Abstract
Dysregulated growth and motility of vascular smooth muscle cells (VSMC) play important role in obstructive vascular diseases. We previously reported that gene transfer of thymidine phosphorylase (TP) into rat VSMC inhibits cell proliferation and attenuates balloon injury induced neointimal hyperplasia; however, the mechanism remains unclear. The current study identified a signaling pathway that mediates effect of TP inhibited VSMC proliferation with a TP activity-dependent manner. Rat VSMC overexpressing human TP gene (C2) or control empty vector (PC) were used. Serum stimulation induced constitutive STAT3 phosphorylation at tyrosine705 in C2 cell but not in PC, which was independent of JAK2 signaling pathway. Inhibition of Src family kinases activity inhibited STAT3 phosphorylation in C2 cells. Lyn activity was higher in C2 cell than in PC. SiRNA based gene knockdown of Lyn significantly decreased serum induced STAT3 phosphorylation in C2 and dramatically increased proliferation of this cell, suggesting that Lyn plays a pivotal role in TP inhibited VSMC proliferation. Unphosphorylated STAT3 (U-STAT3) expression was significantly increased in C2 cells, which may be due to the increased STAT3 transcription. Gene transfection of mouse wild type or Y705F mutant STAT3 into PC cell or mouse primary cultured VSMC significantly reduced proliferation of these cells, suggesting that overexpression of U-STAT3 inhibits VSMC proliferation. We conclude that Lyn mediates TP induced STAT3 activation, which subsequently contributes to upregulate expression of U-STAT3. The U-STAT3 plays a critical role in inhibiting VSMC proliferation.
Keywords: Thymidine phosphorylase, vascular smooth muscle cell proliferation, STAT3
1. Introduction
Dysregulated growth and motility of vascular smooth muscle cells (VSMCs) contribute to neointimal lesion development during the pathogenesis of obstructive vascular diseases, such as atherosclerosis, restenosis after percutaneous transluminal coronary angioplasty, stent graft implantation, and vascular graft failure after coronary artery bypass graft surgery. Therefore, targeting VSMCs to prevent proliferation and migration may be sufficient to produce clinically significant therapeutic effects. Various therapeutic strategies including gene therapies have been employed to decrease VSMCs proliferation and reported to decrease intimal thickening in experimental restenosis [1–3], however, optimal and promising gene therapy strategies are still under investigation.
Thymidine phosphorylase (TP), also known as, platelet-derived endothelial cell growth factor, catalyzes the reversible conversion of thymidine to thymine and 2-deoxy-D-ribose-1-phosphate, and plays a role in maintaining the nuclear pool of these molecules [4]. TP has been widely studied in cancer biology as a proangiogenic factor, however, whether and how it affects the cardiovascular system is still not known. We previously demonstrated that direct injection of a plasmid vector encoding human TP cDNA into ischemic myocardium or hindlimb promotes angiogenesis, arteriogenesis and inhibits apoptosis [5–7]. We further found that gene transfer of TP into VSMCs inhibits proliferation in vitro, and adventitial gene transfer of human TP gene markedly decreased neointima hyperplasia in both balloon injured rat carotid arteries and rabbit venous bypass graft [8, 9]. The mechanism underlying this phenotype is partly related to TP mediated upregulation of heme oxygenase 1 (HO-1) and p27Kip1 expression [8, 9]. Whether this effect is mediated by TP affected intracellular signaling pathway is not clear.
The janus-activated kinase (JAK)/signal transducers and activators of transcription (STAT) pathway is well known to be involved in growth factor or cytokine induced VSMC proliferation and migration. There are 7 members in the STAT family. All of which have been reported to be expressed in VSMCs, and with the exception of STAT2, all have been reported to show increased expression in the balloon injured carotid artery wall [10–14]. A recent study using siRNA showed that STAT1 is necessary for VSMC proliferation [13], and Fludarabine, a chemotherapy drug, significantly abolished VSMC proliferation in vitro and reduced neointimal formation after balloon injury in vivo associated with inhibition of STAT-1 activation [13]. However, other studies also indicated that activation of STAT1 is essential for its inhibitory effect on VSMC proliferation [15, 16]. Interestingly, Bai et al. reported that interferon gamma induced pro-apoptotic molecules XAF1 (X-linked inhibitor of apoptosis associated factor-1) and Noxa in human VSMCs through STAT3 activation and that these proteins sensitized VSMCs in vitro and in vivo to apoptosis triggered via death receptor or mitochondrial mechanisms [17]. These data suggest that STAT proteins may have dual-role on regulating VSMC proliferation and function.
In this manuscript, we show that TP upregulates STAT3 gene expression via up-regulating STAT3 activation in VSMC through the Src family kinase (SFK), Lyn. We found that TP induced expression of STAT3 protein, but not tyrosine phosphorylation contributes to its inhibitory effect on VSMC proliferation. We conclude that regulating TP expression or activity may be a novel strategy in treating ischemic cardiovascular diseases based on its proangiogenic and VSMC inhibitory effects.
2. Materials and methods
2.1. Vascular smooth muscle cell cultures
VSMCs were cultured from aortic arteries of male Sprague-Dawley rats or C57BL6 mice using explant methods as mentioned before [5, 8, 18, 19]. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 100 u/ml penicillin and 100 mg/ml streptomycin. All cells showed “valley and hill” growth pattern when they were confluent and were positively stained for alpha-smooth muscle actin (Sigma), a characteristic marker of VSMC (data not shown). The cells were incubated at 37°C in a 5% CO2 humidified atmosphere and maintained at a sub-confluent stage by subcultured with 0.025% trypsin/1 mmol/L EDTA (Gibco, Carlsbad, CA). Wild-type rat and mouse VSMCs were used from passages 3 to 12. TP overexpressing cell clone number 2 (C2) as well as empty vector-transfected control VSMC cells (PC) has been established as described previously [8], which possess the characters of VSMC as mentioned above.
The use of animals was in compliance with the Guidelines of the Institutional Animal Care and Use Committee of the Faculty of Medical Sciences, University of Fukui, and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All studies have been approved by the ethics committee of University of Fukui.
