Abstract
A workshop to discuss anti-inflammatory approaches in the treatment of CF was held at Novartis Institutes for Biomedical Research (NIBR, Horsham, UK) in March 2008.
Key opinion leaders in the field (Hugo De Jonge, Stuart Elborn, Erich Gulbins, Mike Konstan, Rick Moss, Scott Randell and Adriano Rossi), and NIBR scientists were brought together to collectively address three main aims: (i) to identify anti-inflammatory targets in CF, (ii) to evaluate the pros and cons of targeting specific cell types and (iii) to discuss model systems to profile potential therapeutic agents.
The highlights of the workshop are captured in this review.
Introduction
Cystic fibrosis (CF) is the most common lethal genetic disease in Caucasians affecting approximately 1 in 3000 individuals. It is an autosomal recessive disease involving a mutation in the CFTR (cystic fibrosis transmembrane conductance regulator) chloride ion channel, the gene for which was discovered in 1989 (1). It is a multi-system disorder, with onset in childhood. Morbidity includes pancreatic insufficiency, GI/nutritional deficiencies including an inability to secrete digestive enzymes and critically, pulmonary disease. In the vast majority of cases mortality results from respiratory failure, with the median age of survival having increased to approximately 37 years, while the median age at death has remained at 25.2 years (2).
The classical paradigm for pulmonary morbidity in CF is that as a result of inadequate CFTR function, impaired mucociliary clearance develops which leads to infection and inflammation (3). Inflammation in the CF lung occurs early in life: In foetal and infant airways, with neutrophils predominating in the cellular infiltrate (4). This inflammatory response to infection is excessive relative to the burden of bacteria (5), with the major colonising pathogen in CF being Pseudomonas aeruginosa (Staphylococcus aureus and Haemophilus influenzae can also be present), (6). In addition, viral infections, eg: respiratory syncitial virus, have the potential to impair host defence to bacterial infection in CF patients by damaging the epithelium and further impairing mucociliary clearance. Release of elastase and other proteinases from neutrophils is considered to be a key contributor to lung damage associated with the inflammatory response. In healthy individuals, constitutive anti-proteases complex with proteinases and shield the lung against their activity. In CF, the activity of elastase and other proteinases are significantly elevated and overwhelm this protective mechanism (7). However, the concept that infection precedes inflammation is now being questioned; the Cftr mutation itself may directly induce inflammation prior to infection.
Role of Neutrophils in CF Inflammation
The primary function of the neutrophil is in host defence; phagocytosing and destroying invading pathogens. Specifically the neutrophil generates reactive oxygen species via NADPH oxidase (8) and secretes anti-microbial granular proteins including elastase (six elastase genes are known to exist, with elastase 2 being the most prominent in human neutrophils (9), collagenase, cathepsins and myeloperoxidase (10) to perform this protective role. The release of DNA from neutrophils can form neutrophil extracellular traps (NETS) in which Gram −ve and Gram +ve bacteria can be bound and killed (11;12). However, if this response is not controlled, excessive tissue damage can result, accounting for much of the lung damage and destruction observed in CF. Under the hypoxic conditions found in CF mucus, neutrophils are thought to live longer, and thus their potential to damage the lung is enhanced (13). As a result, strategies to target the neutrophil and its destructive potential have emerged as key therapeutic approaches to CF lung disease. These include inhibition of neutrophil recruitment, activation, release/activation of degranulation products, digestion of DNA and targeted removal of unwanted neutrophils by apoptosis (14;15).
To inhibit pro-inflammatory signalling, the use of broad spectrum anti-inflammatory drugs such as oral corticosteroids (16;17) and non-steroidal anti-inflammatory agents such as ibuprofen (18) have been examined in CF. Disappointingly, the beneficial effects of oral corticosteroids are limited and adverse side-effects are prominent. Inhaled corticosteroids have no demonstrable beneficial effects in CF patients, although these clinical studies were only of a short duration and had small numbers of patients (19). It has been suggested that corticosteroids can delay neutrophil apoptosis (20); an effect which may explain the limited efficacy of corticosteroids in CF. Ibuprofen is used clinically to treat CF and has been demonstrated to slow the rate of decline in FEV1; most dramatically in younger patients (21), but the beneficial effect of this treatment in CF is not widely accepted and there are concerns regarding clinical safety that have hindered its use. In addition to broad spectrum anti-inflammatory agents, the clinical efficacy of several compounds targeting specific neutrophil associated pro-inflammatory mediators have been examined, a notable example of which is the trial of BIIL 284 BS, a LTB4 receptor antagonist. In this trial, CF patients treated for 24 weeks with the drug developed pulmonary exacerbations at twice the frequency of the placebo group, resulting in early termination of the trial (22). Whether or not the occurrence of pulmonary exacerbations reflects the concept that LTB4 inhibition is detrimental in CF, or is a compound-related phenomenon is unclear. Unfortunately no assessment of bacterial counts, inflammatory cells, or mediators have been reported from this study. This highlights the need for detailed biomarker assessment (from sputum, bronchoalveolar lavage (BAL), urine, etc) during clinical studies, in order to monitor factors that may be useful as early indicators of potential exacerbations, and also to gain information related to drug mechanism of action (23). However, selecting samples for biomarker assessment has its challenges. While sputum is generally easy to obtain and non-invasive, it can be difficult to obtain from paediatric patients and biomarker assessment can be variable. BAL is an attractive alternative as samples can be obtained from all age groups, fewer patients are required, and a single lung lobe/segment can be evaluated several times, but it is invasive and expensive to perform and may yield local rather than generalized findings. Serum and urine samples may not be suitable for biomarker assessment if inflammation is confined to the lung. In sputum from CF patients, neutrophil elastase has been demonstrated to provide the best correlate with lung function (24).
