aminopyridine was used to induce interictal activity and seizures in rat neocortical slices and anesthetized rats. We examined the effect of blue light, generated by a small, light-emitting diode (LED), on the frequency and duration of ictal activity in the presence and absence of RuBi-GABA.
Results
Neither blue light alone, nor low concentrations of RuBi-GABA, affected interictal activity or baseline electrical activity in neocortical slices. However, brief, blue illumination of RuBi-GABA, using our LED, dramatically reduced extracellular spikes and bursts. More impressively, illumination of locally applied RuBi-GABA rapidly terminated in vivo seizures induced by topical application of 4-aminopyridine. The RuBi-GABA effect was blocked by the GABAA antagonist picrotoxin, but not duplicated by direct application of GABA.
Interpretation
This is the first example of optical control of in vivo epilepsy, proving that there is sufficient cortical light penetration from an LED and diffusion of caged GABA to quickly terminate intense focal seizures. We are aware that many obstacles need to be overcome before this technique can be translated to patients, but at the moment, this represents a feasible method for harnessing optical techniques to fabricate an implantable device for the therapy of neocortical epilepsy.
The therapy of epilepsy remains inadequate. Despite the introduction of several new antiepileptic drugs over the past 2 decades, close to 30% of patients suffer from poor seizure control even with optimal medical therapy.1 Although surgery has clearly benefited many patients with focal epilepsy, a substantial number of patients do not achieve significant remission after operation, and even those patients who do respond risk neurologic or neuropsychological deficits.2,3 The deficiencies of current therapy have stimulated investigation of invasive but nondestructive epilepsy therapies such as deep brain stimulation, vagus nerve stimulation, focal cooling, and local drug delivery to terminate or even prevent seizures.4–7 However, all these possible therapies have had either limited success in early trials or have yet to be tested in patients.
The relatively disappointing results with electrical stimulation as an epilepsy therapy have made the possibility of optical approaches to seizure control attractive. The transfection of message for light-sensitive chloride pumps into mammalian neurons (optogenetics) suggested a possible way to exploit the temporal and spatial resolution of optical techniques to control the pathological excitation characteristic of epilepsy.8 Unfortunately, optogenetic technology requires permanently altering the genome of transfected neurons and appears to modify their fundamental neurobiology.8
Caged compounds, including caged γ-aminobutyric acid (GABA) analogs, may provide a more practical approach for exploiting the potential of optical techniques to control pathological neuronal excitability. We have already demonstrated that we can use a small, ultraviolet (UV) light-emitting diode (LED) to release sufficient GABA from 1 caged GABA, 4-[([2H-benzopyran-2-one-7-amino-4-methoxy] carbonyl) amino] butanoic acid (BC204), to activate GABAA receptors and suppress interictal activity in cultured neurons and brain slices.9,10 However, no one has yet reported optical suppression of in vivo experimental seizures.
We recently became interested in a new ruthenium-based caged GABA, ruthenium-bipyridine-triphenylphosphine–GABA (RuBi-GABA), which releases GABA in the presence of visible, blue light.11 The lower energy of visible light, compared to UV, results in less light scattering and deeper brain penetration, making RuBi-GABA an attractive compound to test in a model of in vivo epilepsy.11 We report our initial investigation of the antiepileptic properties of RuBi-GABA in rat brain slices and then describe the first example of optical control of in vivo experimental seizures in a severe rodent seizure model. In previous studies, we have shown that Rubi-GABA itself is toxic to cultured neurons after several hours of exposure; however, these experiments demonstrate clear proof of principle of rapid, optical control of in vivo seizures with caged GABA.
Materials and Methods
Animals and Chemicals
We used male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) 2 to 6 weeks old. Animal care and protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. RuBi-GABA was either obtained from the laboratory of Professor Roberto Etchenique of the University of Buenos Aires or purchased from Tocris Bioscience (Ellisville, MO). Diazepam was obtained from Hospira Inc (Lake Forest, IL). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO).
Brain Slice Preparation and Electrophysiology
Rats were anesthetized with isoflurane and rapidly decapitated. Brains were quickly removed and immersed in iced artificial cerebrospinal fluid (ACSF) containing (in millimoles): 124 NaCl, 5 KCl, 2 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 22 NaHCO3, and 10 glucose, continuously bubbled with a 95% O2 and 5% CO2 gas mixture. The cerebellum, brainstem, and brain anterior to the optic chiasm were then removed, allowing the frontal surface to be placed against an agarose block in the vibratome pan. We placed the pan into the vibratome (Vibratome Series 1000 Sectioning System; Technical Products International, St Louis, MO), filled it with iced and oxygenated ACSF, and cooled the vibratome’s well with ice water. Horizontal slices (600µm) were individually cut, and the hemispheres of each slice were separated and incubated in a submerged oxygenated holding chamber at 25°C for at least 1 hour before transfer to a recording chamber for electrophysiology.
Electrophysiologic recordings were made in a submerged chamber (Warner Instruments, Hamden, CT) perfused with oxygenated ACSF at 2ml/min and maintained at 33°C by heating the inflow and the chamber itself. Microelectrodes were made from 1.2mm outer diameter/0.68mm inner diameter borosilicate glass (WPI, Sarasota, FL) and had resistances of 4 to 6 MΩ when filled with ACSF. The recording electrode was placed in the entorhinal cortex, and the extracellular signals were fed into a conventional direct current amplifier (Axoclamp 2A; Axon Instruments, Union City, CA), digitized at 2kHz, and stored on a personal computer using a commercially available analog/digital converter and software (Digidata 1322 and pClamp 9; Axon Instruments). We provoked very frequent rhythmic interictal activity by adding the convulsant 4-aminopyridine (4-AP; 100µM) and removing magnesium from the extracellular perfusate. This interictal activity was quantified 3 ways:
We measured the standard deviation of the baseline voltage recording over the 4.5 seconds immediately preceding, and coinciding with RuBi-GABA uncaging (see below); the light was activated for 5 seconds every 10 minutes, and we only analyzed the 4.5 seconds before and after illumination.
