Abstract
Although it is well known that depression is associated with poorer medical outcomes, the association between depression- and liver transplant (LTX)-specific outcomes has not been investigated. We identified three trajectories of depressive symptoms evolving within the first post-LTX year in a cohort of 167 patients transplanted for alcoholic cirrhosis: a group with consistently low depression levels at all time points (group 1, n = 95), a group with initially low depression levels that rose over time (group 2, n = 41), and a group with consistently high depression levels (group 3, n = 31). Controlling for medical factors associated with poorer survival, recipients with increasing depression or persisting depression were more than twice as likely to die (all cause mortality) within the subsequent years. At 10 years post-LTX the survival rate was 66% for the low depression group, but only 46% and 43%, respectively, for the increasing depression and high depression groups. Except for a paradoxically higher percentage of malignancies in the low depression group, the causes of death and other specific LTX outcomes were not different between groups. Whether treatment of depression will improve survival rates is an area for research.
Keywords: Depression, survival, transplant psychology
Introduction
Depression, one of the most common mental health disorders, is especially relevant to liver transplant (LTX) patients due to its higher prevalence in medically ill populations and its association with poorer medical outcomes. Although the rates of depression are known to be high following liver transplantation (nearly 30% of recipients) (1), few studies have considered the association between depression and outcomes following transplantation. In one study of wait-listed LTX candidates 63% were identified as depressed based on a beck depression inventory (BDI) score >10 (2). Depressed LTX candidates were more likely to die while awaiting transplantation than nondepressed candidates even after controlling for severity of illness (2). While pre-LTX depression was not associated with 1-and 6-month survival after LTX, post-LTX depression was not assessed (2) leaving the question of the association between post-transplant depression and outcomes unanswered. In a subsequent study of post-LTX recipients Singh et al. (3) found that hepatitis C (HCV) infected recipients who had recurrence of their HCV were by 1-year post-LTX more likely to be depressed than others but the association between depression and outcomes was not investigated.
Given the significant medical and psychological stressors created by the burden of end-stage liver disease and the subsequent LTX surgery and recovery, we hypothesized that not only would depressive symptoms be common in this population, but that depression occurring within the critical first year of post-LTX recovery would increase the risk for poor short and long-term medical outcomes. Thus, as part of an investigation of medical outcomes in patients receiving liver transplants for alcoholic liver disease (ALD) we collected prospective repeated measures of depressive symptoms occurring within the first post-LTX year to examine the onset of these symptoms. With these repeated assessments we were additionally able to examine the patterns or trajectories of depression evolving within that first year. Finally with these depressive trajectories we were able to investigate whether they predicted long-term survival and medical outcomes.
Subjects
We conducted a prospective longitudinal study of patients transplanted for ALD to investigate their outcomes following transplant. This cohort provided an opportunity to additionally investigate the association between prospectively measured depressive symptoms and long-term survival and outcomes. Our cohort consisted of patients with ALD who were transplanted at the Starzl Transplant Institute (STI) from May 1998 to July 2003. At the time of enrollment patients needed to be at least 3 months post-transplant and discharged from the medical facility. After signing informed consent, they were voluntarily enrolled in our study. During the period of study recruitment, 226 transplant recipients had either a primary or secondary diagnosis of ALD. Of these, 177 participated (30 died before enrollment, 14 refused to participate and 5 were too ill to be enrolled (e.g. still institutionalized). Four participants did not complete any of their first year questionnaires and were not included in the analyses. Given the goal of examining depression trajectories during the first year post-LTX as predictors of subsequent outcomes, we also excluded the six subjects who died before 1-year post-LTX.
The pre-LTX diagnosis of ALD was determined by a consensus diagnosis from interviews and examinations by our transplant surgeons (PF and MdV), hepatologists and psychiatry team (AD and MGF). Psychiatric diagnoses of alcohol dependence or alcohol abuse were made by the psychiatry team using a structured clinical interview and the diagnostic and statistical manual of mental disorders IV (4) criteria. Patients with ALD had a history of excessive alcohol use defined as ≥ 20 g of ethanol/day for women or ≥ 60 g ethanol/day for men (5) and the majority (>80%) had consumed this amount for 10 years or longer.