Another approach to targeting inflammation in CF is the neutralization of neutrophil activation products. Potential therapeutic agents which have been proposed include anti-elastases, other protease inhibitors (25), DNAse and antioxidants (26). The DNAse Pulmozyme is used clinically in CF patients to reduce mucous viscosity and thus aid mucociliary clearance. Indeed there is clinical evidence to suggest that this therapy improves lung function and decreases the incidence of pulmonary exacerbations (27;28).
Novel strategies to promote neutrophil apoptosis are also emerging. For example, cyclin-dependent kinase (CDK) inhibitor drugs, such as R-roscovitine, can induce caspase-dependent apoptosis per se and importantly inhibit the delay in neutrophil apoptosis induced by survival agents in vivo (29). Indeed, in three mouse models, a carrageenan model of acute pleurisy, a bleomycin model of lung inflammation and a passively-induced arthritis model, R-roscovitine has been demonstrated to promote the resolution of neutrophillic inflammation (29). Roscovitine has yet to be profiled in bacterial infection models.
In summary, the neutrophil is a promising target in CF due to its significant contribution towards the lung pathology of the disease, its tractable nature and because much is known about its behaviour and function. However, the difficulty of selectively targeting this cell type may be challenging. In addition, other cell types clearly contribute to the inflammation in CF lung disease and thus selective inhibition of the neutrophil alone may not be sufficiently efficacious. There are also inevitable concerns about compromising host defence in CF patients, given that this is the primary function of this cell type.
Role of Epithelial Cells in CF Inflammation
The airway epithelium actively participates in innate immunity and orchestrates inflammatory responses. This is in addition to its well-characterised role in regulating airway surface liquid volume and hydration, which is essential for normal mucus clearance. In CF, due to inadequate chloride ion transport and the resultant increased activity of the epithelium sodium channel (ENaC), there is inadequate airway surface liquid hydration (30).
Over-expression of the βENaC subunit in the mouse (Scnn1b) results in animals that exhibit some of the key abnormalities of cystic fibrosis including airway obstruction (as a consequence of increased mucus secretion and impaired mucociliary clearance) and the development of spontaneous neutrophilic lung inflammation and prolonged eosinophilia (31). These findings support the concept that ENaC inhibition may be a potential therapy in CF and therefore suggest that CFTR is not the only ion channel target in the epithelium.
The airway epithelium recognises and responds to inflammatory stimuli via the interaction of various ligands with innate immune system receptors including the tolllike receptors (TLRs); TLR2 (ligands include lipopeptides, peptidoglycan, lipoteichoic acid (32), CXCR1 fragments (33) and TLR5 (only known ligand is flagellin (34) are considered to be of particular importance. The role of airway epithelial TLR4 (potential endogenous ligands include lipopolysaccharide and heat shock proteins (35; 36) is controversial, although sentinel cells such as macrophages and dendritic cells may respond strongly to TLR4 ligands and secrete factors affecting epithelial cells, creating dynamic interactions. P. aeruginosa products acutely activate TLRs although chronic exposure of epithelial cells to this pathogen leads to adaptation and tolerance (37). The induction of mRNA for pro-inflammatory cytokines, including IL-8, ENA-78, MCP-1, MIP-3α and GRO-α decreases after chronic bacterial product exposure. However, cytokine protein production from epithelial cells following repeated P. aeruginosa challenge does not necessarily decrease, which may explain sustained inflammation in the CF airway. Pro-inflammatory cytokine secretion is also enhanced in proportion to the degree of mechanical wounding in vitro, and epithelial damage suffered during a pulmonary exacerbation will likely increase airway inflammation.
