Abstract
Chromatin is a complex assembly that compacts DNA inside the nucleus while providing the necessary level of accessibility to regulatory factors conscripted by cellular signaling systems. In this superstructure, DNA is the subject of mechanical forces applied by variety of molecular motors. Rather than being a rigid stick, DNA possesses dynamic structural variability that could be harnessed during critical steps of genome functioning. The strong relationship between DNA structure and key genomic processes necessitates the study of physical constrains acting on the double helix. Here we provide insight into the source, dynamics, and biology of DNA topological domains in the eukaryotic cells and summarize their possible involvement in gene transcription. We emphasize recent studies that might inspire and impact future experiments on the involvement of DNA topology in cellular functions.
Keywords: DNA topoisomerases, DNA topological domain, DNA topology, torsional stress, transcription
Introduction
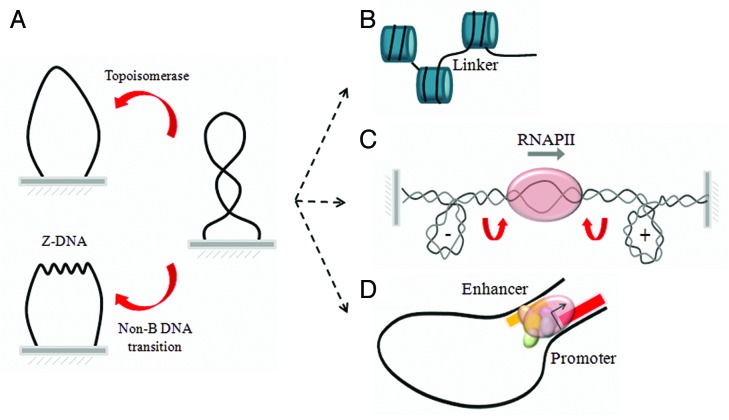
By definition, the formation of a topological domain requires topologically constrained DNA. The constraint might result from a pair of physical clamps attaching the DNA at specialized sites or from restrained rotation of one strand of the double helix around the other in the viscous cellular environment.1 Consequently, DNA within a topological domain can be subjected to torsional tension or supercoiling (Fig. 1A, right). Topological constraints in chromatin DNA could arise at many hierarchical levels of genome organization.
Figure 1. Topological domains of DNA during cellular processes. (A) By definition a topological domain requires topologically constrained DNA ends. Within a domain, genetic transactions can distort the structure of the double helix and generate supercoilings, eventually removed through the relaxation activity of DNA topoisomerases or the transitions of regular B-DNA into non-B DNA conformations (Z-DNA is shown as example). Topological domains can be identified at many hierarchical levels: (B) in the linker region between two adjacent nucleosome; (C) during transcriptional elongation where the RNA polymerase constitutes a moving node between fixed ends; and (D) during enhancer-promoter interaction.
Many cellular processes including transcription require the cumulative action of multiple protein-DNA interactions. Once a protein binds to DNA, it can serve as a foundation for further protein-DNA complexes setting up a composite net of topologically constrained DNA, but unfortunately we know very little about this phenomenon.2 At the basic level of chromatin organization the pattern of DNA topology seems simpler. In the cell nucleus, strong and repetitive interactions between DNA and histones form nucleosomes. The nucleosome is the fundamental unit of chromatin in which 147 DNA base pairs are wrapped around a core of histone proteins.3 Short stretches of naked DNA—linker regions—connect adjacent nucleosomes. Linker DNA ranges between 20 and 90 bp in length and varies between different species and even within a single cellular genome.4,5 Because the exit and entry sites of a linker region are fixed by the embracing nucleosomes, the linker DNA could be viewed as topological domain of the same length (Fig. 1B). Torsional tension of DNA between adjacent nucleosomes, together with linker length, defines the spatial orientation, boundaries, and interactions between nucleosomes and may even orchestrate their structural rearrangements.6
Domain boundaries can also result from the tracking of molecular machinery (i.e., transcription complexes) along the DNA double helix (Fig. 1C). As originally conceived in the twin-domain-model, during transcriptional elongation, DNA rotation is prevented by frictional force or anchoring of the polymerase complex to a nuclear structure.7 Un-twisting or over-twisting of DNA corkscrewing through the transcribing RNA polymerase results in the wave of dynamic supercoiling which might be exploited to participate in the regulation of a variety of DNA transactions.8
In eukaryotic genomes, large number of elements dispersed in linear genome has an impact on transcriptional control. The current belief is that the main mechanism by which these regulatory elements communicate with their target genes is through chromatin looping.9,10 Using approaches based mainly on the chromosome conformation capture technique (3C),11 looping constraints are inferred from interaction frequencies between a point of interest and distal loci of the genome (Fig. 1D). Looping brings distal elements into close spatial proximity to each other and generates the so-called “topological domain”.12 However, this term may often be misleading: the loop-organization of genomes does not necessary means that DNA of any given “topological domain” is indeed topologically isolated from their neighborhood. Co-localization can be the result of specific constraints between two loci mediated by protein complexes, or it can be a nonspecific result of chromatin fiber packing in the crowded nucleus.