We counted the number of spikes in the 4.5-second intervals immediately preceding and coinciding with light application; spikes were defined as voltage excursions from baseline >3× the baseline standard deviation.
We measured the number of bursts in the 4.5-second intervals immediately preceding and coinciding with light application; bursts were defined as clusters of spikes with no return to baseline voltage for >500 milliseconds.
This analysis algorithm was developed because it is difficult to produce true seizures in submerged brain slices. Even in slices obtained from young animals, we have observed consistent electrographic seizures only in interface chambers, which are unsuitable for the pharmacology experiments described in this paper.12 Our analysis algorithm eliminated the potential for investigator bias, because the analyzed segments were preselected prior to any investigator examination, and a control segment immediately preceding illumination was always compared to the illuminated segment that followed. The 10-minute interval between slice illumination also ensured that fresh RuBi-GABA was available for uncaging. We ignored the first 0.5 seconds (we analyzed only 4.5 seconds) after illumination, because there was sometimes a stimulation artifact due to electrical interference from the LED current. Slice uncaging was accomplished by placing a high-intensity blue LED (455nm; Luxeon Star LEDs, Brantford, Ontario, Canada) on a copper pedestal 1mm below the slice coverslip.10 A 5V pulse automatically activated the LED power supply every 10 minutes throughout the entire experiment. We did not measure light output in lumens, but anticipate that the lumen output will rise somewhat superlinearly with current. This effect will probably cause no more than a 10% departure from linearity under the conditions we are using.
Neocortical Seizure Model and Electroencephalography
Four- to 6-week-old male Sprague-Dawley rats were anesthetized with isoflurane (4%) and then placed on a heating pad in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). A 4mm craniotomy was performed over the anterior left hemisphere using a dental drill. We created a reservoir around the cranial window using dental cement, which we filled with our drug-containing solutions. A 2mm slit was placed in the dura, which allowed drug or control ACSF direct access to the pial surface of the anterior left hemisphere. We placed 2 screw electrodes symmetrically over each hemisphere and differentially recorded the electroencephalogram (EEG) between them.6 The EEGs were recorded using standard amplifiers (Grass-Telefactor, West Warwick, RI), digitized (200Hz), and stored using personal computer-based commercial hardware and software (Digidata and Axoscope; Axon Instruments). Control and experimental rats were pretreated by applying either 200µl ACSF or ACSF containing RuBi-GABA ± picrotoxin (PTX), respectively, in the dural reservoir. After 45 minutes of pretreatment, seizures were induced by exchanging reservoir solutions with a new solution containing 4-AP (500µm) and reducing the isoflurane level to <1%. In some control experiments, GABA (to a final concentration of 100µm) was added to the reservoir over 5 seconds at seizure onset, to determine whether GABA could mimic the RuBi-GABA effect. Seizure onset and termination were readily recognizable as abrupt changes in EEG frequency and amplitude.6 We used the blue LED, mounted on to the end of a copper rod and positioned 3 to 4mm above the brain surface and above the reservoir, to uncage Rubi-GABA that had diffused from the reservoir into cortex. The blue light is capable of penetrating through the intact rat dura, not only through the slit, and into brain. As soon as a seizure was observed, a transistor–transistor logic pulse could be manually triggered to activate the LED. We evaluated the effect of 30 and 60 seconds of illumination (500mA) on seizure durations in the absence or presence of RuBi-GABA. All of the experiments involving RuBi-GABA were carried out in a darkened room with only occasional red lamp illumination.
Temperature Measurements
The effect of illumination on slice surface temperature was monitored by a miniature thermocouple (5TC-TT-T-36-36; Omega, Stamford, CT) connected to a digital thermometer (CN1000, Omega). For the in vivo experiments, a thermocouple was inserted through the cranial window and placed on the brain surface to measure the temperature change before and during the LED illumination. The in vitro and in vivo temperature signals were digitized (Digidata and Axoscope) and stored on hard disk.
Statistics
All data are presented as the mean ± standard deviation (SD). We used paired t tests for comparisons of seizure variables within single slices and repeated measures analysis of variance (ANOVA) with multiple comparison tests for analysis of seizure durations in individual rats. ANOVA was used to compare seizure durations between groups of rats.
Results
Effect of RuBi-GABA Uncaging on Interictal Bursts in a Brain Slice
We have previously shown that uncaging Rubi-GABA generates GABAergic currents in mammalian brain slices and that Rubi-GABA does not antagonize GABAergic currents.11 We first wanted to determine whether sufficient GABA could be released by a blue LED to alter interictal discharges in a slice. Neocortical slices were perfused with ACSF containing 4-AP and lacking extracellular magnesium, which triggers paroxysmal activity. We compared the SD of the baseline voltage, number of spikes, and number of bursts over the 4.5 seconds immediately preceding light application and the 4.5 seconds after illumination, allowing 0.5 seconds for stimulation artifacts. In each group, we analyzed data from 10 separate slices, which were each illuminated between 2 and 8×. In the presence of 4-AP and absence of magnesium, LED illumination had no effect on any parameter in the absence of RuBi-GABA (p > 0.05 for all 3 parameters, paired t test; Figs 1 and 2). However, in the presence of RuBi-GABA (10µM), blue light illumination (200mA) significantly reduced baseline SD, diminished spikes, and decreased bursts. With less intense illumination (current 100mA), spikes and bursts were still significantly reduced, with baseline SD relatively less affected. When the RuBi-GABA concentration was reduced from 10 to 3µM, spikes, bursts, and baseline SD all decreased, but the reduction did not reach statistical significance (p > 0.05 for all 3 parameters, paired t test; see Fig 2). We did not observe any increase in spikes or bursting in slices exposed to Rubi-GABA with 4-AP (lacking extracellular magnesium) compared to 4-AP alone (lacking magnesium), indicating that Rubi-GABA does not block spontaneous inhibitory transmission. The RuBi-GABA effect disappeared when PTX (100µM) was present in the ACSF, consistent with RuBi-GABA releasing GABA, which then bound to GABAA receptors (see Figs 1C and 2).