Airway epithelium controls lung inflammation and injury through the NF-κB pathway, as demonstrated in a mouse modular transgenic system in which NF-κB activation resulted in inflammation and progressive lung injury (38). Also, inhibition of NF-κB attenuated LPS-induced inflammation and epithelial damage (39). Targeting this signalling pathway in the epithelium may be an effective anti-inflammatory strategy. However a caveat of this approach is that NF-κB is a key pathway in the induction of immune responses to P. aeruginosa and its inhibition could therefore impact host defence (40).
Key questions remain regarding anti-inflammatory approaches in CF. When is inflammation greater than necessary to confine infection, and can we exploit intrinsic anti-inflammatory regulatory mechanisms to develop novel and more specific therapies?
Role of Lymphocytes in CF Inflammation
Although the neutrophil is widely recognised as a key player in CF inflammation, a lymphocytic infiltrate is also seen in the CF lung (41;42). In CF, lymphocytes are activated at all stages of the disease and in people of all ages, and increased B-cell aggregates (CD20+) are found in the peribronchiolar tissue together with increased CD3+ T-cells in distal bronchiolar tissue and parenchyma from CF patients versus healthy lung tissue (42). CFTR is functionally expressed in lymphocytes, and in cells from CF patients the characteristic defect in the CFTR channel is observed; this can be reversed by anti-sense or wt CFTR (43-45). These cells also possess an alternative to the cAMP-dependent pathway for CFTR activation, which is nitric oxide/cGMP-dependent (46).
The finding that mitogen-activated CF T cell clones are skewed towards a Th2 phenotype compared to controls implicates Th2 pathways in CF. These activated T cell clones secrete 50% less IL-10 compared to controls (47) and also ∼80% less IFNγ following T cell receptor activation (48). Interestingly IL-10 but not IFN-y secretion is inhibited by chloride channel blockade. Moreover, activated peripheral blood mononuclear cells from P. aeruginosa infected CF patients have reduced IFNγ and increased IL-4 secretion compared to uninfected CF patients (49). In CF patients chronically infected with P. aeruginosa, there is an increase in CD4+CCR4+ Th2-cells in the lung compared to CF patients without P. aeruginosa infection (50). Based on preclinical models and correlative clinical data it was hypothesized that treatment of CF patients with the Th1 cytokine, IFNγ might reduce the inflammatory response to P. aeruginosa; however a controlled clinical trial with inhaled IFNγ1b showed no significant improvement in FEV1 or alteration in sputum cytokines (51).
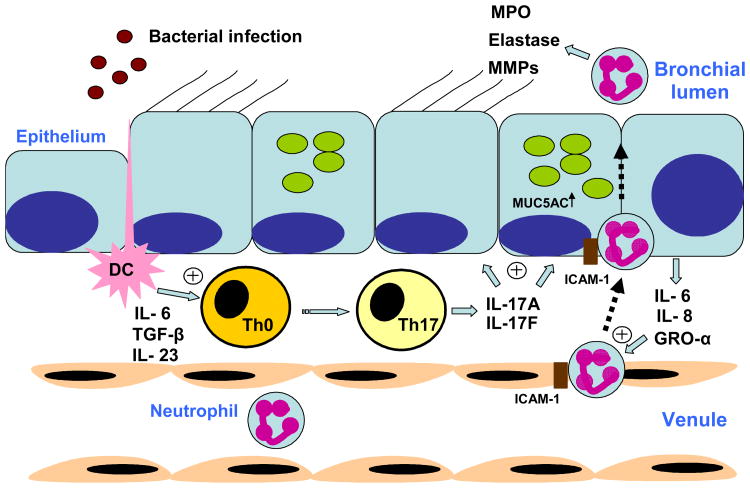
With the description of a Th17 T cell population, the question has arisen as to whether CF is in fact a Th17-mediated disease. Indeed, IL-17A released from CD4+ T cells is crucial for neutrophil recruitment in response to gram negative bacterial infection (Figure 1). This is in part via induction of CXC chemokines (42). Additionally, IL-23p19, IL-22, IL-17A and IL-17F levels have been demonstrated to be elevated in sputum from adult and pediatric CF patients undergoing pulmonary exacerbation (53;54). IL-17A and IL-17F may regulate matrix metalloproteinases, and speculatively this may underlie bronchiectasis in CF.
Figure 1. IL-17-induced neutrophil recruitment in CF.
Production of Interleukin (IL)-6, transforming growth factor (TGF)-β and IL-23 by airway dendritic cells (DC) following activation by bacterial pathogens promotes Th17 cell differentiation. The release of IL-17A and IL-17F from these cells acts on airway epithelial cells to release neutrophil chemokines IL-6. IL-8 and growth related oncogene (GRO)-α. ICAM-1, intracellular adhesion molecule-1; MPO, myeloperoxidase; MMP, matrix metalloprotease.