The proteins that bind and manipulate the DNA of a true topological domain necessarily impart torsional stress which redistributes over constrained DNA regions and has a significant influence on global conformation of double helix. DNA topoisomerases—the enzymes which modulate the topological state of genome—are vital and common to all cellular organisms.13,14 Topoisomerases introduce a transient break in the DNA backbone and allow the release of mechanical stress from the double helix (Fig. 1A, left). In addition, when the stress is high enough, it can be released as strain in a variety of DNA structural transition: from B-DNA to Z-DNAs, cruciforms, quadruplexes, etc.15-18
Proper gene expression occurs largely through the regulation of RNA polymerase transcription which arises at multiple stages: nucleosome remodeling and promoter selection, early transcription from the melting of DNA by the transcriptional machinery through the release of RNA polymerase from promoter-proximal pause sites, transcript elongation, promoter-enhancer interaction, 3-D genomic architecture, and antisense activity.19-25 Although there are no steps purely managed by DNA topology, a growing body of evidence indicates that DNA topology is an important player in the biochemical team. In this review our goal is to dissect the key regulatory steps of transcription, highlighting the importance and the consequences of topological changes within different types of genomic domains.
DNA Topology and Initiation of Transcription
Cells use transcription factors to regulate transcription initiation by RNA polymerase II, but transcription requires disruption of a repressive chromatin context at promoters. Torsional tension of DNA could have profound effect on the local operation of DNA binding proteins, including nucleosomes, components of general transcriptional machinery, and a variety of chromatin organizers. Nucleosomes are dynamic structures that must be modified to allow transcription. It has been shown that chromatin remodeling factors facilitate these conformational dynamics.21,26 Both in vitro and in vivo studies have revealed that as pre-requisite for gene activation all Snf2p-related nucleosome remodelers generate torsional tension in the DNA.27 This DNA topology-based mechanism provides a powerful way to disrupt repressive chromatin structures at the promoters. ATPase generated torque is even capable of inducing transitions of regular B-DNA into non-B DNA conformations.28 Transcription from the promoters that form Z-DNA may require the establishment of a boundary between a nucleosome and Z-DNA,29 as was shown for the promoter of CSF1 gene that adopts a Z-DNA conformation when transcription is activated by BRG1.30
Core histone rearrangement and/or acetylation by factors such as p300/CBP release some of the negative supercoils previously restrained by the nucleosomes.31 Consequently, the changes induced in the chromatin structure result in the topological stress of linker DNA that may create a friendlier neighborhood for general transcription factors and RNA polymerase. Topological coupling between chromatin remodeling and transcription factors binding was inferred from the observation that interaction of the TATA-box Binding Protein is enhanced for supercoiled DNA.32 Accordingly, pre-initiation complex formation and transcription initiation are assisted by torsional stress in vitro.33 This view is also supported by studies that suggested the importance of histone acetylation for transcriptional initiation, but not for elongation, and showed that this modification is often observed on the flanking regions of genes.34,35 In addition, topoisomerase activity is directly required for efficient disassembly of nucleosomes at active promoters.36
The next step in transcription initiation is the transition of the closed complex into an open complex, with local melting of the promoter DNA. This transition depends on the recruitment of TFIIH which contains the ATPase activity required for promoter opening and transition to the open complex.37 To investigate how RNA polymerase responds to DNA supercoiling, a single-molecule approach monitored polymerase-dependent DNA unwinding in torsionally stressed DNA.38 It was demonstrated that the DNA topology influences the rate of formation and stability of the open complex. Negative supercoiling weakens base stacking interactions, thereby promoting the formation of the transcription bubble. Thus the open complex formation is controlled at least in part by DNA topology. Accordingly, in vitro transcription is more efficient and does not require TFIIH if the promoter resides in a negatively supercoiled plasmid.33 Therefore, optimal transcription necessitates a delicate balance between topology of the promoters and transcriptional output. Indeed, recent genome-wide experiments suggest that cells have elaborate mechanisms to coordinate the rates of transcription and the DNA relaxing activity of topoisomerases to adjust supercoiling in the promoter regions of differentially expressed genes.39,40
The mechanics of promoter melting by TFIIH subunits has been a long standing question. Because the ATPase has helicase activity in vitro, it was thought that TFIIH directly separates the two strands of the DNA double helix to form the transcriptional bubble.41 However, recent studies have shown that TFIIH translocates along the double helix and rotates DNA inside of the topologically closed domain established by TFIIH-TFIIE interaction.42 This rotation results in DNA torsional stress, which is relieved by promoter melting.37,43 Thus, the key step in the formation of an open complex is based on DNA topology properties which could help to explain TFIIH-related diseases.44
