Abstract
For most bones, elongation is driven primarily by chondrogenesis at the growth plates. This process results from chondrocyte proliferation, hypertrophy, and extracellular matrix secretion and is carefully orchestrated by complex networks of local paracrine factors and modulated by endocrine factors. We review here recent advances in the understanding of growth plate physiology. These advances include new approaches to study expression patterns of large numbers of genes in the growth plate, using microdissection followed by microarray. This approach has been combined with genome-wide association studies to provide insights into the regulation of the human growth plate. We also review recent studies elucidating the roles of bone morphogenetic proteins, fibroblast growth factors, C-type natriuretic peptide, and suppressor of cytokine signaling in the local regulation of growth plate chondrogenesis and longitudinal bone growth.
Introduction
In the postnatal mammal, elongation of tubular bones occurs at the growth plate. This cartilaginous structure comprises three zones which contain chondrocytes at different stages of differentiation (Kronenberg 2003). The zone closest to the epiphysis is termed the resting zone. The resting zone is thought to contain chondrocytes that serve as progenitor cells which can generate new clones of rapidly proliferating chondrocytes (Abad et al. 2002). Each derivative clone forms a cell column aligned parallel to the long axis of the bone. As these cells replicate, the two daughters line up parallel to the long axis, to maintain the columnar organization. The chondrocytes farther from the epiphysis undergo termination differentiation, in which they cease proliferating and enlarge to form the hypertrophic zone. Throughout the growth plate, chondrocytes secrete proteins and proteoglycans that form the cartilage extracellular matrix. In the resting and proliferative zone, collagen II represents a major component of this matrix, whereas in the hypertrophic zone, there is a shift to production of collagen X (Kronenberg 2003). The hypertrophic chondrocytes farthest from the epiphysis undergo cell death. This cell death has been attributed to apoptosis, but more recent evidence challenges this conclusion (Emons et al. 2009). This region is then invaded from the metaphyseal bone by blood vessels and differentiating osteoblasts and osteoclasts, which remodel the cartilage into bone tissue. The net result of this chondrogenesis and ossification is the formation of new bone underneath the growth plate and therefore bone elongation.
The integrated processes of chondrocyte differentiation, proliferation, cartilage matrix secretion, cell death, and of vascular and bone cell invasion are regulated and coordinated by a complex array of paracrine signaling molecules, which includes insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), Indian hedgehog (IHH) and parathyroid hormone-related protein (PTHrP), bone morphogenic proteins (BMPs), WNTs, and vascular endothelial growth factors (VEGFs). In addition, the rate of endochondral bone formation at the growth plate is regulated by an array of endocrine signals, including growth hormone (GH), IGF-I, thyroid hormone, glucocorticoids, androgens, and estrogens. One of the principal apparent functions of this endocrine system is to allow rapid growth only when the organism is able to take in plentiful nutrients.
Because the growth plate requires so many paracrine and endocrine signaling pathways to function normally, mutations in many genes involved in these signaling pathways lead to bones that are short, which in humans presents as short stature, and often malformed, which presents as a skeletal dysplasia. Thus, mutations in more than 200 genes cause distinct skeletal dysplasias (Warman et al. 2011).
Although there has been remarkable progress recently in our understanding of these signaling pathways that regulate the postnatal growth plate, much remains to be learned. In this review, we present some recent studies giving new insights into these control systems. The number of studies to be reviewed had to be limited, and therefore not all important areas of progress could be included.
Delineating gene expression patterns in the mammalian postnatal growth plate
In the past, gene expression within the growth plate has typically been studied by in situ hybridization, which provides much useful information but necessarily involves studying one candidate gene at a time. However, recently, methods have been developed to study expression patterns of large numbers of genes in the growth plate, using microdissection, followed by microarray (Nilsson et al. 2007). Frozen sections of the growth plate are first microdissected into their constituent zones after which RNA is isolated and mRNA patterns are assessed by microarray. Presumably, the method could readily be modified to use RNA sequencing in place of microarray.
