Abstract
Altered glycosylation is a universal feature of cancer cells and altered glycans can help cancer cells escape immune surveillance, facilitate tumor invasion, and increase malignancy. The goal of this study was to identify specific glycoenzymes, which could distinguish prostate cancer cells from normal prostatic cells. We investigated enzymatic activities and gene expression levels of key glycosyl- and sulfotransferases responsible for the assembly of O- and N-glycans in several prostatic cells. These cells included immortalized RWPE-1 cells derived from normal prostatic tissues, and prostate cancer cells derived from metastasis in bone (PC-3), brain (DU145), lymph node (LNCaP), and vertebra (VCaP). We found that all cells were capable of synthesizing complex N-glycans and O-glycans with the core 1 structure, and each cell line had characteristic bio-synthetic pathways to modify these structures. The in vitro measured activities corresponded well to the mRNA levels of glycosyltransferases and sulfotransferases. Lectin and antibody binding to whole cells supported these results, which form the basis for the development of tumor cell-specific targeting strategies.
Keywords: Glycosyltransferase activities, N-glycosylation, O-glycosylation, Real-time PCR, Prostate cancer cells
Introduction
Prostate cancer (CaP) is the most commonly diagnosed cancer, and the second leading cause of cancer death in men in most regions of the world. Altered glycosylation in cancer cells [1–4] can change antigenicity, facilitate the escape of cancer cells from the immune surveillance, and promote invasion and metastasis [5–13]. In particular, sialic acid is a cell surface carbohydrate involved in multiple functions such as cell adhesion and immunity, and is often found in increased amounts in cancer cells [13, 14].
The prostate produces many glycoproteins, including the N-glycosylated prostate specific antigen (PSA) [15, 16] and highly O-glycosylated secreted and cell surface-bound mucins [17–19]. PSA glycoforms from the serum of healthy people have mainly bi-antennary complex chains while PSA in the sera of CaP patients has many tri- and tetra-antennary N-glycans, suggesting an increase in branching GlcNAc-transferases (GnT) in CaP [4, 16, 20–22]. Significant changes were observed at the nonreducing ends of the oligosaccharide chains of PSA. The glycosylation of MUC1 mucin is also altered in CaP [2, 3].
The selectin ligand sialyl-Lewisx, Neu5Acα2-3Galβ1-4 (Fucα1-3)GlcNAcβ-R (SLex), and related antigens play a critical role in cancer cell migration [23–26], and are associated with a low survival rate [27]. The O-glycan branching enzyme core 2 β6-GlcNAc-transferase (C2GnT1) synthesizes core 2, GlcNAcβ1-6(Galβ1-3)GalNAc-, which forms a scaffold structure for SLex in leukocytes, and may play a role in controlling the adhesion of cancer cells to the endothelium and their invasiveness [22–24, 28]. The expression of C2GnT1 was found to increase with progression of CaP [29]. C2GnT1 also contributes to galectin-1-mediated cell death in LNCaP cells derived from lymph node metastasis [30].
The mechanisms underlying altered glycosylation of cancer glycoproteins involve characteristically abnormal glycosyltransferase activities [7, 31–35]. In the current study, we conducted a comprehensive analysis of the activities and gene expression levels of key enzymes involved in the pathways of N- and O-glycans in four different cultured metastatic CaP cell lines in comparison to cells derived from normal prostate. We correlated transferase activities with mRNA expression levels of transferase genes. We showed that some of the glycosylation pathways are characteristic for a given cancer cell type. In addition, we found that alterations in several enzymes as well as in cell surface carbohydrate structures were common to all of the CaP cell lines. Some of these enzyme patterns resembled those found in colon and breast cancer cells. These studies provide a solid basis for targeting specific transferases and for further work on the biological roles of cancer-associated glycans.
Materials and methods
Materials
Reagents were from Sigma, unless indicated otherwise. Enzyme substrates were also prepared and donated by Hans Paulsen (University Hamburg, Germany) and Khushi Matta (Roswell Park Cancer Institute, Buffalo, NY). The β1,4-Galactosyltransferase (β4GalT) inhibitor 612 was prepared by Walter Szarek (Queen’s University, Canada). Lectins were obtained from Vector Labs. Antibodies anti-SLex (CSLEX1) was from BD Pharmingen; anti-Ley, anti-Tn, anti-Lea and anti-Sialyl-Tn antibodies were from Abcam.
Cell cultures
Human colon cancer Caco-2 cells were obtained from John MacLeod (Queen’s University) and grown in Dulbecco’s Modified Eagle’s medium. The immortalized human prostate cell line RWPE-1 was from ATCC, and cells were grown in Keratinocyte serum free medium. Metastatic CaP cell lines (from ATCC) were kindly donated by Jeremy Squire (Queen’s University) and were grown as recommended by ATCC. PC-3 cells, derived from bone metastasis (stage IV), were grown in F-12 K medium from Invitrogen. DU145 cells, derived form brain metastasis, were grown in Eagle’s Minimum Essential medium. LNCaP cells, derived from left supraclavicular lymph node metastasis, were grown in RPMI-1640 medium. VCaP cells, derived from vertebral metastasis, were grown in DMEM. All cell lines were grown at 37 °C with 5 % CO2 and growth media contained 10 % fetal bovine serum (FBS) (except for RWPE-1), as well as 100 U Penicillin/ml and 100 μg Streptomycin/ml.
Preparation of cell homogenates
For glycosyltransferase and sulfotransferase assays, cells were harvested immediately after reaching confluence. Cells were detached with 0.25 % trypsin-EDTA (Invitrogen), washed once in growth medium followed by two washes with phosphate-buffered saline (PBS, Invitrogen). Pellets were stored at −80 °C, then hand homogenized on ice in 0.25 M sucrose (1 ml sucrose/l08 cells) and stored in small aliquots at −80 °C. Protein concentrations of homogenates were determined by the Bio-Rad (Bradford) protein assay method using bovine serum albumin as the standard.
Glycosyltransferase assays
Glycosyltransferases were assayed at standard conditions using fresh aliquots of frozen cell homogenates immediately after thawing [36–44]. Enzymes were kept on ice until the time of incubation. Assays were carried out in duplicate with variations between assays of<10 %. Enzyme products were isolated by AG1x8 or Sep-Pak C18, followed by separation with reversed-phase HPLC. Table 1 lists the enzymes and substrates used as well as the HPLC conditions in every assay. The acceptor substrate for β1,2-N-acetylglucosaminyltransferase I (GnT-I) was Manα1-6(Manα1-3)Manβ-octyl (Toronto Research Chemicals). Manα1-6 (GlcNAcβ1-2Manα1-3)Manβ-octyl was enzymatically prepared from Manα1-6(Manα1-3)Manβ-octyl, using recombinant GnT-I (donated by H. Schachter, University of Toronto, Canada), and was purified by reversed-phase HPLC.
Table 1.
