Abstract
The first enantioselective synthesis of a potent GlyT1 inhibitor is described. A 3-nitroazetidine donor is used in an enantioselective aza-Henry reaction catalyzed by a bis(amidine)-triflic acid salt organocatalyst, delivering the key intermediate with 92% ee. This adduct is reductively denitrated and converted to the target through a short sequence, thereby allowing assignment of the absolute configuration of the more potent enantiomer.
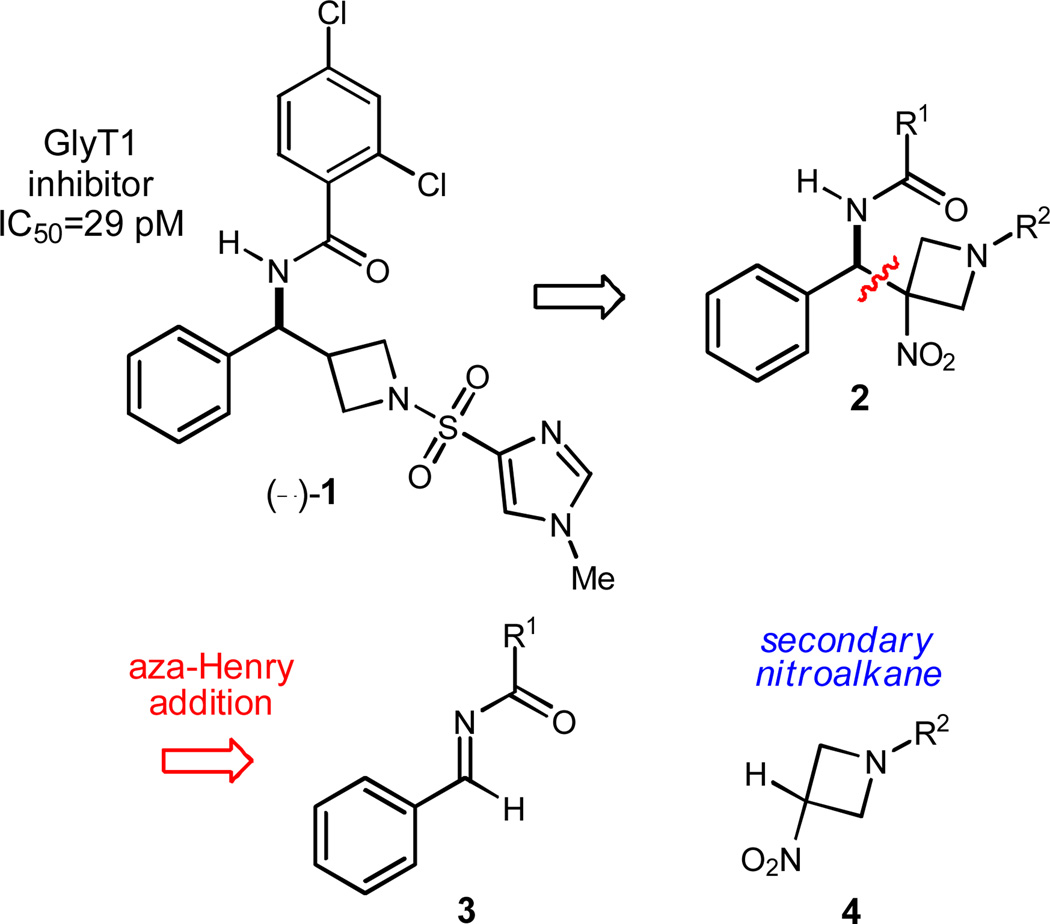
Azetidine 1 is a potent GlyT1 inhibitor first prepared by Lindsley1 as part of a program aimed at the discovery of novel, potent GlyT1 inhibitors. Inhibition of GlyT1 can increase glycine levels and NMDA signaling, thereby providing a promising therapeutic target for the treatment of schizophrenia.2 The racemate was prepared by a short synthetic sequence, and chromatographic separation of enantiomers using a chiral stationary phase led to the determination that one enantiomer is not only a potent inhibitor (IC50=29 pM), but also inhibits glycine reuptake 104 times better than its antipode.1 We established three goals: 1) develop an enantioselective preparation of the substituted aminomethyl azetidine core, 2) synthetically convert this core to the potent GlyT1 inhibitor 1, and in so doing 3) assign the absolute configuration of the more potent enantiomer of 1. A motivation to achieve the first of these goals was the opportunity to develop an enantioselective addition of 3-nitro azetidines to imines using Bis(AMidine) [BAM] based chiral proton catalysis.