Measures and Variables
Every 3 months for the first post-LTX year we repeatedly assessed depressive symptoms using the BDI, a widely used instrument to assess the severity of symptoms of depression (6). It contains 21 multiple choice questions, each answer scored on a scale of 0–3 and summed to a total score. Higher total scores indicate more severe depressive symptoms. Cronbach’s alpha in our sample across all time points was 0.86–0.95 demonstrating very good internal consistency between the affective and physical symptoms in the BDI.
Psychiatric and psychosocial variables were extracted from the medical record from structured psychiatric evaluations performed at the pre-LTX assessment. Demographic and socioeconomic variables were provided by participants on their first survey assessment.
Medical variables and survival information were obtained from the medical records. The model for end-stage liver disease (MELD) score was calculated at the point of transplant. Transplant recipients provide blood samples weekly for the first 3 months following transplant, then monthly to 1 year and then every 2–3 months thereafter. We collected all data on liver enzymes (alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyltransferase (GGTP)) and renal functioning (creatinine) out to the furthest post-LTX time point for each participant. According to the standard normal values at our chemistry lab (ALT <63 IU/L, AST <41 IU/L, GGTP <41 IU/L and creatinine < 1.5 mg/dL) we calculated the percentage of each individuals’ labs that were abnormal 50% or more of the time. Patients undergo biopsies based on clinical picture and need. We similarly calculated the percentage of biopsies that were read by our pathologists as demonstrating acute rejection, recurrent HCV or as having steatohepatitis. For our outcomes we considered those who had 20% or more of biopsies with these findings. All tissue pathology data were reviewed by trained evaluators in our STI Transplant Pathology department. Each biopsy is rated on specific features including the presence of fat, bile duct and portal inflammation, venous endothelial inflammation and lobar architecture and inflammation. Acute rejection required both a pathologist’s descriptive report of acute rejection and a rejection activity index score ≥ 3 (7).
The Charlson comorbidity index is a commonly used index of medical comorbidity developed based on 1-year mortality data (8). The index encompasses 22 comorbid medical conditions predicting mortality where mortality for each disease was converted to a relative risk of death within 12 months. A summary score is created from the weighted relative risks of the comorbid illnesses. The Charlson comorbidity index was calculated from all of the medical co-morbidities documented in the medical record up to 1 year following transplant.
In a prior analysis we determined the amounts of daily alcohol consumption for participant’s post-LTX course using a calendar method of data collection. These data allowed us to compute the total yearly amounts of alcohol consumption (9). For these analyses as we were examining survival following the first post-LTX year, we used the total amount of alcohol consumed in the first year as a predictor of outcomes.
Statistical Analyses
We examined BDI scores over the four time points within the first post-LTX year. Two data procedures were performed to prepare these data for further analysis. First because cluster analysis requires complete data we identified any missing BDI values. We applied unbalanced repeated measures modeling to impute the missing scores (10, 11). Ninety-four percent had two or more data points and those with incomplete data were no different than others on any parameter assessed (including age, gender, race, sociodemographic variables, severity of liver disease or pre-LTX depression, anxiety or substance use history). Those with any incomplete data (vs. none) also did not differ on levels of depression observed when the BDI was able to be assessed. Thus, the missingness made criteria to be considered as ignorably missing (12,13) and imputation was thus appropriate. Next because we hypothesized that specific levels of depression would be associated with poorer outcomes, we applied standard threshold cut points (6) to classify each subject’s score as showing low (0–9.5), moderate (9.51–16.5) and high (16.51 and above) depressive symptoms at each of the four time points.
Hierarchical agglomerative cluster analysis (14,15) was then applied to subjects’ depressive symptom levels across the four waves of assessment to identify if subjects could be categorized into discrete groups depending on their unique trajectories of change in depression levels over time. The analysis used the unweighed pair-group method, with arithmetic averages and squared Euclidean distance coefficients. A three-cluster solution (i.e. describing three distinct groups of subjects) provided the optimal fit to the data, based on examination of standard metrics (e.g. amalgamation coefficient).
We examined predictors of depression trajectory membership considering pre-LTX demographic (age, gender, race, marital status, educational level, occupation), psychiatric history variables (alcohol dependence diagnosis, family history of alcohol disorder, years of heavy drinking, length of sobriety, other substance use, history of depression or anxiety) and early post-LTX medical variables (transplant hospitalization length of stay (LOS), intensive care unit (ICU) LOS, Charlson comorbidity index at 1-year post-LTX, MELD score at LTX, hepatocellular carcinoma, donor age, and number of inpatient admission and number of clinic visits within the first post-LTX year and total amount of alcohol in standard drinks consumed in the first post-LTX year). Amounts of alcohol consumed were natural log transformed prior to analyses due to skewedness. Differences between groups were identified using cross tabulation for categorical variables or analysis of variance for continuous variables. Fisher’s exact test was used for categorical variables when the number of cases was small.
