Abstract
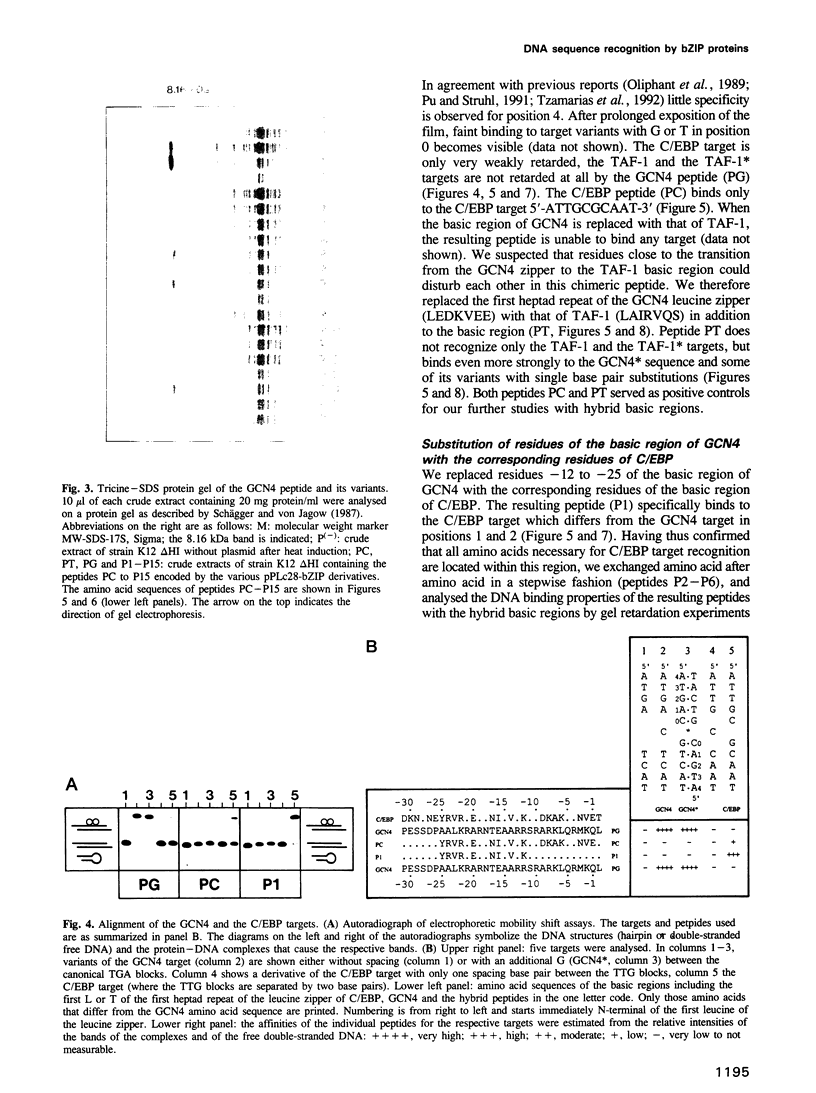
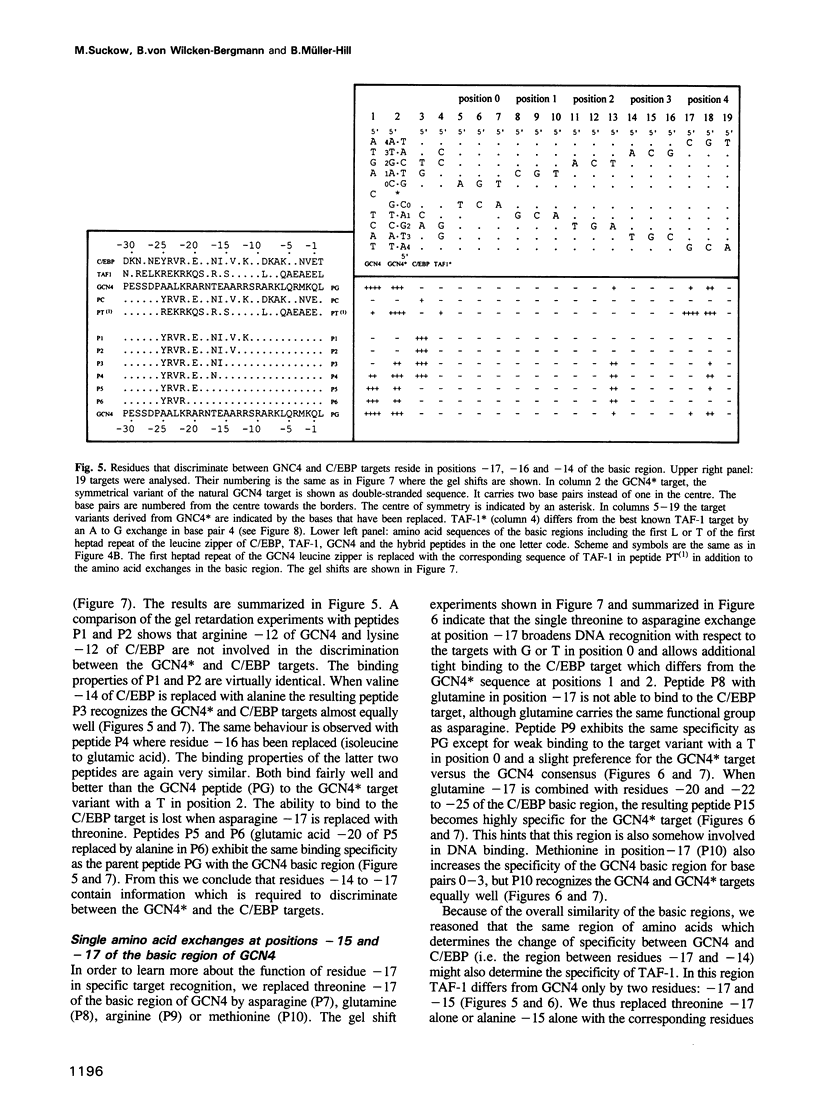
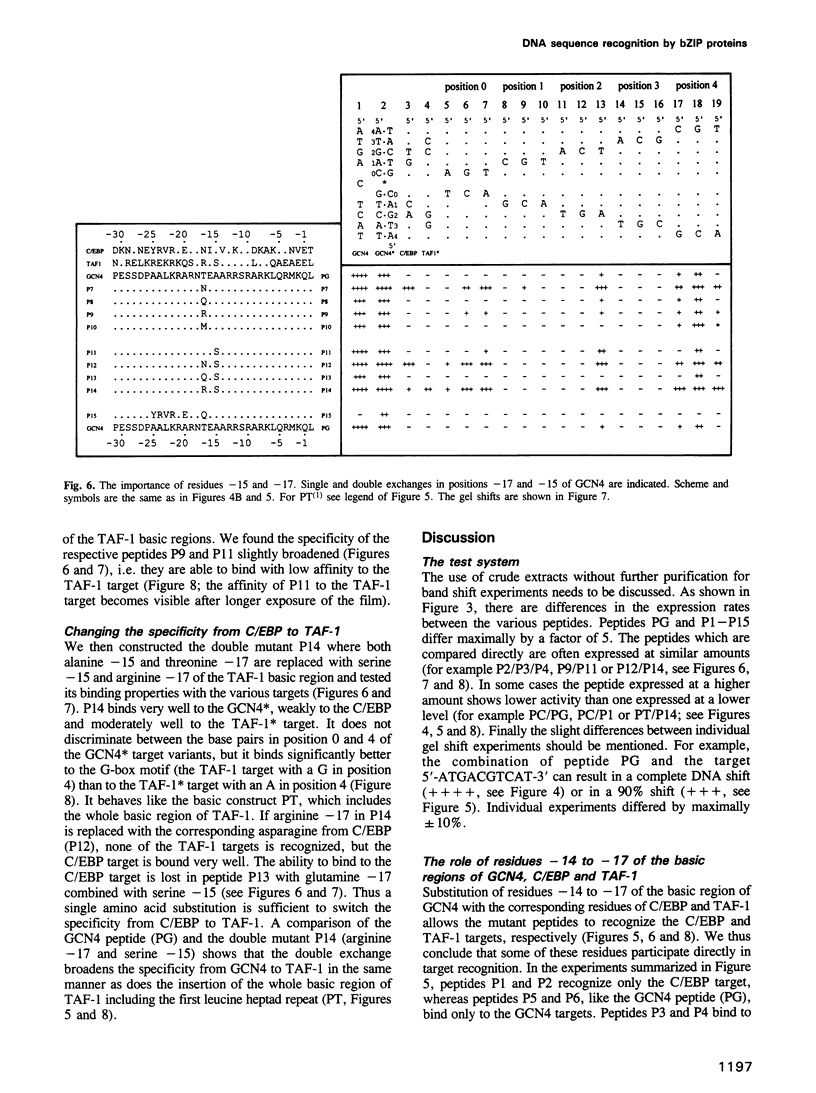
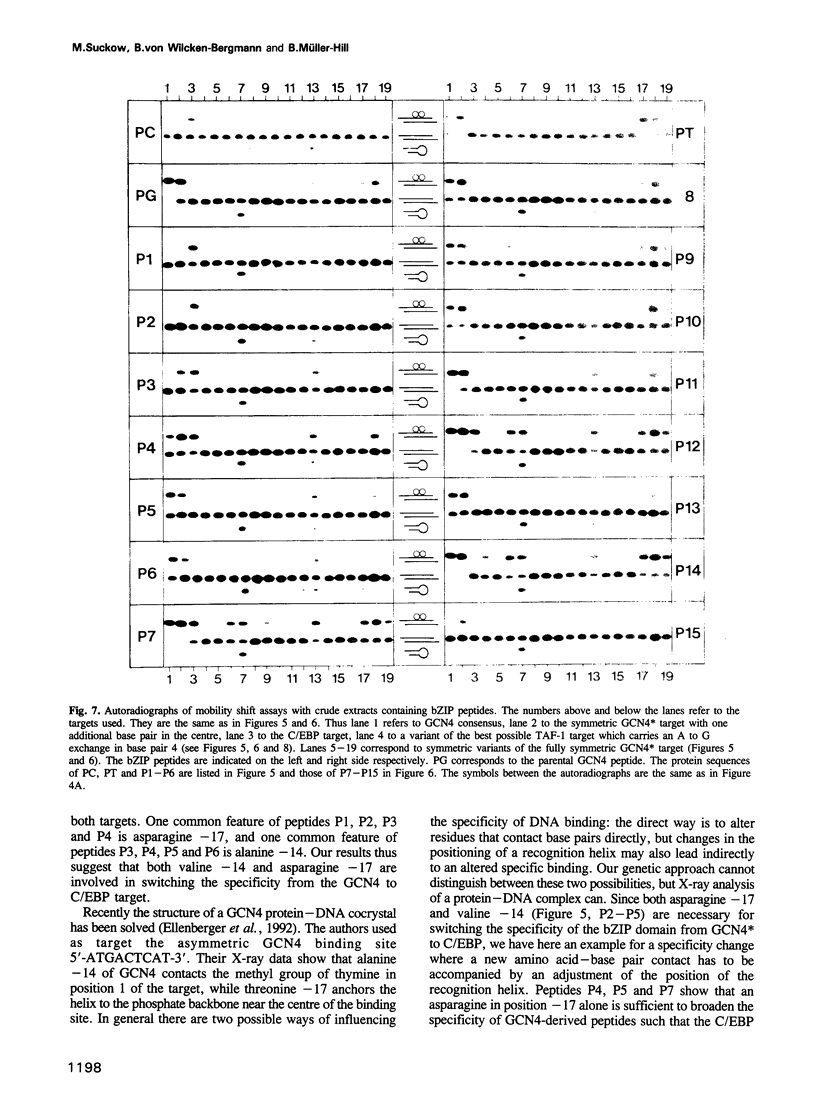
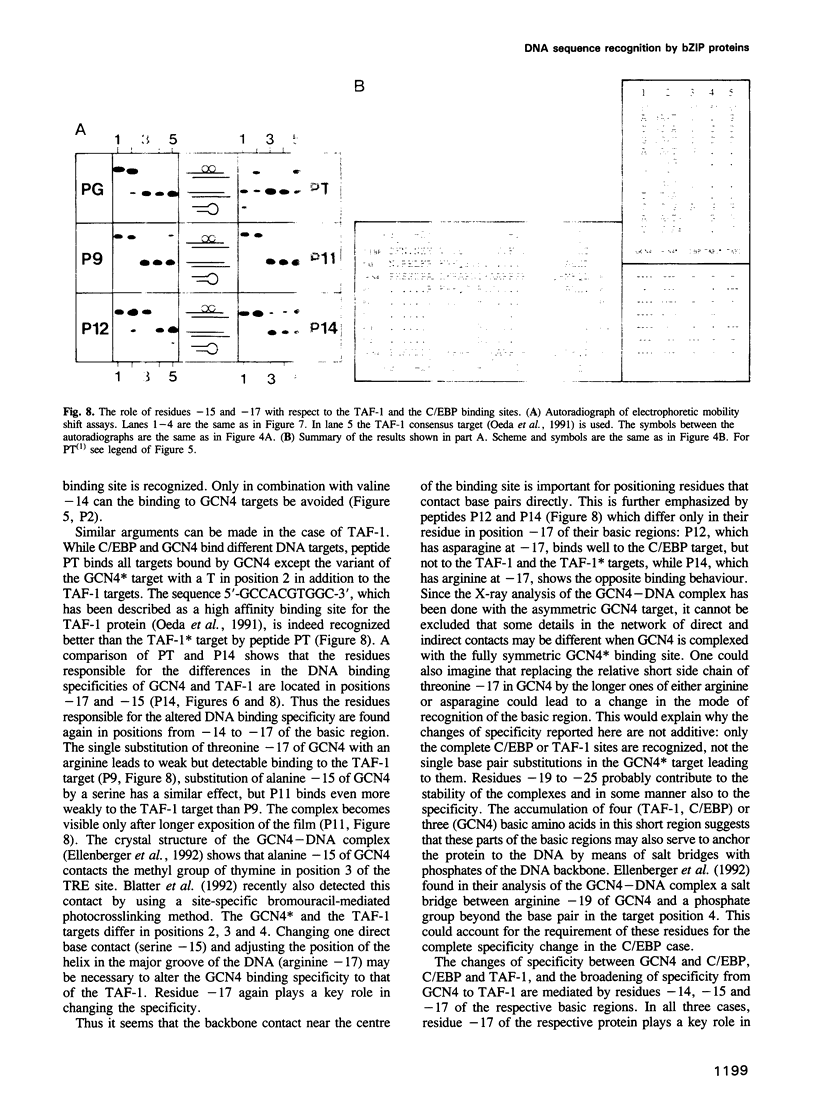
The bZIP regions of the eukaryotic transcription factors GCN4 and C/EBP have similar protein sequences but they recognize different DNA sequences. In order to understand their specificity, a vector was constructed which permits overexpression in Escherichia coli of those domains of GCN4 that are necessary and sufficient for specific DNA binding i.e. the basic region and the leucine zipper. Specific DNA binding was monitored with gel shift experiments. The residues of the basic region of GCN4 were systematically replaced by those of C/EBP to transform GCN4 into C/EBP with respect to DNA binding. Residues -17, -16 and -14 were found to be responsible for switching GCN4 to C/EBP binding specificity (we define as residue +1 the first leucine of the first leucine heptad repeat of GCN4). We broadened the specificity of GCN4 to TAF-1 by replacing residues -15 and -17 and we changed the specificity of C/EBP to TAF-1 by swapping residue -17 of a particular hybrid. Thus residues positioned from -14 to -17 of the basic region play a key role in recognizing specific DNA sequences.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Johnson P. F., McKnight S. L. Cognate DNA binding specificity retained after leucine zipper exchange between GCN4 and C/EBP. Science. 1989 Nov 17;246(4932):922–926. doi: 10.1126/science.2530632. [DOI] [PubMed] [Google Scholar]