2.2. Gene transfection and knockdown
Phagemid vector pBK-RSV encoding human TP as well as K115E (Lys-115-->Glu), L148R (Leu-148-->Arg) and R202S (Arg-202-->Ser) mutant TP cDNA [20] were reversely transfected into 105 mouse VSMC in 6-well plate using Lipofectamine LTX Plus Regent (Invitrogen). The cells were cultured for 72 hrs and then cell proliferation was assessed by cell counting. Mouse wild-type (wt) stat3 and tyrosine 705 to phenylalanine mutant stat3 (Y705F-stat3) were cloned into pcDNA6/Myc-His B as mentioned before [21]. These plasmid vectors were transfected into PC cells using Lipofectamine LTX Plus Regent and cell clones was generated by selection with Blasticidin (10 µg/ml). To further determine the function of STAT3 on VSMC, primary cultured mouse VSMC cells (passages 3) were seeded at a concentration 1.5 × 105/well in 6-well plate, and cultured overnight. The wt-stat3 and Y705F-stat3 as well as empty plasmid vector pCDNA5/Myc-His B were then transfected into the cells using Lipofectamine LTX Plus Regent and cell numbers were assessed 72 hrs later. The parent VSMCs were used as control.
Pre-designed siRNA targeting rat Lyn gene was purchased from Qiagen (Valencia, CA); and Silencer™ siRNA Transfection II Kit was purchased from Applied Biosystems. A final concentration of 30 nmol/L siRNA was transfected into 106 C2 cells in 10-cm plate using a reverse transfection method based on the instruction of the manufacturer. The cells were allowed to grow for 24 hrs, serum starved for 24 hrs, and then re-stimulated with 10% FCS for another 24 hrs. The cells were washed with cold PBS and then harvested for Western blot assay. In some experiments, Lyn siRNA was transfected into C2 cell in 24-well plate, cultured for 3 days and cell proliferation was assessed by 3-[4,5- dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide (MTT; Sigma Chemical, St. Louis, MO) assay.
2.3. Cell proliferation and survival assays
The cell proliferation and survival was assessed by MTT as previously described and elsewhere [8, 22], or by trypan blue staining based direct cell counting using a hemocytometer, or counted with Z™ Series COULTER COUNTER® (Beckman Coulter, Inc.) as indicated in the results.
In some experiment, mouse VSMC were seeded in 12-well plate (104/well), cultured overnight and then subjected to serum starvation for 24 hrs. The cells were then stimulated with DMEM containing 1% FCS in the presence of C2 or PC cell lysates at a final protein concentration of 40µg/ml. The cells were cultured for 48 hrs and cell proliferation was assessed with MTT assay. C2 and PC lysates were prepared in sterile PBS by 3 frozen-thaw cycles, and protein concentration was assessed with Bio-Rad Protein Assay kit (Bio-Rad).
2.4. Western blot assay
Cells were seeded at 106 per 10-cm plate and allowed to grow for 24 hrs. The cells were synchronized with serum free media for 24 hrs and then stimulated with DMEM containing 10% FCS for the indicated times. TP has no specific cell surface receptor. There was no intracellular signaling pathway that mediates effect of TP has been demonstrated. We thus used serum as a stimulator as it has multiple simultaneous effects on kinase cascades. In some experiments, synchronized cells were treated with Src inhibitor PP2 or its analog control PP3 or AG490 in the stimulating media for indicated times. Cells were lysed in the ice-cold lysis buffer containing (in mmol/L): 20 Tris, pH 7.0, 2 EGTA, 5 EDTA, 30 NaF, 60 β-glycerophosphate, pH 7.2, 20 Na4P2O7.10H2O, 1 Na3VO4, 1% Triton X-100 and proteinase inhibitor cocktail (Roche R&D). To study STAT3 nuclear translocation, cells were harvested with trypsin/EDTA, and cytosolic and nuclear proteins were extracted with a Nuclear/Cytosol Fractionation kit (BioVision, CA) as described before [18]. Thirty to fifty microgram of extract was subjected for immunoblot assay using antibodies to total STAT3, phospho-tyrosine705-STAT3, phosphor-serine727-STAT3, phospho-JAK2, JAK2, phospho-Src (Tyr416), phospho-Src (Tyr 527) as well as Fyn (all are from Cell Signaling), or to Lyn (SC-7274, Santa Cruz), actin (SC-1616R), alpha-tubulin (SC-8035), and TP (SC-9523), or to TATA-binding protein (abcam) and 4G10 Anti-phosphotyrosine (Millipore) antibodies. Synchronized and serum stimulated cells were also subjected for immunoprecipitation-immunoblotting assay as mentioned anywhere [21, 23, 24].
2.5. Real-time PCR–based mRNA quantitative assay
Synchronized VSMCs were treated with 10% FCS for 3 hrs and then total RNA was extracted from VSMCs using RNeasy Mini Kit (QIAGEN). One µg of the total RNA was treated with DNase I, and used for cDNA construction using AMV First Strand cDNA Synthesis Kit for RT-PCR (Roche). Real-time PCR for STAT3 was performed using SYBR Green PCR Master Mix (Applied Biosystems) with an iCycler iQ Real-Time PCR detection system [18, 25]. The forward primer for rat STAT3 was: 5’-GAG CTG GCT GAC TGG AAG AGG-3’, and the reverse primer was: 5’-TTG TTG GCG GGT CTG AAG TTG-3’.
2.6. Statistical analysis
All of the presented data were from at least 3 independent experiments, except where indicated in the text. Results are expressed as the mean ± SEM. Statistical analysis was performed using One-way ANOVA (StatView 5.0) or unpaired t test as appropriate. A value of p<0.05 was considered statistically significant.
3. Results
3.1. TP activity is necessary for TP inhibited VSMC proliferation
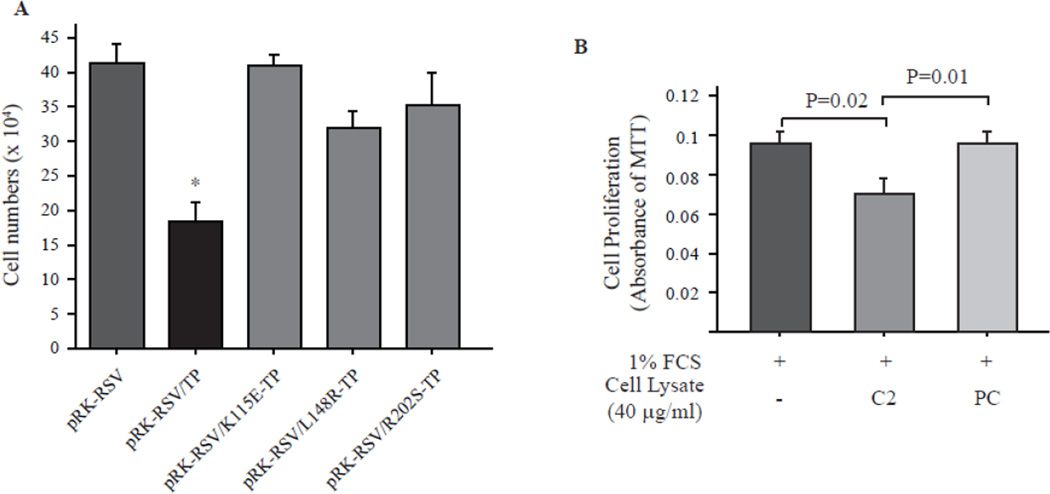
We previously have shown that TP inhibitor, TPI, increase C2 proliferation but had no effect on PC, suggesting that TP activity is necessary for its inhibitory effect on VSMC. To exclude the potentiality that the slowed growth of C2 is due to an artificial effect of constitutive gene overexpression, mouse primary cultured VSMC was transfected with phagemid vector encoding human TP cDNA or K115E-, L148R- and R202S-mutant TP cDNA for 72 hrs and then cell numbers were counted with Z™ Series COULTER COUNTER®. The mutations of TP on these sites have been reported to completely diminish TP activity [20]. As shown in Fig. 1A, in comparing to the empty vector transfected VSMC, all of the TP mutants did not have significant effect on cell proliferation. However, similar to rat VSMC, wild type TP transfection dramatically inhibited mouse VSMC growth. We also treated mouse VSMC with culture media in the presence of C2 or PC cell lysates. In comparing to serum alone, PC lysate had no effect on mouse VSMC growth; however, C2, which has high TP activity [8], dramatically inhibited cell proliferation (Fig. 1B). These data is in line with our previous finding [8], clearly demonstrated that the inhibitory effect of TP on VSMC proliferation is dependent on its enzymatic activity.