After promoter melting, RNA polymerase initiates RNA synthesis at the transcription start site. Increasing evidence show that RNA polymerase II often halts just after promoter clearance, typically ∼20–60 nucleotides downstream.45,46 In fact, recent genome-wide studies suggest that paused RNA polymerase II is a common feature of gene regulation, especially in development.47 Release of the paused polymerase is emerging as one of the major mechanisms of gene control.48 Promoter escape depends on the phosphorylation of the large subunit of RNA polymerase II by TFIIH and other general transcription factors such as the Mediator complex and the positive transcription elongation factor b (P-TEFb).23 However the full mechanism(s) of polymerase pausing and release is still pending. In the recent high resolution mapping of DNA supercoiling near promoters of a human cell line it was shown that paused genes have higher level of torsional tension localized near their transcription start sites compared with elongating genes.39 Moreover, experiments with topoisomerase inhibitors imply that the level of this tension is topoisomerase I dependent. These observations are in good accordance with the finding that treatment of cells with topoisomerase I inhibitor elicits a redistribution of RNA polymerase along transcribed genes and enhances the escape from pausing sites.40,49,50 This suggests that the pause-release of the transcriptional machinery may be influenced by the specific DNA supercoiling balance at promoters. The precise mechanism still remains to be established, however one might hypothesize that the activity of pause-regulated factors and/or the processivity of transcriptional machinery are coupled to local DNA topology around the pause site. Indeed, in a refined set of experiments monitoring RNA polymerase translocating in real-time along supercoiled DNA, it was shown that the arrest of polymerase imposed by accumulating supercoils was relieved upon release of the opposing torque.51
DNA Topology and Transcription Elongation
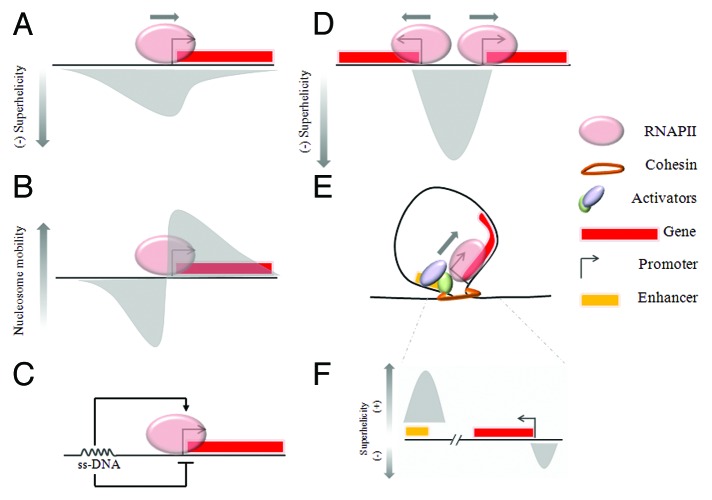
Transcription elongation results in severe topological perturbation of DNA.52 During the movement of the transcriptional machinery along chromatin template, nucleosomes should be redistributed, thus releasing or rearranging the negative supercoils they constrain. Additionally, screwing DNA through the RNA polymerase imposes dynamic torsional tension on the template (Fig. 2A).51,53 In vitro, even a single nucleosome strongly inhibits elongation of transcription, but evidently this is not a problem in vivo.54 It is crucial to understand how RNA polymerase traverses through nucleosome arrays to account for the efficiency of transcription in vivo.
Figure 2. Transcription is associated with dynamic perturbation of DNA. (A) Negative DNA supercoiling occurs at upstream promoter regions of every transcribed gene. (B) Nucleosome mobilization potential is differentially affected upstream and downstream of transcribing RNA polymerase. (C) Non-B DNA formed as result of ongoing transcription has the capacity to regulate the promoter output in real-time. (D) The activity of divergent closely juxtaposed promoters may be mechanically coupled through dynamic supercoiling. (E) Enhancer transcription could be required to generate torsional stress which results in reorganization of local chromatin structure and favors enhancer-promoter communication.
Nucleosome mobilization in front of polymerase is a possible mechanism to achieve high elongation efficiency. A surprising observation is that gene activation decreases nucleosome occupancy along the full body of the gene well before the first polymerase even approaches the end of the gene.55 Furthermore, in vitro negative supercoiling promotes nucleosome assembly, whereas positive supercoiling inhibits it.56 The reorganization of a multiprotein complex such as a chromatin fiber by the diffusion of the chromatin remodelers should be a rather slow process. Torsional tension in DNA can propagate in a much faster fashion suggesting that positive supercoiling generated in front of elongating RNA polymerase could help to de-condense chromatin for more efficient transcription.57 Though poly-ADP ribose polymerase (PARP) was shown to mediate this process because it can associate with topoisomerases, involvement of DNA topology remains a viable mechanistic consideration for gene body opening.58,59 To investigate how nucleosomes respond to different topological environments, nucleosomes on DNA under tension and torque were studied using an elegant single-molecule approach. Dramatic loss of H2A/H2B dimers was observed at the physiologically relevant level of positive torsional tension suggesting that DNA topology can be a potent regulator of nucleosomes mobilization during transcription.60 A recent in vivo study also supports this idea (Fig. 2B, right). Using a sequencing-based assay to determine DNA torsional states with high resolution, it was shown that accumulation of positive supercoiling results in increased nucleosome turnover within gene bodies.40