This approach was applied to the proximal tibiae of 1-week old rats and the resulting expression data were analyzed using bioinformatics algorithms (Lui et al. 2010). Expression in the resting and the proliferative zone was compared to identify pathways involved in the differentiation of resting zone to proliferative zone chondrocytes. This analysis implicated vitamin D receptor / retinoid × receptor (VDR/RXR) activation, platelet-derived growth factor (PDGF) signaling, BMP signaling, and notch signaling. Similar analysis of the proliferative to hypertrophic differentiation step implicated p53 signaling, ephrin receptor signaling, oncostatin M signaling, and BMP signaling (Lui et al. 2010).
Evidence for a BMP signaling gradient across the growth plate
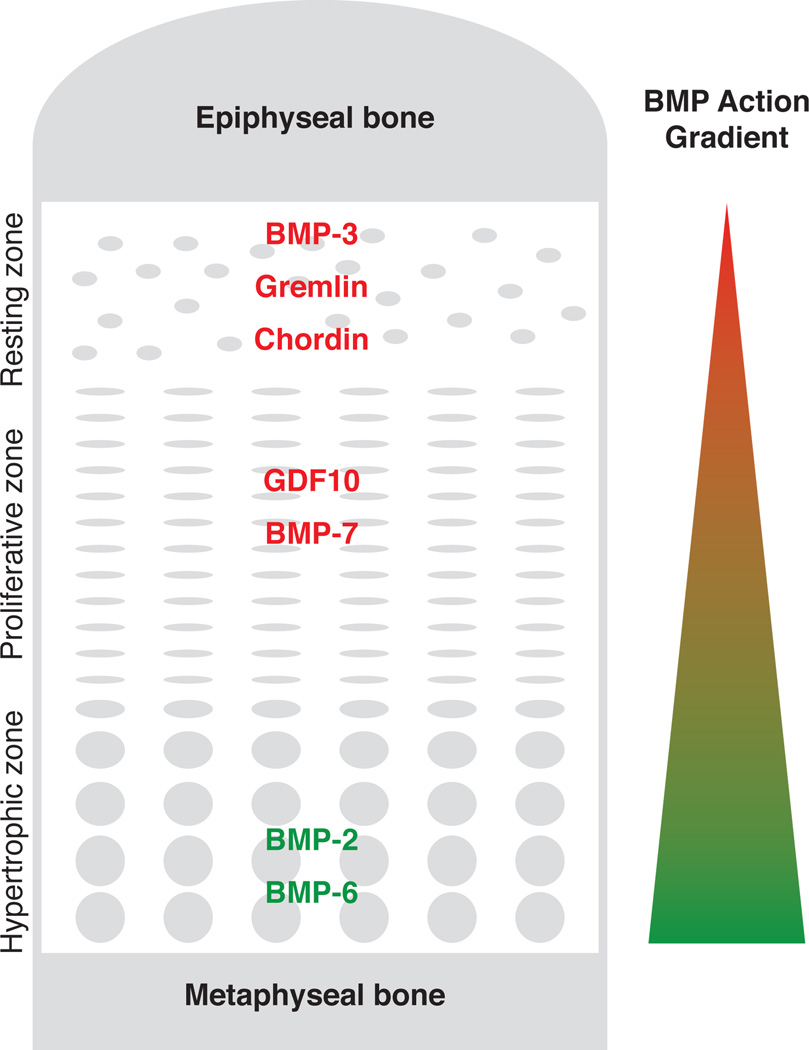
As noted above, microarray analysis implicated BMP signaling in both the differentiation of resting zone chondrocytes to proliferative zone chondrocytes and of proliferative zone chondrocytes to hypertrophic zone chondrocytes. More extensive analysis of the BMP signaling pathway using micodissection followed by real-time PCR has shown evidence for a BMP signaling gradient across the growth plate with the greatest BMP signaling occurring in the hypertrophic zone and the least in the resting zone (Nilsson et al. 2007). Consistent with this concept, immunolocalization of phosphorylated SMAD-1, - 5, and -8 in the growth plate increases with increasing distance from the epiphysis (Yoon et al. 2006).