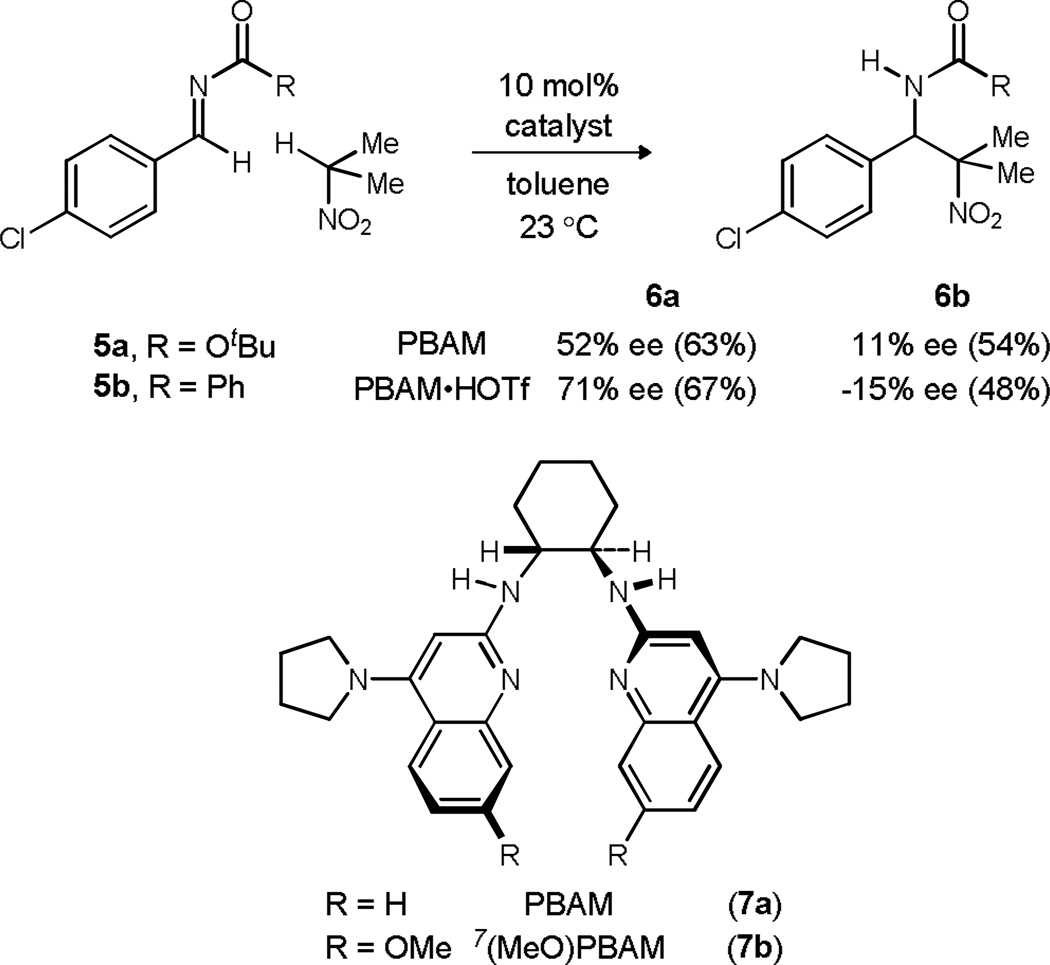
Our broader interest in the application of Brønsted acid catalysis to the development of therapeutics3 motivated an approach to this molecule using an asymmetric aza-Henry reaction between an imine (3) and nitroazetidine (4) (Scheme 1). Subsequent denitration of the resulting tertiary nitroalkane 2 might then give the underlying structural foundation of target 1. The catalyzed, enantioselective addition of secondary nitroalkanes is rare and remains limited to 2-nitropropane additions to N-Boc imines.4,5,6 In the case of BAM catalysis, 2-nitropropane was used to initially evaluate the feasibility of the approach (Scheme 2).7 Catalyzed addition of 2-nitropropane to N-Boc imine 5a at 23 °C delivered the addition product (6a) with 71% ee using PBAM·HOTf (7a·HOTf) (67% yield). The free base form of the catalyst (7a) provided the addition product with lower enantioselection (52% ee, 63% yield).8 A more direct application of this synthetic approach to 1 would involve an appropriate N-acyl imine, and the feasibility of this was investigated using N-benzoyl imine 5b. Unfortunately, this electrophile resulted in an addition product (6b) with low enantioselection, regardless of the protonation