RNA polymerase is an highly processive enzyme with velocity in vivo up to 70 bases per second.61 If elongation proceed without rotation of the transcriptional apparatus, 1 negative supercoil should be generated for each turn of double helix and almost 1500 supercoils per averaged 15 kb human gene length.62,63 Therefore it is reasonable to wonder how transcription might exploit this enormous level of supercoiling or whether this is just a by-product, eventually cleaned-up by topoisomerases. However, topoisomerases do not instantaneously relax these supercoils and high torsional stress could drive transition of B-DNA into non-B conformation at the susceptible sequences.39,53 In fact, identification of non-B DNA structures upstream of the active promoters is the classical way to estimate the level of supercoiling generated during transcription elongation. The formation of alternative DNA conformations might expose specific DNA binding sites and engage transcription factors, as occurs on the human MYC promoter, the best studied example of this regulatory mechanism.64
Single-molecule fluorescent in situ hybridization approaches, which allow individual RNA molecules to be measured, have shown that transcription is an intrinsically noisy process.65 Cell-to-cell variability in gene expression is likely to be dangerous in case of short-life, low-abundance mRNAs of key genes and should be minimized.66,67 Even if the same promoter in different cells received a signal at the same time, there would be cell-to-cell variability due to the stochastic recruitment of transcription factors and engagement of RNA polymerase. It was shown that due to activated transcription, non-B DNA could form in vivo as far as 1.5 kb upstream of the promoters (Fig. 2c). This unusual structure recruited transcriptional factors essential for the fine and tight regulation of MYC proto-oncogene output. When this real-time feed-back system is compromised, cells exhibit striking cell-to-cell heterogeneity in MYC levels that could predispose to disease.53,66,67 In addition to promoting synchronous patterns of gene transcription among groups of cells, it is possible that this mechanism might also help to equilibrate transcript levels for genes encoding subunits of protein complexes.
Though a similar regulatory mode has also been reported on the USP29 gene, it is still unknown how widely this cooperation between DNA topology and DNA conformation-sensitive proteins might be used in cells.68 There are no reasons to believe that USP29 and MYC are special. Considering that abnormal oncogene expression is a common feature of malignancy, the deregulation of the regulatory pathway described above might occur at the promoters of oncogenes and tumor suppressors.8,69 Recent genome-wide approaches have been used for a fine-analysis of DNA topology in human cell lines revealing that dynamic supercoiling transmitted at least 2 kb upstream from transcription start sites is a characteristic of virtually every transcribed gene (Fig. 2A).39,70 High-resolution mapping of single-stranded DNA also revealed that these structures are a more frequent genomic feature than previously thought.44 These findings highlight that transcription is inevitably coupled with dynamic perturbations of the double helix and that such perturbations may have the capacity of regulatory feedback in real time.
Negative supercoiling transmitted into the upstream promoter region may also stabilize chromatin fiber by reducing nucleosome mobility.56 A stable, dominant configuration of promoter chromatin will mask particular sites preventing binding of sequence-specific transcriptional factors that do not participate in the ongoing transcriptional program (Fig. 2B, left). Again, this type of feedback might decrease stochastic patterns of transcription by reducing the number of unwanted activation and/or repression events.
Finally, negative supercoiling upstream of transcription start sites could be reinforced if promoters in divergent orientation are transcribed, providing an additional level of regulation (Fig. 2D). Divergent promoters represent more than 10% of all human genes, but what is more important is that transcription initiation even from the single promoter is not obligatorily unidirectional. By using different experimental techniques, it was shown that most active promoters support divergent initiation with productive elongation efficiently occurring in the bodies of the coding genes.71,72 The frequency of this promoter arrangement suggests that it might be used in regulatory pathways.25 In model systems it has been demonstrated both in vitro and in vivo that supercoiling generated between divergently transcribing RNA polymerases18,53 is high enough to result in non-B DNA formation, imposing real-time co-regulation of transcription activity as discussed above. Negative supercoiling could also directly facilitate DNA melting during open complex formation. This regulatory mechanism could help to bypass multiple abortive initiation events and to regulate—inhibit or promote—transcriptional noise due to these rate-limiting steps.19
DNA Topology and Genome Architecture
It was realized a long time ago that enhancers which may reside at a large linear distance away from the promoter are required for proper gene expression. Master transcription factors bind enhancer regions and by recruiting Mediator activate much of the gene expression programs necessary for cell identity. Recent studies show that active promoter-enhancer communication is accompanied by looping of the intervening DNA in the chromatin complex to juxtapose the enhancer and the target promoter (Fig. 1D). The mechanism(s) that establishes this proximity largely remain undescribed, though available data suggest that inter-nucleosomal interactions involving the histone tails are important.73