- Blatter E. E., Ebright Y. W., Ebright R. H. Identification of an amino acid-base contact in the GCN4-DNA complex by bromouracil-mediated photocrosslinking. Nature. 1992 Oct 15;359(6396):650–652. doi: 10.1038/359650a0. [DOI] [PubMed] [Google Scholar]
- Boelens R., Lamerichs R. M., Rullmann J. A., van Boom J. H., Kaptein R. The interaction of lac repressor headpiece with its operator: an NMR view. Protein Seq Data Anal. 1988;1(6):487–498. [PubMed] [Google Scholar]
- Cunningham A. M., Reed R. R. A sense of smell. Curr Biol. 1992 Mar;2(3):116–118. doi: 10.1016/0960-9822(92)90239-7. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Brandl C. J., Struhl K., Harrison S. C. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted alpha helices: crystal structure of the protein-DNA complex. Cell. 1992 Dec 24;71(7):1223–1237. doi: 10.1016/s0092-8674(05)80070-4. [DOI] [PubMed] [Google Scholar]
- Gentz R., Langner A., Chang A. C., Cohen S. N., Bujard H. Cloning and analysis of strong promoters is made possible by the downstream placement of a RNA termination signal. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4936–4940. doi: 10.1073/pnas.78.8.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. E., Hope I. A., Macke J. P., Struhl K. Saturation mutagenesis of the yeast his3 regulatory site: requirements for transcriptional induction and for binding by GCN4 activator protein. Science. 1986 Oct 24;234(4775):451–457. doi: 10.1126/science.3532321. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell. 1985 Nov;43(1):177–188. doi: 10.1016/0092-8674(85)90022-4. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. GCN4, a eukaryotic transcriptional activator protein, binds as a dimer to target DNA. EMBO J. 1987 Sep;6(9):2781–2784. doi: 10.1002/j.1460-2075.1987.tb02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. DNA bending by Fos and Jun: the flexible hinge model. Science. 1991 Nov 22;254(5035):1210–1214. doi: 10.1126/science.1957173. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kisters-Woike B., Lehming N., Sartorius J., von Wilcken-Bergmann B., Müller-Hill B. A model of the lac repressor-operator complex based on physical and genetic data. Eur J Biochem. 1991 Jun 1;198(2):411–419. doi: 10.1111/j.1432-1033.1991.tb16030.x. [DOI] [PubMed] [Google Scholar]
- Kolkhof P., Teichmann D., Kisters-Woike B., von Wilcken-Bergmann B., Müller-Hill B. Lac repressor with the helix-turn-helix motif of lambda cro binds to lac operator. EMBO J. 1992 Aug;11(8):3031–3038. doi: 10.1002/j.1460-2075.1992.tb05373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lehming N., Sartorius J., Kisters-Woike B., von Wilcken-Bergmann B., Müller-Hill