Cox proportional hazard modeling was used to generate survival curves and investigate which pre- and early post-transplant variables predicted survival beyond 1 year. The data on survival for each patient were included until the end of the observation period or until the event occurred. Based on the results of the cluster analyses we used the BDI trajectory group assignment to predict survival. We also examined medical variables commonly associated with poorer post-LTX survival; LTX hospitalization and ICU LOS, Charlson comorbidity index, MELD score, HCV, pre-LTX hepatocellular carcinoma, recipient age, donor age and gender. The values for MELD scores, length of hospital and ICU stay were natural log transformed prior to further analyses due to skewedness. The length of the hospital and ICU stays was highly correlated so only the length of the hospital stay was included in the analyses. Correlations between the BDI scores and Charlson comorbidty index across all time points were small (Pearson correlation coefficients <0.28) suggesting that the BDI is capturing the symptoms of depression independent of physical morbidity.
Outcomes and causes of death were dichotomized as present or not and differences between trajectory groups were identified using cross tabulation. Fisher’s exact test was used when the number of cases was small.
Continuous variables are presented as the mean ± standard deviation (SD), and categorical variables are presented as proportions. A p-value less than 0.05 was considered statistically significant. All analyses were performed using statistical package for the social sciences (SPSS) version 18.0.
Results
Sample demographics
The 167 participants, who survived the first year and were included in the analyses, were predominately European American males, reflecting the demographics of the transplant population and of patients with end-stage ALD. Table 1 shows their pretransplant demographic, psychiatric and early post-LTX medical characteristics. The majority (75%) of participants met pre-LTX lifetime Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for alcohol dependence and most had an additional lifetime psychiatric diagnosis, mostly depressive disorders (20%). In addition, nearly 40% had used substances other than alcohol and 27% had used injected drugs (over 60% of those who used other substances). A majority of the patients (55%) had not participated in any form of addiction rehabilitation prior to transplantation. Most (63%) identified a first-degree biologic relative who also had problems with alcohol use.
Table 1.
Cohort demographic, psychiatric and medical characteristics (n = 167)
| Demographics | |
| Gender, % male | 84 |
| Age, M (SD) | 50 (8) |
| Race, % white | 95 |
| Education, % > high school | 48 |
| Occupation, % blue collar | 75 |
| Marital status, % married | 52 |
| Alcohol and psychiatric history | |
| Years heavy drinking, M (SD) | 20 (9) |
| Months of sobriety, M (SD)b | 40 (46) |
| Average drinks/week, M(SD)b | 107 (109) |
| Alcohol rehabilitation, % yes | 45 |
| Family history alcoholism, % yes | 63 |
| Alcohol dependence diagnosis, % yes | 75 |
| Other substance use, % yes | 38 |
| Depressive disorder, % yes | 20 |
| Anxiety disorder, % | 13 |
| Medical variables | |
| Presence of hepatitis C, % yes | 47 |
| MELD score, M (SD)a | 17 (7) |
| Charlson comorbidity score (SD) | 7.15 (1.5) |
Log transformed to reduce skewness prior to statistical test. Untransformed means and SDs are presented to facilitate interpretation.
Depression trajectories
We identified three clusters of trajectories of depressive symptoms within that first year: a group who showed consistently low depression levels at all time points (group 1, n = 95), a group with initially low depression levels that rose over time (group 2, n = 41) and a group with consistently high depression levels (group 3, n = 31). The low depression group had 60% or more with low BDI scores over all time points. The increasing depression group had nearly 60% moderate to high BDI scores at the 3 month time point but 100% by the 12 month assessment. The high depression group had nearly 100% across all time points with moderate to high BDI scores (see Figure 1 for the percentage of groups by level of BDI scores at each time point).
Figure 1.