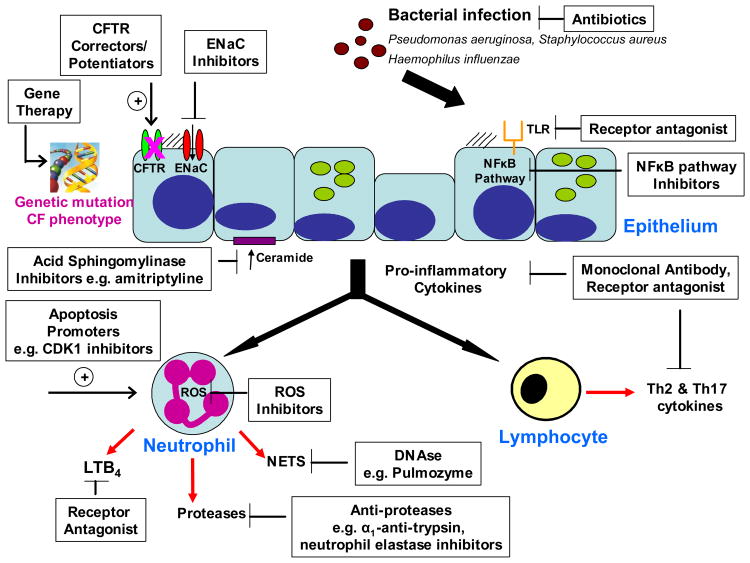
The pathogenesis of inflammation and subsequent lung injury in CF, involves crosstalk between epithelium, T lymphocytes and neutrophils, all of which are potential targets for selective pro-inflammatory pathway inhibitors (Figure 2).
Figure 2. Overview of potential anti-inflammatory strategies for the treatment of cystic fibrosis.
CF, cystic fibrosis; CFTR cystic fibrosis transmembrane conductance regulator; ENaC, epithelium sodium channel; TLR toll-like receptor, NFκB, nuclear factor kappa B; LTB4, leukotriene B4; ROS, reactive oxygen species; NETS, neutrophil extracellular traps; CDK1, cyclin dependent kinase 1.
Model systems for profiling anti-inflammatory agents in CF
Model systems for profiling anti-inflammatory compounds in CF include cultured primary cells or cell lines, human lung xenografts in severe combined immunodeficiency (SCID) mice and CF animal models in species such as the mouse, ferret and pig.
In cultured cells and cell lines, it is questionable as to whether the CF inflammatory state can be fully reproduced. In addition cultured cells and cell lines can respond differently to stimuli compared to primary cells. Although both cultured cells and cell lines can demonstrate spontaneous activation of NFκB (55), higher expression of pro-inflammatory mediators (56-58) and a greater inflammatory response to P. aeruginosa exposure (59), there are often minimal differences in the gene expression profile between CF and non-CF cells. In addition, increased expression and secretion of pro-inflammatory mediators are not always present or inducible by P. aeruginosa (60). These discrepancies may arise due to selection and passaging of cells and also differences in P. aeruginosa strain variants, exposure times etc. Nasal brushings obtained from patients or healthy volunteers are a source of primary cells that can be cultured to create pseudostratified mucociliary epithelium (61). This strategy has demonstrated phenotypic differences between airway epithelial cells from healthy volunteers and COPD patients, with greater IL-8 release in response to LPS treatment from the latter.
Grafting of human foetal airways into SCID mice has shown characteristics of the CF airway, such as increased IL-8 content in airway surface liquid and an influx of mouse leukocytes to the airway epithelium (62). Although these characteristics make it an attractive model, it is limited by the poor availability of foetal CF lungs.