Transcriptome analyses by RNA deep-sequencing show that many enhancers are transcribed.74 The function of RNA transcripts derived from enhancer sites (eRNA) is unknown, but the transcripts and/or ongoing transcription are required for enhancer action: eRNA has been used as a marker of active enhancers.75,76 Efficient sliding of interacting chromatin fibers which brings together enhancer and promoter require dynamic rearrangement of chromatin structure. The simple act of RNA polymerase II transcription is sufficient to alter the local chromatin environment and this process likely reflects dynamic supercoiling emanating from the transcribed enhancer independent of gene transcription (Fig. 2E).77-79 Contraction of decondensed chromatin due to “depletion attraction forces” could also contributes to the loop formation.80 In favor of this idea, it was shown that formation of a chromatin loop topologically isolating the enhancer from the target promoter is sufficient to inhibit enhancer’s activity in vivo.81 Involvement of DNA topology in transcription regulation through enhancers was also elucidated from studies which have shown the presence of topoisomerases at enhancer regions.82,83
Dense complexes of DNA and transcription factors in the vicinity of promoters and enhancers likely require a complex architecture of multiple interactions between molecular partners. It is plausible that many of these interactions will be not permissive without strongly bent, twisted or otherwise stressed double stranded DNA, suggesting that cells have evolved a way to modulate the stiffness of DNA.84 Strategic placement of non-B DNA forming sequences, which could flip into the more flexible conformation in comparison with double helix, might be a means to defeat the rigidity of DNA.64 Accordingly, in silico analyses showed a high enrichment of these sequences at regulatory regions of the genome and, notably, at the promoters of a wide range of oncogenes.85-88 In addition, several conformation-sensitive proteins with regulatory function bind and stabilize non-B DNAs, suggesting an important role of these structures in transcriptional output.64 Another way to make genetic processes more tolerant to the constrained DNA topology is weakening the DNA through introducing double strand breaks or nicks.84 Locally targeted DNA damage mediated by topoisomerases, which induce a permissive chromatin setting, has been implicated in transcriptional activation by nuclear hormone receptors.89-91
In recent years the combination of advanced imaging techniques and new molecular approaches revealed that the eukaryotic genome is organized into sub-megabase loops, termed topological domains.12 The domain organization is invariant between different cell types, suggesting that there is no direct relevance to the differential transcriptional activity or development programs. Domain bases are enriched for the proteins such as CTCF and cohesin which are known for their potential to act as a supercoiling boundary. The purpose of such universal large-scale chromatin organization is still to be uncovered, but recent findings hint to the importance of the DNA topology in possible function of these domains. It was shown that topoisomerases facilitate the expression of a large number of long genes with sub-megabase length.49 Topoisomerase inhibitors affect the expression of long genes in all investigated cell types to date, implying that this DNA topology-dependent effect is common to all mammalian cells. The data rule out any secondary effects on inhibition such as DNA damage and formation of covalent complexes and suggest that topoisomerases have distinct effect on transcription depending on the topology of domain which hosts each particular gene.
The factors that create and maintain this domain organization are presently unknown. CTCF and cohesin might be involved in establishment or maintenance of topological domains in the mammalian genome as their binding sites are enriched at the domain boundaries.12 However, in a recent study of the topological organization of the genome, it was surprisingly observed that the topological domain structure remains intact in the human cells even after cohesin and CTCF depletion or destruction.92 Though the proteins responsible for domain formation and maintenance remain to be fully enumerated. Two recent papers suggest that DNA topology itself may be an important player. By using dynamic simulations, it was shown that including supercoiling into models of topological domain organization can qualitatively and quantitatively reproduce experimental 3C data obtained in eukaryotic cells.93 Another work points to supercoiling and transcriptional activity as critical determinants of domain formation in bacteria. It was revealed that bacterial genome is composed of regions with increased frequency of contacts, highly reminiscent of topological domains in eukaryotic chromosomes.79 Overall these studies suggest that individual topological domains in eukaryotic cells could be transcription- and topoisomerases-dependent and composed of supercoiled regions forming plectonemes.
Conclusion
In the last few years, new approaches have reinforced the strong relationship between DNA topology and transcription. Together, they suggest that DNA topology provides an additional level of transcriptional regulation and must be precisely controlled. Our understanding of this phenomenon is far from being fundamental and demands the aggressive development of innovative experimental approaches. Exciting times for this area of research are coming.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1.Vinograd J, Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966;49:103–25. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–19. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 4.Woodcock CL, Skoultchi AI, Fan Y. Role of linker histone in chromatin structure and function: H1 stoichiometry and nucleosome repeat length. Chromosome Res. 2006;14:17–25. doi: 10.1007/s10577-005-1024-3. [DOI] [PubMed] [Google Scholar]