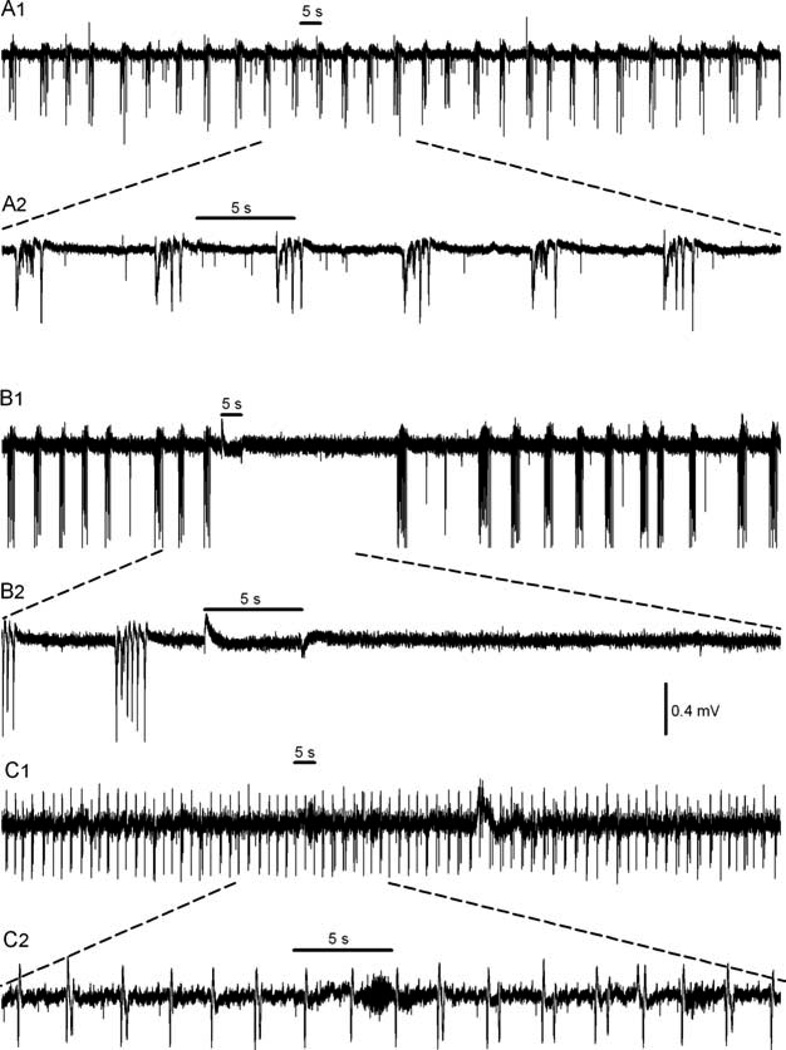
FIGURE 1.
Effect of ruthenium-bipyridine-triphenylphosphine–γ-aminobutyric acid (RuBi-GABA) on interictal bursts in a brain slice. (A) Interictal bursts induced by 4-aminopyridine were not affected by illumination in the absence of RuBi-GABA. A2 shows the illuminated portion of A1 on an expanded scale. (B) When 10µm RuBi-GABA was present, illumination (light-emitting diode [LED] current 200mA) suppressed individual spikes and bursts. B2 shows the illuminated portion of B1 on an expanded scale. The spike and burst suppression greatly outlasts the slice illumination in B because of GABA persistence after uncaging. (C) After the addition of picrotoxin (100µm) to the perfusion fluid, illumination of RuBi-GABA no longer prevented spikes and bursts. C2 shows the illuminated portion of C1 on an expanded scale. The horizontal bars in A, B, and C represent 5 seconds of LED illumination.
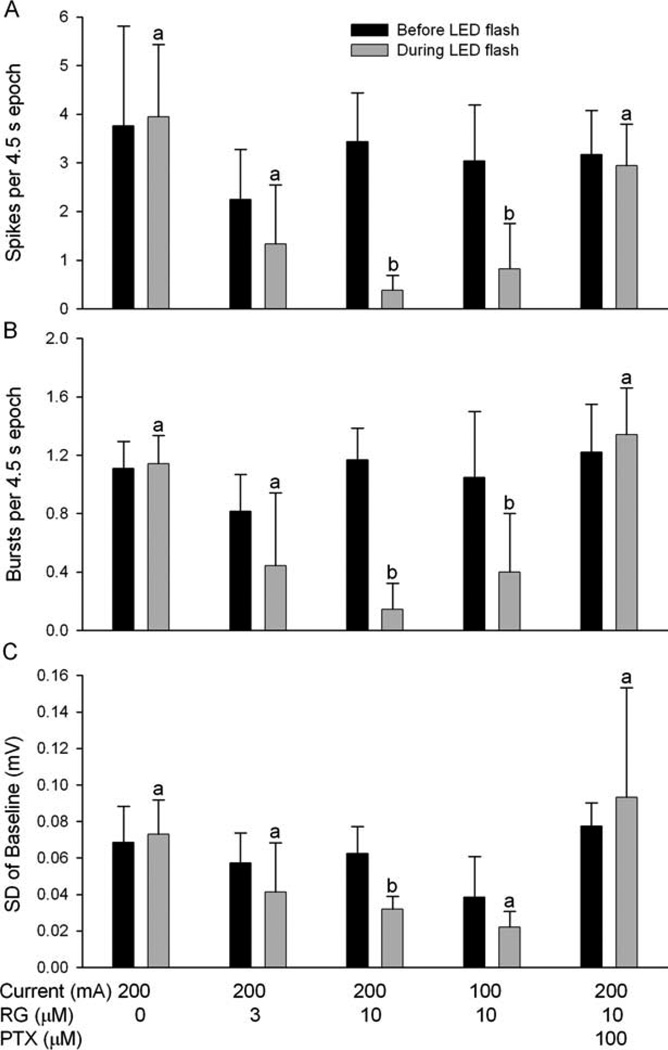
FIGURE 2.