Pig and ferret have 91-92% CFTR sequence homology and similar lung structure and physiology with human. The pig in particular has been successfully used to model many diseases and may provide an interesting opportunity to gain new insights into the pathogenesis of CF (63). However, despite the many CF-relevant similarities between human and pig lungs (anatomy, histology, electrolyte transport, submucosal gland function and immune and inflammatory responses), it is still unclear whether a CF pig will develop lung disease with features similar to that seen in human CF (63). The utility of this species may also be limited by expense and logistical implications. While the mouse has only 78% sequence homology to human, mouse models are available for all classes of Cftr mutation except class V. Mouse models are also easier to produce/acquire than pig and ferret models, however there are a number of issues relating to using the mouse. For example, there are differences between mouse and human epithelial cell morphology and physiology, specifically the distribution of submucosal glands. In addition CF mice rarely display spontaneous lung inflammation, although there have been notable exceptions in animals overexpressing the βENaC subunit (31) and those homozygous for the ΔF508 Cftr mutation (64). Compared to wild type control animals, the latter also exhibited increased susceptibility to infection induced by intratracheal instillation of lipopolysaccharide from P. aeruginosa (65). The use of some CF mice is further hampered by poor fertility and high mortality. Indeed, despite the βENaC overexpressing mouse exhibiting many of the key abnormalities of CF, a postnatal mortality of ∼50% at 4 weeks of age may limit the use of these animals for the evaluation of potential therapeutic interventions. Additionally, in a more recent publication overexpression of βENaC has been shown to cause emphysema (66)
Cross-breeding of CF mice with other genetically modified mice has highlighted the potential importance of other gene products, such as acid-sphingomyelinase (67), in the pathogenesis of CF. In this study, compared to wild type animals, epithelial cells from Cftr deficient mice were found to have higher levels of ceramide, which increased with age and were predominantly found in large airways. Ceramide accumulation in intracellular vesicles, as well as at the apical membrane of epithelial cells, occurs as a result of elevated pH resulting in the net increased production of ceramide via an imbalance of the acid sphingomyelinase and acid ceramidase activities. It is proposed that elevated ceramide levels lead to airway inflammation due to apoptosis of epithelial cells and deposits of DNA and mucus, as well as the release of pro-inflammatory mediators. This in turn leads to increased susceptibility to P. aeruginosa infections. By crossing these Cftr deficient mice with an acid sphingomyelinase heterozygous mouse, lung ceramide levels were normalized. In addition treatment of the mice with amitriptyline, a known acid sphingomyelinase inhibitor, had the same result. In Cftr deficient mice, an increase in bacterial load in the lung is observed 2 hours post P. aeruginosa infection versus wild type. This increased bacterial load is reduced by treatment with amitriptyline and also in Cftr deficient mice crossed with acid sphingomyelinase heterozygous mice (67). It is possible that normalisation of ceramide levels may represent a novel means of preventing bacterial infection in CF patients. In the future, cross-breeding of CF mice with NF-κB reporter mice, which permit the detection of NF-kB activation by bioluminescence (68;69), may provide further insight into the pro-inflammatory state of mouse CF models, the impact of bacterial infection and the efficacy of anti-inflammatory therapies.
Proof of Concept study designs
Drug development is becoming an increasingly expensive and challenging undertaking (70), and the “Proof of Concept” (PoC) study is occupying an increasingly important place in the drug development paradigm. Proof of concept can be thought of as demonstration of a meaningful biological activity in man. A successful proof of concept study generates confidence in the ultimate therapeutic potential of a compound and often triggers additional development activities such as large-scale production, additional preclinical safety studies, and later-phase clinical studies. By performing a proof of concept study early in the development of a compound there is also an opportunity to halt development in light of a negative outcome, allowing transfer of resources and activities to more promising compounds and therapeutic approaches.
Demonstration of biological activity in a proof of concept study is achieved through measurement of a biomarker. A biomarker is a characteristic that is objectively measured as an indicator of normal biological processes, pathogenic processes, or a pharmacological response to a therapeutic intervention (71). Biomarkers can be observable characteristics (e.g. height), quantifiable changes at the protein, mRNA, or DNA level, or readily measurable clinical parameters. Successful registration of a therapeutic compound requires demonstration of improvement in a clinical endpoint, a characteristic or variable that reflects how a patient feels, functions or survives. A surrogate endpoint, a biomarker that is intended to substitute for a clinical endpoint, is expected to predict clinical benefit (or harm or lack of benefit/harm) based on epidemiologic, therapeutic, pathophysiologic or other scientific evidence.
There are several characteristics of an ideal proof of concept study that increase its appeal in the drug development paradigm. The study should be of short duration, such as a single dose or at most a few weeks of repeated dosing. The target population should be focused, with a small sample size. Measurable, robust biomarkers that correlate with a relevant clinical (registration) outcome are highly desired. Finally, the study should enable intelligent Go/No Go decisions for the compound. Thus a successful proof of concept study design results in limited exposure of human subjects to the compound while providing a rapid readout for decision making.
There are several design elements that should be considered when planning a proof of concept study for an anti-inflammatory compound in cystic fibrosis. These include the duration of dosing, the patient population, the choice of endpoints (biomarkers) that reflect the mechanism of action and correlate with a relevant clinical outcome, and the selection of safety biomarkers. The ideal proof of concept study would involve two to four weeks of dosing in a limited number of patients with a change in an inflammatory biomarker predictive of clinical efficacy serving as the primary outcome measure. The likelihood of success in employing a study design such as this can be predicted by a thorough preclinical understanding of the pharmacokinetic-pharmacodynamic relationship of the compound, although as noted above there are several shortcomings in current preclinical models of CF that reduce the ability to optimize this prediction.