- 5.Godde JS, Widom J. Chromatin structure of Schizosaccharomyces pombe. A nucleosome repeat length that is shorter than the chromatosomal DNA length. J Mol Biol. 1992;226:1009–25. doi: 10.1016/0022-2836(92)91049-U. [DOI] [PubMed] [Google Scholar]
- 6.Barbi M, Mozziconacci J, Victor JM, Wong H, Lavelle C. On the topology of chromatin fibres. Interface Focus. 2012;2:546–54. doi: 10.1098/rsfs.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987;84:7024–7. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baranello L, Levens D, Gupta A, Kouzine F. The importance of being supercoiled: how DNA mechanics regulate dynamic processes. Biochim Biophys Acta. 2012;1819:632–8. doi: 10.1016/j.bbagrm.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Laat W, Duboule D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature. 2013;502:499–506. doi: 10.1038/nature12753. [DOI] [PubMed] [Google Scholar]
- 10.Deng W, Lee J, Wang H, Miller J, Reik A, Gregory PD, Dean A, Blobel GA. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–44. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miele A, et al. Mapping chromatin interactions by chromosome conformation capture. Curr Protoc Mol Biol, 2006. Chapter 21: p. Unit 21 11. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–80. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 14.Forterre P, Gadelle D. Phylogenomics of DNA topoisomerases: their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res. 2009;37:679–92. doi: 10.1093/nar/gkp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacolla A, Wells RD. Non-B DNA conformations, genomic rearrangements, and human disease. J Biol Chem. 2004;279:47411–4. doi: 10.1074/jbc.R400028200. [DOI] [PubMed] [Google Scholar]
- 16.Sun D, Hurley LH. The importance of negative superhelicity in inducing the formation of G-quadruplex and i-motif structures in the c-Myc promoter: implications for drug targeting and control of gene expression. J Med Chem. 2009;52:2863–74. doi: 10.1021/jm900055s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich A, Zhang S. Timeline: Z-DNA: the long road to biological function. Nat Rev Genet. 2003;4:566–72. doi: 10.1038/nrg1115. [DOI] [PubMed] [Google Scholar]
- 18.Kouzine F, Liu J, Sanford S, Chung HJ, Levens D. The dynamic response of upstream DNA to transcription-generated torsional stress. Nat Struct Mol Biol. 2004;11:1092–100. doi: 10.1038/nsmb848. [DOI] [PubMed] [Google Scholar]
- 19.Michel M, Cramer P. Transitions for regulating early transcription. Cell. 2013;153:943–4. doi: 10.1016/j.cell.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 20.Smolle M, Workman JL. Transcription-associated histone modifications and cryptic transcription. Biochim Biophys Acta. 2013;1829:84–97. doi: 10.1016/j.bbagrm.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat Rev Genet. 2008;9:15–26. doi: 10.1038/nrg2206. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Pelechano V, Järvelin AI, Steinmetz LM. Functional consequences of bidirectional promoters. Trends Genet. 2011;27:267–76. doi: 10.1016/j.tig.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak H, Lis JT. Control of transcriptional elongation. Annu Rev Genet. 2013;47:483–508. doi: 10.1146/annurev-genet-110711-155440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Sharp PA. Divergent transcription: a driving force for new gene origination? Cell. 2013;155:990–6. doi: 10.1016/j.cell.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–93. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 26.Varga-Weisz PD, Becker PB. Regulation of higher-order chromatin structures by nucleosome-remodelling factors. Curr Opin Genet Dev. 2006;16:151–6. doi: 10.1016/j.gde.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Havas K, Flaus A, Phelan M, Kingston R, Wade PA, Lilley DM, Owen-Hughes T. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell. 2000;103:1133–42. doi: 10.1016/S0092-8674(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 28.Mulholland N, Xu Y, Sugiyama H, Zhao K. SWI/SNF-mediated chromatin remodeling induces Z-DNA formation on a nucleosome. Cell Biosci. 2012;2:3. doi: 10.1186/2045-3701-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong B, Chen S, Kwon JA, Rich A. Characterization of Z-DNA as a nucleosome-boundary element in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007;104:2229–34. doi: 10.1073/pnas.0611447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Mulholland N, Fu H, Zhao K. Cooperative activity of BRG1 and Z-DNA formation in chromatin remodeling. Mol Cell Biol. 2006;26:2550–9. doi: 10.1128/MCB.26.7.2550-2559.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales V, Richard-Foy H. Role of histone N-terminal tails and their acetylation in nucleosome dynamics. Mol Cell Biol. 2000;20:7230–7. doi: 10.1128/MCB.20.19.7230-7237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn JD. Topological effects of the TATA box binding protein on minicircle DNA and a possible thermodynamic linkage to chromatin remodeling. Biochemistry. 2000;39:3520–4. doi: 10.1021/bi992263f. [DOI] [PubMed] [Google Scholar]
- 33.Parvin JD, Sharp PA. DNA topology and a minimal set of basal factors for transcription by RNA polymerase II. Cell. 1993;73:533–40. doi: 10.1016/0092-8674(93)90140-L. [DOI] [PubMed] [Google Scholar]
- 34.Roberge M, O’Neill TE, Bradbury EM. Inhibition of 5S RNA transcription in vitro by nucleosome cores with low or high levels of histone acetylation. FEBS Lett. 1991;288:215–8. doi: 10.1016/0014-5793(91)81037-9. [DOI] [PubMed] [Google Scholar]