Summary of ruthenium-bipyridine-triphenylphosphine–γ-aminobutyric acid (RG) effect on (A) spikes, (B) interictal bursts, and (C) baseline standard deviation (SD). The black bars represent the values of the parameters in the 4.5 seconds immediately preceding illumination, and the gray bars represent the values of the same parameters in the 4.5 seconds during illumination, allowing a 0.5-second window for illumination electrical artifact. Illumination (light-emitting diode [LED] current 200mA) had no effect on any of the 3 parameters in the absence of RG. However, in the presence of 10µm RG, 5 seconds of illumination (current 200mA) significantly reduced all 3 parameters (p < 0.01, paired t test). When the LED current was reduced to 100mA, the spikes and bursts were still diminished (p < 0.01, paired t test), but baseline SD was not significantly reduced (p > 0.05). At 3µM RuBi-GABA, spikes, bursts, and baseline SD were reduced, but not significantly (p > 0.05). In the presence of 10µm RG plus picrotoxin (PTX; 100µm), 5 seconds of illumination no longer reduced any of the 3 parameters (p > 0.05, paired t test or Wilcoxin signed rank) (a, p > 0.05; b, p < 0.01).
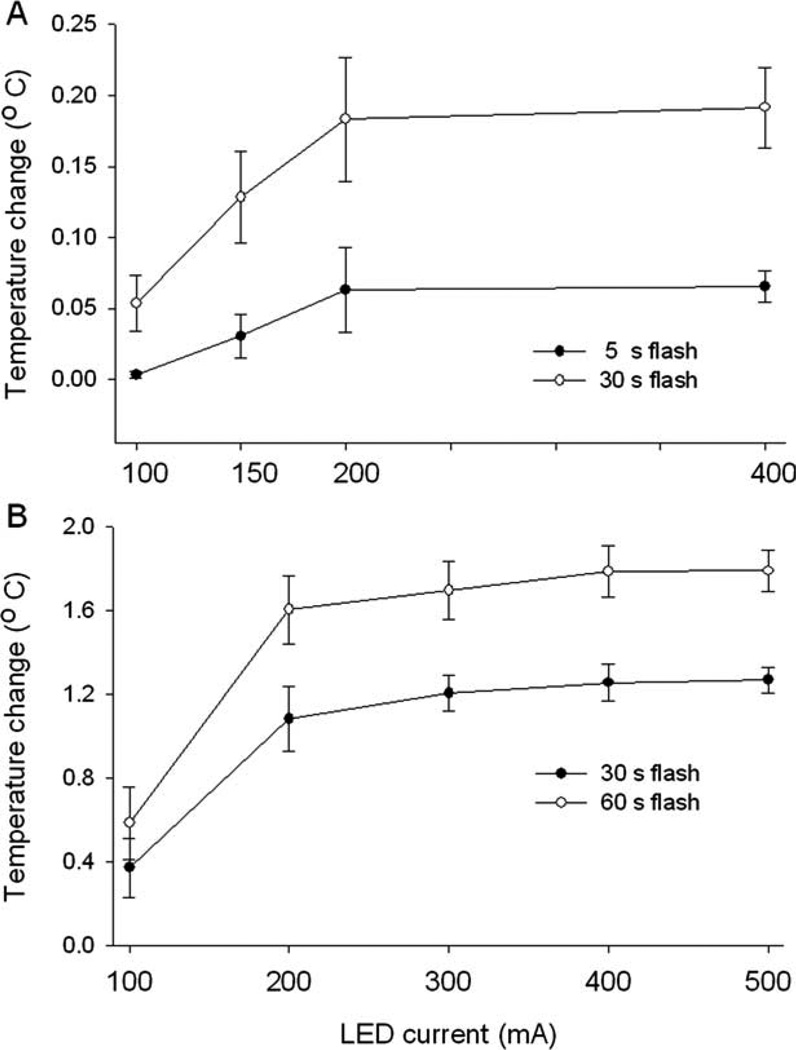
Because LED activation could heat the slice and alter neuron activity, we monitored slice surface temperature during 5 and 30 seconds of illumination, using LED currents between 100 and 400mA. After 30 seconds of illumination at the highest current, the temperature rose by only 0.2°C (Fig 3A). This is not enough to alter seizurelike activity, based upon our prior control experiments.
FIGURE 3.
Effect of blue light-emitting diode (LED) illumination on slice and brain surface temperature. (A) Upper plot shows the maximum temperature change found in a brain slices during a 30-second period of blue LED illumination (n = 8 measurements at each current). The lower plot shows the maximum temperature change during the 5-second illumination period (n = 8 measurements at each current power level). A 400mA LED current raised slice temperature <0.2°C after 30 seconds. (B) The top line represents the maximum temperature increase recorded from the cortical surface after 60 seconds of blue light illumination (n = 6 measurements at each current). The bottom line shows the maximum temperature change after 30 seconds of light flash.