state of the electron rich BAM ligand (11% ee and −15% ee, Scheme 2). Our approach therefore relied on the use of an N-Boc imine electrophile which would offer the advantage of providing the fundamental aminomethyl azetidine backbone should alternative derivatives be desired for further medicinal chemistry studies.
Scheme 1.
Retrosynthetic analysis of GlyT1 inhibitor 1
Scheme 2.
Enantioselective 2-nitropropane additions
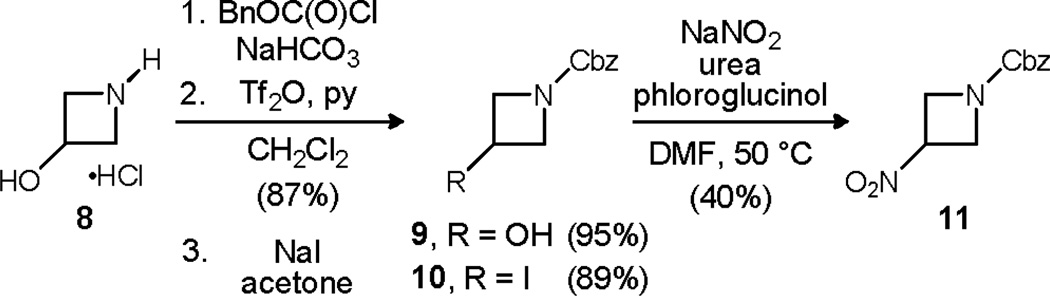
Preparation of a protected 3-nitroazetidine was targeted next. 3-Hydroxyazetidine is a relatively inexpensive, commercially available substance as its hydrochloride salt (8),9 and it was converted to 9 in 95% yield using Cbz-Cl under basic conditions. For reasons not clear, conversion of N-Cbz derivative 9 to the corresponding bromide or iodide using triphenyl phosphine and carbon tetrabromide or iodine, respectively, failed. And while the mesylate was readily prepared, it was not a competent precursor to the iodide or nitroazetidine through substitution. Nitroazetidine 11 was ultimately prepared by triflation of the alcohol (87% yield), conversion of the triflate to iodide 10 (89% yield), and substitution using the Kornblum protocol (40% yield, Scheme 3).10
Scheme 3.
