Abstract
The proneural genes of Drosophila are required for the segregation of neural precursors from the ectoderm. One of the proneural genes, achaete (ac), is responsible for the formation of a subset of larval and adult sense organs. We have examined the pattern of expression of ac and its regulation during embryogenesis. ac is expressed at particular locations of the ectoderm according to a highly dynamic and complex pattern. At each location the expression of ac occurs in two stages. First, ac is expressed in a cluster of cells ('competent' stage). This pattern of expression depends on an array of cis-regulatory sites which promote the expression of ac in clusters of cells at particular locations. Second, one cell of each cluster accumulates ac transcripts at a high level ('determined' stage) and becomes the neural progenitor cell. The restriction of the determined stage to one cell of the cluster requires the gene Notch. The transactivation of ac by sc (scute) or by other genes of the achaete-scute complex (AS-C) plays no role in defining the initial pattern of expression of ac, but might play a role in the shift from the competent to the determined level. We propose that the requirements of particular sense organs for different AS-C genes reflect the organization of this gene complex, rather than functional differences between the genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Alonso M. C., Cabrera C. V. The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J. 1988 Aug;7(8):2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells L., Modolell J., Ruiz-Gómez M. A unitary basis for different Hairy-wing mutations of Drosophila melanogaster. EMBO J. 1988 Dec 1;7(12):3899–3906. doi: 10.1002/j.1460-2075.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer R., Carretto R., Jan Y. N. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989 Jul;3(1):21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C. Transcriptional activation by heterodimers of the achaete-scute and daughterless gene products of Drosophila. EMBO J. 1991 Oct;10(10):2965–2973. doi: 10.1002/j.1460-2075.1991.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V. Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development. 1990 Sep;110(1):733–742. [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992 Jun;8(6):202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Cubas P., de Celis J. F., Campuzano S., Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. Genes Dev. 1991 Jun;5(6):996–1008. doi: 10.1101/gad.5.6.996. [DOI] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Jamet E., Burri M., Bopp D., Basler K., Hafen E., Dumont N., Spielmann P., Ghysen A., Noll M. The paired box gene pox neuro: a determinant of poly-innervated sense organs in Drosophila. Cell. 1992 Apr 3;69(1):159–172. doi: 10.1016/0092-8674(92)90127-x. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Kuner J. M., Theis J., O'Farrell P. H. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell. 1985 Nov;43(1):59–69. doi: 10.1016/0092-8674(85)90012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A. Genetic Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1979 Mar;91(3):491–520. doi: 10.1093/genetics/91.