List of enzymes, reaction substrates and HPLC conditions Assays for the enzymes listed were carried out under conditions described in Materials and methods. The gene names (according to NCBI) indicate specific genes known to be responsible for the activities, and/or genes examined for mRNA expression. Enzyme products were isolated using the acceptor substrates listed, and a C18 column and acetonitrile (AN) - water mixtures as the liquid phase; % AN acetonitrile concentration in the liquid phase, Bn benzyl, Gn GlcNAc, M mannose; pnp p-nitrophenyl
| Enzyme | Short Name | Gene Name | Acceptor Substrate and Assay Concentration (mM) | HPLC (%AN) | |
|---|---|---|---|---|---|
| Polypeptide-N-acetylgalactosaminyltransferase | ppGalNAcT | AQPTPPP | 0.5 | ||
| Core 1 β1,3-galactosyltransferase | C1GalT | C1GALT1 | GalNAcα-Bn | 0.5 | 14 |
| T-synthase | |||||
| Core 2 β1,6-N-acetylglucosaminyltransferase | C2GnT1 | GCNT1 | Galβ3GalNAcα-Bn | 0.5 | 13 |
| C2GnT3 | GCNT4 | ||||
| Core 3 β1,3-N-acetylglucosaminyltransferase | C3GnT | GalNAcα-Bn | 1.0 | 14 | |
| Core 3 synthase | B3GNT6 | ||||
| Core 4 β1,6-N-acetylglucosaminyltransferase | C2GnT2 | GCNT3 | Gnβ3GalNAcα-pnp | 0.5 | 12 |
| β1,3-N-acetylglucosaminyltransferase | Extension | Galβ4Gnβ-Bn | 1.0 | 13 | |
| β3GlcNAcT | B3GNT1 | ||||
| iGnT | |||||
| β1,3-N-acetylglucosaminyltransferase | Elongation | Galβ3(6-deoxy)GalNAcα-Bn | 1.0 | 18 | |
| β3GlcNAcT | B3GNT3 | ||||
| β1,3/4-N-acetylgalactosaminyltransferase | β4GalNAcT | B4GALNT3 | Gnβ3GalNAcα-pnp | 0.5 | 13 |
| β3GalNAcT | Gnβ-Bn | 0.5 | 12 | ||
| β1,3/4-Galactosyltransferase | β4GalT | B4GALT1-6 | Gnβ-Bn | 0.5 | 12 |
| β3GalT5 | B3GALT5 | Gnβ3GalNAcα-pnp | 0.5 | 13 | |
| β3GalT | B3GALT1-2 | ||||
| β1,2-N-acetylglucosaminyltransferase I | GnT-I | MGAT1 | Mα6(Mα3)Mβ-octyl | 0.5 | 24 |
| β1,4-N-acetylglucosaminyltransferase II | GnT-II | MGAT2 | Mα6(Gnβ2Mα3)Mβ-octyl | 0.5 | 24 |
| N-acetylglucosaminyltransferases III-V | GnT-III-V | Gnβ2Mα6(Gnβ2Mα3)Mβ-octyl | 0.5 | 24 | |
| β1,6-N-acetylglucosaminyltransferase V | GnT-V | MGAT5 | Gnβ2Mα6(Gnβ2[4-deoxy]Mα3) 4-O-methyl-Mβ-Octyl | 1.0 | 24 |
| α1,2-Fucosyltransferase | α2FUT1 | FUT1 | Galβ-Bn | 2.0 | 11 |
| α1,2-Fucosyltransferase | α2FUT2 | FUT2 | Galβ3GalNAcα-Bn | 2.0 | 13 |
| α1,3-Fucosyltransferase | α3FUT3–9 | FUT3-9 | GalNAcβ4Gnβ-Bn | 2.0 | 13 |
| α1,4-Fucosyltransferase | α3/4FUT3 | FUT3 | 2-O-Methyl-Galβ3Gnβ-Bn | 2.0 | 13 |
| α2,3-Sialyltransferase | ST3Gal | ST3GAL1 | Gal(6-deoxy)β3GalNAcα-Bn | 1.0 | 12 |
| α2,3-Sialyltransferase | ST3Gal3-6 | ST3GAL3-6 | |||
| α2,6-Sialyltransferase | ST6Gal | ST6GAL1 | |||
| α2,6-Sialyltransferase | ST6GalNAc1-4 | ST6GALNAC1-4 | |||
| Galactosyl-3-O-sulfotransferase | Gal3ST | GAL3ST1-4 | Gal(6-deoxy)β3GalNAcα-Bn | 2.0 | 13 |
| N-acetylglucosaminyl-6-O-sulfotransferase | GlcNAc6ST | CHST1-4 | Gnβ-Bn | 2.0 | 12 |
GalNAc-transferases
Polypeptide α-GalNAc-transferase (ppGalNAcT) [42] and β1,3/4 N-acetylgalactosaminyltransferase (β3/4GalNAcT) activities were determined in a total volume of 40 μl containing 0.125 M MES (N-morpholino-ethanesulfonic acid) pH 7, 0.125 % Triton X-100, 10 mM AMP, 12.5 mM MnCl2, 0.9 mM UDP-[3H]GalNAc (2,000–4,000 cpm/nmol), 0.5 mM AQPTPPP (for ppGalNAcT) or GlcNAcβ1-3GalNAcα-p-nitrophenyl or GlcNAcβ-Bn (for β3/4GalNAcT), and 10 μl cell homogenate (80 to 150 μg protein). Samples were incubated at 37 °C for 1 h and product determined using AG1x8 and HPLC as described [42].
Glycosyltransferases that synthesize core 1 to 4 glycans
The activities (Table 1) of the T-synthase (C1GalT) and core 3 synthase (C3GnT) that act on GalNAcα-Bn, C2GnT1 that acts on Galβ1-3GalNAcα-Bn or C2GnT2 that acts on both Galβ1-3GalNAcα-Bn and GlcNAcβ1-3GalNAcα-p-nitro-phenyl (Toronto Research Chemicals) to synthesize core 4, GlcNAcβ1-6(GlcNAcβ1-3)GalNAcα-, were determined as described [42].
GlcNAc-transferases-I to V
The assay mixtures for GlcNAc-transferases (Table 1) that synthesize N-glycan branches contained 0.9 mM UDP-[3H] GlcNAc (1,600 cpm/nmol) and 0.5 mM Manα6(Manα3)-Manβ-octyl (for GnT-I, Toronto Research Chemicals), Manα6(GlcNAcβ2Manα3)Manβ-octyl (for GnT-II), GlcNAcβ2-Manα6(GlcNAcβ2Manα3)Manβ-octyl (for GnT-III to V) or 1 mM GlcNAcβ2Manα6(GlcNAcβ2[4-deoxy-]Manα3)[4-O-methyl-]Manβ-octyl in the absence of MnCl2 (for GnT-V) (Table 1). Enzyme parameters for GnT-V were determined using PC-3 cell homogenates. Which exhibited high activity of this enzyme, and the non-linear regression program Origin-Pro 8.1.
Galactosyltransferases
GalT assays were carried out as described [40], using 1 mM UDP-[3H]Gal (1,600 cpm/nmol) and 0.5 mM GlcNAcβ-Bn or GlcNAcβ1-3GalNAcα-p-nitrophenyl as acceptor substrates. Inhibition assays of β4GalT assays were carried out in the presence of 0.5 mM substrates and 0.5 mM GlcNBu-S-2-naphthyl (612). Since this inhibitor was dissolved in methanol, the same amount of methanol (10 %) was present in all assays.
Sialyltransferases
The α2,3-sialyltransferase (ST3Gal) activities (Table 1) were determined in a total volume of 40 μl containing 0.1 M Tris, pH 7, 0.125 % Triton X-100, 12.5 mM MnCl2, 0.8 mM CMP-[3H]sialic acid (2,400 cpm/nmol), 1 mM Galβ1-3(6-deoxy)GalNAcα-Bn as substrate and 10 μl cell homogenates (80 to 150 μg protein). Samples were incubated at 37 °C for 1 h and reaction mixtures applied to Sep-Pak C18 columns. Enzyme product eluted with methanol was analyzed by reversed-phase HPLC.
Sulfotransferases
Sulfotransferase (Gal3ST and GlcNAc6ST, Table 1) activities were determined in a total volume of 40 μl containing 50 mM MES pH 7, 0.1 % Triton X-100, 2 mM ATP, 10 mM NaF, 10 mM 2, 3-mercaptopropanol, 10 mM MnCl2, 0.8 mM 3′-phosphoadenosine 5′-phospho[35S]sulfate (PAP35S) (1,300 cpm/nmol), 2 mM Galβ1-3(6-deoxy)Gal-NAcα-Bn or GlcNAcβ-Bn (for Gal3ST or GlcNAc6ST, respectively) and 10 μl cell homogenate (80 to 150 μg protein). Samples were incubated at 37 °C for 1 h and the reaction mixtures were eluted through Sep-Pak C18 column, followed by HPLC analysis (C18 column).
Fucosyl-transferases
Assays for α2FUT, α3FUT and α3/4FUT (Table 1) were carried out as described for GalT assays, except that the donor substrate was 1.25 mM GDP-[3H]Fuc (1,000 cpm/nmol) or 1 mM GDP-[14C]Fuc (2,600 cpm/nmol) and the acceptor was either 2 mM Galβ-Bn, Galβ1-3GalNAcα-Bn or GalNAcβ1-4GlcNAcβ-Bn (Table 1). Mixtures were separated by AG1x8 and reversed-phase HPLC.