Synthesis of 3-nitroazetidine from 3-hydroxyazetidine
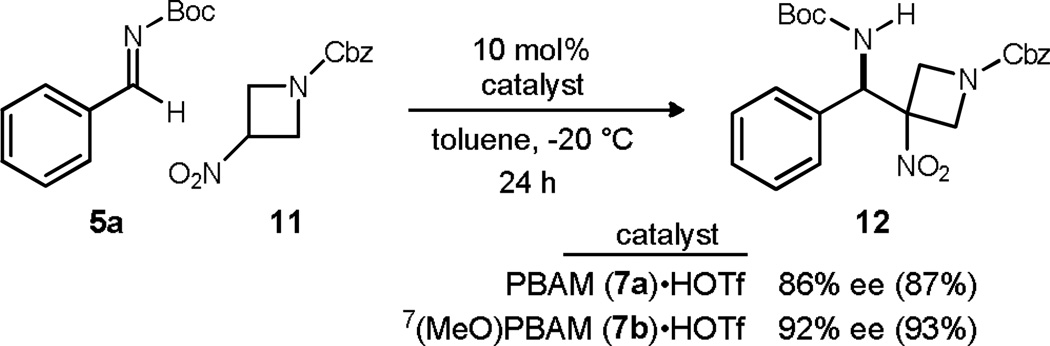
With the desired nitroalkane in hand, conditions analogous to those in Scheme 2 were applied. Use of PBAM·HOTf at room temperature provided the addition product (12) with good enantioselection (78% ee). The time to completion of this reaction was noted to be very short (70 minutes) relative to the addition of 2-nitropropane to aryl N-Boc imines (reaction times of ~24 hours). The improved reactivity provided the opportunity to lower the reaction temperature as a means to increase the observed enantioselection. In the event, the addition product could be acquired with 86% ee at −20 °C and a one day reaction time. An additional BAM catalyst was evaluated in this context to further improve enantioselection (Scheme 4). The 7-methoxy quinoline-derived PBAM catalyst7(MeO)PBAM·HOTf (7b·HOTf) led to appreciably higher enantioselection at the 92% ee level with excellent yield.
Scheme 4.
Development of highly enantioselective 3-nitroazetidine additions
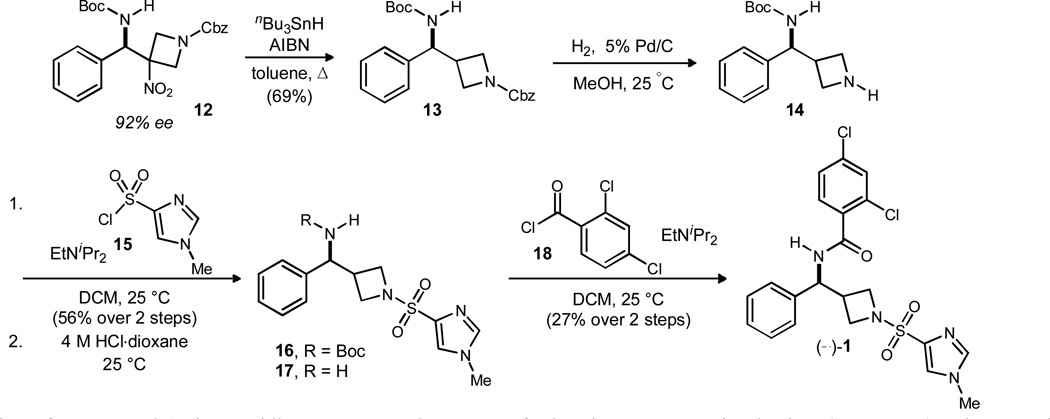
With enantiomerically enriched aza-Henry product 12 in hand, a stannane-mediated reductive denitration was attempted (Scheme 5).11,12,6b This reaction proceeded smoothly to furnish denitrated product 13 in 69% yield. The preparation of 13 provides the key scalemic substituted aminomethyl azetidine scaffold common to target 1 as well as any number of derivatives through subsequent derivatization.13 To establish the validity of the former, the Cbz group of 13 was removed by hydrogenolysis before acylation with sulfonyl chloride 15 to provide 16 (56% yield over two steps). Acid-promoted Boc deprotection and subsequent acylation with 18 provided the GlyT1 inhibitor (−)-1 in 27% yield over two steps (unoptimized). Material prepared in this way, using (R,R)-catalyst 7b, delivers the levorotatory enantiomer. Using HPLC retention times for comparison, this material corresponds to the faster eluting, and incidently more potent inhibitor.1
Scheme 5.