Fig. 1. TP activity is necessary for its inhibitory effect on VSMC proliferation.
A. Phagemid vector encoding human wild type TP or K115E (Lys-115-->Glu), L148R (Leu-148-->Arg) and R202S (Arg-202-->Ser) mutant TP were reversely transfected into 105 mouse primary cultured VSMC in 6-well plate using Lipofectamine LTX Plus Regent. The cell numbers were counted 72 hrs later with Z™ Series COULTER COUNTER®. N=3 for each transfection. *p<0.01, pBR-RSV/TP vs. others. B. 104 primary cultured mouse VSMC was seeded in 12-well plate, cultured for overnight and then subjected for serum starvation for 24 hrs. The cells were stimulated with 1% FCS in the presence C2 or PC cell lysate for 24 hrs and the cell proliferation was assessed with MTT assay. N=4 for each condition.
3.2. TP increased STAT3 phosphorylation and expression in VSMC
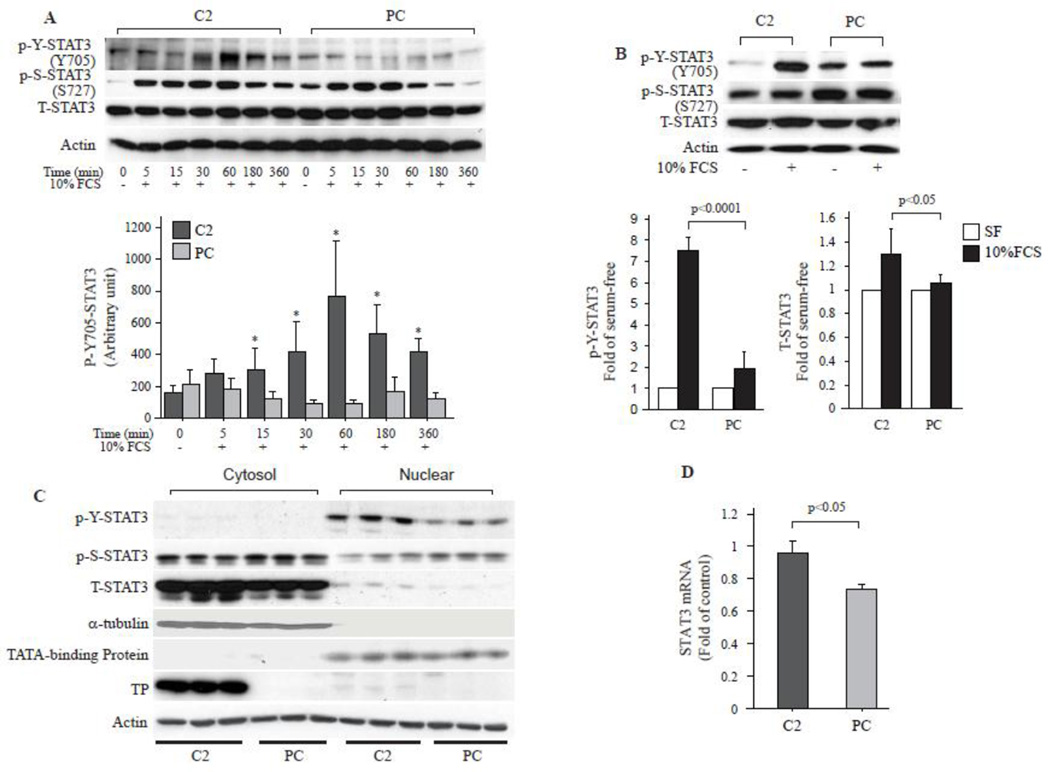
Since STAT3 signaling is involved in growth factor and cytokine induced VSMC proliferation and migration, we examined whether TP has effects on signaling pathway involving STAT3. Serum starvation and re-stimulation induced STAT3 tyrosine-705 phosphorylation (p-Y-STAT3) as well as serine-727 phosphorylation (p-S-STAT3) were examined. P-Y-STAT3 was in same level in C2 and PC cells after serum starvation for 24 hrs (Fig. 2A, 0 min). Serum did not influence p-Y-STAT3 in PC cells, however, unexpectedly, significantly increased p-Y-STAT3 in C2 cells. The p-Y-STAT3 was increased within 5 min, reached to maximal level after 1 hr, and then gradually decreased. The level of p-Y-STAT3 was higher in C2 than in PC at all of the examined time points (Fig. 2A and Fig. 2B). P-S-STAT3 was higher in PC than in C2 after serum-starvation for 24 hrs (4–5 folds). Serum stimulation significantly induced p-S-STAT3 in both cells as early as 5 min and the relative fold changes were markedly higher in C2 than in PC (Supplementary Fig. IA). However, the total amount at each time point was similar between C2 and PC. The p-S-STAT3 reached to peak in both cells around 15–30 min after stimulation and then gradually decreased and returned to the basal level in both cells after 24 hrs (Fig. 2B). There were no differences in T-STAT3 expression after the short term serum stimulation (Fig. 2A and Supplementary Fig. IB), but it significantly increased in C2 cell than in PC 24 hrs later (Fig. 2B). Nuclear and cytosolic fractionation revealed that p-Y-STAT3 in C2 nuclei was far greater than in PC after serum stimulation for 24 hrs (Fig. 2C). The P-S-STAT3 was similar between C2 and PC in the nucleus. While there was no significant difference in detectable phosphorylated STAT3 (Y705 or Ser727) in the cytosolic fractions between cells 24 hrs after serum stimulation, but the T-STAT3 was dramatically higher in C2 than in PC (Fig. 2C and Supplementary Fig. IC), suggesting that unphosphorylated STAT3 (U-STAT3) was increased in C2 cells. Real-time PCR based quantification assay demonstrated that serum-stimulated expression of STAT3 was also higher in C2 than in PC in mRNA level (Fig. 2D).
