Abstract
A major thrust in type 1 diabetes research is stopping the destruction of β cells that leads to type 1 diabetes. Research over the past thirty years has defined genetic factors and evidence of autoimmunity that have led to the development of robust prediction models in those at high risk of type 1 diabetes. The ability to identify those at risk and the development of new agents and of collaborative research networks has led to multiple trials aimed at preventing β cell loss. Trials at all stages of beta cell loss have been conducted: primary prevention - prior to the development of autoimmunity, secondary prevention – after autoantibodies are found, and tertiary prevention – intervening after diagnosis to maintain remaining β cells. Studies have shown mixed results with evidence of maintained insulin secretion after the time of diagnosis described in a number of studies and primary and secondary prevention proving to be elusive. Much has been learned from the increasing number of studies in the field in terms of network creation, study design and choice of intervention that will facilitate new avenues of investigation.
Keywords: Type 1 diabetes, autoimmunity, prevention, immunology, genetics, clinical trials
Introduction
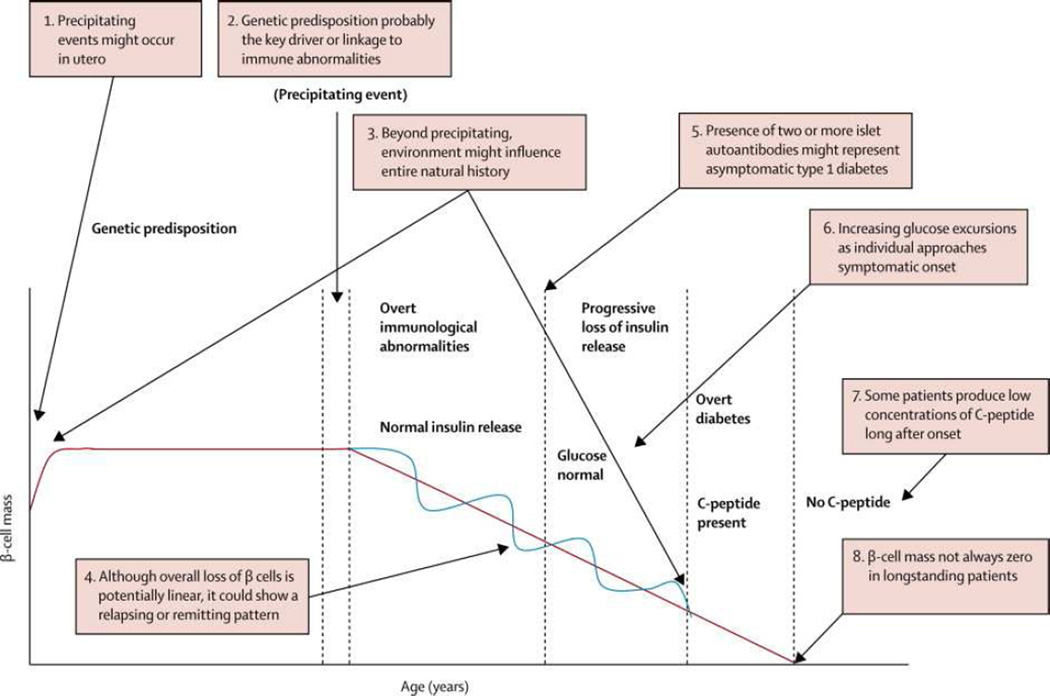
The number of studies aimed at prevention of beta cell loss prior to or soon after the development of type 1 diabetes has accelerated in recent years. Evidence developed over the past 30 years shows that type 1 diabetes is the result of a chronic autoimmune process that leads to beta cell destruction and insulin dependence. Eisenbarth initially described the main features and stages in the pathogenesis of type 1 diabetes in 1986 and he and colleagues updated this model in 2014 (Figure 1) (1, 2).
Genetic susceptibility is a critical factor in initiation of autoimmunity with the majority of risk being found in the HLA class II region, with smaller effects of many other genes (see below).
Precipitating events such as exposure to environmental factors are then thought to initiate β cell destruction. Despite intensive searches for environmental triggers, strongly conclusive evidence for any particular factor remains to be identified.
Autoimmunity directed at β cells is then found. The autoimmune attack is likely facilitated by immune dysregulation which may be due to genetic susceptibility factors. The immune response is thought to be mediated by T lymphocytes, but the most easily detected finding is the presence of islet autoantibodies.