Completion of the synthesis of GlyT1 inbibitor (−)-1
In summary, the more potent enantiomer of 1 was prepared using an aza-Henry addition for strategic convergency. This step proceeded with good enantioselection (92% ee) in a rare example of a secondary nitroalkane addition to an imine. This key intermediate, following reductive denitration, was converted to the desired small molecule inhibitor in four steps and used to assign the absolute configuration of (−)-1 as depicted. Differentially protected, constrained 1,3-diamine 13 may also find broader use as a chiral nonracemic small molecule now readily accessible.
Supplementary Material
Acknowledgments
We are grateful for the generous financial support provided by the NIH (GM 084333). This work is dedicated to our colleague Craig Lindsley with respect and admiration.
Footnotes
Electronic Supplementary Information (ESI) available: full preparative and analytical details for all new compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.Lindsley CW, Conn PJ, Williams R, Jones CK, Sheffler DJ. 2010/0256186. US. 2010:A1.
- 2.Pinard E, Alanine A, Alberati D, Bender M, Borroni E, Bourdeaux P, Brom V, Burner S, Fischer H, Hainzl D, Halm R, Hauser N, Jolidon S, Lengyel J, Marty H-P, Meyer T, Moreau J-L, Mory R, Narquizian R, Nettekoven M, Norcross RD, Puellmann B, Schmid P, Schmitt S, Stalder H, Wermuth R, Wettstein JG, Zimmerli D. J. Med. Chem. 2010;53:4603–4614. doi: 10.1021/jm100210p. [DOI] [PubMed] [Google Scholar]; Mezler M, Hornberger W, Mueller R, Schmidt M, Amberg W, Braje W, Ochse M, Schoemaker H, Behl B. Mol. Pharmacol. 2008;74:1705–1715. doi: 10.1124/mol.108.049312. [DOI] [PubMed] [Google Scholar]; Lindsley CW, Wolkenberg SE, Kinney GG. Current Topics in Medicinal Chemistry. 2006;6:1883–1896. doi: 10.2174/156802606778249784. [DOI] [PubMed] [Google Scholar]; Hopkins CR. ACS Chemical Neuroscience. 2011;2:685–686. doi: 10.1021/cn200108z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis TA, Johnston JN. Chem. Sci. 2011;2:1076–1079. doi: 10.1039/C1SC00061F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon TP, Jacobsen EN. Angew. Chem. Int. Ed. 2005;44:466–468. doi: 10.1002/anie.200461814. [DOI] [PubMed] [Google Scholar]; Gomez-Bengoa E, Linden A, Lopez R, Mugica-Mendiola I, Oiarbide M, Palomo C. J. Am. Chem. Soc. 2008;130:7955–7966. doi: 10.1021/ja800253z. [DOI] [PubMed] [Google Scholar]
- 5.Many procedures using unactivated primary nitroalkanes beyond nitromethane are often based on the use of 5–10 equivalents of nitroalkane. Selected examples: Chang YW, Wang JJ, Dang JN, Xue YX. Synlett. 2007:2283–2285. Yoon TP, Jacobsen EN. Angew. Chem. Int. Ed. 2005;44:466–468. doi: 10.1002/anie.200461814. Trost BM, Lupton DW. Org. Lett. 2007;9:2023–2026. doi: 10.1021/ol070618e. Wang C-J, Dong X-Q, Zhang Z-H, Xue Z-Y, Teng H-L. J. Am. Chem. Soc. 2008;130:8606–8607. doi: 10.1021/ja803538x. Rueping M, Antonchick AP. Org. Lett. 2008;10:1731–1734. doi: 10.1021/ol8003589. Xu X, Furukawa T, Okino T, Miyabe H, Takemoto Y. Chem. Eur. J. 2006;12:466–476. doi: 10.1002/chem.200500735. Rampalakos C, Wulff WD. Adv. Synth. Catal. 