At present, the most critical impediment to designing an ideal proof of concept study for an anti-inflammatory therapy in CF is the lack of an inflammatory biomarker that has been shown to be predictive of clinical efficacy. This is not surprising given the limited anti-inflammatory therapeutic arsenal employed in CF care. Oral corticosteroid therapy in CF was preceded by clinical studies that examined clinical endpoints rather than biomarkers (72), and subsequent studies also failed to identify a relevant biomarker while revealing toxicity that limited widespread use (11). Ibuprofen is another anti-inflammatory therapy with clinical utility in CF (12, 15). While ibuprofen therapy has been shown to decrease the influx of neutrophils in oral wash samples (73), this potential biomarker, and indeed the therapy itself, has not been widely adopted. Examples of potential inflammatory biomarkers in CF that could be considered in designing a proof of concept study include neutrophil count and cytokine levels in induced sputum (23;24), cytokine levels or C-reactive protein in serum (74), and desmosine in urine (75), although none of these have been shown to predict clinical efficacy for a therapeutic compound. Continued efforts to identify informative inflammatory biomarker(s) in CF are thus sorely needed. Finally, the need for reliable safety biomarkers in trials of anti-inflammatory therapies in CF is highlighted by the report of an increased incidence of pulmonary exacerbations in CF patients receiving BIIL284-BS, an oral leukotriene B4 receptor antagonist (22). This report underscores the potential risks of anti-inflammatory therapy in this patient population.
Summary
Inflammation in cystic fibrosis involves multiple inflammatory cells types, including neutrophils, lymphocytes and epithelial cells. The use of broad-spectrum anti-inflammatory agents is one approach to modulate inflammation in CF, however corticosteroids show only limited improvements in FEV1. Also noteworthy is the modest improvement in FEV1 observed in patients treated with the broad spectrum macrolide antibiotic, azithromycin (76) which has been shown to have anti-inflammatory effects, although the mechanism(s) underlying this activity remain unclear (77). There are however, several proposed mechanisms for the anti-inflammatory effects of macrolides including: suppression of production and secretion of pro-inflammatory cytokines (via ERK1/2 inhibition and activation), down-regulation of adhesion molecules and promoting inflammatory cell apoptosis (78).
Targeting the neutrophil specifically is a promising approach due to the lung pathology seen in CF, which is clearly associated with an excessive neutrophil influx and activation, coupled with poor neutrophil clearance. Conversely however, this strategy has the potential to compromise host defence given the key role that neutrophils contribute to innate host defence. Targeting the epithelial cell or lymphocyte may also be beneficial given their potential roles in orchestrating the inflammatory response in the CF lung.
In order to profile potential novel anti-inflammatory targets appropriate model systems are required. Cell lines are useful to screen compounds against certain targets, however a caveat is that only certain aspects of the CF inflammatory state can be reproduced. For target validation and screening of the most promising strategies, the mouse and potentially in the future the ferret and pig could provide suitable disease models. Translation of these preclinical findings into effective anti-inflammatory therapies in the clinic will ultimately benefit from continued attempts to identify inflammatory translational biomarkers predictive of clinical efficacy.
Targeting inflammation in CF would seem to be an attractive approach to the treatment of this disease, but obtaining sufficient anti-inflammatory efficacy versus impairing host defence remains a key challenge.