Real-time PCR analysis of the mRNAs of glycosyl-and sulfotransferase genes
RNA from cultured prostate cells was isolated by TRI-REAGENT according to the manufacturer’s instruction. To prepare cDNA, 2 μg RNA were used in a 20 μl reaction mixture using a Verso reverse transcriptase kit (Thermo scientific) as follows: 5 min at r.t., 60 min at 42 °C, and 2 min at 95 °C. Quantitative real-time PCR was performed in 10 μl reaction volume in a 96-well plate using 2 μl of diluted (1:1) cDNA with SYBR® Premix Ex Taq™ (TAKARA BIO INC.) on a Mastercycler Epgradient realplex (Eppendorf AG, Hamburg, Germany). The PCR conditions included 1 cycle at 95 °C for 2 min followed by 45 cycles at 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 45 s. The data were analyzed using Eppendorf realplex software, version 1.5 (Eppendorf). The amounts of glycosyltransferase transcripts (see Table 1 for gene names) were normalized to the amount of GAPDH transcript in the same cDNA sample. Relative fold differences in transcript expression were determined using the following comparative CT method: CT method: 2−[ΔCt (Target)]×100 where ΔCt=(Target)=Ct (Target)−Ct (GAPDH) as described previously [43]. The results were expressed as the amount (%) relative to that (100 %) of GAPDH and plotted as mean relative amount± SEM. Primer sequences used for expression analysis of all genes including GAPDH are summarized in Supplementary Table 1.
Lectin staining assays of whole cells
Lectins were used to determine cell surface glycosylation. Lectin staining was carried out as described [44] with cells fixed on 96 well microtitre plates. Confluent cells (7.2×104 to 1.04×105) were incubated with biotinylated lectins followed by alkaline phosphatase-conjugated Avidin and nitrophenyl-phosphate reaction substrate. The color change was measured with a microplate reader at 405 nm. The intensities were normalized to those of 1.0×105cells. The differences between means were compared using the Student’s t-test. In all cases, p<0.01 was considered statistically significant. Each sample was analyzed at least seven times.
Enzyme linked immunosorbent assays (ELISA) of whole cells
Antibodies were also used to assess cell surface glycans. ELISA was carried out as described [45]. Cells were grown to confluency in 96-well plates, and were then fixed. Confluent cells (7.2×104 to 1.2×105 cells) were incubated with anti-SLex (CSLEX1), anti-Ley, anti-Tn, anti-Lea or anti-Sialyl-Tn antibodies followed by alkaline phosphatase-linked secondary antibodies and nitrophenyl-phosphate reaction substrate. Samples were analyzed as described above.
Results
The enzymatic activities of several glycosyltransferases and sulfotransferases were compared in homogenates from four different metastatic CaP cell lines and a normal prostatic cell line, as well as the human colon cancer cell line Caco-2 [39] and rat colon mucosal homogenate, which served as positive controls. Enzyme nomenclature and substrates used are listed in Table 1. The activities were correlated to the mRNA expression of isoenzymes and the cell surface expression of lectin and anti-carbohydrate antibody binding sites. The cell surface carbohydrates showed distribution patterns that appeared to be specific for each prostatic cell line.
Synthesis of complex N-glycans
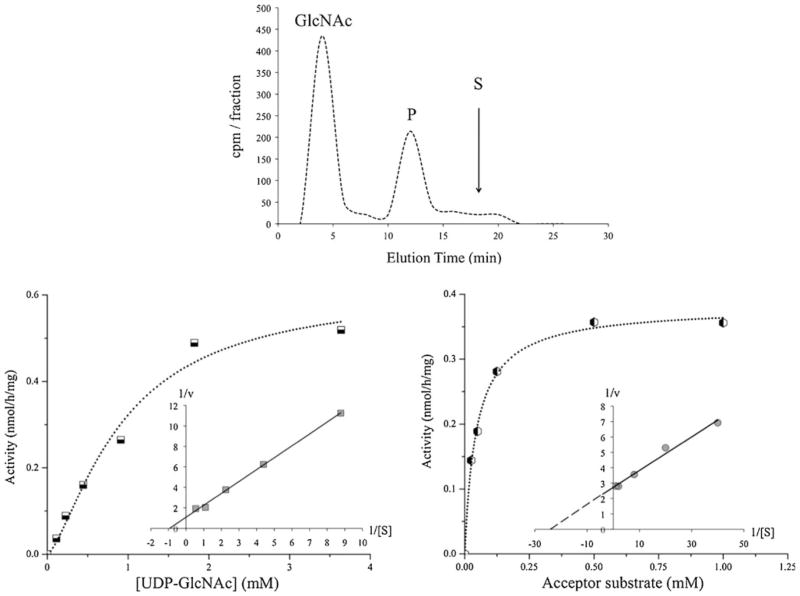
As shown in Table 2, all prostatic cell lines contained GlcNAc-transferase (GnT-I and II) activities involved in the synthesis of bi-antennary N-glycans. The activity of GnT-I which synthesizes the first antenna of N-glycans, i.e. GlcNAcβ1-2 linked to the Manα1-3 branch, was reduced in all CaP cells up to 85 % when compared to prostatic RWPE-1 cells. GnT-II, which adds GlcNAc in a β 1-2 linkage to the Manα1-6 arm was active in all prostatic cells. The combined activities of GnT-III, IV and V using a biantennary substrate were low (<0.5 nmol/h/mg) in all cells (data not shown). GnT-V which synthesizes the GlcNAcβ1-6 branch of tetra-antennary N-glycans was also assayed using an acceptor substrate specific for GnT-V (Fig. 1a). The activities of GnT-V were higher in all CaP cells compared to RWPE-1 cells. PC-3 and LNCaP cells exhibited the highest GnT-V activities (0.31 and 0.35 nmol/h/mg, respectively). The reaction rate was linear with respect to protein concentrations up to 0.29 mg/ml and incubation times up to 2 h. Because of the low GnT-V activity in normal prostatic cells it was not possible to obtain an accurate KM value. For GnT-V in PC-3 cell homogenates, the apparent KM value for UDP-GlcNAc was 0.93 mM and Vmax was 0.62 nmol/h/mg (Fig. 1b). For the acceptor substrate, the apparent KM and Vmax values were 0.05 mM and 0.38 nmol/h/mg, respectively (Fig. 1c). This KM value was similar to that previously found for a purified hamster kidney GnT-V (0.06 mM) [41].
Table 2.
GlcNAc-transferase activities that synthesize N-glycan antennae. Assays were carried out by HPLC as described in Material and Methods. The results were calculated as the average of duplicates that differ by<10 %. Abbreviations are as in Table 1. Caco-2 cells served as positive controls. RWPE-1 cells were derived from normal prostate. PC-3, DU145, LNCaP and VCaP cells are metastatic prostate cancer cells
| Enzyme | Activity (nmol/h/mg)
|
|||||
|---|---|---|---|---|---|---|
| RWPE-1 | PC-3 | DU145 | LNCaP | VCaP | Caco-2 | |
| GnT-I | 7.21 | 1.65 | 1.10 | 3.94 | 2.60 | 3.45 |
| GnT-II | 3.40 | 1.19 | 1.55 | 5.64 | 2.79 | 2.44 |
| GnT-V | 0.03 | 0.31 | 0.05 | 0.35 | 0.23 | 0.08 |
Fig. 1.