These patterns suggest that a BMP signaling gradient across the growth plate may contribute to the progressive differentiation of resting to proliferative to hypertrophic chondrocytes (Fig.1). Low levels of BMP signaling in the resting zone may help maintain the progenitor cell state. Farther from the epiphysis, greater BMP signaling may induce differentiation to proliferative chondrocytes and, even farther from the epiphysis, yet greater BMP signaling may induce terminal differentiation to hypertrophic chondrocytes. Functional studies support this model. Bmp2 stimulates resting zone chondrocytes to proliferate and stimulates proliferative zone chondrocytes to hypertrophy in an organ culture model (De Luca et al. 2001). In vivo overexpression of constitutively active Bmpr1a in mice has no effect on proliferation but accelerates hypertrophic differentiation (Kobayashi et al. 2005). Recent evidence specifically implicates Bmp2 in this process. In mice, conditional targeted ablation of Bmp2 causes severe defects in chondrocyte proliferation and differentiation through a mechanism involving Runx2 protein levels (Shu et al. 2011). The effects of BMPs on the growth plate appear to involve the canonical BMP signaling pathway in that combined loss of regulatory Smad1 and Smad5 in mice causes a severe skeletal dysplasia with impaired proliferation and hypertrophic differentiation (Retting et al. 2009). Although this review focuses on the function of the postnatal growth plate, it is important to recognize that BMP signaling affects embryonic development of the cartilaginous skeleton and thus genetic manipulations in mice may have combined embryonic and postnatal effects.
Figure 1. Hypothesized BMP action gradient in the growth plate.
Based on previous microarray and real-time PCR data, BMP agonists (green) and antagonists (red) were expressed primarily in the hypertrophic zone and resting zone, respectively. These findings suggest a BMP signaling gradient across the growth plate that may be important for spatial control of chondrocyte differentiation within the growth plate.
In addition to BMPs, other paracrine systems also appear to form gradients across the growth plate. Of these, the best studied involves parathyroid hormone-related protein (PTHrP). In the embryonic skeleton, PTHrP is secreted by periarticular chondrocytes of long bones (Kronenberg 2003). PTHrP diffuses across the growth cartilage maintaining chondrocytes in the proliferative state (Hirai et al. 2011). Cells more distant from the source of PTHrP undergo hypertrophic differentiation. The prehypertrophic and hypertrophic chondrocytes then secrete Indian hedgehog (Ihh), which has a negative-feedback effect on PTHrP production and also independent effects on chondrocyte differentiation. More recent evidence suggests that the Ihh–PTHrP system is maintained in postnatal growth plate but the PTHrP source shifts to the resting zone (Chau et al. 2011; Hirai et al. 2011; Koziel et al. 2005).
Genome-wide association studies provide insights into the regulation of the human growth plate
A recent large meta-analysis of genome-wide association (GWA) studies identified at least 180 loci that influence adult height (Lango et al. 2010). Some of the genes within these loci likely affect height through endocrine mechanisms, such as GH1, which encodes GH, and GHSR, which encodes the GH secretagogue receptor. However, other genes likely affect height through a direct, local effect in the growth plate, such as ACAN, which encodes aggrecan, a critical proteoglycan component of the cartilage matrix. Thus GWA studies of height have the potential to provide important insights into the molecular pathways regulating the human growth plate.
However, one challenge in the analysis of GWA data is to identify the causative gene(s) in each locus. At most loci, there are multiple genes that are sufficiently close to account for the linkage to adult stature, and thus additional information is needed to determine which of these genes modulates height and which are merely located close to the causative genes. We therefore used a mouse knockout phenotype database and human disease databases to identify genes within the GWA loci that are likely required for normal growth plate function. We also used expression microarray studies of mouse and rat growth plate to identify genes that have higher expression in growth plate cartilage than in other tissues, genes that are spatially regulated across different zones in the growth plate and/or genes that are temporally regulated in the growth plate during postnatal life, as growth plate function declines.