Fig. 2. TP induces STAT3 phosphorylation and overexpression.
A. 106 cells cultured in 10-cm plate were synchronized in serum free media for 24 hrs and then stimulated with serum for the indicated times. Western blot analyses were performed to examine the STAT3 phosphorylation status. P-Y-STAT3 indicates tyrosine phosphorylated STAT3, P-S-STAT3 indicates serine phosphorylated STAT3, and T-STAT3 represents total STAT3. Actin was blotted as loading control. Bar graph represents 3 independent experimental analyses of P-Y-STAT3 bands density that were analyzed by ATTO densitometry (ATTO, Tokyo Japan). * p<0.01, C2 vs. PC at the same time point. B. Western blot analysis of p-Y-STAT3, p-S-STAT3 and T-STAT3 after serum stimulation for 24 hrs. Bar graph represents 3 independent experimental analyses of P-Y-STAT3 and T-STAT3. C. Synchronized cells were stimulated with 10% FCS for 24 hrs and then the nuclear and cytosolic proteins were extracted. Western blot assay was performed for p-Y-STAT3, p-S-STAT3 and T-STAT3 in the nuclear and cytosolic fractions. Alpha-tubulin and TATA-binding protein were used as nuclear and cytosol purity control. TP was blotted to show its expression in the two cell lines. Actin was blotted as loading control for whole blot. N=3. D. Real-time PCR analysis of STAT3 mRNA expression after serum stimulation for 3 hrs. Data were adjusted to actin expression and further compared to the serum-free controls (n=6).
3.3. TP induced STAT3 phosphorylation is independent to JAK2
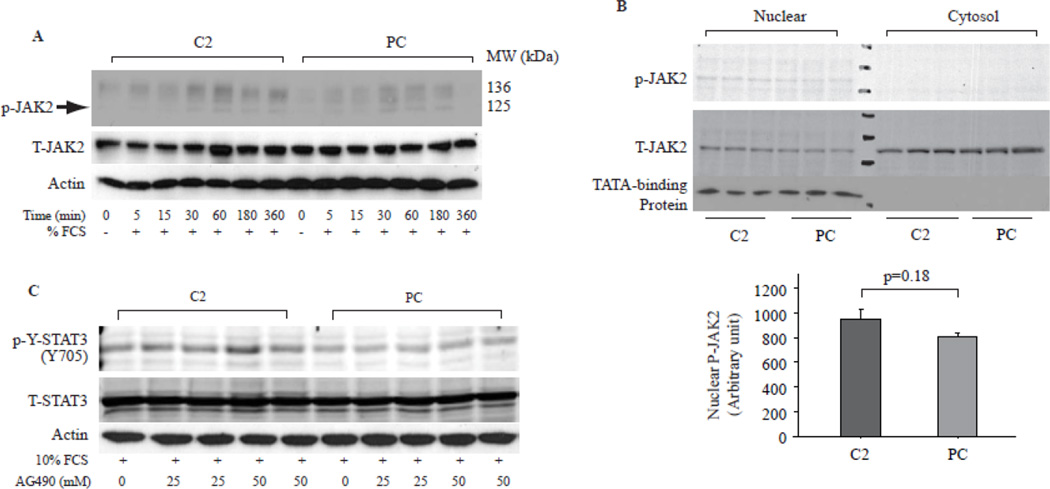
JAK2 plays important role in activation of STATs, and recent reports demonstrated a nuclear localization of JAK1 and JAK2, which is associated with high rates of cell growth [26, 27]. Serum only induced weak JAK2 phosphorylation in both C2 and PC cells; however, there was no difference at each examined time points between cells (Fig. 3A). There was no difference in JAK2 phosphorylation between C2 and PC cells in nuclear fraction after serum stimulation for 24 hrs (Fig. 3B). Treatment of cells with AG490, a JAK2 inhibiter, also did not alter serum stimulated STAT3 phosphorylation (Fig. 3C), suggesting that TP induced p-Y-STAT3 was JAK2 independent.
Fig. 3. JAK2 was not involved in TP induced STAT3 activation.
A. Cells treated as in Fig. 2A were subjected for Western blot assay to assess JAK2 activation and expression. P-JAK2 indicates phosphorylated Jak2, T-Jak2 indicates total JAK, actin was used as loading control. B. Western blot assay of JAK2 in the nuclear and cytosolic fractions of C2 and PC cells as prepared in Fig. 2C. TATA-binding protein was used as purity control. Bar graphs showed cumulative data of Western blot band densities of nuclear p-JAK2. N=3. C. Synchronized cells were stimulated with 10% FCS in the presence of AG490, a JAK2 inhibitor for 1 hr and then the cell lysates were subjected for Western blot assay with antibodies to p-Y-STAT3 and T-STAT3, Blot for actin was used as loading control.