β1,6-N-acetylglucosaminyltransferase Venzymatic activity and kinetics. GnT-V enzymatic activity in homogenates from PC-3 cells (derived from bone metastasis) was measured as described in Materials and Methods. GnT-V enzymatic activity was found to be increased in all prostatic cancer cells compared to normal prostatic cells. a Enzyme reaction product was separated by HPLC using 24 % acetonitrile in H2O as the mobile phase. The radioactivity (cpm) of collected 2 min fractions is shown. The elution of radioactive GlcNAc and specific acceptor substrate (S), GlcNAcβ2Manα6(GlcNAcβ2[4-deoxy-]Manα3)[4-O-methyl-]Manβ-octyl, is indicated by the arrow; this was well separated from the radioactive enzyme product (P). b The apparent KM for UDP-GlcNAc was 0.93 mM and Vmax was 0.62 nmol/h/mg (with 0.5 mM acceptor substrate). The inset shows the Lineweaver-Burk plot. c The apparent KM for the acceptor substrate was 0.05 mM and the apparent Vmax was 0.38 nmol/h/mg (with 1 mM UDP-GlcNAc). The inset shows the Lineweaver-Burk plot
Glycosyl- and sulfotransferase activities that assemble O-glycans
Prostatic cancer cells produce highly O-glycosylated mucins that are abnormally expressed and glycosylated [3, 12, 18]. We therefore measured the enzymatic activities of glycosyl-and sulfotransferases that participate in the synthesis of O-glycans. The addition of the first sugar in the O-glycosylation pathways is catalyzed by a family of ppGalNAcT. Using a peptide acceptor with only one O-glycosylation site (AQPTPPP), high activities (3.0 to 12.5 nmol/h/mg) of ppGal-NAcT were detected in all prostatic cell homogenates (Table 3). This suggests that glycoproteins and mucins in these prostatic cells are potentially highly O-glycosylated, which corresponds to the expression of the HP lectin epitope (Supplementary Figure 1) and the Tn antigen (Supplementary Figure 2) on cell surfaces. All of these prostatic cells contained the activity of T-synthase, which synthesizes core 1, Galβ1-3GalNAc-, the T antigen recognized by PNA lectin (Supplementary Figure 1). However, the subsequent processing of core 1 appeared to be quite different among these cells. The C2GnT activity responsible for the synthesis of branched core 2 O-glycans was not detected in PC-3 and DU145 cells. C2GnT activity was low in normal prostatic RWPE-1 cells (0.3 nmol/h/mg), but was four to ten-fold higher in VCaP and LNCaP cells, respectively. These cells therefore can synthesize complex branched O-glycan structures.
Table 3.
Activities of glycosyltransferases that synthesize O-glycan core structures. Assays were carried out by HPLC as described in Material and methods. Abbreviations are as in Tables 1 and 2
| Enzyme | Activity (nmol/h/mg)
|
|||||
|---|---|---|---|---|---|---|
| RWPE-1 | PC-3 | DU145 | LNCaP | VCaP | Positive control | |
| ppGalNAcT | 11.6 | 3.0 | 5.7 | 11.7 | 12.5 | 18.0a |
| C1GalT | 3.0 | 2.3 | 2.3 | 2.2 | 1.0 | 3.4a |
| C2GnT1 | 0.3 | < 0.1 | < 0.1 | 3.1 | 1.2 | 56.1b |
| C3GnT | 0.3 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 1.2b |
| C2GnT2 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | 54.0b |
Activity in Caco-2 cell homogenates
activity in rat colon mucosal homogenates
The activity of core 3 synthase, B3GNT6, that synthesizes core 3, GlcNAcβ1-3GalNAc, was not detected in any of these CaP cells while normal prostatic cells exhibited a low activity (0.3 nmol/h/mg). Therefore, core 3 structure is expected to be produced primarily in RWPE-1 cells. None of the prostatic cells appeared to be capable of synthesizing core 4 from core 3 [7].
Extension and elongation glycosyltransferases
The activities of extension β1,3-N-acetylglucosaminyltransferases (β3GlcNAcT, iGnT) and β1,3/4-N-acetylgalactosaminyltransferases [32, 46] were low in prostatic cells (Table 4). In contrast, high activities of β3/4GalT were observed in all prostatic cells (Table 4). The β4GalT1 inhibitor 612 (N-butyryl-glucosamine-1-thio-2-naphthyl) [40, 47], inhibited GalT activity in prostate cell lines by 65 to 99 % (data not shown). In addition, prostatic cells consistently showed lower GalT activities using O-glycan core 3 substrate, which is a preferred substrate for β3GalT5, as compared to the GlcNAcβ-Bn substrate. These results suggest that the majority of the GalT activity in prostatic cells was due to β4GalT although there was also substantial β3GalT activity.
Table 4.
Activities of extension and termination glycosyltransferases and sulfotransferases. Assays were carried out by HPLC as described in Material and methods. Abbreviations are as in Table 1 and 2
| Enzyme | Activity (nmol/h/mg)
|
|||||
|---|---|---|---|---|---|---|
| RWPE-1 | PC-3 | DU145 | LNCaP | VCaP | Positive control | |
| Extension β3GlcNAcT | 0.1 | 0.3 | 0.1 | 0.3 | < 0.1 | 1.1b |
| Elongation β3GlcNAcT | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 1.4b |
| β3/4GalNAcT (Gnβ3GalNAcα-pnp substrate) | < 0.1 | < 0.1 | 0.2 | 0.4 | 0.2 | 15.3b |
| β3/4GalNAcT (Gnβ-Bn substrate) | < 0.1 | < 0.1 | < 0.1 | 0.1 | < 0.1 | 0.2b |
| β3/4GalT (Gnβ-Bn substrate) | 25.9 | 9.3 | 31.7 | 39.7 | 17.2 | 38.4a |
| β3/4GalT (Gnβ3GalNAcα-pnp substrate) | 6.3 | 3.7 | 6.9 | 13.3 | 8.4 | 13.1a |
| α2FUT1 | 0.9 | < 0.1 | 0.2 | 0.6 | 0.7 | >10b |
| α2FUT2 | 0.8 | 0.2 | 0.8 | 0.3 | 0.7 | >10b |
| α3FUT | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 3.1b |
| α4FUT3 | 1.1 | < 0.1 | < 0.1 | < 0.1 | 0.1 | 0.8b |
| ST3Gal | 0.4 | 17.3 | 0.2 | 7.7 | 1.3 | 7.0c |
| Activity (pmol/h/mg) | ||||||
| Gal3ST | 3.2 | 0.5 | 0.7 | 0.6 | 1.6 | 148.7b |
| GlcNAc6ST | 5.3 | 0.2 | 0.1 | < 0.1 | < 0.1 | 0.5b |
Enzyme activity in Caco-2 cell homogenates
activity in rat colon mucosal homogenates
ST3Gal activity in mouse intestine homogenates from ST3Gal1 overexpressing mice [61]
Terminal modification by glycosyltransferases and sulfotransferases
Fuc-transferase (FUT) activities were compared between normal prostatic and CaP cells (Table 4). The activities of α2FUT1, which synthesizes the Fucα1-2Gal linkage in histo-blood group substances and α2FUT2 activity, which synthesizes the Fucα1-2Gal linkage in secretory blood group substances appeared to be variable and low (Table 4). The activities of α3FUT and α4FUT3 involved in the synthesis of Lewis blood group structures were generally low, but highest in RWPE-1 cells (Table 4).
All cells exhibited activities of α2,3-sialyltransferases (ST3Gal) (Table 4). A 3-fold higher activity of ST3Gal was seen in VCaP cells, compared to normal prostatic cells, and a dramatic 43- and 19-fold increase was seen in PC-3 and LNCaP cells, respectively. Thus in PC-3 cells, core 1 is expected to be highly α2-3-sialylated and in LNCaP and VCaP cells both cores 1 and 2 are α2-3-sialylated. The high ST3Gal activities are mirrored in the MAAII binding of cell surfaces (Supplementary Figure 1).
The Gal and GlcNAc residues in N- and O-glycans can be sulfated. The sulfotransferase activities (Gal3ST) that synthesize the Gal-3-O-sulfate ester of core 1 were reduced in CaP cells to 16 to 50 % of the activity in RWPE-1 cells. The sulfotransferase activities that synthesize the GlcNAc-6-O-sulfate ester (GlcNAc6ST) were reduced to 2 and 4 %, in DU145 and PC-3 cells, respectively, relative to the activity in RWPE-1 cells, and were undetectable in LNCaP and VCaP cells (Table 4). Thus, sulfotransferase activities are down regulated in CaP cells, similar to colon cancer or tumorigenic polyposis coli cells [7, 37].