Footnotes
Authors are listed alphabetically
Invited speakers: Mike Konstan - Overview of inflammation in cystic fibrosis
Adriano Rossi - Role of the neutrophil
Rick Moss - Role of the lymphocyte
Scott Randell - Role of the epithelial cell
Stuart Elborn – Inflammation and anti-inflammatory treatment in cystic fibrosis
Hugo De Jonge - Model systems for profiling anti-inflammatory agents
Erich Gulbins - Model systems for profiling anti-inflammatory agents
Dave Waltz and Mike Konstan – Proof of concept study designs
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda MD. Patient registry 2006 annual data report. Cytsic Fibrosis Foundation; 2007. [Google Scholar]
- 3.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24(2):137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160(1):186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 6.Govan JR, Nelson JW. Microbiology of lung infection in cystic fibrosis. Br Med Bull. 1992;48(4):912–930. doi: 10.1093/oxfordjournals.bmb.a072585. [DOI] [PubMed] [Google Scholar]
- 7.Balfour-Lynn IM. The protease-antiprotease battle in the cystic fibrosis lung. J R Soc Med. 1999;92(Suppl 37):23–30. doi: 10.1177/014107689909237s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: Oxidants myeloperoxidase and bacterial killing. Blood. 1998;92(9):3007–301. [PubMed] [Google Scholar]
- 9.http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=retrieve&dopt=fullreport&list_uids=1991&log$=databasead&logdbfrom=protein
- 10.Borregaard N, Coweland JB. Granules of the human neutrophilc polymorphonuclear leukocyte. Blood. 1997;89:3503–3521. [PubMed] [Google Scholar]
- 11.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5(8):577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 13.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NFκB. J Exp Med. 2005;200(1):105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstan MW, Davis PB. Pharmacological approaches for the discovery and development of new anti-inflammatory agents for the treatment of cystic fibrosis. Adv Drug Deliv Rev. 2002;54(11):1409–1423. doi: 10.1016/s0169-409x(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 15.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28(2):331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Dovey M, Aitken ML, Emerson J, McNamara S, Waltz DA, Gibson RL. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest. 2007;132(4):1212–1218. doi: 10.1378/chest.07-0843. [DOI] [PubMed] [Google Scholar]
- 17.Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126(4):515–523. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 18.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 19.Balfour-Lynn IM, Klein NJ, Dinwiddie R. Randomised controlled trial of inhaled corticosteroids (fluticasone propionate) in cystic fibrosis. Arch Dis Child. 1997;77(2):124–130. doi: 10.1136/adc.77.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McColl A, Michlewska S, Dransfield I, Rossi AG. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. ScientificWorldJournal. 2007;7:1165–1181. doi: 10.1100/tsw.2007.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–1089. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstan MW, Doring G, Lands LC, et al. Results of a phase II clinical trial of BIIL 284 BS (an LTB4receptor antagonist) for the treatment of CF lung disease. Pediatr Pulmonol. 2005;(28):125–126. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4(4):406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Hamblett N, Aitken ML, Accurso FJ, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(8):822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElvaney NG, Hubbard RC, Birrer P, et al. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991;337(8738):392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- 26.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis. Conclusions from the CF antioxidant workshop, Bethesda, Maryland, November 11-12, 2003. Free Radic Biol Med. 2007;42(1):15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suri R. The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis. BioDrugs. 2005;19(3):135–144. doi: 10.2165/00063030-200519030-00001. [DOI] [PubMed] [Google Scholar]
- 28.Shah PL, Hodson ME. Dornase alfa. BioDrugs. 1997;8(6):439–445. doi: 10.2165/00063030-199708060-00004. [DOI] [PubMed] [Google Scholar]
- 29.Rossi AG, Sawatzky DA, Walker A, et al. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12(9):1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132(5):1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 31.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10(5):487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 32.Zähringer U, Lindner B, Inamura S, et al. TLR2 – promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213(3-4):205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Hartl D, Latzin P, Hordijk P, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13(12):1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 34.Gewirtz TA, Navas TA, Lyons S, et al. Cutting Edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial pro-inflammatory gene expression. J Immunol. 2001;167:1882–1887. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 35.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. Journal of leukocyte biology. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 36.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annual review of biochemistry. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 37.Wu Q, Lu Z, Verghese MW, Randell SH. Airway epithelial cell tolerance to Pseudomonas aeruginosa. Respir Res. 2005;6:26. doi: 10.1186/1465-9921-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantano C, Ather JL, Alcorn JF, et al. Nuclear factor-kappaB activation in airway epithelium induces inflammation and hyperresponsiveness. Am J Respir Crit Care Med. 2008;177(9):959–969. doi: 10.1164/rccm.200707-1096OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng DS, Han W, Chen SM, et al. Airway epithelium controls lung inflammation and injury through the NF-kappa B pathway. J Immunol. 2007;178(10):6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 40.Sadikot RT, Zeng H, Joo M, Everhart MB, et al. Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 2006;176(8):4923–4930. doi: 10.4049/jimmunol.176.8.4923. [DOI] [PubMed] [Google Scholar]