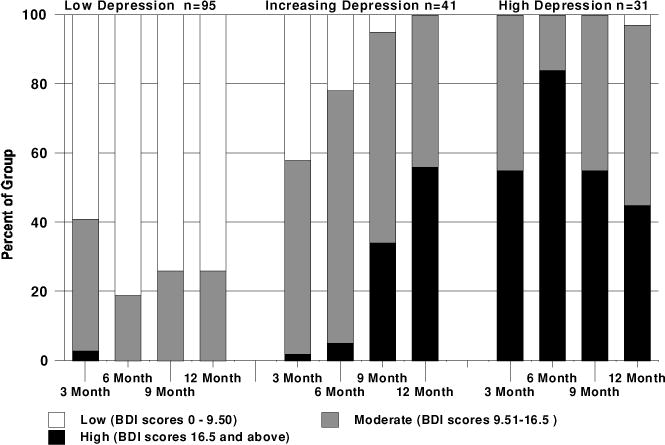
Percentage of cluster groups with low, moderate and high BDI scores at each time point.
We considered pre-LTX psychiatric, demographic and early post-LTX medical factors as predictors of the depression trajectories (Table 2). The low depression group was significantly older (mean 52 vs. 48 years), more likely to be married or have a partner, less likely to have had a pretransplant history of depression or other substance use, less likely to have HCV and had more years of heavy drinking. There were no other differences between groups on demographic, medical or psychiatric history variables.
Table 2.
Comparison of pre-LTX and early post-LTX variables between trajectory groups
| Pre- and early post-LTX variables | Low depression | Increasing depression | High depression | Test statistica, p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age, mean (SD) | 52 (8) | 48 (7) | 48 (8) | 5.9, 0.003 |
| Gender (% male) | 87 | 73 | 87 | ns |
| Race (% white) | 96 | 95 | 94 | ns |
| Married or had partner (% yes) | 61 | 44 | 36 | 7.5, 0.02 |
| Education (% beyond HS) | 53 | 40 | 43 | ns |
| Psychosocial | ||||
| Alcohol dependence dx (% yes) | 72 | 73 | 87 | ns |
| Family hx alcoholism (% yes) | 62 | 58 | 72 | ns |
| Prior hx of depression (% yes) | 12 | 32 | 26 | 0.046* |
| Other substance use (% yes) | 28 | 42 | 61 | 11, 0.004 |
| Years of heavy drinking, mean (SD) | 22 (9) | 18 (8) | 18 (9) | 3.2, 0.04 |
| Length of sobriety, months (SD) | 41 (47) | 39 (43) | 41 (45) | ns |
| Alcohol amount consumed in first year, mean (SD)b | 8 (38) | 12 (42) | 6 (17) | ns |
| Medical | ||||
| HCV infection (% yes) | 38 | 54 | 65 | 7.7, 0.02 |
| HCC (% yes) | 16 | 7 | 19 | ns |
| MELD, mean (SD) | 17 (7) | 17 (7) | 16 (8) | ns |
| Length of hospital stay, m days (SD) | 24 (24) | 25 (23) | 24 (24) | ns |
| ICU length of stay, m days (SD) | 14 (21) | 15 (18) | 17 (24) | ns |
| Donor age, mean years (SD) | 46 (17) | 47 (15) | 41 (18) | ns |
| Charlson comorbidity index at 1 year, m (SD) | 6.8 (1.2) | 7.2 (1.7) | 7.4 (1.4) | ns |
| Inpatient admissions within first year, m (SD) | 2.3 (2) | 1.9 (1.7) | 2.7 (2.8) | ns |
| Transplant clinic visits within first year, m (SD) | 30 (12) | 31 (12) | 26 (14) | ns |
Exact p-value is reported because the number of cases is small (2-sided Fisher’s exact p).
χ2(2) for dichotomous variables, F (2,167) for continuous variables.
For purposes of comparison between types of alcohol consumed, alcohol consumption was reported in standard alcohol drink units (i.e. one 12-ounce beer, 5–6 ounces of wine or a 1-ounce ‘shot’ of hard liquor). Untransformed data are presented for clarity.
Depression trajectory outcomes
We found that those in the increasing depression group and the high depression group had significantly poorer survival beyond the first post-LTX year (χ2 = 34, p = 0.000) compared to those in the low depression group even when controlling for age and other medical variables commonly associated with poorer survival: hospital length of stay, Charlson comorbidity index, severity of liver disease at the point of LTX (MELD score), HCV, hepatocellular carcinoma and donor age (see Table 3 Variables in the survival analysis). In fact at 10 years post-LTX the survival rate was 66% for the low depression group, but only 46% and 43%, respectively, for the increasing depression and the high depression groups (Figure 2). Only three variables reached significance, age, the Charlson comorbidity index and depression, and depression had the strongest impact on survival. Compared to low depression patients, the increasing and high depression groups were more than twice as likely to die (all cause mortality) over the subsequent years (hazard ratios 2.25, CI 1.2–4.3 and HR 2.38, CI 1.2–4.7, respectively).