Expression profiles of glycosyl- and sulfotransferase genes
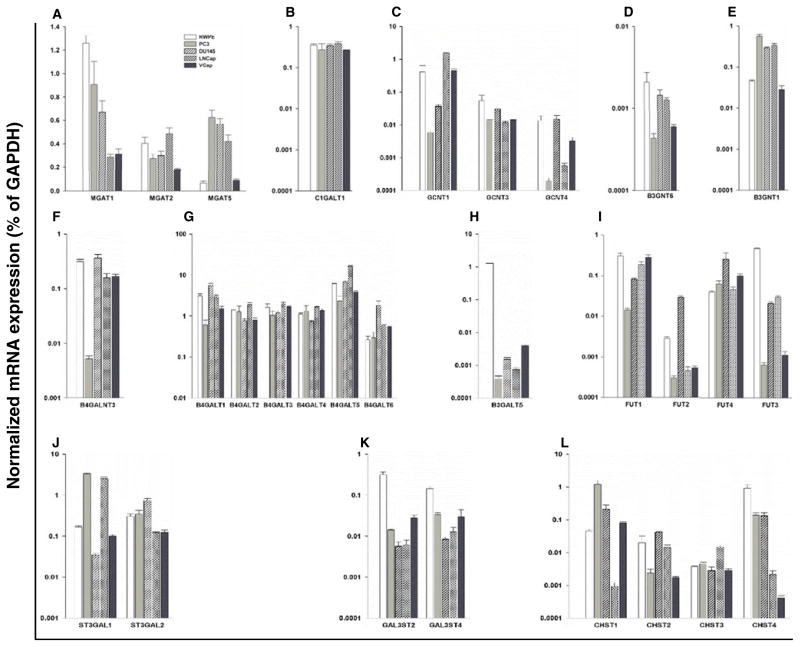
To identify specific glycosyltransferase and sulfotransferase isoenzymes responsible for the enzyme activities measured in prostatic cells, and to determine if the activities can be predicted from the mRNA levels of glycosyltransferase and sulfotransferase genes we carried out real-time PCR (Fig. 2, Supplementary Figure 3). Values for transferase gene expression were related to those of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which based on the Ct values varied by <7 % in the different cell lines.
Fig. 2.
Quantitative real-time PCR analysis of the mRNAs of glycosyltransferase and sulfotransferase genes in normal and cancerous prostatic cells. The expression levels of glycosyltransferase and sulfotransferase genes are shown, which corresponded to the enzymatic activities measured in this study. The gene expression levels were calculated by the ΔCt method as described in Materials and Methods and expressed as relative amount to that of GAPDH (100 %). The enzyme names are listed in Table 1. Results are shown for a MGAT1, 2, 5; (GnT-I, II, V); b C1GALT1 (C1GalT); c GCNT1,3,4 (C2GnT1-3); d B3GNT6 (Core 3 Synthase); e B3GNT1 (Extension β3GlcNAcT); f B4GALNT3 (β4GalNAcT); g B4GALT1-6 (β4GalT); h B3GALT5 (β3GalT5); i FUT1-4; j ST3GAL1-2; k sulfotransferases GAL3ST2 & 4; and l sulfotransferases CHST1-4. The data were obtained from three independent experiments and expressed as mean ± SEM
N-glycan transferases
The expression level of the GnT-I gene (MGAT1) was highest in RWPE-1 cells and lower in all CaP cells (Fig. 2a), which reflected the GnT-I activities measured in these cells (Table 2). The expression levels of the GnT-II (MGAT2) gene were consistent with the enzyme activity profile (Fig. 2a, Table 2). The GnT-V gene (MGAT5) was expressed highest in PC-3 cells, followed by DU145, LNCaP, and then VCaP, and was lowest in RWPE-1 cells (Fig. 2a). Thus, both the enzymatic activities and the mRNA levels of GnT-V were higher in all CaP cells than in RWPE-1 cells.
O-glycan transferases
The C1GALT1 gene was expressed at similar levels in all five prostatic cells (Fig. 2b), which paralleled the activities (Table 3). The GCNT1 gene, encoding one of the enzymes that synthesize core 2, was expressed highest in LNCaP cells (Fig. 2c), matching the high C2GnT activity in these cells (Table 3). This was followed by VCaP and RWPE-1 cells. The activity was not detectable in DU145 and PC-3 cells, which corresponded to low expression levels of this gene (Fig. 2c). The expression levels of GCNT3 involved in core 4 synthesis were also low, explaining why the activity was below the level of detection in our assays.
As could be predicted from the low core 3 synthase activity in RWPE-1 cells and the lack of the activity in CaP cells, the B3GNT6 gene was expressed at extremely low levels in CaP while RWPE-1 cells were the highest expressors (Fig. 2d). The expression levels of the B3GNT1 gene (Fig. 2e) correlated well with β3GlcNAcT enzymatic activities (Table 4). Additional members of the β3GlcNAcT gene family (B3GNT2-5) were also expressed in prostatic cells (Supplementary Figure 3). The B4GALNT3 gene involved in the synthesis of GalNAcβ1-4GlcNAc extension was expressed in all prostatic cells (Fig. 2f). This gene plays a role in cell growth and invasion of colon cancer cells [48]. The combined gene expression levels of isoenzymes of the β4GalT family (B4GALT1-6), which synthesize type 2 chains (N-acetyllactosamines) were high (Fig. 2g) and paralleled the high activities. In contrast, the transcript level of the B3GALT5 gene was low in all CaP cells and higher in RWPE-1 cells (Fig. 2h).
Figure 2i shows the gene expression levels of FUT1 and FUT2 that matched the patterns of enzymatic activities (Table 4). While α3FUT4 gene expression was similar in all prostatic cells, the α3/4FUT3 expression pattern agreed with the highest enzymatic activity detected in normal prostatic cells (Table 4). The expression levels of these enzymes contribute to the synthesis of Lea and Ley epitopes and correspond to the high SLex expression on normal prostatic cells (Supplementary Figure 2).
Results from the ST3Gal enzyme activities (Table 4) predicted a high expression level of ST3GAL1, which synthesizes the sialylα2-3Gal linkages on cores 1 and 2, in PC-3 and LNCaP cells. The mRNA levels of the ST3GAL1 gene corresponded to the high activities (Fig. 2j). Several other ST3GAL genes (2 to 6) were also found to be expressed with minor variations among CaP and normal prostatic cells (Supplementary Figure 3). These sialyltransferases may participate in SLex synthesis in all prostatic cells (Supplementary Figure 2).
The α2,6-sialyltransferases that synthesize the sialylα2-6GalNAc linkage on O-glycans to form sialyl-Tn and sialyl-T antigens were variably expressed (Supplementary Figure 3) although the activities were not measured. The expression level of ST6GALNAC1 responsible for the synthesis of the STn epitope, as well as anti-STn antibody binding was lowest in normal prostatic cells (Supplementary Figure 2). The ST6GAL1 gene involved in adding sialic acid in α2-6 linkage to terminal Gal residues in N-glycans was variably expressed (Supplementary Figure 3). These enzymes provide the ligands for SNA lectin binding (Supplementary Figure 1).
The expression levels of the 3-O-sulfotransferase GAL3ST2&4 genes, responsible for the synthesis of the Gal-3-O-sulfate ester linkage in cores 1 and 2, were highest in RWPE-1 cells (Fig. 2k). Among the four N-acetylglucosaminyl-6-O-sulfotransferase (CHST1-4) genes responsible for the synthesis of the sulfate-6-O-GlcNAc linkage, CHST4 was expressed highest in RWPE-1 cells. These expression profiles of these genes matched the pattern of enzyme activities measured in prostatic cells (Table 4).
Discussion
Altered glycosylation of glycoproteins and mucins has been reported in prostate cancer but the enzymes responsible for alterations have not been identified. In this study, we compared the enzymatic activities and the gene expression profiles of glycosyl- and sulfotransferases involved in N- and O-glycan synthesis in normal and cancerous prostatic cells. We showed that the activities corresponded well to the mRNA expression levels for most transferases. We found that each prostatic cell line exhibits characteristic biosynthesis pathways. In addition, we have identified a number of transferases that may in this combination potentially serve as markers for CaP. This includes high GnT-V activity and gene expression, undetectable core 3 synthase activity and low enzymatic activity of sulfotransferases in CaP cells, as well as an upregulation of C2GnT1 and ST3Gal in a selected number of CaP cells. However, this remains to be confirmed with prostate tissue from patients with prostate cancer. The pathways in these cell lines partly correspond to the lectin and antibody binding patterns of prostatic cell surfaces. A lack of correlation may be explained by the selected exposure of glycans on the cell surface, or possibly, by differences in glycosylation of secreted glycoproteins or mucins and cell surface molecules that include glycolipids.