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A., Santamaria P. Developmental Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1978 Mar;88(3):469–486. doi: 10.1093/genetics/88.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrell J., Campuzano S. The helix-loop-helix domain: a common motif for bristles, muscles and sex. Bioessays. 1991 Oct;13(10):493–498. doi: 10.1002/bies.950131002. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989 Aug;5(8):251–255. doi: 10.1016/0168-9525(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Ghysen A., O'Kane C. Neural enhancer-like elements as specific cell markers in Drosophila. Development. 1989 Jan;105(1):35–52. doi: 10.1242/dev.105.1.35. [DOI] [PubMed] [Google Scholar]
- González F., Romani S., Cubas P., Modolell J., Campuzano S. Molecular analysis of the asense gene, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J. 1989 Dec 1;8(12):3553–3562. doi: 10.1002/j.1460-2075.1989.tb08527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriely A., Dumont N., Dambly-Chaudière C., Ghysen A. The determination of sense organs in Drosophila: effect of the neurogenic mutations in the embryo. Development. 1991 Dec;113(4):1395–1404. doi: 10.1242/dev.113.4.1395. [DOI] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989 Oct;107(2):389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991 Mar 22;64(6):1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Campos-Ortega J. A. Genes in subdivision 1B of the Drosophila melanogaster X-chromosome and their influence on neural development. J Neurogenet. 1987 Jun;4(4):179–200. [PubMed] [Google Scholar]
- Martínez C., Modolell J. Cross-regulatory interactions between the proneural achaete and scute genes of Drosophila. Science. 1991 Mar 22;251(5000):1485–1487. doi: 10.1126/science.1900954. [DOI] [PubMed] [Google Scholar]
- Muller H. J., Prokofyeva A. A. The Individual Gene in Relation to the Chromomere and the Chromosome. Proc Natl Acad Sci U S A. 1935 Jan;21(1):16–26. doi: 10.1073/pnas.21.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Rodríguez I., Hernández R., Modolell J., Ruiz-Gómez M. Competence to develop sensory organs is temporally and spatially regulated in Drosophila epidermal primordia. EMBO J. 1990 Nov;9(11):3583–3592. doi: 10.1002/j.1460-2075.1990.tb07569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Modolell J. The achaete-scute complex is expressed in neurogenic regions of Drosophila embryos. EMBO J. 1987 Jul;6(7):2085–2092. doi: 10.1002/j.1460-2075.1987.tb02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez M., Modolell J. Deletion analysis of the achaete-scute locus of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1238–1246. doi: 10.1101/gad.1.10.1238. [DOI] [PubMed] [Google Scholar]
- Skeath J. B., Carroll S. B. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991 Jun;5(6):984–995. doi: 10.1101/gad.5.6.984. [DOI] [PubMed] [Google Scholar]
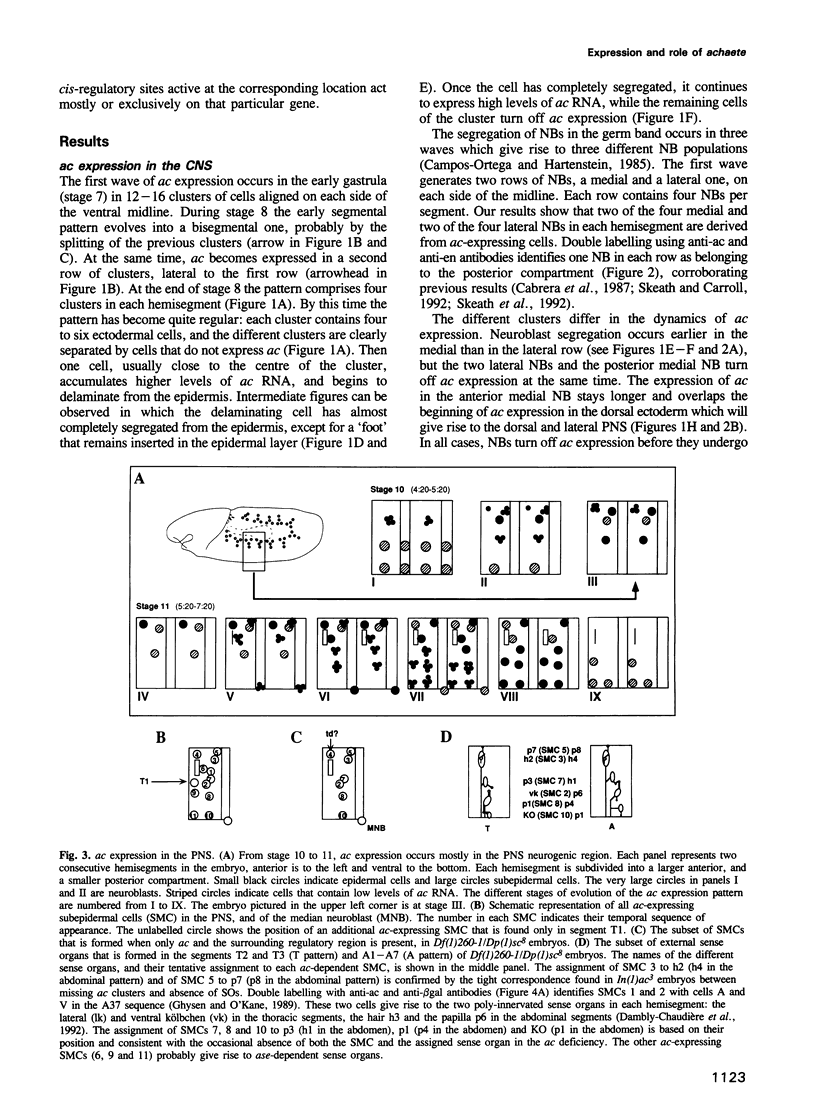
- Skeath J. B., Carroll S. B. Regulation of proneural gene expression and cell fate during neuroblast segregation in the Drosophila embryo. Development. 1992 Apr;114(4):939–946. doi: 10.1242/dev.114.4.939. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]