With progressive loss of beta cells, the first metabolic abnormality, reduced insulin secretion in response to a glucose challenge is found (3). Early glucose abnormalities such as impaired glucose tolerance during an oral glucose tolerance test then occur.
Ongoing loss of insulin secretion eventually leads to overt symptoms of diabetes and the diagnosis is made. In most patients, a honeymoon phase with improved insulin secretion follows. Beta cell loss continues so that many individuals lose all insulin secretion but recent evidence suggests that small amounts of insulin secretion may remain many years after diagnosis (4).
Figure 1.
The Natural History of Type 1 Diabetes
Reprinted from The Lancet, 383, Mark A. Atkinson, George S Eisenbarth, Aaron W Michels, Type 1 Diabetes, 69–82., Copyright 2014, with permission from Elsevier.
Interventions targeting the loss of beta cells at all stages of the process have been carried out. These studies depend on the understanding of the epidemiology, genetics and prediction of type 1 diabetes.
Epidemiology
The incidence of type 1 diabetes varies widely on a global level. The highest reported incidence is in Finland at 57.6 new cases per 100,000 population per year in those 0 to 14 years of age. Other European countries with high incidence are Sweden, Norway and United Kingdom, all at > 25 new cases per 100,000 per year. Outside Europe, the countries with large populations of those of European background with the highest incidence are Canada (25.9/100,000/yr) and Australia (22.5/100, 000 per year). Of the non-European ancestry countries, Saudi Arabia and Kuwait have the highest incidence at 31.4 and 22.3 respectively. Most African, Asian and South American countries have lower incidence at less than 8.5 cases/100,00 per year (5). The overall incidence of diabetes is rising at approximately 3% per year, particularly in those with a lower genetic susceptibility, indicating that the role of susceptibility genes is complex and changing (6, 7).
Genetics of Type 1 Diabetes
T1D is approximately 15 times more common in family members of those with T1D, with the general population prevalence of approximately 0.3% and the family prevalence of approximately 6%, making family members the logical target population for studies of interventions to prevent diabetes (8, 9). More than 40 genetic loci have been associated with T1D with the HLA region accounting for approximately 50% of genetic risk (10). Haplotypes most strongly associated with T1D include: DRB1*03:01-DQA1*05:01-DQB1*02:01 (DR3) and DRB1*04:01/02/04/05/08-DQA1*03:01-DQB1*03:02/04 (DR4) (11). The DRB1*15:01-DQA1*01:02-DQB1*06:02 (DR2) haplotype is dominantly and almost completely protective for T1D, (11). Of the other associated regions, a region in the regulatory region of the insulin gene, the PTPN22 (protein tyrosine phosphatase non-receptor type 22) gene, and the interleukin 2 receptor α (IL2RA) have the greatest contribution (10). The majority of the genetic loci identified have a function in the immune system, providing new understanding of disease pathogenesis and potential targets for intervention.
Environmental Factors
Identification of highly specific environmental factors critical to the development of T1D remains a challenge. The lack of complete concordance in monozygotic twins has provided evidence that environmental factors play a significant role. Multiple factors such as viral and bacterial infections (or lack thereof), food exposures, increasing obesity rates, psychosocial stress and the gut microbiome have been implicated. The effort to better identify environmental factors is currently being led by the Environmental Determinants of Diabetes in the Young (TEDDY) Study. This study screened 421,000 infants and enrolled 8,600 infants with high risk HLA genotypes for serial assessment of islet autoimmunity and environmental exposures such as diet, infectious diseases and immunizations (12). A recent TEDDY publication reported that there was no difference in the rate of the detection of viruses prior to the development of islet antibodies in those who progressed to the development of diabetes within 6 months (13).
Targets for prevention or early intervention
Prevention of T1D requires interventions aimed at avoiding or altering exposure to environmental trigger(s) prior to the development of autoimmunity – primary prevention; interfering with the autoimmune process that causes β cell destruction – secondary prevention; or halting or reversing β cell loss after clinical presentation of T1D – tertiary prevention.
PRIMARY PREVENTION
Primary prevention studies are targeted at those who do not show any evidence of autoimmunity, in that no islet autoantibodies are present. Thus far, all studies have been done in newborns at high risk of diabetes, those with a first degree relative with diabetes and high risk HLA haplotypes, and have involved a dietary intervention or supplement.