The combined phenotype-expression-GWA analysis implicated 78 genes in human growth plate function (Lui et al. 2012). Of these, some were already known to function in the human growth plate because human mutations affect the growth plate. In addition, many of the implicated genes participate in molecular pathways that have previously been implicated in the regulation of the growth plate chondrocyte proliferation and differentiation in the mouse, such as the IHH-PTHrP system (GLI2, IHH, HHIP, PTCH1, and PTHLH lie within GWAS loci), BMP/TGF superfamily signaling (TGFB2, BMP6, LTBP3, NOG, BMP2, GDF5), C-type natriuretic peptide signaling (NPPC, PRKG2, NPR3), GH-IGF-I signaling (IGF2BP2, IGF2BP3, IGF1R), and FGF signaling (FGF18). This analysis suggests that these pathways are important not only in the mouse but also in the human growth plate.
In addition, the method implicates many genes not previously known to regulate either the mouse or human growth plate (Lui et al. 2012). For example, the analysis implicates IGF2BP2 and IGF2BP3 based on presence in the GWA loci and expression patterns in the growth plate. These mRNA binding proteins have previously been implicated in mRNA localization, turnover, and translational control (Christiansen et al. 2009), and mRNA targets include Igf2, H19, c-myc, beta-actin, and Gdf1. Although neither IGF2BP2 nor IGF2BP3 has a recognized mouse or human phenotype, targeted ablation of the third member of the gene family, IGF2BP1, impairs bone growth and advances mineralization (Hansen et al. 2004). Thus, the data suggest that this family of proteins regulates growth plate chondrogenesis in both mice and humans.
Loss-of-function mutations of CNP impair and gain-of-function mutations stimulate bone growth
One interesting pathway implicated by the combined microarray-GWAS analysis and by previous studies is C-type natriuretic peptide (CNP, or NPPC) signaling (Lui et al. 2012). CNP belongs to a family of three natriuretic peptides, with ANP and BNP being the other two members (Potter et al. 2006). Unlike the other two members, CNP does not stimulate “natriuresis” at physiological concentrations. Instead, CNP is found in high concentration in cartilage (Hagiwara et al. 1994) and functions primarily as a local cartilage growth factor to stimulate growth plate chondrocytes (Pejchalova et al. 2007). Interestingly, homozygous loss-of-function mutations of the CNP receptor, natriuretic peptide receptor B (NPR-B, or NPR2), which is also highly expressed in the growth plate, cause acromesomelic dysplasia type Maroteaux in humans (Bartels et al. 2004), while heterozygous mutations of NPR2 are associated with short stature (Olney et al. 2006; Vasques et al. 2013). Conversely, activating mutation of NPR2 (Miura et al. 2012; Hannema et al. 2013) and overexpression of NPPC (Agoston et al. 2007) in humans both cause overgrowth disorders. These growth phenotypes have been replicated in knockout and transgenic mice, with Nppc or Npr2 knockout causing severe short stature (Chusho et al. 2001; Tsuji & Kunieda 2005) and transgenic expression of activated Npr2 causing tall stature (Miura et al. 2012). At the cellular level, CNP stimulates chondrocyte proliferation, chondrocyte hypertrophy, and cartilage matrix production (Mericq et al. 2000; Agoston et al. 2007). At the molecular level, CNP inhibits the extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathways (Ozasa et al. 2005), therefore counteracting the growth-inhibitory downstream signaling of fibroblast growth factor (FGF) in the growth plate (Yasoda et al. 2004), which will be discussed in the next section. Due to its potent effect on offsetting FGF signaling, the use of CNP in treating achondroplasia (ACH) caused by activating mutation of FGF receptor 3 (FGFR3) is under active investigation. It is yet unclear if all the growth-stimulating effects of CNP on chondrocytes are dependent on FGF signaling.