Effect of RuBi-GABA Uncaging on In Vivo Seizures
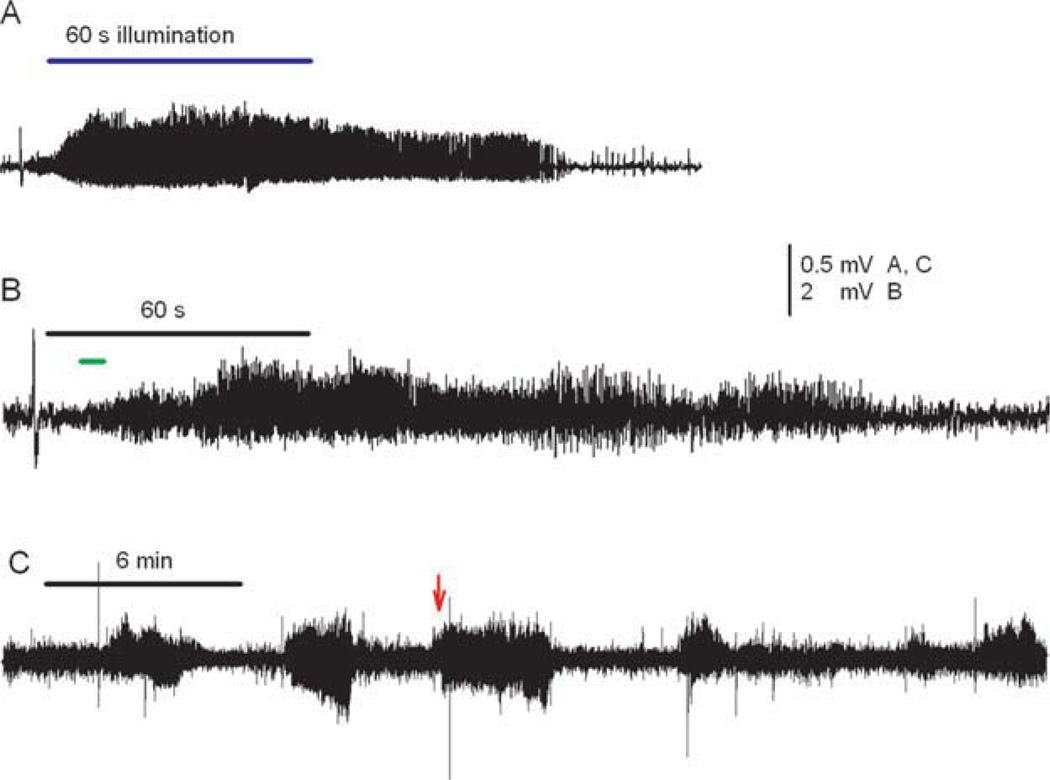
After about 45 minutes, dural application of ACSF containing 4-AP (500µm) on the brain surface consistently induced recurrent seizures lasting about 2 to 4 minutes. These seizures were easy to identify electrographically by their discrete onset, stereotypically evolving amplitude and frequency, and abrupt cessation (Fig 4A).
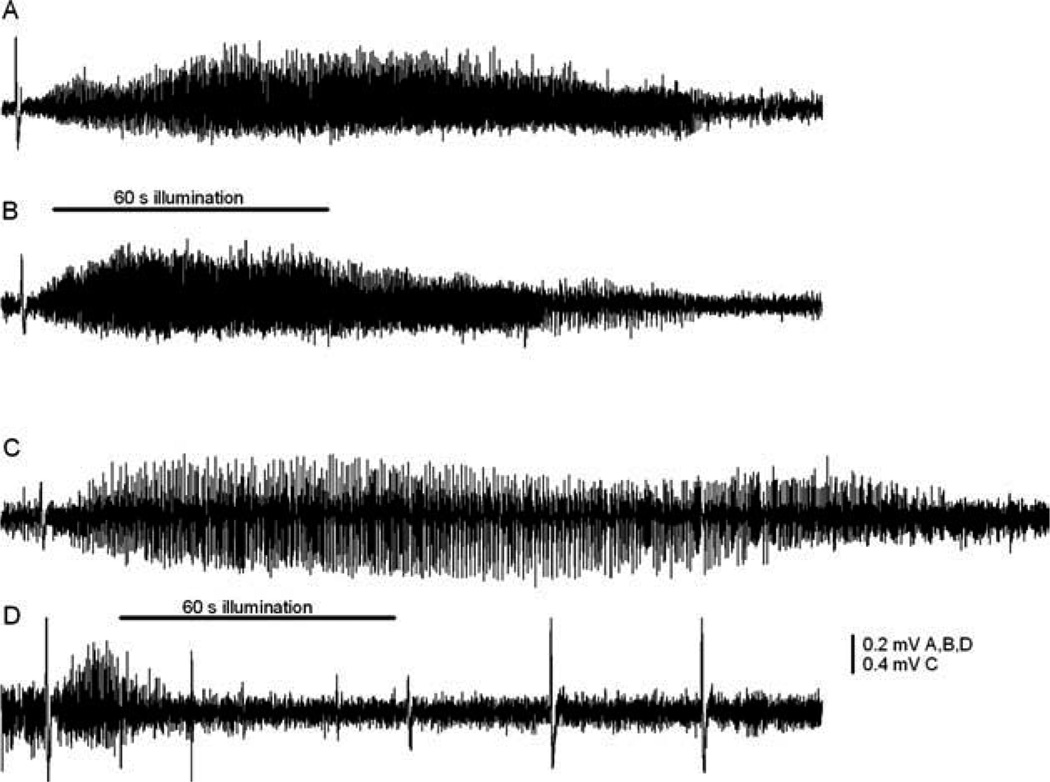
FIGURE 4.