- 35.Barth TK, Imhof A. Fast signals and slow marks: the dynamics of histone modifications. Trends Biochem Sci. 2010;35:618–26. doi: 10.1016/j.tibs.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Durand-Dubief M, Persson J, Norman U, Hartsuiker E, Ekwall K. Topoisomerase I regulates open chromatin and controls gene expression in vivo. EMBO J. 2010;29:2126–34. doi: 10.1038/emboj.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grünberg S, Warfield L, Hahn S. Architecture of the RNA polymerase II preinitiation complex and mechanism of ATP-dependent promoter opening. Nat Struct Mol Biol. 2012;19:788–96. doi: 10.1038/nsmb.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314:1139–43. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kouzine F, Gupta A, Baranello L, Wojtowicz D, Ben-Aissa K, Liu J, Przytycka TM, Levens D. Transcription-dependent dynamic supercoiling is a short-range genomic force. Nat Struct Mol Biol. 2013;20:396–403. doi: 10.1038/nsmb.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Teves SS, Henikoff S. Transcription-generated torsional stress destabilizes nucleosomes. Nat Struct Mol Biol. 2014;21:88–94. doi: 10.1038/nsmb.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzder SN, Sung P, Bailly V, Prakash L, Prakash S. RAD25 is a DNA helicase required for DNA repair and RNA polymerase II transcription. Nature. 1994;369:578–81. doi: 10.1038/369578a0. [DOI] [PubMed] [Google Scholar]
- 42.He Y, Fang J, Taatjes DJ, Nogales E. Structural visualization of key steps in human transcription initiation. Nature. 2013;495:481–6. doi: 10.1038/nature11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim TK, Ebright RH, Reinberg D. Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science. 2000;288:1418–22. doi: 10.1126/science.288.5470.1418. [DOI] [PubMed] [Google Scholar]
- 44.Kouzine F, Wojtowicz D, Yamane A, Resch W, Kieffer-Kwon KR, Bandle R, Nelson S, Nakahashi H, Awasthi P, Feigenbaum L, et al. Global regulation of promoter melting in naive lymphocytes. Cell. 2013;153:988–99. doi: 10.1016/j.cell.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min IM, Waterfall JJ, Core LJ, Munroe RJ, Schimenti J, Lis JT. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 2011;25:742–54. doi: 10.1101/gad.2005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwak H, Fuda NJ, Core LJ, Lis JT. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science. 2013;339:950–3. doi: 10.1126/science.1229386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13:720–31. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine M. Paused RNA polymerase II as a developmental checkpoint. Cell. 2011;145:502–11. doi: 10.1016/j.cell.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King IF, Yandava CN, Mabb AM, Hsiao JS, Huang HS, Pearson BL, Calabrese JM, Starmer J, Parker JS, Magnuson T, et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature. 2013;501:58–62. doi: 10.1038/nature12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baranello L, Bertozzi D, Fogli MV, Pommier Y, Capranico G. DNA topoisomerase I inhibition by camptothecin induces escape of RNA polymerase II from promoter-proximal pause site, antisense transcription and histone acetylation at the human HIF-1alpha gene locus. Nucleic Acids Res. 2010;38:159–71. doi: 10.1093/nar/gkp817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma J, Bai L, Wang MD. Transcription under torsion. Science. 2013;340:1580–3. doi: 10.1126/science.1235441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman LA, Garrard WT. DNA supercoiling in chromatin structure and gene expression. Crit Rev Eukaryot Gene Expr. 1992;2:165–209. [PubMed] [Google Scholar]
- 53.Kouzine F, Sanford S, Elisha-Feil Z, Levens D. The functional response of upstream DNA to dynamic supercoiling in vivo. Nat Struct Mol Biol. 2008;15:146–54. doi: 10.1038/nsmb.1372. [DOI] [PubMed] [Google Scholar]
- 54.Izban MG, Luse DS. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked DNA but very poorly on chromatin templates. J Biol Chem. 1992;267:13647–55. [PubMed] [Google Scholar]
- 55.Petesch SJ, Lis JT. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell. 2008;134:74–84. doi: 10.1016/j.cell.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta P, Zlatanova J, Tomschik M. Nucleosome assembly depends on the torsion in the DNA molecule: a magnetic tweezers study. Biophys J. 2009;97:3150–7. doi: 10.1016/j.bpj.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meiners JC, Quake SR. Femtonewton force spectroscopy of single extended DNA molecules. Phys Rev Lett. 2000;84:5014–7. doi: 10.1103/PhysRevLett.84.5014. [DOI] [PubMed] [Google Scholar]
- 58.Ju BG, Rosenfeld MG. A breaking strategy for topoisomerase IIbeta/PARP-1-dependent regulated transcription. Cell Cycle. 2006;5:2557–60. doi: 10.4161/cc.5.22.3497. [DOI] [PubMed] [Google Scholar]
- 59.Thomas CJ, Kotova E, Andrake M, Adolf-Bryfogle J, Glaser R, Regnard C, Tulin AV. Kinase-mediated changes in nucleosome conformation trigger chromatin decondensation via poly(ADP-ribosyl)ation. Mol Cell. 2014;53:831–42. doi: 10.1016/j.molcel.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheinin MY, Li M, Soltani M, Luger K, Wang MD. Torque modulates nucleosome stability and facilitates H2A/H2B dimer loss. Nat Commun. 2013;4:2579. doi: 10.1038/ncomms3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh J, Padgett RA. Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol. 2009;16:1128–33. doi: 10.1038/nsmb.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol. 2007;14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavelle C. Forces and torques in the nucleus: chromatin under mechanical constraints. Biochem Cell Biol. 2009;87:307–22. doi: 10.1139/O08-123. [DOI] [PubMed] [Google Scholar]