Table 3.
Variables in survival analyses
| 95.0% CI for hazard ratio
|
|||||
|---|---|---|---|---|---|
| Variables | B | Sig. | Hazard ratio | Lower | Upper |
| Low depression (referent) | 0.014 | ||||
| Increasing depression | 0.812 | 0.012 | 2.25 | 1.19 | 4.26 |
| High depression | 0.867 | 0.012 | 2.38 | 1.21 | 4.67 |
| Charlson score at 1 year | 0.277 | 0.001 | 1.32 | 1.12 | 1.55 |
| Age | 0.038 | 0.045 | 1.04 | 1.00 | 1.08 |
| MELD score at LTX | −0.756 | 0.345 | 0.47 | 0.10 | 2.25 |
| Hospital length of stay | −0.139 | 0.670 | 0.87 | 0.46 | 1.65 |
| HCV | 0.107 | 0.711 | 1.12 | 0.63 | 1.95 |
| HCC | 0.118 | 0.745 | 1.13 | 0.55 | 2.28 |
| Donor age | 0.008 | 0.327 | 1.00 | 0.99 | 1.02 |
| Alcohol use (total consumption first year) | 0.00 | 0.310 | 1.00 | 1.00 | 1.00 |
Figure 2.
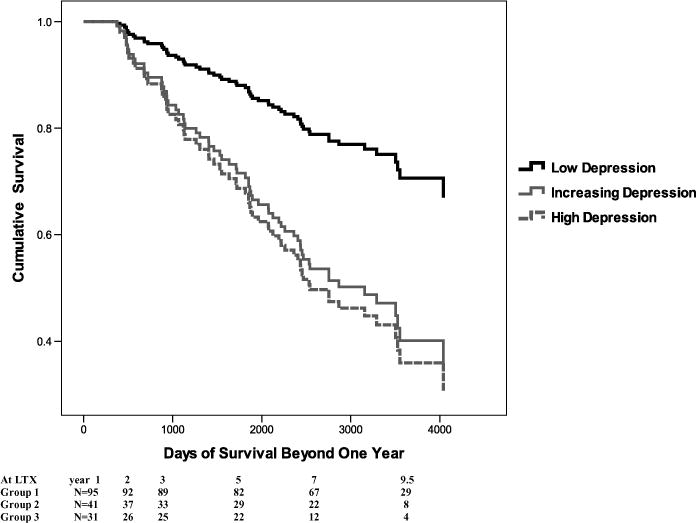
Survival for three groups of BDI trajectories.
We also preliminarily investigated outcomes and causes of death between the three groups. The depression groups did not differ on percentage of abnormal ALT, AST or creatinine but the two depression groups were more likely to have elevated GGTP. Interestingly there were no differences between groups with respect to graft loss, or occurrence of rejection episodes on biopsy. There also was no difference between groups on death due to recurrent HCV. This is especially interesting given that a larger percentage of those with HCV were in the increasing and high depression groups. However there were more deaths in the low depression group due to malignancies compared to the two other groups (see Table 4 for comparison of group outcomes). There was one death from suicide at 1.5 years following transplant and this patient was in the low depression group.
Table 4.