Examples of 4-aminopyridine (4-AP)-induced neocortical seizures and effect of ruthenium-bipyridine-triphenylphosphine–γ-aminobutyric acid (RuBi-GABA). (A) Control seizure induced by 4-AP lasted 140 seconds. (B) Blue light illumination (500mA) had no effect on seizure duration in the absence of RuBi-GABA. (C) Without illumination, local administration of RuBi-GABA (5µM) did not shorten seizure durations. (D) The seizure duration significantly diminished with pairing of the light flash (500mA) and RuBi-GABA (5µM). The horizontal bar represents 60 seconds of blue light-emitting diode illumination (B and D).
We assessed the severity of this model by determining its sensitivity to parenteral diazepam. Others have described rapid attenuation of rodent status epilepticus after administration of a single diazepam dose.13 When we administered diazepam (20mg/kg; intraperitoneal), after the initial appearance of seizures in our model, seizure duration was only reduced from 230 ± 79 seconds to 122 ± 39, 180 ± 55, 148 ± 125, and 213 ± 121 seconds at 15, 30, 45, and 60 minutes, respectively, following the diazepam injection (n = 3 rats). This implies that 4-AP–induced focal seizures are very intense.
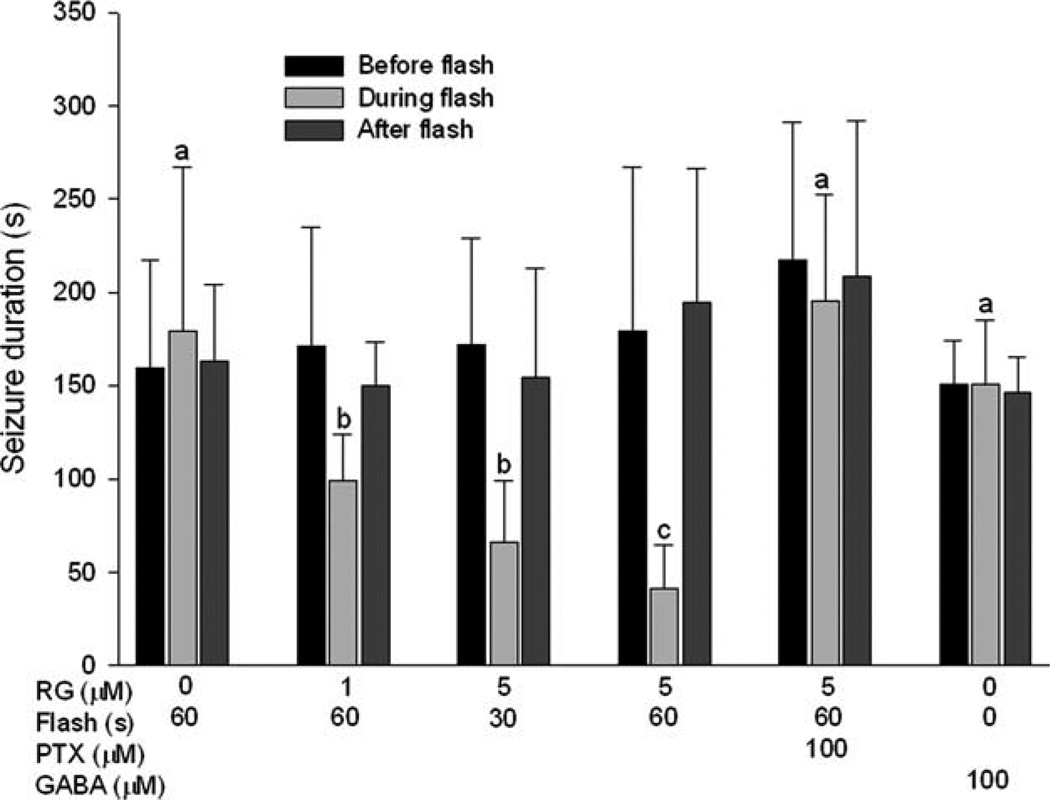
To test whether RuBi-GABA uncaging had an effect on experimental neocortical seizures, we examined the influence of Rubi-GABA on seizure durations in the presence and absence of blue LED illumination. Illumination (500mA) had no effect on seizure duration in the absence of RuBi-GABA (see Fig 4B). Alternately, RuBi-GABA did not alter seizure durations in the absence of illumination (Fig 4C). However, seizures were dramatically shortened when the cortex was illuminated for 30 to 60 seconds (500mA) with Rubi-GABA present at concentrations as low as 1µm (see Figs 4D and 5). The effect of Rubi-GABA was apparent within a few seconds, and if anything, was artificially delayed because we allowed a few seconds for seizures to develop before we activated the LED (see Fig 4D). Some large depolarizations, detected after Rubi-GABA had terminated seizures, could be the GABAergic field potentials previously described by others.14
FIGURE 5.
Photorelease of γ-aminobutyric acid (GABA) from ruthenium-bipyridine-triphenylphosphine–GABA (RG) significantly reduces the duration of neocortical seizures. For each animal, the seizure durations before, during, and after blue light illumination were compared. Seizure duration was not affected by illumination in the absence of RG (a, p > 0.4, 1-way repeated measures analysis of variance [ANOVA] on ranks). However, illumination (30 or 60 seconds) significantly reduced the duration of seizures in the presence of different concentrations of RG (b, c, p < 0.05 and p < 0.001, respectively, compared to preillumination and postillumination durations; 1-way repeated measures ANOVA followed by Student-Newman-Keuls; n = 7 or 8 animals in each group). Picrotoxin (PTX; 100µm) eliminated the effect of RG illumination (n = 4 animals). Focal dural reservoir application of GABA at seizure onset also had no effect on seizure durations (n = 8 animals). There was no significant difference in seizure durations before and after illumination across all the groups, indicating no effect of RuBi-GABA on seizure durations in the absence of illumination (p > 0.4 by ANOVA). The shaded bars for focal GABA administration represent before, during, and after GABA application, not illumination.