3.4. TP induces STAT3 activation via Lyn
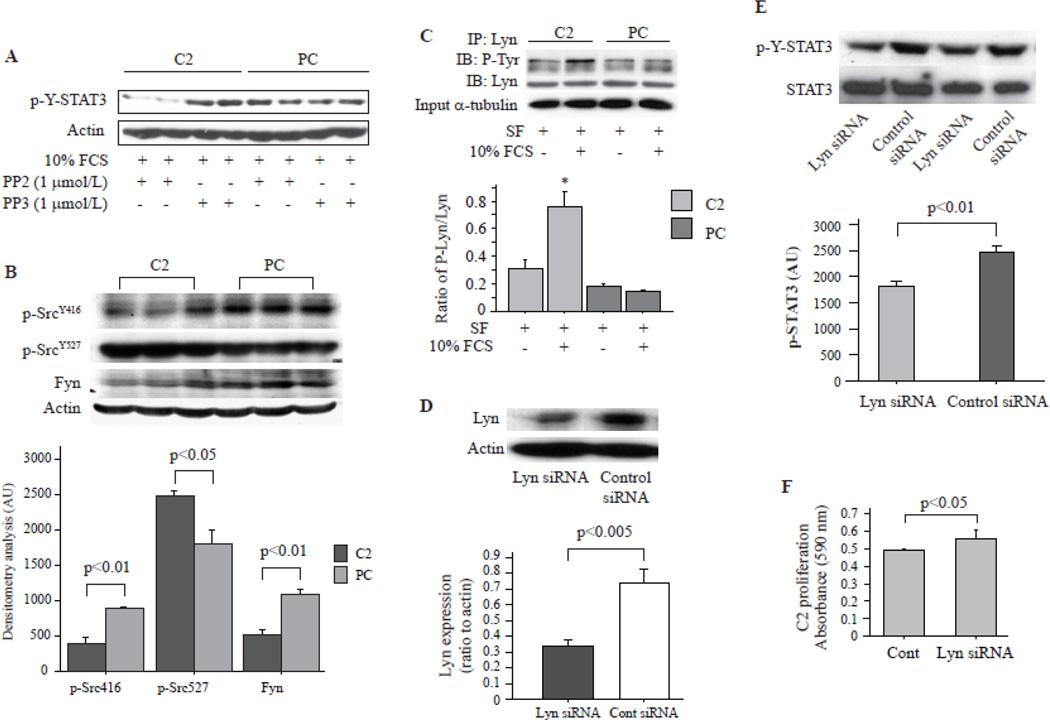
SFK has been reported to directly activate STAT3 [28–30]. To determine whether SFK is responsible for the STAT3 activation in C2 cells, synchronized cells were stimulated with 10% FCS in the presence of PP2, a potential SFK inhibitor, for 24 hrs. PP3, an analog of PP2, treated cells were used as controls. As shown in Fig. 4A, PP3 did not influence serum stimulated STAT3 phosphorylation, however, PP2 significantly decreased p-Y-STAT3 in C2 cell, but has no effect on PC cells. Src activity is regulated by tyrosine phosphorylation at two sites with opposing effects. Phosphorylation of Tyrosine (Tyr)416 in the activation loop of the kinase domain by carboxy-terminal Src kinase (Csk) upregulates enzyme activity. Phosphorylation of Tyr527 in the carboxy-terminal tail renders the enzyme less active [31, 32]. By immunoblotting assays we demonstrated that Src was phosphorylated at Tyr527 but not at Tyr416 site in C2, suggesting that its activity was lower in C2 than in PC cells (Fig. 4B). Therefore Src should not be involved in TP induced STAT3 tyrosine phosphorylation. Total amount of Fyn was also lower in C2 than in PC (Fig. 4B). We thus immunoprecipitated Lyn, another SFK member, and performed immunoblot assay using anti-phosphotyrosine antibody (4G10) to determine its phosphorylation status. Serum had no effect on Lyn phosphorylation in PC cells, but significantly increased tyrosine phosphorylated Lyn in C2 cells (Fig. 4C). These data suggested that Lyn may be involved in the TP induced STAT3 tyrosine phosphorylation. To test this hypothesis, a specific Lyn siRNA was used to knockdown Lyn expression in C2 cells (Fig. 4D). Interestingly, knockdown of Lyn expression significantly decreased serum induced STAT3 tyrosine phosphorylation (Fig. 4E), and increased C2 cell proliferation (Fig. 4F). This finding suggested that Lyn plays a critical role in TP inhibited VSMC proliferation.
Fig. 4. TP induced STAT3 activation via src family kinase, Lyn.
A. Src family kinases inhibitor PP2 decreased p-Y-STAT3 in C2 cells. Serum starved cells were stimulated with 10% serum in the presence of PP2 or PP3 for 24 hrs, and total cell lysates were used for immunoblotting assay. Actin was re-blotted as loading control. B. Synchronized cells were stimulated with 10% FCS for 24 hrs and then total cell lysate was used for Western blot assay of tyrosine phosphorylated Src as well as Fyn expression. Actin was blotted as loading control. C. The cells treated as mentioned in B were used for immunoprecipitation (IP) of lyn and the IPs were subjected for immunoblotting (IB) assay using antibodies to phosphotyrosine (4G10) and Lyn. Bar graph showed ratio of phosphorylated Lyn to total Lyn, N=4. * p<0.001 vs. others. Alpha-tubulin was used as input loading control. D. SiRNA targeting rat Lyn was transfected into C2 cells, and Lyn expression was assessed 72 hrs later, n=3. E. Lyn siRNA was transfected into C2 cells for 48 hrs. The cells were serum starved for 24 hrs and then stimulated with 10% FCS for another 24 hrs. Whole cell lysate was analyzed by Western blot assay using indicated antibodies. Bar graph represents cumulative data of 4 different experiments. F. 2000 C2 cells in 24-well plate were reversely transfected with Lyn siRNA for 72 hrs and cell proliferation was assessed with MTT assay. N=4.
3.5. STAT3 protein but not STAT3 phosphorylation is necessary for TP inhibited VSMC proliferation
Recent studies have demonstrated that U-STAT3 regulates a set of genes and plays important roles in regulating pathophysiological responses [21, 33–35]. U-STAT3 also possesses anti-inflammatory effects, suggesting that U-STAT3 might be a natural antagonist of the pro-inflammatory phospho-STAT3 [36]. We thus hypothesized that U-STAT3 might inhibit VSMC proliferation. To examine this hypothesis, mouse wt-stat3 gene or Y705F-stat3 gene, which cannot be phosphorylated at the 705-tyrosine site [21], were transfected into PC cells. Two cell clones that over expressed wt-stat3 and Y705F-stat3 and had similar level of total STAT3 expression (Fig. 5A) were used for proliferation assay. Surprisingly, either direct cell counting or MTT assay demonstrated that both the wt and Y705F-stat3 gene transfection markedly decreased PC proliferation to the level of C2 cells (Fig. 5B and C). These data suggest that TP induced accumulation of U-STAT3 may contribute to the decreased proliferation in C2 cells, which is independent to Y705 phosphorylation status. This finding was further confirmed by transfection of the above plasmid vectors into mouse primary cultured VSMCs. As shown in Fig. 5D, in comparing to parent VSMCs (No transfection), empty vector pCDNA6B did not affect VSMCs proliferation. However, pCDNA6B/wt-stat3 or pcDNA6B/Y705F-stat3 dramatically inhibited mouse VSMCs proliferation.
Fig. 5. Unphosphorylated STAT3 inhibits VSMC proliferation.