TRIGR (Trial to Reduce IDDM in the Genetically at Risk) is testing the hypothesis that a hydrolyzed diet that avoids early cow’s milk protein exposure will protect high risk newborns from initiation of the β cell autoimmunity, as evidenced by the development of islet autoantibodies and prevent T1D (14). It is based on studies showing that the early introduction of cow’s milk may be a risk factor for T1D (15). Infants were randomly assigned to feeding up to 6–8 months of age with a regular cow’s milk-based formula or a casein hydrolysate formula. Breast feeding was encouraged and accounted for in the study design. The major outcomes are the frequency of T1D-associated autoantibodies and/or development of diabetes by age 6 years and the development of diabetes by age 10 years. Screening for TRIGR began in 2002, with final enrollment completed in 2006. Thus, the 6 year outcome will be available shortly and the final outcomes in 2017. The Finnish TRIGR pilot study reported a reduction in the development of islet autoantibodies in the infants randomized to the hydrolyzed formula (16). Another similar trial that assessed the impact of complete avoidance of bovine insulin in infants was conducted in the Finland, FINDIA. It randomized high risk infants to cow’s milk formula, whey-based hydrolyzed formula, or whey-based formula free of bovine insulin during the first 6 months of life whenever breast milk was not available. A reduced rate of development of one islet autoantibody was seen in the bovine insulin free group (17).
A double-blind placebo-controlled pilot study of omega 3 fatty acid supplementation with Docosahexaenoic acid (DHA) to prevent islet autoimmunity was carried out by the Type 1 Diabetes TrialNet study group (18). Diets higher in omega-3 fatty acids have been associated with lower risk of islet autoimmunity and diabetes (19). DHA has an anti-inflammatory effect. Entry to the study was during the third trimester in pregnant mothers or during the first 5 months of life in infants, both with a first-degree relative with T1D. At birth, HLA typing was done on cord blood and those with high risk alleles continued DHA supplementation or newly identified infants were randomized. The pilot study was designed to assess feasibility and effects on inflammatory cytokines Thus far, no effect on autoimmunity has been seen.
A feasibility study, BABYDIET, of delay of introduction of gluten to prevent islet autoimmunity in infants with a first degree relative with T1D and high risk HLA genotypes was conducted in Germany. The timing of introduction of cereals to infants has been associated with diabetes (20). Infants were randomized to introduction of gluten at 6 or 12 months with follow-up every 3 months to 36 months of age. Results showed no difference in islet autoantibodies or the development of diabetes (21).
SECONDARY PREVENTION
The goal of secondary prevention studies is to prevent the progression from islet autoimmunity to overt T1D. Robust prediction models are required to conduct these studies to allow identification of those with clear evidence of islet autoimmunity and to design studies with a clear understanding of the underlying risk of diabetes in the selected population. These models use islet autoantibodies and measures of glucose tolerance to stratify risk. Autoantibodies typically develop years before onset of diabetes. These antibodies include islet cell antibodies (ICA), insulin autoantibodies (IAA), and antibodies to glutamic acid decarboxylase (GAD), tyrosine phosphatase (IA-2/ICA512) and zinc transporter 8 (ZnT8) (22). The presence of two or more antibodies indicates a significantly increased risk of developing diabetes. A recent publication pooling data from 3 longitudinal studies following those at high risk from birth, DAISY, DIPP and BABYDIAB/BABYDIET, showed that the 10 year risk of diabetes was 70% in those with multiple autoantibodies, 14.5% in those with one autoantibody and 0.4% in those with no autoantibodies (23). Other factors such as age, BMI, and glucose and insulin responses to oral glucose tolerance tests have been utilized to develop a risk score that can further define those at increased risk (24). Type 1 Diabetes TrialNet, an international study group carrying out studies of the prevention and early treatment of type 1 diabetes, is conducting a large longitudinal observational study of relatives of those with type 1 diabetes to further refine prediction models and identify those at increased risk (25).