All prostatic cells are capable of producing biantennary chains and show similar binding of ConA. Higher enzymatic activities and mRNA levels of GnT-V in CaP cells compared to those in normal prostatic cells suggest that CaP cells have a greater potential to generate the GlcNAcβ6 (GlcNAcβ2)Manα6 branch of N-glycans, which has been shown to contribute to cancer cell invasion and metastasis [49, 50]. This finding corresponds to the higher branching pattern found in PSA glycans from prostate cancer patients [4, 16, 21, 22].
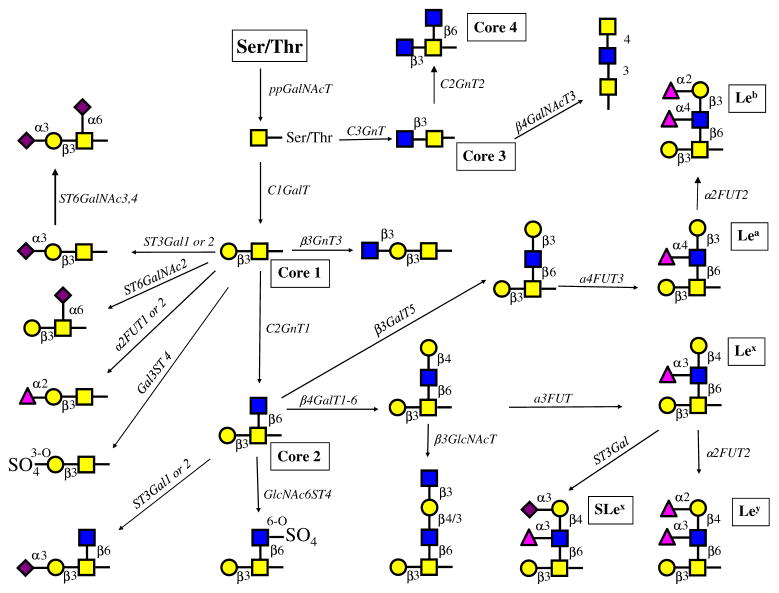
While N-glycans share a common core (Man)3(GlcNAc)2 structure, O-glycans have a number of different core structures and are more heterogeneous. Figure 3 depicts the biosynthesis pathways of a great variety of the major O-glycan structures in prostatic cells, predicted based on enzymatic activities and mRNA levels of glycogenes obtained from these cells. Many more extended and branched structures may be synthesized in normal and cancerous prostatic cells.
Fig. 3.
Proposed major O-glycosylation pathways in normal and prostatic cancer cells. Based on the major enzymatic activities and the relative gene expression levels of glycosyltransferases and sulfotransferases, the proposed major O-glycosylation pathways in prostate cells were constructed (nomenclature is shown in Table 1). Many other minor pathways are possible. Real-time PCR showed the expression of isoenzymes. RWPE-1 cells can synthesize core 1, 2 and 3 structures. Which can be extended and modified by the addition of sugar residues or sulfate esters and Lewis antigens Lea, Leb, Lex, Ley and SLex Only RPWE-1 cells showed the activity that synthesizes core 3 but not the activity that synthesizes core 4 although the enzyme (C2GnT2) was expressed at low levels. Both PC-3 (from bone metastasis) and DU145 (from brain metastasis) lack C2GnT1 activity and GCNT1 expression, which results in shorter O-glycan chains with mainly sialylated core 1 structures. DU145 cells exhibit a low ST3Gal enzymatic activity, whereas PC-3 cells exhibit a high enzymatic activity and a high gene expression of ST3GAL1. The prostatic cancer cells appear to be unable to synthesize core structures 3 and 4 although significant amounts of mRNA for the respective enzyme genes were found. LNCaP cells (from lymph node metastasis) contain high enzymatic C2GnT activity and gene expression level of GCNT1 and can form complex core 1 and core 2, but not core 3 O-glycans. ST3Gal enzymatic activity and gene expression are high in LNCaP cells. These glycans can be modified to form a variety of complex structures with extension enzymes as well as α1,2-and α1,3FUT4 and Gal3ST, but not α3/4FUT3. Although the activities of α2,6-sialyltransferases were not directly measured, the gene expression levels suggest that sialylα2-6GalNAc- and sialylated core 1 structures can be synthesized. Sulfotransferases using GlcNAc-R substrate are expressed although the activities were not detected. However, sulfotransferases using core 1 substrate were active. VCaP cells differ from LNCaP in that the expression of sulfotransferases Gal3ST1-4 and the enzymatic activity of Gal3ST is also higher in VCaP cells. α3/4FUT3 and α3FUT4 are active in VCaP cells and can synthesize various Lewis antigens
Enzymatic activity assays are often limited by their low sensitivity, and their difficulty to discriminate among different isoenzymes due to the fact that enzymes share substrates. To solve this problem, sensitive real-time PCR analysis of the expression profile of specific isoenzymes was carried out. In our study, most of the enzymatic activities corresponded well with their gene expression profiles, although clearly the complex control of biosynthesis and mechanisms that display carbohydrates on cell surfaces are still not well understood.
Normal prostatic RWPE-1 cells are capable of forming many complex O-glycans with core 1, 2 and 3 structures and a variety of terminal epitopes that may resemble those found in human colonic mucins [31]. PC-3 and DU145 cells are expected to have only core 1-based O-glycans and appear to be restricted in their ability to form branched chains since the activity of C2GnT1 was undetectable in spite of mRNA expression. In contrast, LNCaP cells are capable of synthesizing a multitude of O-glycan structures with core 1 and 2 structures [51].
With the exception of LNCaP cells, all prostatic cells show a higher Tn-antigen expression compared to normal prostatic cells, which is typically found in cancer cells [31]. The mechanism of this Tn antigen exposure is not clear. The cancer-associated sialyl-Tn antigen, which may play a role in the tumorigenic properties of cancer cells [52] was also exposed in all CaP cells at higher levels compared to normal prostatic cells. This correlated with higher expression levels of ST6GALNAC1. The expression of ST6GAL acting on N-glycans has been shown to be increased in colon cancer [53], and in VCaP cells correlated with high SNA binding.
LNCaP cells have a high potential to form the branched core 2 structure and to α2-3-sialylated core 1 and 2 structures. Since C2GnT1 and ST3Gal compete in vivo for the common core 1 substrate [54] the high ST3Gal activity can limit the conversion of core 1 to core 2 by C2GnT1.
Although core 2 structures have been shown to be important scaffolds for SLex, related to the invasive and meta-static potential of a number of cancer cell types [23, 24, 55], SLex levels expressed in CaP cells did not correlate with C2GnT1 expression, or with the activities or expression levels of the enzymes that directly synthesize SLex. Thus, the regulation of glycan synthesis remains to be further investigated.
Core 3 synthase activity was clearly detected in RWPE-1 cells but not in any of the cancer cells. A decrease in core 3 synthesis has been shown in colon cancer tissues [36] and appears to be a characteristic of colon cancer cell lines [37, 38]. This suggests that the absence of core 3 may be a marker for cancer cells, including CaP cells. PC-3 cells from bone metastasis and LNCaP cells transfected with the C3GNT6 gene showed reduced ability of migration and invasion through extracellular matrix components, and suppressed tumor formation and metastasis in mice [56, 57]. Most of these findings have been reproduced in a C3GNT6 gene knockout mouse model [58] suggesting that core 3-associated glycans may have a protective function in the normal prostatic tissue.
Because of the enzymatic activity and gene expression levels of ST3GAL1, PC-3 cells are expected to synthesize O-glycans with sialylated core 1 structures, similar to those found on breast cancer cells T47D [59]. In breast cancer, the high ST3Gal enzymatic activity or ST3GAL1 expression level may be used as a biomarker [33] and appears to protect cancer cells in vivo [60–62]. However, high ST3Gal enzymatic activity in CaP is cell-specific and is not a general feature of all metastatic CaP cells.