Without illumination, we did not see any difference between seizure durations in the absence or presence of Rubi-GABA, consistent with prior observations that Rubi-GABA does not antagonize GABAA receptors at low concentration (Fig 5).11 When PTX (100µm) was added with RuBi-GABA, illumination no longer affected seizure durations, confirming that the in vivo effect of RuBi-GABA was mediated through GABA release (Figs 5 and 6A). We failed to see a rapid effect on seizure durations when GABA (final concentration 100 µm) was directly added to the reservoir during the first 5 seconds after seizure onset (see Figs 5 and 6B, C). Seizures were eventually attenuated, but the effect took several minutes, as reported by others who have used topically applied GABA or muscimol to terminate experimental cortical seizures (Fig 6C).15,16
FIGURE 6.
Rapid seizure termination after illumination of ruthenium-bipyridine-triphenylphosphine–γ-aminobutyric acid (GABA) is blocked by picrotoxin (PTX) and not duplicated by local GABA application. (A) In the presence of PTX, illumination of caged GABA for 60 seconds (bar) no longer stopped seizures. (B) When GABA was quickly applied into the dural reservoir (short, lower bar), the seizures were also unaffected. (C) A longer electroencephalographic recording shows 2 control seizures, followed by a third seizure that was unaffected by rapid application of GABA (arrow). The duration of a fourth seizure that came 3 minutes later was shorter than the 3 prior seizures, suggesting that GABA application eventually had an effect. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
It is unlikely that cortical heating from the LED accounts for the antiepileptic effect of in vivo uncaging. The maximum rise in brain surface temperature was 1.3 ± 0.1 and 1.8 ± 0.1°C for 30 and 60 seconds of illumination, respectively, with an LED current of 500mA (see Fig 3B). Illumination alone at this intensity had no effect on seizure durations (see Figs 4B and 5). The cortical temperature rise probably exceeded the slice temperature rise because slice perfusion with saline was more effective than cortical blood flow in stabilizing tissue temperature.
Discussion
Although we and others have used optical methods to reduce seizurelike activity in vitro, to our knowledge, this is the first report of optical control of in vivo seizures.8–10,17 There is clearly great interest in exploiting optical methods to control neuronal activity and inhibit pathological neuronal excitation. However, at present, this has not been achieved using any of the other optical methods in living animals, and there are good reasons to believe that each will have serious drawbacks. Although it is possible that optogenetic methods could be manipulated to allow targeting of neurons in the seizure onset zone, there are substantial obstacles to overcome before it will be possible to transfect postmitotic human neurons with the genes for light-sensitive plant ion pumps. At least 1 report has already documented alterations in neuronal current–voltage relationships in rodent neurons expressing 1 of the channel rhodopsins, so they may not be benign insertions.8 Similarly, the organic photoswitches that alter channel permeability would also be expected to modify the basic biophysical properties of those channels.18 The light-sensitive neurosteroid derivatives that potentiate GABA currents also potentiate NMDA responses, making them unattractive as antiepileptic agents.17,19
One of the most encouraging aspects of our results with RuBi-GABA is that it successfully stopped very intense focal seizures that were not stopped by doses of intraperitoneal diazepam known to terminate other types of rat status epilepticus. Thus, direct activation of GABA receptors by low concentrations of uncaged GABA in this paradigm is more effective than modulation of GABA receptors by a benzodiazepine. The positive findings in both the slice and in vivo models with such low concentrations of RuBi-GABA were an initial surprise. They probably reflect the high quantum efficiency of RuBi-GABA as well as GABA action at very high-affinity, extrasynaptic receptors.20,21 In view of the severity of both models employed in this study, it is possible that antiepileptic concentrations of RuBi-GABA in more realistic seizure models or clinical epilepsy could be <1µm, making toxicity less unlikely. Similarly, we may be able to reduce the LED intensity if real in vivo seizures are milder and less resistant to therapy.
RuBi-GABA was the first caged compound that we tested that is sensitive to light in the visible range. The ability to uncage beyond the ultraviolet range, where most other caged compounds are illuminated, is attractive, because longer wavelengths lead to less light scattering and penetrate further into brain. The lower energy of longer wavelengths also makes them potentially less toxic than ultraviolet.
Other groups are developing methods for focal application of GABA or related inhibitory transmitter analogs to try to prevent focal seizures.16 Caged GABA offers at least 4 clear advantages. First, optical activation is extremely fast. In our examples, seizures were terminated in several seconds despite that we allowed them to progress for up to 10 seconds prior to activating the LED. The effect of direct GABA application was much slower, not appearing until several minutes after GABA was added to the dural reservoir, as seen by others who have used cortical application of GABA or muscimol to treat experimental seizures.15,16 Second, uncaging obviates direct injection of drugs into the cortex, which will be physically destructive, especially if repeated over many seizures. Third, uncaging allows spatial flexibility, because the LEDs are so small that a flexible array of LEDs could be placed over the seizure onset zone. This would permit testing different patterns of spatial illumination to determine the optimal illumination pattern for seizure control. Direct injection of drug into large, irregular, or discontinuous cortical regions is not feasible. Fourth, the GABA concentration transient released by uncaging will be low and last for only a few seconds before it is removed by reuptake systems. If focally applied, the concentration of GABA or other inhibitory agonists needs to be much higher (>25mM for GABA) for a sufficiently high concentration to reach dendritic GABAA receptors.15 These high levels will diffuse from the application site and persist for many seconds, disrupting normal synaptic activity, as shown by the EEG.15,16 This is what we also observed when GABA was placed in the reservoir, and seizures persisted for minutes until they slowly diminished.