Three large multi-centre trials of diabetes prevention in autoantibody positive relatives have been completed. The European Nicotinamide Diabetes Intervention Trial (ENDIT), studied nicotinamide in ICA-positive relatives. The study was based on findings from animal models showing that treatment could prevent diabetes, in vitro studies that showed protection from beta cell death and a pilot study showing positve results (26). ENDIT found no difference in the development of T1D in those treated with nicotinamide compared to placebo (27). In the Diabetes Prevention Trial-Type 1 (DPT-1), insulin was given either orally or parenterally to alter the immune response toward insulin and reduce islet destruction (28, 29). Oral insulin had been found to be effective in prevention of diabetes in young NOD mice (30). Parenteral insulin similarly was found to reduce diabetes in the NOD model and a pilot study in high risk individuals had shown promising results (31, 32). Subjects at high risk (greater than 50% risk of developing diabetes over 5 years due to the presence of ICA and low first phase insulin response) received parenteral insulin. Those with a lower risk (25 to 50% over 5 years with ICA and IAA but normal first phase insulin secretion) received daily oral insulin. Parenteral insulin did not show an effect on the development of T1D (29). The analysis of the primary endpoint of oral insulin treatment did not show any benefit but post hoc analysis suggested a beneficial effect in the subgroup with high titers of insulin autoantibodies (28). In the Type 1 Diabetes Prediction and Prevention Study (DIPP), newborns from the general population and siblings of those with diabetes had HLA genotyping done on cord blood and those high risk HLA alleles were followed prospectively (33). Those who developed two or more islet antibodies were treated with nasal insulin or placebo. The study was stopped as the treatment had no effect. The lack of benefit seen in these studies is disappointing, but the feasibility of large scale prevention studies has been proven and significant knowledge and experience to guide future studies has been gained.
There are currently a number of secondary diabetes prevention trials underway. The Type 1 Diabetes TrialNet study, “Oral Insulin for Prevention of Diabetes in Relatives at Risk for Type 1 Diabetes Mellitus” is further investigating the post hoc finding of benefit of oral insulin in the DPT-1 subjects with high IAA titers. Relatives with normal glucose tolerance, insulin autoantibodies and one of ICA, GAD, ICA512 or ZnT8 antibodies are randomized to oral insulin or placebo, with an endpoint of the development of diabetes. Recruitment began in 2007 and is ongoing. TrialNet is also studying Abatacept, (CTLA-4 Ig) in relatives with two or more islet autoantibodies and normal glucose tolerance. This study’s primary endpoint is the development of impaired glucose tolerance. This design will allow earlier assessment of the impact of the therapy on the loss of insulin production. It is based on the finding that abatacept delays the loss of insulin secretion in recent onset diabetes and the drug’s effectiveness in treating another autoimmune disease, rheumatoid arthritis (34). TrialNet’s third prevention study is assessing the impact of teplizumab, (modified anti-CD3) on progression to diabetes in relatives with 2 or more islet antibodies and abnormal glucose tolerance. Teplizumab has been studied in recent onset diabetes in a number of studies and has been shown to preserve insulin secretion as described below (35).
The Pre-POINT trial is an international multicentre study that is examining intervention with oral insulin in children age 18 months to 7 years who have a sibling or two or more relatives with T1D and high risk HLA alleles to examine the impact on the prevention of the development of islet antibodies (36). Recruitment was completed in 2013 with results anticipated shortly. The Intranasal Insulin Trial (INIT) is based in Australia and New Zealand and is assessing the effect of intranasal insulin in first and second degree relatives ages 4 to 30 years who are at increased risk of diabetes based on data from an earlier pilot study and on positive findings in the NOD mouse (37). Randomization was completed recently and results are anticipated in 2 years. The Swedish DiAPREV-IT trial has randomized 50 children with GAD Ab and one additional islet antibody to 2 injections of GAD formulated in alum or placebo (38). Treatment with the diabetes antigen, GAD, in the NOD model has been shown to prevent diabetes (39). Follow up will continue for 5 years with results expected in 2015.
TERTIARY PREVENTION
After the diagnosis of diabetes and initiation of insulin treatment, many patients have some recovery of insulin secretion and relatively easily controlled blood glucose levels. Prolongation of the honeymoon period has the potential to have significant beneficial effects. In the Diabetes Control and Complications Trial, those subjects with sustained C-peptide production were found to have rates of nephropathy, retinopathy and hypoglycemia that were half that found in those without any residual insulin (40). Measurement of C-peptide production in response to a stimulus such as a mixed meal allows an assessment of residual insulin secretion and can be used as a marker of the efficacy of interventions (41). Studies in recent onset diabetes are typically initiated within 6 to 12 weeks of diagnosis and follow subjects to assess the decline in C-peptide production over 1 to 2 years.
Several drugs, including, cyclosporine, azathioprine and prednisone, were studied in the 1980’s for their ability to induce a remission after the diagnosis of diabetes (42, 43). Despite some positive findings, side effects limited further use of these agents. Many new humanized immunomodulatory antibodies have been developed in recent years, both in the diabetes arena and in other autoimmune conditions.