Comparison of outcomes between the three BDI trajectory groups
| Outcomes | Low depression | Increasing depression | High depression | Exact p* |
|---|---|---|---|---|
| ≥20% of biopsies with steatohepatitisa | 8%, n = 7 | 8%, n = 3 | 13%, n = 4 | p = 0.69 |
| ≥20% of biopsies with acute rejectionb | 31%, n = 27 | 36%, n = 14 | 27%, n = 8 | p = 0.73 |
| Graft failure | 37%, n = 10 | 55%, n = 11 | 31%, n = 5 | p = 0.32 |
| ≥ 50% GGTP values abnormal | 68% n = 65 | 85% n = 35 | 87% n = 27 | p = 0.03 |
| ≥ 50% AST values abnormal | 38% n = 36 | 51% n = 21 | 36% n = 11 | p = 0.28 |
| ≥ 50% ALT values abnormal | 58% n = 55 | 73% n = 30 | 74% n = 23 | p = 0.13 |
| ≥ 50% creatinine values abnormal | 44% n = 42 | 49% n = 20 | 48% n = 15 | p = .86 |
| Causes of death | N = 27c | N = 20 | N = 16 | |
| Alcoholic liver disease | 7%, n = 2 | 5%, n = 1 | 6%, n = 1 | p = 1.0 |
| Recurrent HCV | 22%, n = 6 | 35%, n = 7 | 25%, n = 4 | p = 0.67 |
| Other causes of liver failured | 7%, n = 2 | 15%, n = 3 | 12%, n = 2 | p = 0.69 |
| Malignancye | 37%, n = 10 | 5%, n = 1 | 6%, n = 1 | p = 0.009 |
| Cardiac eventsf | 4%, n = 1 | 10%, n = 2 | 12%, n = 2 | p = 0.59 |
| Infection or sepsis | 7%, n = 2 | 15%, n = 3 | 19%, n = 3 | p = 0.50 |
| Unknown | 7%, n = 2 | 5%, n = 1 | 12%, n = 2 | p = 0.71 |
| Accident/trauma | 7%, n = 2 | 5%, n = 1 | 0% | p = 0.78 |
| Other | 4%, n = 1 | 5%, n = 1 | 6%, n = 1 | p = 1.00 |
Exact p-values are reported because the numbers of cases are small (2-sided Fisher’s exact p).
Ten participants did not get biopsies.
Rejection Activity Index score ≥ 3 on biopsy.
Total number adds to more than 27 as two persons died of a combination of recurrent HCV and alcohol related liver disease.
Includes portal vein thrombosis, hepatic artery thrombosis, acute/chronic rejection, infarction.
Includes hepatocellular carcinoma, lung and skin.
Includes myocardial infarction, heart failure, arrhythmia.
While we had hypothesized that depression and resumed alcohol consumption would be associated, there was no difference between groups in the amounts of alcohol consumed within that first year post-LTX as the depression trajectories were evolving. Approximately 50% of all three depression groups were abstainers. Groups also did not differ on the percentage of biopsies showing steatohepatitis (fat commonly due to recurrent alcohol use) nor were there differences between groups on death due to recurrent ALD.
Conclusions
We found distinct trajectories of depressive symptom levels, as measured by BDI scores, can emerge during the first post-LTX year. From these trajectories we found both persistent and incipient depression in the early post-LTX period are significantly associated with poorer long-term survival independent of medical predictors of poorer survival. In fact depression was the strongest predictor of survival conferring a two times higher risk for death. Younger, unmarried patients with HCV and pre-LTX histories of other substance use and depression were more likely to experience these problematic trajectories. Those in the rising and high depression groups while more likely to be infected with HCV were not more likely to die of HCV recurrence. While HCV is commonly associated with poorer survival, the effect size noted in very large cohorts (16) is small (HR 1.14) and while HCV had a similar effect size in our study (HR 1.12) the size of our cohort may not have allowed this to reach significance.
Most importantly these trajectories reveal temporal patterns within the first post-LTX year that would not have been identified on a single assessment. Single time points can reveal high or low symptoms but not whether the individual is better or worse. As is the standard in clinical practice, monitoring symptoms over time is essential to discover trends of persisting symptoms that require clinical attention. Untreated depression can worsen as may have been evidenced by our increasing depression trajectory. Interestingly we did not find a decreasing depression trajectory. However, about 40% of the low depression group had moderate and a small percentage had high symptoms of depression at the 3 month time point but at subsequent time points only about 20% had moderate symptoms. The low depression group may represent some who were initially depressed but largely recovered as the year progressed. Thus these findings have important implications for clinicians who assess patients during the first post-LTX year and who should be watchful for both the onset and evolution of depressive symptoms. While the creation of depression trajectories is not clinically feasible, we demonstrate the use of patient rated clinical measures can provide critical information for the assessment and monitoring of depressive symptoms. Depression is a readily treatable disorder and many pharmacologic and therapeutic interventions exist that are acceptable for LTX recipients.
The depressed groups had several important risk factors for depression. They were more likely to have had a prior history of depression. Depression can often reactivate in times of stress such as undergoing LTX. They were also more likely to be infected with HCV which is also associated with depression. Lastly social support is critical to early recovery and those who were not married and had no partner were more likely to develop depression, perhaps reflecting the essential benefit of support in the early post-LTX recovery period.