- 41.Azzawi M, Johnston PW, Majumdar S, Kay AB, Jeffery PK. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis. 1992;145(6):1477–1482. doi: 10.1164/ajrccm/145.6.1477. [DOI] [PubMed] [Google Scholar]
- 42.Hubeau C, Lorenzato M, Couetil JP, et al. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124(1):69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bubien JK. CFTR may play a role in regulated secretion by lymphocytes: a new hypothesis for the pathophysiology of cystic fibrosis. Pflugers Arch. 2001;443(Suppl 1):S36–S39. doi: 10.1007/s004240100641. [DOI] [PubMed] [Google Scholar]
- 44.Chen JH, Schulman H, Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989;243(4891):657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- 45.McDonald TV, Nghiem PT, Gardner P, Martens CL. Human lymphocytes transcribe the cystic fibrosis transmembrane conductance regulator gene and exhibit CF-defective cAMP-regulated chloride current. J Biol Chem. 1992;267(5):3242–3248. [PubMed] [Google Scholar]
- 46.Dong YJ, Chao AC, Kouyama K, et al. Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 1995;14(12):2700–2707. doi: 10.1002/j.1460-2075.1995.tb07270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moss RB, Bocian RC, Hsu YP, Dong YJ, et al. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106(2):374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;120(3):518–525. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108(5):329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 50.Hartl D, Griese M, Kappler M, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117(1):204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 51.Moss RB, Mayer-Hamblett N, Wagener J, et al. Randomized, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatr Pulmonol. 2005;39(3):209–218. doi: 10.1002/ppul.20152. [DOI] [PubMed] [Google Scholar]
- 52.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubin PJ, McAllister F, Kolls JK. Is cystic fibrosis a TH17 disease? Inflamm Res. 2007;56(6):221–227. doi: 10.1007/s00011-007-6187-2. [DOI] [PubMed] [Google Scholar]
- 54.McAllister F, Henry A, Kreindler JL, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175(1):404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L71–L78. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- 56.Kammouni W, Figarella C, Marchand S, Merten M. Altered cytokine production by cystic fibrosis tracheal gland serous cells. Infect Immun. 1997;65(12):5176–5183. doi: 10.1128/iai.65.12.5176-5183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Johnson XD, Iazvovskaia S, Tan A, Lin A, Hershenson MB. Signaling intermediates required for NF-kappa B activation and IL-8 expression in CF bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L307–L315. doi: 10.1152/ajplung.00086.2002. [DOI] [PubMed] [Google Scholar]
- 58.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104(1):72–78. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 59.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L493–L502. doi: 10.1152/ajplung.2001.280.3.L493. [DOI] [PubMed] [Google Scholar]
- 60.Aldallal N, McNaughton EE, Manzel LJ, et al. Inflammatory response in airway epithelial cells isolated from patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(9):1248–1256. doi: 10.1164/rccm.200206-627OC. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien GJ, Riddell G, Elborn JS, Ennis M, Skibinski G. Staphylococcus aureus enterotoxins induce IL-8 secretion by human nasal epithelial cells. Respir Res. 2006;7:115. doi: 10.1186/1465-9921-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tirouvanziam R, de Bentzmann S, Hubeau C, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol. 2000;23(2):121–127. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 63.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118(4):1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guilbault C, Martin P, Houle D, et al. Cystic fibrosis lung disease following infection with Pseudomonas aeruginosa in Cftr knockout mice using novel non-invasive direct pulmonary infection technique. Lab Anim. 2005;39(3):336–352. doi: 10.1258/0023677054306944. [DOI] [PubMed] [Google Scholar]
- 65.Legssyer R, Huaux F, Lebacq J, et al. Azithromycin reduces spontaneous and induced inflammation in DeltaF508 cystic fibrosis mice. Respir Res. 2006;7:134. doi: 10.1186/1465-9921-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mall MA, Harkema JR, Trojanek JB, et al. Development of chronic bronchitis and emphysema in beta-epthithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177(7):730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teichgraber V, Ulrich M, Endlich N, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14(4):382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 68.Blackwell TS, Yull FE, Chen CL, et al. Multiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 69.Everhart MB, Han W, Sherrill TP, et al. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176(8):4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 70.Sollano J, Kirsch J, Bala M, Chambers M, Harpole L. The Economics of Drug Discovery and the Ultimate Valuation of Pharmacotherapies in the Marketplace. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.117. [DOI] [PubMed] [Google Scholar]
- 71.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 72.Auerbach HS, Williams M, Kirkpatrick JA, Colten HR. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;2(8457):686–688. doi: 10.1016/s0140-6736(85)92929-0. [DOI] [PubMed] [Google Scholar]
- 73.Konstan MW, Krenicky JE, Finney MR, et al. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther. 2003;306(3):1086–1091. doi: 10.1124/jpet.103.052449. [DOI] [PubMed] [Google Scholar]
- 74.Levy H, Kalish LA, Huntington I, et al. Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(3):256–262. doi: 10.1002/ppul.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Downey DG, Martin SL, Dempster M, et al. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(3):216–220. doi: 10.1002/ppul.20553. [DOI] [PubMed] [Google Scholar]
- 76.Saiman L, Mayer-Hamblett N, Campbell P, Marshall BC. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(8):1008–1012. doi: 10.1164/rccm.200502-218OC. [DOI] [PubMed] [Google Scholar]
- 77.Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31(1):12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications:proposed mechanisms of action. Pharmacol Thera. 2008;117(3):393–405. doi: 10.1016/j.pharmthera.2007.11.001. [DOI] [PubMed] [Google Scholar]