We believe that it is not unrealistic to envision an integrated device that includes a pump for the delivery of caged GABA into the subarachnoid space, an array of 1 or more LEDs embedded in a flexible matrix covering possible seizure onset zone(s) in the cortex, and an amplifier capable of activating the LEDs when a seizure is detected. For over 2 decades, neurosurgeons have implanted pumps that deliver baclofen into the subarachnoid space, so the technology for long-term drug administration directly into the subarachnoid space is well established.22 A clinical trial of a responsive, implantable stimulation system for epilepsy has already validated automatic human seizure detection and the long-term reliability of subdural electrode arrays.23 There is also substantial interest in improving the efficiency and spectral specificity of LEDs, which would permit smaller batteries and allow LEDs to be better matched with the light absorption of newer caged compounds.24
Several practical problems still have to be solved before this approach can be considered for clinical development, including assessment of caged GABA stability, toxicity, and cortical penetration. Our preliminary observations suggest that RuBi-GABA is toxic to cultured neurons, so RuBi-GABA itself is unlikely to be the caged GABA developed as a clinical drug. Nevertheless, our positive results are an important proof of principle that GABA uncaging can terminate focal seizures and should encourage synthesis of new caged compounds that could be developed as clinical drugs.
Acknowledgment
Supported by a CURE Multidisciplinary Award (X.Y., D.L.R., and S.M.R.), National Eye Institute RO1 EY11787 (D.S.P. and R.Y.), and the Kavli Institute (D.S.P. and R.Y.).
We thank R. Miller for help with LED assembly and D. Qin for assistance with device fabrication.
Footnotes
Potential Conflicts of Interest
R.Y.: employment, HHMI; patents, coinventor of RuBi-GABA but has received no royalties. S.M.R.: employment, University of Minnesota; grants/grants pending, NIH, CURE.
References
- 1.Sander J. The natural history of epilepsy in the era of new antiepileptic drugs and surgical treatment. Epilepsia. 2003;44(suppl 1):17–20. doi: 10.1046/j.1528-1157.44.s.1.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Tellez-Zenteno J, Dhar R, Hernandez-Ronquillo L, et al. Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain. 2007;130:334–345. doi: 10.1093/brain/awl316. [DOI] [PubMed] [Google Scholar]
- 3.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 4.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 5.Eder H, Jones D, Fisher R. Local perfusion of diazepam attenuates interictal and ictal events in the bicuculline model of epilepsy in rats. Epilepsia. 1997;38:516–521. doi: 10.1111/j.1528-1157.1997.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang X-F, Rothman S. Focal cooling rapidly terminates experimental neocortical seizures. Ann Neurol. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- 7.Nakken K, Henriksen O, Roste G, et al. Vagal nerve stimulation—the Norwegian experience. Seizure. 2003;12:37–41. doi: 10.1016/s1059131102001383. [DOI] [PubMed] [Google Scholar]
- 8.Tonnesen J, Sorensen A, Deisseroth K, et al. Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A. 2009;106:12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman S, Perry G, Yang X, et al. Optical suppression of seizure-like activity with an LED. Epilepsy Res. 2007;74:201–209. doi: 10.1016/j.eplepsyres.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Schmidt B, Rode D, et al. Optical suppression of experimental seizures in rat brain slices. Epilepsia. 2010;51:127–135. doi: 10.1111/j.1528-1167.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 11.Rial Verde E, Zayat L, Etchenique R, et al. Photorelease of GABA with visible light using an inorganic caging group. Front Neural Circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill M, Wong M, Amarakone A, et al. Rapid cooling aborts seizure- like activity in rodent hippocampal-entorhinal slices. Epilepsia. 2000;41:1241–1248. doi: 10.1111/j.1528-1157.2000.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 13.Kapur J, Macdonald R. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABA(A) receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uva L, Avoli M, de Curtis M. Synchronous GABA-receptor-dependent potentials in limbic areas of the in-vitro isolated adult guinea pig brain. Eur J Neurosci. 2009;29:911–920. doi: 10.1111/j.1460-9568.2009.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John J, Baptiste S, Sheffield L, et al. Transmeningeal delivery of GABA to control neocortical seizures in rats. Epilepsy Res. 2007;75:10–17. doi: 10.1016/j.eplepsyres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Ludvig N, Baptiste S, Tang H, et al. Localized transmeningeal muscimol prevents neocortical seizures in rats and nonhuman primates: therapeutic implications. Epilepsia. 2009;50:678–693. doi: 10.1111/j.1528-1167.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 17.Eisenman L, Shu H, Akk G, et al. Anticonvulsant and anesthetic effects of a fluorescent neurosteroid analog activated by visible light. Nat Neurosci. 2007;10:523–530. doi: 10.1038/nn1862. [DOI] [PubMed] [Google Scholar]
- 18.Fortin D, Banghart M, Dunn T, et al. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenman L, Shu H, Wang C, et al. NMDA potentiation by visible light in the presence of a fluorescent neurosteroid analogue. J Physiol. 2009;587:2937–2947. doi: 10.1113/jphysiol.2009.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scimemi A, Semyanov A, Sperk G, et al. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation ofGABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 22.Albright A, Turner M, Pattisapu J. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233–239. doi: 10.3171/ped.2006.104.4.233. [DOI] [PubMed] [Google Scholar]
- 23.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 24.Schubert E, Kim J. Solid-state light sources getting smart. Science. 2005;308:1274–1278. doi: 10.1126/science.1108712. [DOI] [PubMed] [Google Scholar]