A. Mouse wt-stat3 and Y705F mutant stat3 were transfected into PC cells and transfected gene overexpressing cell clones were generated by blasticidin (10 µg/ml) and G418 (1 mg/ml) double selection. Whole cell lysates were subjected for Western blot assay using antibody to total STAT3. Actin was re-blotted as loading control. B. 104 cells were planted into 6-well plate, and cultured under normal conditions. The cell numbers were counted daily up to 3 days, n=3. * p<0.01, PC vs. others at the same day. C. 104 cells were planted into 12-well plates and cultured under normal conditions. Culture media was changed every 2 days. Cell proliferation was assessed by MTT assay at the indicated days. Data were represented as fold change of Day 0, which MTT assay was performed 6 hrs later when the cells completely adhered to the plate. n=3. * p<0.01, PC vs. others at the same day. D. 1.5 × 105 mouse primary cultured VSMCs were planted into 6-well plate and transfected with indicated plasmid vectors. Normal cultured parent VSMC (No transfection) was used as control. The cell numbers was analyzed with Z™ Series COULTER COUNTER®. N=3.
4. Discussion
Obstructive and thrombotic cardiovascular diseases are the most common cause of death and disability in the developed world. The etiology of these disorders is multi-factorial and involves complex interplay between lifestyle and fixed causative factors [37]. An initiating step is often vascular injury induced by cellular oxidative stress precipitated by the production of damaging reactive oxygen species by many cell types including VSMCs and monocytes/macrophages [37], and finally induced both thrombotic and non-thrombotic occlusion of the vessel. VSMCs are the major cell population in the arterial wall, and changes in their behavior, function and redox status contribute to alterations in vascular remodeling and cell signaling. An extensive body of experimental medicine has demonstrated that targeting VSMC proliferation and migration may achieve a significant therapeutic effect in these diseases [1–3, 8, 9, 38]. However, developing novel mechanism-based targets for intervention in these diseases remains a high priority.
TP has been found in atherosclerotic plaque [39], and its expression is suggested to correlate with the plaque angiogenesis which might contribute to plaque growth, and risk of rupture. However, plaque angiogenesis may not in fact be a requirement for atherogenesis, but rather a physiological response to the pathophysiological state of the arterial wall [40]. By treating the mouse VSMCs with high TP activity containing cell lysate or gene transfection of lose-function of mutant TP into VSMCs (Fig. 1), we clearly demonstrated that high TP activity inhibits VSMC proliferation. Our data suggest that the higher TP activity in atherosclerotic plaque may contribute to attenuate vessel wall VSMC response to the pathophysiological stimulation.
TP has noticeable effects on angiogenesis that has been clearly demonstrated. One concern raised here is how TP inhibits VSMC proliferation but enhances angiogenesis. VSMC are necessary for arteriogenesis (collateral vessel development) as well as vascular maturation. The mechanisms by how the vessel wall formation and lumen formation are controlled are only partially understood. Therapeutic stimulation of arteriogenesis with cytokines or genes has been successfully performed in experimental animal models. Translation into clinical practice, however, has hitherto been problematic. The reasons are multiple. Uncontrolled smooth muscle cell proliferation induces vascular occlusion and is lethal [41], which might be one of the reasons that caused the failure of these novel developed therapeutic strategies. Balanced and controlled VSMC proliferation might be a key control point in atherosclerosis and arteriogenesis. Our previous studies have demonstrated that TP gene injection enhanced both angiogenesis and arteriogenesis in ischemic hearts and hind limbs [5–7]. TP may play a role in balancing and controlling the VSMC growth during arteriogenesis as well as vascular maturation.
In this study, we discovered that TP induced not only STAT3 activation but also total STAT3 accumulation in VSMCs (Fig. 2). Cell lysates containing high TP activity is sufficient to induce STAT3 tyrosine phosphorylation (Supplementary Fig. II), but dramatically inhibited VSMC proliferation (Fig. 1B). In contrary to this finding, a recent study showed that inhibition of STAT3 signaling prevented VSMC proliferation and inhibited neointimal formation [42]. WP1066, a compound induces VSMC apoptosis associated with inhibition of STAT3 signaling, was used to examine effect of STAT3 inhibition on VSMC proliferation in vitro [42]. In addition, a C57Bl6 mouse strain, which has been widely known to be resistance to guide wire induced neointima formation [43], was used to examine the effect of WP1066 on neointimal hyperplasia in vivo. Therefore, data generated from this study should be interpreted with caution. STAT3 has GAS motif in its promoter region, which is a binding site of p-Y-STAT3. Serum stimulation also increased expression of STAT3 in RNA level, suggesting that TP induced accumulation of U-STAT3 protein was resulted from the phospho-STAT3 upregulated transcription of STAT3 gene. Phosphorylation on Ser727 is indispensable for STAT3 maximal transcriptional activity [44]. Although the fold change of p-S-STAT3 was higher in C2 after serum stimulation, it returned to the basal level 24 hrs later and showed a relative lower level of total amount in the whole cell lysate of C2, suggesting that its activity is not related to the decreased C2 proliferation.
STAT proteins participate in a wide variety of physiological processes and direct seemingly contradictory responses, such as proliferation and apoptosis. The classical model for STAT activation has been identified and characterized in which STATs bind through phospho-tyrosine/SH2-domain interactions and are then phosphorylated by JAK2 [45]. The phosphorylated STATs then dimerize and translocate to the nucleus to regulate expression of their targeting genes. However, our data suggested that JAK2 is not involved in TP induced p-Y-STAT3, but Lyn plays a priority role. Src family tyrosine kinases are signaling intermediates in a diverse array of cellular events including cell differentiation, motility, proliferation, and survival [46]. Lyn has been described to have an inhibitory role in myeloid lineage proliferation [47]; however, surprisingly, little is known about its effect on VSMC. Here, we demonstrated that Lyn may negative regulate cell proliferation in TP overexpressing VSMCs. Many phospho-STAT3 targeting genes are known, including genes encoding the anti-apoptotic proteins (e.g. Bcl-xl, Mcl-1, and Bcl-2), proliferation associated proteins (e.g. Cyclin D1 and Myc), and proangiogenic factor (e.g. VEGF) [48–55], as well as antioxidant enzymes such as HO-1 [56], which has been found to be upregulated by TP in our previous studies [8, 9]. Therefore, the current paper supports our previous finding and that TP induced phospho-STAT3 may contribute to the higher HO-1 expression in C2 cells. In contrast to its proliferative and developmental effects, STAT3 also enhances apoptosis in response to death stimulation [17], suggesting the intricacy of STATs family in influencing VSMC function. However, this pro-apoptotic effect could not explain our finding as we did not find an enhanced apoptosis under normal culture condition as well as any of the mentioned treatments in both cells.