Anti-CD3 Therapies
The humanized monoclonal antibody hOKT3γ1 (Ala-Ala), known as teplizumab, interferes with T cell activation by binding the T cell receptor, CD3 (44). It has been extensively studied in NOD mice and has been one of the only agents shown to reverse diabetes in this model (44). Herold et al showed that this modified anti-CD3 antibody slowed the loss of C-peptide production over two years after one course of administration in newly diagnosed patients within 6 weeks of diagnosis with a reduction in HbA1c and lower insulin doses (45, 46). A European multicenter trial showed that a single course of a very similar modified anti-CD3 antibody, ChAglyCD3, otelixizumab, in newly diagnosed patients resulted in higher C-peptide production and reduced insulin doses for 18 months following treatment when compared to placebo (47). These results were most pronounced among patients with C-peptide production at or above 50th percentile. Another study of teplizumab in new onset subjects between 3 and 30 years of age within 8 weeks of diagnosis gave 2 doses, one at the initiation of the study and a second dose one year later. Results showed that C-peptide production was 75% higher in the treated group at 2 years (48). In addition, the manufacturers of both of these modified CD3 molecules carried out large international trials of these agents, the DEFEND and Protégé studies, in recent onset patients. The DEFEND study of otelixizumab did not show any benefit, likely due to the significantly lower dose used than in the earlier studies (49). The Protégé study did not meet its primary endpoint of HbA1c <6.5% and insulin dose <0.5units/kg, but showed improved C-peptide production in those treated with the largest dose (50).
Immunoactive Antibodies
The TrialNet study group assessed the role of mycophenolate mofetil (MMF) and daclizumab (DZB) in maintaining C-peptide production. Both MMF and DZB have been shown to be effective in transplantation regimens. Daclizumab targets CD25, the alpha subunit of the interleukin -2 receptor, resulting in reduced activation of T lymphocytes. This study was stopped early when analysis of C-peptide production at one year revealed no difference between the groups (51). TrialNet also investigated the use of the anti-B lymphocyte monoclonal antibody, rituximab, in preserving C-peptide production. Rituximab depletes mature B cells thereby reducing antigen presentation to T cells. Study results showed a slower loss of C-peptide with lower insulin doses and lower HbA1c in the treated group (52). CTLA-4, a costimulatory molecule expressed on T cells is an important negative regulator of T cell activation. A 2 year TrialNet study of CTLA-4 Ig (Abatacept) in recent onset diabetes showed improved C-peptide at 2 years (34). Interleukin 1β is a pro-inflammatory cytokine and has been implicated in the pathogenesis of T1D. Two studies in new onset diabetes, one using the anti-IL1β antibody, canakinumab, and the other using the IL1 receptor antagonist, anakinra, did not alter the course of C-peptide decline (53). A small study of alefacept, a drug that blocks the interaction between CD2 and CD58 that is required for T lymphocyte costimulation, given in two 12 week courses, did not show a difference in C-peptide loss at 12 months (54). Surprisingly, treatment with anti-thymocyte globulin (ATG), a potent T lymphocyte depleting antibody, over 4 days did not show any effect on C-peptide loss at 1 year post treatment (55).
Antigen-Based Treatment
The concept of altering autoimmunity by treatment with a target antigen has existed for many years. Glutamic acid decarboxylase (GAD) is an important autoantigen in type 1 diabetes. An early small study in recent onset children showed a slowed the fall of C-peptide production over the first 30 months after initiation of therapy (56). Two larger studies, one by TrialNet and one international study did not find any difference in C-peptide loss at 12 to 15 months (57, 58).
It is clear that highly effective prevention of β cell loss remains an elusive target. Given that safety is primary in all interventions in this area, the intensity of immunotherapy used in other autoimmune conditions is not appropriate. Therapies aimed at the specific immune response to the β cells represent an ideal option but translation of the fundamental science in this area to clinical trials is challenging. Lessons learned from studies conducted thus far point to the ongoing need for appropriately powered studies, the maintenance of clinical trial networks that can rapidly carry out these studies and facilitate collaboration between fundamental and clinical scientists, and the need for more robust understanding and assessment of the ongoing immune response and the effect of therapies on it. Much has been learned over the past 10 years with the significant increase in clinical trials in this area but much more remains to be done.