B. Mutant lac repressors with new specificities hint at rules for protein--DNA recognition. EMBO J. 1990 Mar;9(3):615–621. doi: 10.1002/j.1460-2075.1990.tb08153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Sartorius J., Niemöller M., Genenger G., v Wilcken-Bergmann B., Müller-Hill B. The interaction of the recognition helix of lac repressor with lac operator. EMBO J. 1987 Oct;6(10):3145–3153. doi: 10.1002/j.1460-2075.1987.tb02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., Hoess R. H., DeGrado W. F. Design of DNA-binding peptides based on the leucine zipper motif. Science. 1990 Aug 17;249(4970):774–778. doi: 10.1126/science.2389143. [DOI] [PubMed] [Google Scholar]
- Oeda K., Salinas J., Chua N. H. A tobacco bZip transcription activator (TAF-1) binds to a G-box-like motif conserved in plant genes. EMBO J. 1991 Jul;10(7):1793–1802. doi: 10.1002/j.1460-2075.1991.tb07704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant A. R., Brandl C. J., Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol. 1989 Jul;9(7):2944–2949. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W. T., Struhl K. Highly conserved residues in the bZIP domain of yeast GCN4 are not essential for DNA binding. Mol Cell Biol. 1991 Oct;11(10):4918–4926. doi: 10.1128/mcb.11.10.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Sartorius J., Lehming N., Kisters-Woike B., von Wilcken-Bergmann B., Müller-Hill B. The roles of residues 5 and 9 of the recognition helix of Lac repressor in lac operator binding. J Mol Biol. 1991 Mar 20;218(2):313–321. doi: 10.1016/0022-2836(91)90714-h. [DOI] [PubMed] [Google Scholar]
- Sartorius J., Lehming N., Kisters B., von Wilcken-Bergmann B., Müller-Hill B. lac repressor mutants with double or triple exchanges in the recognition helix bind specifically to lac operator variants with multiple exchanges. EMBO J. 1989 Apr;8(4):1265–1270. doi: 10.1002/j.1460-2075.1989.tb03500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sellers J. W., Vincent A. C., Struhl K. Mutations that define the optimal half-site for binding yeast GCN4 activator protein and identify an ATF/CREB-like repressor that recognizes similar DNA sites. Mol Cell Biol. 1990 Oct;10(10):5077–5086. doi: 10.1128/mcb.10.10.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D., Pu W. T., Struhl K. Mutations in the bZIP domain of yeast GCN4 that alter DNA-binding specificity. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2007–2011. doi: 10.1073/pnas.89.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Tils D., Sartorius J., Auerswald E. A., Schröder W., Müller-Hill B. A synthetic operon containing 14 bovine pancreatic trypsin inhibitor genes is expressed in E. coli. EMBO J. 1986 Dec 1;5(12):3219–3225. doi: 10.1002/j.1460-2075.1986.tb04632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