By using conditional knockout mice, STAT3 has been revealed playing pleiotropic roles, and showing that there is an essential Stat3-dependent function that is not mediated by any of the known Stat3-activating cytokines or their receptors [57]. Recent studies demonstrated that unphosphorylated STATs can enter nuclei and induce “renegade” gene expression [21, 33, 35, 58]. Though nuclear-cytoplasmic shuttling of unphosphorylated STAT1 has been known for a decade [59], and U-STAT3 has been found to be functional about 6 years ago [35], how unphosphorylated STATs regulate gene expression is still unknown. We have found that connective tissue growth factor (CTGF), which promotes VSMC proliferation, is a target of U-STAT3 [21], and U-STAT3 negatively regulates CTGF expression at protein level in HEK293 cells (YH, unpublished data). However, we don’t know whether CTGF plays a role in U-STAT3 induced inhibition of VSMC proliferation. Further studies are necessary to determine the mechanism and the potential downstream pathways that mediate the effect of U-STAT3 on VSMC proliferation.
In summary, our study first demonstrated that TP induced STAT3 phosphorylation and increased total amount of U-STAT3 in VSMC, which may play a critical role in inhibiting VSMC proliferation. Our data provide novel insights into the role of TP in the cardiovascular system.
Supplementary Material
Highlights.
Inhibition of vascular smooth muscle proliferation benefits atherosclerotic diseases
Thymidine phosphorylase inhibits vascular smooth muscle cell proliferation
We found thymidine phosphorylase impacts STAT3 activity via Lyn
We found an unphophorylated STAT3 inhibits VSMC proliferation
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists, Category A (to W. L, Project No.16689023), from the Ministry of Education, Culture, Sports, Science and Technology, Japan; and American Heart Association Postdoctoral Fellowship Award #0825564D (to H. Y.). The authors thank Dr. Roy L Silverstein for critical reading this paper.
Abbreviations
- VSMC
vascular smooth muscle cell
- TP
Thymidine phosphorylase
- HO-1
heme oxygenase 1
- JAK/STAT
janus-activated kinase/signal transducers and activators of transcription
- SFK
Src family kinase
- FCS
fetal calf serum
- C2
TP overexpressing cell clone number 2
- PC
empty vector-transfected control VSMC cells
- MTT
3-[4,5- dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- p-Y-STAT3
tyrosine-705 phosphorylated STAT3
- p-S-STAT3
serine-727 phosphorylated STAT3
- T-STAT3
total STAT3
- U-STAT3
unphosphorylated STAT3
- IP
immunoprecipitation
- IB
immunoblotting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci U S A. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morishita R, Gibbons GH, Ellison KE, Nakajima M, von der Leyen H, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. Intimal hyperplasia after vascular injury is inhibited by antisense cdk 2 kinase oligonucleotides. J Clin Invest. 1994;93:1458–1464. doi: 10.1172/JCI117123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons M, Edelman ER, DeKeyser JL, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 4.Barton GJ, Ponting CP, Spraggon G, Finnis C, Sleep D. Human platelet-derived endothelial cell growth factor is homologous to Escherichia coli thymidine phosphorylase. Protein Sci. 1992;1:688–690. doi: 10.1002/pro.5560010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Tanaka K, Ihaya A, Fujibayashi Y, Takamatsu S, Morioka K, Sasaki M, Uesaka T, Kimura T, Yamada N, Tsuda T, Chiba Y. Gene therapy for chronic myocardial ischemia using platelet-derived endothelial cell growth factor in dogs. Am J Physiol Heart Circ Physiol. 2005;288:H408–H415. doi: 10.1152/ajpheart.00176.2004. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Tanaka K, Morioka K, Takamori A, Handa M, Yamada N, Ihaya A. Long-term effect of gene therapy for chronic ischemic myocardium using platelet-derived endothelial cell growth factor in dogs. J Gene Med. 2008;10:412–420. doi: 10.1002/jgm.1156. [DOI] [PubMed] [Google Scholar]
- 7.Yamada N, Li W, Ihaya A, Kimura T, Morioka K, Uesaka T, Takamori A, Handa M, Tanabe S, Tanaka K. Platelet-derived endothelial cell growth factor gene therapy for limb ischemia. J Vasc Surg. 2006;44:1322–1328. doi: 10.1016/j.jvs.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Tanaka K, Morioka K, Uesaka T, Yamada N, Takamori A, Handa M, Tanabe S, Ihaya A. Thymidine phosphorylase gene transfer inhibits vascular smooth muscle cell proliferation by upregulating heme oxygenase-1 and p27KIP1. Arterioscler Thromb Vasc Biol. 2005;25:1370–1375. doi: 10.1161/01.ATV.0000168914.85107.64. [DOI] [PubMed] [Google Scholar]
- 9.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566–1574. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Baetta R, Soma M, De-Fraja C, Comparato C, Teruzzi C, Magrassi L, Cattaneo E. Upregulation and activation of Stat6 precede vascular smooth muscle cell proliferation in carotid artery injury model. Arterioscler Thromb Vasc Biol. 2000;20:931–939. doi: 10.1161/01.atv.20.4.931. [DOI] [PubMed] [Google Scholar]
- 11.Kundumani-Sridharan V, Wang D, Karpurapu M, Liu Z, Zhang C, Dronadula N, Rao GN. Suppression of activation of signal transducer and activator of transcription-5B signaling in the vessel wall reduces balloon injury-induced neointima formation. Am J Pathol. 2007;171:1381–1394. doi: 10.2353/ajpath.2007.061258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusch A, Tkachuk S, Haller H, Dietz R, Gulba DC, Lipp M, Dumler I. Urokinase stimulates human vascular smooth muscle cell migration via a phosphatidylinositol 3-kinase-Tyk2 interaction. J Biol Chem. 2000;275:39466–39473. doi: 10.1074/jbc.M003626200. [DOI] [PubMed] [Google Scholar]
- 13.Torella D, Curcio A, Gasparri C, Galuppo V, De Serio D, Surace FC, Cavaliere AL, Leone A, Coppola C, Ellison GM, Indolfi C. Fludarabine prevents smooth muscle proliferation in vitro and neointimal hyperplasia in vivo through specific inhibition of STAT-1 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2935–H2943. doi: 10.1152/ajpheart.00887.2006. [DOI] [PubMed] [Google Scholar]
- 14.Wang TH, Xiang QL, Chen JW, Pan H, Cui YH. Raloxifene plus 17beta-estradiol inhibits proliferation of primary cultured vascular smooth muscle cells and human mammary endothelial cells via the janus kinase/signal transducer and activator of transcription3 cascade. Eur J Pharmacol. 2007;561:7–13. doi: 10.1016/j.ejphar.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Kusch A, Tkachuk S, Tkachuk N, Patecki M, Park JK, Dietz R, Haller H, Dumler I. The tight junction protein ZO-2 mediates proliferation of vascular smooth muscle cells via regulation of Stat1. Cardiovasc Res. 2009;83:115–122. doi: 10.1093/cvr/cvp117. [DOI] [PubMed] [Google Scholar]