The levels of many glycosyltransferase activities and mRNA expression were shown to be cell type-specific in prostatic cells. It remains to be shown if this is due to variations in cancer cell phenotypes or due to the metastatic sites. The results obtained from cultured cells as models for prostate tumors and metastasis need to be validated in primary tumor tissues and cancer tissues from different meta-static sites.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Prostate Cancer Fight Foundation, Motorcycle Ride for Dad (to I.B.), and grants from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA 1I1BX000985), the National Institutes of Health (1R21HL097238 and 2RO1HL48282) and the State of Nebraska (LB506)(to P.W.C.).
Abbreviations
- FUT
fucosyltransferase
- Gal3ST
3-O-sulfotransferase
- GalT
galactosyltransferase
- GalNAcT
GalNAc-transferase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GnT GlcNAcT
GlcNAc-transferase
- GlcNAc6ST
N-acetylglucosaminyl-6-O-sulfotransferase
- HPLC
high pressure liquid chromatography
- PCR
polymerase chain reaction
- ppGalNAcT
polypeptide GalNAc-transferase
- PSA
prostate specific antigen
- SLex
sialyl-Lewisx
- ST3Gal
α2,3-sialyltransferase
- ST6Gal(NAc)
α2,6-sialyltransferase
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10719-012-9428-8) contains supplementary material, which is available to authorized users.
Contributor Information
Yin Gao, Email: Yin.Gao@queensu.ca, Department of Medicine, Division of Rheumatology, and Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, Ontario, Canada.
Vishwanath B. Chachadi, Email: vchachadi@unmc.edu, VA Nebraska-Western Iowa Health Care System, Research Service, Omaha, NE, USA. Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, Omaha, NE, USA
Pi-Wan Cheng, Email: pcheng@unmc.edu, VA Nebraska-Western Iowa Health Care System, Research Service, Omaha, NE, USA. Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, Omaha, NE, USA.
Inka Brockhausen, Email: brockhau@queensu.ca, Department of Medicine, Division of Rheumatology, and Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, Ontario, Canada.
References
- 1.Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354–1367. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 2.Burke PA, Gregg JP, Bakhtiar B, Beckett LA, Denardo GL, Albrecht H, De Vere White RW, De Nardo SJ. Characterization of MUC1 glycoprotein on prostate cancer for selection of targeting molecules. Int J Oncol. 2006;29:49–55. [PubMed] [Google Scholar]
- 3.Garbar C, Mascaux C, Wespes E. Expression of MUC1 and sialyl-Tn in benign prostatic glands, high-grade prostate intraepithelial neoplasia and malignant prostatic glands: a preliminary study. Anal Quant Cytol Histol. 2008;30:71–77. [PubMed] [Google Scholar]
- 4.Tabarés G, Radcliffe CM, Barrabés S, Ramírez M, Aleixandre RN, Hoesel W, Dwek RA, Rudd PM, Peracaula R, de Llorens R. Different glycan structures in prostate-specific antigen from prostate cancer sera in relation to seminal plasma PSA. Glycobiology. 2006;16:132–145. doi: 10.1093/glycob/cwj042. [DOI] [PubMed] [Google Scholar]
- 5.Barthel SR, Gavino JD, Wiese GK, Jaynes JM, Siddiqui J, Dimitroff CJ. Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology. 2008;18:806–817. doi: 10.1093/glycob/cwn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci U S A. 2009;106:19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carraway KL, Fregien N, Carraway CA. Tumor sialomucin complexes as tumor antigens and modulators of cellular interactions and proliferation. J Cell Sci. 1992;103(Pt 2):299–307. doi: 10.1242/jcs.103.2.299. [DOI] [PubMed] [Google Scholar]
- 9.David L, Nesland JM, Clausen H, Carneiro F, Sobrinho-Simões M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992;27:162–172. [PubMed] [Google Scholar]
- 10.Hoff SD, Matsushita Y, Ota DM, Cleary KR, Yamori T, Hakomori S, Irimura T. Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 1989;49:6883–6888. [PubMed] [Google Scholar]
- 11.Kojima N, Handa K, Newman W, Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem Biophys Res Commun. 1992;182:1288–1295. doi: 10.1016/0006-291x(92)91872-n. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan P, Lin MF, Cheng PW. Elevated expression of L-selectin ligand in lymph node-derived human prostate cancer cells correlates with increased tumorigenicity. Glycoconj J. 2009;26:75–81. doi: 10.1007/s10719-008-9167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano R, Muchmore E, Dennis JW. Sialylation and malignant potential in tumour cell glycosylation mutants. Glycobiology. 1994;4:665–674. doi: 10.1093/glycob/4.5.665. [DOI] [PubMed] [Google Scholar]
- 14.Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66:1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Janković MM, Kosanović MM. Glycosylation of urinary prostate-specific antigen in benign hyperplasia and cancer: assessment by lectin-binding patterns. Clin Biochem. 2005;38:58–65. doi: 10.1016/j.clinbiochem.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Peracaula R, Tabarés G, Royle L, Harvey DJ, Dwek RA, Rudd PM, de Llorens R. Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology. 2003;13:457–470. doi: 10.1093/glycob/cwg041. [DOI] [PubMed] [Google Scholar]
- 17.Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ, Li Y. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis. 2005;22:565–573. doi: 10.1007/s10585-005-5376-z. [DOI] [PubMed] [Google Scholar]
- 18.Singh AP, Chauhan SC, Bafna S, Johansson SL, Smith LM, Moniaux N, Lin MF, Batra SK. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66:421–429. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 19.Wu GJ, Peng Q, Fu P, Wang SW, Chiang CF, Dillehay DL, Wu MW. Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene. 2004;327:201–213. doi: 10.1016/j.gene.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Bélanger A, van Halbeek H, Graves HC, Grandbois K, Stamey TA, Huang L, Poppe I, Labrie F. Molecular mass and carbohydrate structure of prostate specific antigen: studies for establishment of an international PSA standard. Prostate. 1995;27:187–197. doi: 10.1002/pros.2990270403. [DOI] [PubMed] [Google Scholar]
- 21.Meany DL, Zhang Z, Sokoll LJ, Zhang H, Chan DW. Glycoproteomics for prostate cancer detection: changes in serum PSA glycosylation patterns. J Proteome Res. 2009;8:613–619. doi: 10.1021/pr8007539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohyama C, Hosono M, Nitta K, Oh-eda M, Yoshikawa K, Habuchi T, Arai Y, Fukuda M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amur-ensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology. 2004;14:671–679. doi: 10.1093/glycob/cwh071. [DOI] [PubMed] [Google Scholar]
- 23.St Hill CA, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, Walcheck B. The high affinity selectin glycan ligand C2-O-SLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Kat-suyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 25.Hanski C, Klussmann E, Wang J, Böhm C, Ogorek D, Hanski ML, Krüger-Krasagakes S, Eberle J, Schmitt-Gräff A, Riecken EO. Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue. Glycoconj J. 1996;13:727–733. doi: 10.1007/BF00702336. [DOI] [PubMed] [Google Scholar]
- 26.Ito H, Hiraiwa N, Sawada-Kasugai M, Akamatsu S, Tachikawa T, Kasai Y, Akiyama S, Ito K, Takagi H, Kannagi R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int J Cancer. 1997;71:556–564. doi: 10.1002/(sici)1097-0215(19970516)71:4<556::aid-ijc9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 28.Machida E, Nakayama J, Amano J, Fukuda M. Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 2001;61:2226–2231. [PubMed] [Google Scholar]