2008;350:1785–1790. doi: 10.1002/adsc.200800214. Robak MT, Trincado M, Ellman JA. J. Am. Chem. Soc. 2007;129:15110–15111. doi: 10.1021/ja075653v. Petrini M, Torregiani E. Tetrahedron Lett. 2006;47:3501–3503. Palomo C, Oiarbide M, Laso A, Lopez R. J. Am. Chem. Soc. 2005;127:17622–17623. doi: 10.1021/ja056594t. Nugent BM, Yoder RA, Johnston JN. J. Am. Chem. Soc. 2004;126:3418–3419. doi: 10.1021/ja031906i. Okino T, Nakamura S, Furukawa T, Takemoto Y. Org. Lett. 2004;6:625–627. doi: 10.1021/ol0364531. (syn-selective) Handa S, Gnanadesikan V, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2007;129:4900-+. doi: 10.1021/ja0701560. Reviews: Westermann B. Angew. Chem. Int. Ed. 2003;42:151–153. doi: 10.1002/anie.200390071. Akiyama T, Itoh J, Fuchibe K. Adv. Synth. Catal. 2006;348:999–1010.
- 6.Fully substituted nitroalkanes can be employed if one of the substituents increases the nitroalkane acidity: Singh A, Yoder RA, Shen B, Johnston JN. J. Am. Chem. Soc. 2007;129:3466–3467. doi: 10.1021/ja068073r. Shen B, Johnston JN. Org. Lett. 2008;10:4397–4400. doi: 10.1021/ol801797h. Singh A, Johnston JN. J. Am. Chem. Soc. 2008;130:5866–5867. doi: 10.1021/ja8011808. Wilt JC, Pink M, Johnston JN. Chem. Commun. 2008:4177–4179. doi: 10.1039/b808393b. Han B, Liu Q-P, Li R, Tian X, Xiong X-F, Deng J-G, Chen Y-C. Chem. Eur. J. 2008;14:8094–8097. doi: 10.1002/chem.200801170. Shen B, Makley DM, Johnston JN. Nature. 2010;465:1027–1032. doi: 10.1038/nature09125. Metal-organic catalysts can also provide heightened reactivity: Chen Z, Morimoto H, Matsunaga S, Shibasaki M. J. Am. Chem. Soc. 2008;130:2170–2171. doi: 10.1021/ja710398q. Knudsen KR, Jorgensen KA. Org. Biomol. Chem. 2005;3:1362–1364. doi: 10.1039/b500618j.
- 7.Dobish MC, Johnston JN. Org. Lett. 2010;12:5744–5747. doi: 10.1021/ol1025712. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davis TA, Wilt JC, Johnston JN. J. Am. Chem. Soc. 2010;132:2880–2882. doi: 10.1021/ja908814h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis TA, Dobish MC, Schwieter KE, Chun AC, Johnston JN. Org. Synth. 2012;89:380–393. doi: 10.15227/orgsyn.089.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy VVRMK, Babu KK, Ganesh A, Srinivasulu P, Madhusudhan G, Mukkanti K. Org. Process Res. Dev. 2010;14:931–935. [Google Scholar]
- 10.Kornblum N, Larson HO, Blackwood RK, Mooberry DD, Oliveto EP, Graham GE. J. Am. Chem. Soc. 1956;78:1497–1501. [Google Scholar]
- 11.Ono N, Miyake H, Tamura R, Kaji A. Tetrahedron Lett. 1981;22:1705–1708. [Google Scholar]
- 12.Tanner DD, Blackburn EV, Diaz GE. J. Am. Chem. Soc. 1981;103:1557–1559. [Google Scholar]
- 13.Brandi A, Cicchi S, Cordero FM. Chem. Rev. 2008;108:3988–4035. doi: 10.1021/cr800325e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.