- 16.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23:177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 17.Bai Y, Ahmad U, Wang Y, Li JH, Choy JC, Kim RW, Kirkiles-Smith N, Maher SE, Karras JG, Bennett CF, Bothwell AL, Pober JS, Tellides G. Interferon-gamma induces X-linked inhibitor of apoptosis-associated factor-1 and Noxa expression and potentiates human vascular smooth muscle cell apoptosis by STAT3 activation. J Biol Chem. 2008;283:6832–6842. doi: 10.1074/jbc.M706021200. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Febbraio M, Reddy SP, Yu DY, Yamamoto M, Silverstein RL. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. The Journal of clinical investigation. 2010;120:3996–4006. doi: 10.1172/JCI42823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue H, Lee JD, Shimizu H, Uzui H, Mitsuke Y, Ueda T. Effects of magnesium on the production of extracellular matrix metalloproteinases in cultured rat vascular smooth muscle cells. Atherosclerosis. 2003;166:271–277. doi: 10.1016/s0021-9150(02)00390-8. [DOI] [PubMed] [Google Scholar]
- 20.Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, Akiyama S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995;55:1687–1690. [PubMed] [Google Scholar]
- 21.Yue H, Li W, Desnoyer R, Karnik SS. Role of nuclear unphosphorylated STAT3 in angiotensin II type 1 receptor-induced cardiac hypertrophy. Cardiovasc Res. 2010;85:90–99. doi: 10.1093/cvr/cvp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circulation research. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Li W, Major J, Rahaman SO, Febbraio M, Silverstein RL. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood. 2011;117:5744–5750. doi: 10.1182/blood-2009-01-201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Tanaka K, Chiba Y, Kimura T, Morioka K, Uesaka T, Ihaya A, Sasaki M, Tsuda T, Yamada N. Role of MMPs and plasminogen activators in angiogenesis after transmyocardial laser revascularization in dogs. Am J Physiol Heart Circ Physiol. 2003;284:H23–H30. doi: 10.1152/ajpheart.00240.2002. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson J, Bjursell G, Kannius-Janson M. Nuclear Jak2 and transcription factor NF1-C2: a novel mechanism of prolactin signaling in mammary epithelial cells. Molecular and cellular biology. 2006;26:5663–5674. doi: 10.1128/MCB.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zouein FA, Duhe RJ, Booz GW. JAKs go nuclear: Emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth factors. 2011;29:245–252. doi: 10.3109/08977194.2011.614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mlinaric-Rascan I, Yamamoto T. B cell receptor signaling involves physical and functional association of FAK with Lyn and IgM. FEBS letters. 2001;498:26–31. doi: 10.1016/s0014-5793(01)02474-7. [DOI] [PubMed] [Google Scholar]
- 29.Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21:3334–3358. doi: 10.1038/sj.onc.1205398. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Kurosaki T, Corey SJ. Engagement of the B-cell antigen receptor activates STAT through Lyn in a Jak-independent pathway. Oncogene. 2007;26:2851–2859. doi: 10.1038/sj.onc.1210092. [DOI] [PubMed] [Google Scholar]
- 31.Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987;49:1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- 32.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18:443–451. doi: 10.1038/cr.2008.41. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 36.Pena G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol. 2010;40:2580–2589. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J Mol Cell Cardiol. 2009;47:372–381. doi: 10.1016/j.yjmcc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 39.Boyle JJ, Wilson B, Bicknell R, Harrower S, Weissberg PL, Fan TP. Expression of angiogenic factor thymidine phosphorylase and angiogenesis in human atherosclerosis. J Pathol. 2000;192:234–242. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH699>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 41.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 42.Daniel JM, Dutzmann J, Bielenberg W, Widmer-Teske R, Gunduz D, Hamm CW, Sedding DG. Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation. Basic Res Cardiol. 2012;107:1–12. doi: 10.1007/s00395-012-0261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–260. doi: 10.2174/138945008783755601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lufei C, Koh TH, Uchida T, Cao X. Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene. 2007;26:7656–7664. doi: 10.1038/sj.onc.1210567. [DOI] [PubMed] [Google Scholar]
- 45.Seidel HM, Lamb P, Rosen J. Pharmaceutical intervention in the JAK/STAT signaling pathway. Oncogene. 2000;19:2645–2656. doi: 10.1038/sj.onc.1203550. [DOI] [PubMed] [Google Scholar]
- 46.Pertel T, Zhu D, Panettieri RA, Yamaguchi N, Emala CW, Hirshman CA. Expression and muscarinic receptor coupling of Lyn kinase in cultured human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L492–L500. doi: 10.1152/ajplung.00344.2005. [DOI] [PubMed] [Google Scholar]
- 47.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 48.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 50.Epling-Burnette PK, Zhong B, Bai F, Jiang K, Bailey RD, Garcia R, Jove R, Djeu JY, Loughran TP, Jr, Wei S. Cooperative regulation of Mcl-1 by Janus kinase/stat and phosphatidylinositol 3-kinase contribute to granulocyte-macrophage colony-stimulating factor-delayed apoptosis in human neutrophils. J Immunol. 2001;166:7486–7495. doi: 10.4049/jimmunol.166.12.7486. [DOI] [PubMed] [Google Scholar]
- 51.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 52.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 54.Thirunavukkarasu M, Juhasz B, Zhan L, Menon VP, Tosaki A, Otani H, Maulik N. VEGFR1 (Flt-1+/−) gene knockout leads to the disruption of VEGF-mediated signaling through the nitric oxide/heme oxygenase pathway in ischemic preconditioned myocardium. Free Radic Biol Med. 2007;42:1487–1495. doi: 10.1016/j.freeradbiomed.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 56.Shih RH, Lee IT, Hsieh HL, Kou YR, Yang CM. Cigarette smoke extract induces HO-1 expression in mouse cerebral vascular endothelial cells: involvement of c-Src/NADPH oxidase/PDGFR/JAK2/STAT3 pathway. J Cell Physiol. 2010;225:741–750. doi: 10.1002/jcp.22270. [DOI] [PubMed] [Google Scholar]
- 57.Levy DE, Lee CK. What does Stat3 do? The Journal of clinical investigation. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown S, Zeidler MP. Unphosphorylated STATs go nuclear. Curr Opin Genet Dev. 2008;18:455–460. doi: 10.1016/j.gde.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Meyer T, Begitt A, Lodige I, van Rossum M, Vinkemeier U. Constitutive and IFN-gamma-induced nuclear import of STAT1 proceed through independent pathways. The EMBO journal. 2002;21:344–354. doi: 10.1093/emboj/21.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.