- 29.Hagisawa S, Ohyama C, Takahashi T, Endoh M, Moriya T, Nakayama J, Arai Y, Fukuda M. Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology. 2005;15:1016–1024. doi: 10.1093/glycob/cwi086. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela HF, Pace KE, Cabrera PV, White R, Porvari K, Kaija H, Vihko P, Baum LG. O-glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res. 2007;67:6155–6162. doi: 10.1158/0008-5472.CAN-05-4431. [DOI] [PubMed] [Google Scholar]
- 31.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 32.Brockhausen I. Comprehensive Natural Products II Chemistry and Biology. In: Mander L, Lui H-W, Wang PG, editors. Biosynthesis of complex mucin-type O-glycans. Carbohydrates, nucleosides and nucleic acids. Vol. 6. Elsevier; Oxford: 2010. pp. 315–350. Chapter 11. [Google Scholar]
- 33.Burchell J, Poulsom R, Hanby A, Whitehouse C, Cooper L, Clausen H, Miles D, Taylor-Papadimitriou J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology. 1999;9:1307–1311. doi: 10.1093/glycob/9.12.1307. [DOI] [PubMed] [Google Scholar]
- 34.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–366. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seko A, Ohkura T, Kitamura H, Yonezawa S, Sato E, Yamashita K. Quantitative differences in GlcNAc:beta1–>3 and GlcNAc:beta1–>4 galactosyltransferase activities between human colonic adenocarcinomas and normal colonic mucosa. Cancer Res. 1996;56:3468–3473. [PubMed] [Google Scholar]
- 36.Yang JM, Byrd JC, Siddiki BB, Chung YS, Okuno M, Sowa M, Kim YS, Matta KL, Brockhausen I. Alterations of O-glycan biosynthesis in human colon cancer tissues. Glycobiology. 1994;4:873–884. doi: 10.1093/glycob/4.6.873. [DOI] [PubMed] [Google Scholar]
- 37.Vavasseur F, Dole K, Yang J, Matta KL, Myerscough N, Corfield A, Paraskeva C, Brockhausen I. O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. Eur J Biochem. 1994;222:415–424. doi: 10.1111/j.1432-1033.1994.tb18880.x. [DOI] [PubMed] [Google Scholar]
- 38.Vavasseur F, Yang JM, Dole K, Paulsen H, Brockhausen I. Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology. 1995;5:351–357. doi: 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- 39.Brockhausen I, Romero P, Herscovics A. Glycosyltransferase changes upon differentiation of CaCo-2 human colonic adenocarcinoma Cells. Cancer Res. 1991;51:3136–3142. [PubMed] [Google Scholar]
- 40.Brockhausen I, Benn M, Bhat S, Marone S, Riley JG, Montoya-Peleaz P, Vlahakis JZ, Paulsen H, Schutzbach JS, Szarek WA. UDP-Gal: GlcNAc-R beta1,4-galactosyltransfer-ase–a target enzyme for drug design. Acceptor specificity and inhibition of the enzyme. Glycoconj J. 2006;23:525–541. doi: 10.1007/s10719-006-7153-x. [DOI] [PubMed] [Google Scholar]
- 41.Brockhausen I, Reck F, Kuhns W, Khan S, Matta KL, Meinjohanns E, Paulsen H, Shah RN, Baker MA, Schachter H. Substrate specificity and inhibition of UDP-GlcNAc:GlcNAc beta 1-2Man alpha 1-6R beta 1,6-N-acetylglucosaminyltransferase V using synthetic substrate analogues. Glycoconj J. 1995;12:371–379. doi: 10.1007/BF00731340. [DOI] [PubMed] [Google Scholar]
- 42.Brockhausen I, Dowler T, Paulsen H. Site directed processing: role of amino acid sequences and glycosylation of acceptor glycopeptides in the assembly of extended mucin type O-glycan core 2. Biochim Biophys Acta. 2009;1790:1244–1257. doi: 10.1016/j.bbagen.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Yip J, Harrison M, Brockhausen I. Primary human osteoblasts and bone cancer cells as models to study glycodynamics in bone. Int J Biochem Cell Biol. 2008;40:471–483. doi: 10.1016/j.biocel.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Brown JR, Fuster MM, Li R, Varki N, Glass CA, Esko JD. A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin Cancer Res. 2006;12:2894–2901. doi: 10.1158/1078-0432.CCR-05-2745. [DOI] [PubMed] [Google Scholar]
- 46.Brockhausen I, Williams D, Matta KL, Orr J, Schachter H. Mucin Synthesis III: UDP-GlcNAc:Galβ1-3(GlcNAcβ1-6)Gal-NAc-R (GlcNAc to Gal) β3-N-acetylglucosaminyltransferase, an enzyme in porcine gastric mucosa involved in the elongation of mucin-type oligosaccharides. Can J Biochem Cell Biol. 1983;61:1322–1333. doi: 10.1139/o83-169. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y, Lazar C, Szarek WA, Brockhausen I. Specificity of β4galactosyltransferase inhibitor 2-naphthyl 2-butanamido-2-de-oxy-1-thio-β-D-glucopyranoside. Glycoconj J. 2010;27:673–684. doi: 10.1007/s10719-010-9312-3. [DOI] [PubMed] [Google Scholar]
- 48.Huang J, Liang JT, Huang HC, Shen TL, Chen HY, Lin NY, Che MI, Lin WC, Huang MC. Beta1,4-N-acetylgalactosaminyltransferase III enhances malignant phenotypes of colon cancer cells. Mol Cancer Res. 2007;5:543–552. doi: 10.1158/1541-7786.MCR-06-0431. [DOI] [PubMed] [Google Scholar]
- 49.Yao M, Zhou DP, Jiang SM, Wang QH, Zhou XD, Tang ZY, Gu JX. Elevated activity of N-acetylglucosaminyltransferase V in human hepatocellular carcinoma. J Cancer Res Clin Oncol. 1998;124:27–30. doi: 10.1007/s004320050129. [DOI] [PubMed] [Google Scholar]
- 50.Tsui KH, Chang PL, Feng TH, Chung LC, Sung HC, Juang HH. Evaluating the function of matriptase and N-acetylglucosaminyltransferase V in prostate cancer metastasis. Anticancer Res. 2008;28:1993–1999. [PubMed] [Google Scholar]
- 51.Premaratne P, Wélen K, Damber JE, Hansson G, Bäckström M. O-glycosylation of MUC1 mucin in prostate cancer and the effects of its expression on tumor growth in a prostate cancer xenograft model. Tumor Biol. 2011;32:203–213. doi: 10.1007/s13277-010-0114-9. [DOI] [PubMed] [Google Scholar]
- 52.Pinho S, Marcos NT, Ferreira B, Carvalho AS, Oliveira MJ, Santos-Silva F, Harduin-Lepers A, Reis CA. Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007;249:157–170. doi: 10.1016/j.canlet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Lise M, Belluco C, Perera SP, Patel R, Thomas P, Ganguly A. Clinical correlations of alpha2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma. 2000;19:281–286. doi: 10.1089/027245700429828. [DOI] [PubMed] [Google Scholar]
- 54.Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, Clausen H, Burchell JM, Taylor-Papadimitriou J. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem. 2001;276:11007–11015. doi: 10.1074/jbc.M006523200. [DOI] [PubMed] [Google Scholar]
- 55.Hatakeyama S, Kyan A, Yamamoto H, Okamoto A, Sugiyama N, Suzuki Y, Yoneyama T, Hashimoto Y, Koie T, Yamada S, Saito H, Arai Y, Fukuda M, Ohyama C. Core 2 N-acetylglucosaminyltransferase-1 expression induces aggressive potential of testicular germ cell tumor. Int J Cancer. 2010;127:1052–1059. doi: 10.1002/ijc.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci U S A. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SH, Hatakeyama S, Yu SY, Bao X, Ohyama C, Khoo KH, Fukuda MN, Fukuda M. Core 3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J Biol Chem. 2009;284:17157–17169. doi: 10.1074/jbc.M109.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;240:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur J Biochem. 1995;233:607–617. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- 60.Cazet A, Julien S, Bobowski M, Krzewinski-Recchi MA, Harduin-Lepers A, Groux-Degroote S, Delannoy P. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr Res. 2010;345:1377–1383. doi: 10.1016/j.carres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 61.Mungul A, Cooper L, Brockhausen I, Ryder K, Mandel U, Clausen H, Rughetti A, Miles DW, Taylor-Papadimitriou J, Burchell JM. Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int J Oncol. 2004;25:937–943. [PubMed] [Google Scholar]
- 62.Picco G, Julien S, Brockhausen I, Beatson R, Antonopoulos A, Haslam S, Mandel U, Dell A, Pinder S, Taylor-Papadimitriou J, Burchell J. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 2010;20:1241–1250. doi: 10.1093/glycob/cwq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.