- 64.Kouzine F, Levens D. Supercoil-driven DNA structures regulate genetic transactions. Front Biosci. 2007;12:4409–23. doi: 10.2741/2398. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez A, Golding I. Genetic determinants and cellular constraints in noisy gene expression. Science. 2013;342:1188–93. doi: 10.1126/science.1242975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Kouzine F, Nie Z, Chung HJ, Elisha-Feil Z, Weber A, Zhao K, Levens D. The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression. EMBO J. 2006;25:2119–30. doi: 10.1038/sj.emboj.7601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber A, Liu J, Collins I, Levens D. TFIIH operates through an expanded proximal promoter to fine-tune c-myc expression. Mol Cell Biol. 2005;25:147–61. doi: 10.1128/MCB.25.1.147-161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Chung HJ, Vogt M, Jin Y, Malide D, He L, Dundr M, Levens D. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J. 2011;30:846–58. doi: 10.1038/emboj.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Naughton C, Avlonitis N, Corless S, Prendergast JG, Mati IK, Eijk PP, Cockroft SL, Bradley M, Ylstra B, Gilbert N. Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat Struct Mol Biol. 2013;20:387–95. doi: 10.1038/nsmb.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, Young RA, Sharp PA. Divergent transcription from active promoters. Science. 2008;322:1849–51. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–8. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulaeva OI, Zheng G, Polikanov YS, Colasanti AV, Clauvelin N, Mukhopadhyay S, Sengupta AM, Studitsky VM, Olson WK. Internucleosomal interactions mediated by histone tails allow distant communication in chromatin. J Biol Chem. 2012;287:20248–57. doi: 10.1074/jbc.M111.333104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Santa F, Barozzi I, Mietton F, Ghisletti S, Polletti S, Tusi BK, Muller H, Ragoussis J, Wei CL, Natoli G. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. doi: 10.1371/journal.pbio.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hah N, Murakami S, Nagari A, Danko CG, Kraus WL. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013;23:1210–23. doi: 10.1101/gr.152306.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petesch SJ, Lis JT. Overcoming the nucleosome barrier during transcript elongation. Trends Genet. 2012;28:285–94. doi: 10.1016/j.tig.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim A, Zhao H, Ifrim I, Dean A. Beta-globin intergenic transcription and histone acetylation dependent on an enhancer. Mol Cell Biol. 2007;27:2980–6. doi: 10.1128/MCB.02337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marenduzzo D, Faro-Trindade I, Cook PR. What are the molecular ties that maintain genomic loops? Trends Genet. 2007;23:126–33. doi: 10.1016/j.tig.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Zhu X, Ling J, Zhang L, Pi W, Wu M, Tuan D. A facilitated tracking and transcription mechanism of long-range enhancer function. Nucleic Acids Res. 2007;35:5532–44. doi: 10.1093/nar/gkm595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cockerill PN, Garrard WT. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986;44:273–82. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- 83.Rosenberg M, Fan AX, Lin IJ, Liang SY, Bungert J. Cell-cycle specific association of transcription factors and RNA polymerase ii with the human β-globin gene locus. J Cell Biochem. 2013;114:1997–2006. doi: 10.1002/jcb.24542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baranello L, Kouzine F, Levens D. DNA Topoisomerases: Beyond the standard role. Transcription. 2013;4 doi: 10.4161/trns.26598. [DOI] [PubMed] [Google Scholar]
- 85.Brooks TA, Hurley LH. The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nat Rev Cancer. 2009;9:849–61. doi: 10.1038/nrc2733. [DOI] [PubMed] [Google Scholar]
- 86.Ray BK, Dhar S, Henry C, Rich A, Ray A. Epigenetic regulation by Z-DNA silencer function controls cancer-associated ADAM-12 expression in breast cancer: cross-talk between MeCP2 and NF1 transcription factor family. Cancer Res. 2013;73:736–44. doi: 10.1158/0008-5472.CAN-12-2601. [DOI] [PubMed] [Google Scholar]
- 87.Zhabinskaya D, Benham CJ. Theoretical analysis of the stress induced B-Z transition in superhelical DNA. PLoS Comput Biol. 2011;7:e1001051. doi: 10.1371/journal.pcbi.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawaya S, Bagshaw A, Buschiazzo E, Kumar P, Chowdhury S, Black MA, Gemmell N. Microsatellite tandem repeats are abundant in human promoters and are associated with regulatory elements. PLoS One. 2013;8:e54710. doi: 10.1371/journal.pone.0054710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 90.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, Zhang J, Rose DW, Fu XD, Glass CK, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–83. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perillo B, Ombra MN, Bertoni A, Cuozzo C, Sacchetti S, Sasso A, Chiariotti L, Malorni A, Abbondanza C, Avvedimento EV. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319:202–6. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 92.Zuin J, Dixon JR, van der Reijden MI, Ye Z, Kolovos P, Brouwer RW, van de Corput MP, van de Werken HJ, Knoch TA, van IJcken WF, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci U S A. 2014;111:996–1001. doi: 10.1073/pnas.1317788111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benedetti F, Dorier J, Burnier Y, Stasiak A. Models that include supercoiling of topological domains reproduce several known features of interphase chromosomes. Nucleic Acids Res. 2014;42:2848–55. doi: 10.1093/nar/gkt1353. [DOI] [PMC free article] [PubMed] [Google Scholar]