Table 1.
Studies with Active Enrollment
| Study | Study Population | Primary Endpoint | Study Sites |
|---|---|---|---|
| Natural History Study of the Development of Type 1 Diabetes Sponsor: TrialNet Study Group - |
|
Development of diabetes | TrialNet sites in Canada, US, UK, Italy, Australia and New Zealand |
| Oral Insulin For Prevention of Diabetes In Relatives at Risk for Type 1 Diabetes Mellitus Sponsor: TrialNet Study Group – |
|
Development of diabetes | TrialNet sites in Canada, US, UK, Italy, Australia and New Zealand |
| AntiCD3 mAb (Teplizumab) For Prevention of Diabetes in Relatives At-Risk for Type 1 Diabetes Mellitus Sponsor: TrialNet Study Group |
|
Development of diabetes | TrialNet sites in US, Canada (pending approval) |
| CTLA-4 Ig (Abatacept) for prevention of abnormal glucose tolerance (AGT) and diabetes in relatives at-risk for Type 1 diabetes mellitus |
|
Development of impaired glucose tolerance | TrialNet sites in Canada, US |
| Imatinib Treatment in Recent Onset Type 1 Diabetes Mellitus Sponsor : Juvenile Diabetes Research Foundation |
|
Preservation of C-peptide at 1 year | 5 US sites |
| DIABGAD - Trial to Preserve Insulin Secretion in Type 1 Diabetes Using GAD-Alum (Diamyd) in Combination With Vitamin D and Ibuprofen |
|
Preservation of C-peptide up to 30 months | Sweden |
| A Trial of High Dose Immunosuppression and Autologous Hematopoietic Stem Cell Support Versus Intensive Insulin Therapy in Adults With Early Onset Type I Diabetes Mellitus |
|
Preservation of C-peptide up to 5 years | Chicago |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014 Jan 4;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sosenko JM, Palmer JP, Rafkin LE, et al. Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care. 2010 Mar;33(3):620–625. doi: 10.2337/dc09-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic s-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010 Nov;59(11):2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. IDF Diabetes Atlas. http://www.idf.org/diabetesatlas. [PubMed] [Google Scholar]
- 6.Patterson CC, Dahlquist GG, Gyurus E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009 Jun 13;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 7.Steck AK, Armstrong TK, Babu SR, et al. Stepwise or linear decrease in penetrance of type 1 diabetes with lower-risk HLA genotypes over the past 40 years. Diabetes. 2011 Mar;60(3):1045–1049. doi: 10.2337/db10-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifacio E, Ziegler AG. Advances in the prediction and natural history of type 1 diabetes. Endocrinol Metab Clin North Am. 2010 Sep;39(3):513–525. doi: 10.1016/j.ecl.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia. 2009 Sep;52(9):1820–1828. doi: 10.1007/s00125-009-1427-3. [DOI] [PubMed] [Google Scholar]
- 10.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009 Jun;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble JA, Valdes AM, Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011 Dec;11(6):533–542. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagopian WA, Erlich H, Lernmark A, et al. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011 Dec;12(8):733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HS, Briese T, Winkler C, et al. Next-generation sequencing for viruses in children with rapid-onset type 1 diabetes. Diabetologia. 2013 Aug;56(8):1705–1711. doi: 10.1007/s00125-013-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.TRIGR Study Group. Akerblom HK, Krischer J, et al. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow-up. Diabetologia. 2011 Mar;54(3):627–633. doi: 10.1007/s00125-010-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virtanen SM, Laara E, Hypponen E, et al. Cow's milk consumption, HLA-DQB1 genotype, and type 1 diabetes: a nested case-control study of siblings of children with diabetes. Childhood diabetes in Finland study group. Diabetes. 2000 Jun;49(6):912–917. doi: 10.2337/diabetes.49.6.912. [DOI] [PubMed] [Google Scholar]
- 16.Knip M, Virtanen SM, Seppa K, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med. 2010 Nov 11;363(20):1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaarala OMDD, Ilonen JMDD, Ruohtula TMA, et al. Removal of Bovine Insulin From Cow's Milk Formula and Early Initiation of Beta-Cell Autoimmunity in the FINDIA Pilot Study. Archives of Pediatrics & Adolescent Medicine. 2012;166(7):608–614. doi: 10.1001/archpediatrics.2011.1559. [DOI] [PubMed] [Google Scholar]
- 18.Chase HP, Lescheck E, Rafkin-Mervis L, et al. Nutritional Intervention to Prevent (NIP) Type 1 Diabetes A Pilot Trial. ICAN: Infant, Child, & Adolescent Nutrition. 2009 Apr 1;1(2):98–107. 2009. [Google Scholar]
- 19.Norris JM, Yin X, Lamb MM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007 Sep 26;298(12):1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 20.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003 Oct 1;290(13):1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 21.Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011 Jun;34(6):1301–1305. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007 Oct 23;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013 Jun 19;309(23):2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosenko JM, Skyler JS, Mahon J, et al. Validation of the Diabetes Prevention Trial-Type 1 Risk Score in the TrialNet Natural History Study. Diabetes Care. 2011 Aug;34(8):1785–1787. doi: 10.2337/dc11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009 Apr;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 26.Gale EA. Theory and practice of nicotinamide trials in pre-type 1 diabetes. Journal of Pediatric Endocrinology. 1996 May-Jun;9(3):375–379. doi: 10.1515/jpem.1996.9.3.375. [DOI] [PubMed] [Google Scholar]