We also found that most outcomes including causes of death were not different between groups, but those in the low depression group were more likely to die of a malignancy and nearly half of those deaths (n = 4) were from lung cancer. We did not have smoking data on participants but believe these are likely tobacco related deaths. The mean survival for lung cancer deaths was 4 years suggesting that smoking-related cancers may not develop until the long term and perhaps those in the increasing and high depression trajectories died before developing such a cancer. This underscores the importance of maintaining not only alcohol but tobacco abstinence.
Those in the depression groups were more likely to have elevated GGTP values. While it may seem reasonable to expect that depression would lead to alcohol use as a form of coping or self-medication and thus elevated liver enzymes, we did not find a connection between alcohol use and depression, based on amounts of alcohol consumed within the first year. However we did not assess alcohol consumption beyond the first year and patterns may change over time. The groups also did not differ in deaths as a result of alcoholic liver disease. However, the number of deaths for any specific cause was small and thus these findings remain preliminary. An explanation for the higher GGTP values is unclear; however, depression is sometimes associated with poorer adherence to medical regimens. We did not have adherence data to examine this assumption.
The negative impact of depression and even symptoms of depression on outcomes is well established following myocardial infarction (17–20), stroke (21) and other cardiovascular diseases and even chronic disease states such as diabetes (22). In these studies it was the depression following the medical event or diagnosis that predicted survival. The several studies of depression prior to transplant in liver (2) and lung candidates (23,24) have not found pretransplant depressive symptoms to predict short-term post-transplant survival. Perhaps it is the proximity of the depressive symptoms to outcomes that is critical. So many factors can intervene from the pre- to post-operative phases that the influence of pretransplant depression on post-transplant outcomes may be less relevant.
Increasingly the associations between depression and possible pathophysiologic mechanisms that could worsen medical illness are being identified including increased inflammatory markers (e.g. proinflammatory cytokines), endocrinologic derangements (e.g. increased stress hormones such as cortisol) and cardiovascular abnormalities (e.g. rigid parasympathetic tone, decreased heart rate variability, increased coagulation factors). These mechanisms, if similar for depressed LTX patients, could lead to poorer outcomes. Such systemic mechanisms may lead to earlier death from a wide range of causes which may account for the increased rate in death we observed but not differences in specific causes of death.
It would be important to determine whether our findings can be replicated in other transplant cohorts. This is especially important as our cohort of LTX patients included only those with pre-LTX diagnosis of ALD. Alcohol dependence and major depression are often comorbid psychiatric disorders and thus we may have observed higher rates of depressive symptoms than would be seen in a sample of patients with all liver diseases. These findings should also be replicated in other cohorts of organ transplant recipients. In one study DeVito Dabbs et al. found that lung and heart-lung transplant recipients who had depressive symptoms also experienced greater levels of physical impairment than those who did not and the role of psychological distress was independent of the contribution of actual medical complications to increased physical impairment (25). Dew et al. found that persistently high depressive symptoms during the first year after heart transplantation increased the risk for chronic graft rejection during subsequent years (26). However, we examined acute but not chronic rejection.
An additional limitation of our study is that we assessed symptoms of depression not the actual diagnosis of a clinical depression. While higher BDI scores are suggestive of an actual depressive disorder, further assessment would be needed to establish the diagnosis. However the BDI is the best patient rated instrument having excellent validity and reliability and is used extensively in studies of medically ill populations. As a dimensional measure the BDI can produce a continuum of depressive symptom severity across which the patient’s relative level of distress can be measured. This dimensional approach allowed us to observe changes in the severity of symptoms over time and thus identify the trajectories of symptoms. In addition, a recent meta-analysis found that just having symptoms of depression can increase the risk of death in congestive heart failure (27). Lastly we do not know whether those with significant depressive symptoms were treated and what their treatment response may have been.
In the future researchers should look beyond depressive symptoms to the potential mechanistic underpinnings of how depression may lead to worse outcomes (e.g. increased physiologic stress or proinflammatory cytokines, etc.). These studies could lead to improved identification perhaps even before depressive symptoms evolve. Screening and treating patients for depression throughout the early post-LTX period targeting those most at risk for depressive symptoms is recommended. Whether interventions to treat depression early on will improve long-term survival remains unknown.
Acknowledgments
This research is funded by grant nos. K23 AA0257 from the National Institute of Alcohol Abuse and Alcoholism and R01 DK066266 from the National Institute of Digestive Disorders and Kidney Diseases Rockville, MD, USA.