- 27.Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004 Mar 20;363(9413):925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 28.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial--Type 1. Diabetes Care. 2005 May;28(5):1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Trial--Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002 May 30;346(22):1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(22):10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkinson MA, Maclaren NK, Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990 Aug;39(8):933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- 32.Keller RJ, Eisenbarth GS, Jackson RA. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet. 1993;341(8850):927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- 33.Nanto-Salonen K, Kupila A, Simell S, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008 Nov 15;372(9651):1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 34.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Jul 30;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daifotis AG, Koenig S, Chatenoud L, et al. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clinical Immunology. 2013 Dec;149(3):268–278. doi: 10.1016/j.clim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Achenbach P, Barker J, Bonifacio E, et al. Modulating the natural history of type 1 diabetes in children at high genetic risk by mucosal insulin immunization. Curr Diab Rep. 2008 Apr;8(2):87–93. doi: 10.1007/s11892-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 37.Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004 Oct;27(10):2348–2355. doi: 10.2337/diacare.27.10.2348. [DOI] [PubMed] [Google Scholar]
- 38.Larsson HE, Lernmark A, Larsson HE, Lernmark A. Does immune-tolerance treatment with Alum-formulated GAD65 protect insulin-production in the pancreatic islet beta cells? Hum. 2011 Jan 1;7(1):45–49. doi: 10.4161/hv.7.1.14488. [DOI] [PubMed] [Google Scholar]
- 39.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 40.Steffes MW, Sibley S, Jackson M, et al. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003 Mar;26(3):832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 41.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004 Jan;53(1):250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 42.Feutren G, Papoz L, Assan R, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986 Jul 19;2(8499):119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- 43.Silverstein J, Maclaren N, Riley W, Spillar R, Radjenovic D, Johnson S. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N Engl J Med. 1988 Sep 8;319(10):599–604. doi: 10.1056/NEJM198809083191002. [DOI] [PubMed] [Google Scholar]
- 44.Chatenoud L, Bluestone JA, Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nature Reviews Immunology. 2007 Aug;7(8):622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 45.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005 Jun;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002 May 30;346(22):1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 47.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005 Jun 23;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 48.Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013 Nov;62(11):3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clinical Immunology. 2013 Dec;149(3):268–278. doi: 10.1016/j.clim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011 Aug 6;378(9790):487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care. 2010 Apr;33(4):826–832. doi: 10.2337/dc09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009 Nov 26;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran A, Bundy B, Becker DJ, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013 Jun 1;381(9881):1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. The Lancet Diabetes & Endocrinology. 2013;1(4):284–294. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gitelman SE, Gottlieb PA, Rigby MR, et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. The Lancet Diabetes & Endocrinology. 2013;1(4):306–316. doi: 10.1016/S2213-8587(13)70065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ludvigsson J, Faresjo M, Hjorth M, et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med. 2008 Oct 30;359(18):1909–1920. doi: 10.1056/NEJMoa0804328. [DOI] [PubMed] [Google Scholar]
- 57.Ludvigsson J, Krisky D, Casas R, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012 Feb 2;366(5):433–442. doi: 10.1056/NEJMoa1107096. [DOI] [PubMed] [Google Scholar]
- 58.Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. The Lancet. 2011 Jul 29;378(9788):319–327. doi: 10.1016/S0140-6736(11)60895-7. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]