This publication was made possible by grant number 5UL1 RR024153-04 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov.
Abbreviations
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BDI
Beck Depression Inventory
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- GGTP
gamma-glutamyltransferase
- HCV
hepatitis C
- ICU
intensive care unit
- LOS
length of stay
- LTX
liver transplant
- M
mean
- MELD
model for end-stage liver disease
- SD
standard deviation
- STI
Starzl Transplant Institute
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.DiMartini A, Dew MA, Trzepacz PT. Chapter 31-Organ transplantation. In: Levenson J, editor. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Press; 2004. pp. 775–803. [Google Scholar]
- 2.Singh N, Gayowski T, Wagener MM, Marino IR. Depression in patients with cirrhosis: Impact on outcome. Digest Dis Sci. 1997;42:1421–1427. doi: 10.1023/a:1018898106656. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Gayowski T, Wagener MM, Marino IR. Quality of life, functional status, and depression in male liver transplant recipients with recurrent viral hepatitis C. Transplantation. 1999;67:69–72. doi: 10.1097/00007890-199901150-00011. [DOI] [PubMed] [Google Scholar]
- 4.First Michael., editor. Diagnostic and statistical manual for mental disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 5.Diehl AM. Alcoholic liver disease. Liver Transplant Surg. 1997;3:206–211. [PubMed] [Google Scholar]
- 6.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous Banff schema for grading liver allograft rejection: An International Consensus Document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.DiMartini A, Dew MA, Day N, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant. 2010;10:2305–2312. doi: 10.1111/j.1600-6143.2010.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon WJ, Merdian KL. ANOVA and regression with BMDP 5V. Los Angeles, CA: Dixon Statistical Associates; 1992. [Google Scholar]
- 11.Gibbons RD, Hedeker DR, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Arch Gen Psych. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 12.Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Boston: Pearson/Allyn and Bacon; 2007. [Google Scholar]
- 13.Mazumdar S, Tang G, Houck PR, et al. Statistical analyses of longitudinal psychiatric data with dropouts. J Psych Res. 2007;41:1032–1041. doi: 10.1016/j.jpsychires.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romesburg HC. Cluster analysis for researchers. Belmont, CA: Lifetime Learning Publications; 1984. [Google Scholar]
- 15.Everitt BS, Landau S, Leese M. Cluster analysis. 4. New York: Oxford University Press; 2001. [Google Scholar]
- 16.Thuluvath PJ, Krok KL, Segev DL, Yoo HY. Trends in post-liver transplant survival in patients with hepatitis C between 1991 and 2001 in the United States. Liver Transplant. 2007;13:719–724. doi: 10.1002/lt.21123. [DOI] [PubMed] [Google Scholar]
- 17.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 18.Carney RM, Blumenthal JA, Catellier D, et al. Depression as a risk factor for mortality after acute myocardial infarction. Am J Cardiol. 2003;92:1277–1281. doi: 10.1016/j.amjcard.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Frasure-Smith N, Lesperance F, Juneau M, et al. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 21.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32:696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 22.Lin EBH, Heckbert HR, Rutter CM, et al. Depression and increased mortality in diabetes: Unexpected causes of death. Ann Family Med. 2009;7:414–421. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodman CL, Geist LJ, Vance S, Laxson C, Jones K, Kline JN. Psychiatric disorders and survival after lung transplantation. Psychosomatics. 1999;40:293–297. doi: 10.1016/S0033-3182(99)71221-1. [DOI] [PubMed] [Google Scholar]
- 24.Squier H, Ries A, Kaplan R. Quality of well-being predicts survival in lung transplantation candidates. Am J Respir Crit Care Med. 1995;152:2032–2036. doi: 10.1164/ajrccm.152.6.8520772. [DOI] [PubMed] [Google Scholar]
- 25.De Vito Dabbs A, Dew MA, Stilley CS, et al. Psychosocial vulnerability, physical symptoms and physical impairment after lung and heart—lung transplantation. J Heart Lung Transplant. 2003;22:1268–1275. doi: 10.1016/s1053-2498(02)01227-5. [DOI] [PubMed] [Google Scholar]
- 26.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality 1–3 years after heart transplantation. J Heart Lung Transplant. 1999;18:549–562. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 27.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease:A meta-analysis. Psychosom Med. 2004;66:802–813. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]