In addition to CNP, a related peptide, brain natriuretic peptide (BNP), also has been implicated in growth plate regulation. There is evidence that BNP is transcriptionally regulated by the transcription factor SHOX (Marchini et al. 2007). Because SHOX deficiency underlies the growth plate dysfunction in Leri-Weill, Langer, and Turner syndromes, the findings suggest that decreased BNP expression may play a role in the pathogenesis of these disorders.
Elucidating the role of FGFs in growth plate
FGF signaling is important for growth plate development, as mutations in various FGF receptors (FGFR) can lead to skeletal disease in humans (Chen & Deng 2005). Various in vivo studies suggest that FGFR1 and FGFR3 signaling are growth-inhibiting, while FGFR2 signaling is growth-promoting. Cartilage-specific (Col2a1-Cre) inactivation of Fgfr1 in mice showed a transient increase height in hypertrophic zone, and delayed terminal differentiation of hypertrophic chondrocytes (Jacob et al. 2006). However, increase in adult body length has not been reported. In contrast, inactivation of Fgfr2 in the mesenchymal condensations (Dermo1-cre), which affects both the osteoblast and chondrocyte lineages, resulted in mice with skeletal dwarfism (Yu et al. 2003), suggesting a growth-promoting effect of Fgfr2 signaling. Clinically, FGFR3 signaling is perhaps most relevant to growth plate development, as gain-of-function mutations of FGFR3 in humans cause achondroplasia (ACH), hypochondroplasia, and thanatophoric dysplasia (Rousseau et al. 1994; Shiang et al. 1994; Foldynova-Trantirkova et al. 2012).
Consistently, transgenic mice with activated Fgfr3 in the growth plate show reduced chondrocyte proliferation, decreased numbers of hypertrophic chondrocytes and decreased height of the hypertrophic zone (Chen et al. 1999), while Fgfr3 knockout mice showed increased chondrocyte proliferation, increased height of hypertrophic zone, and increased skeletal growth (Eswarakumar & Schlessinger 2007).
Several signaling pathways downstream of FGFR3 activation have been elucidated, including phosphoinositide 3 kinase-AKT pathway (Priore et al. 2006; Ulici et al. 2010), the extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathway (Krejci et al. 2008; Matsushita et al. 2009), and the signal transducer and activation of transcription (STAT) pathway (Li et al. 1999). These advancements in our understanding of the FGFR3 signaling pathway have contributed to the ongoing development of therapeutics for ACH. For example, growth-plate specific overexpression of CNP (Col2a1-Nppc) or administration of a CNP analogue has been shown to counteract FGF-induced MAPK activation and rescue the growth phenotype of ACH mice (Yasoda et al. 2004; Lorget et al. 2012). Other recently described potential therapeutics of ACH include meclizine, an anti-histaminic drug that promotes chondrocyte proliferation (Matsushita et al. 2013); and a soluble form of human FGFR3 (sFGFR3) that acts as a decoy receptor to interfere with FGF binding and signaling (Garcia et al. 2013).
Expression studies in rodents have provided clues to the physiological ligands for FGF receptors in the growth plate. In growth plates of 1-wk old rats, only Fgf2, 7, 18, and 22 expression was detectable by real-time PCR (Lazarus et al. 2007), whereas expression was far higher in the perichondrium adjacent to the growth plate, particularly for Fgf1,2,6,7,9,18 (Lazarus et al. 2007). In human fetal growth plate expression of FGF1,2,5,8-14,16-19,21 was detected at the mRNA level and FGF1,2,17,19 at the protein level (Krejci et al. 2007). Functional studies in mice suggest signaling by Fgf9 and Fgf18 both contribute to growth plate development. Knockout mouse models of Fgf9 (Hung et al. 2007) and Fgf18 (Liu et al. 2002) suggest that both Fgf9 and Fgf18 promote chondrocyte proliferation during early development of the growth plate, but then function to inhibit chondrocyte proliferation and promote hypertrophic differentiation at later stages of development.
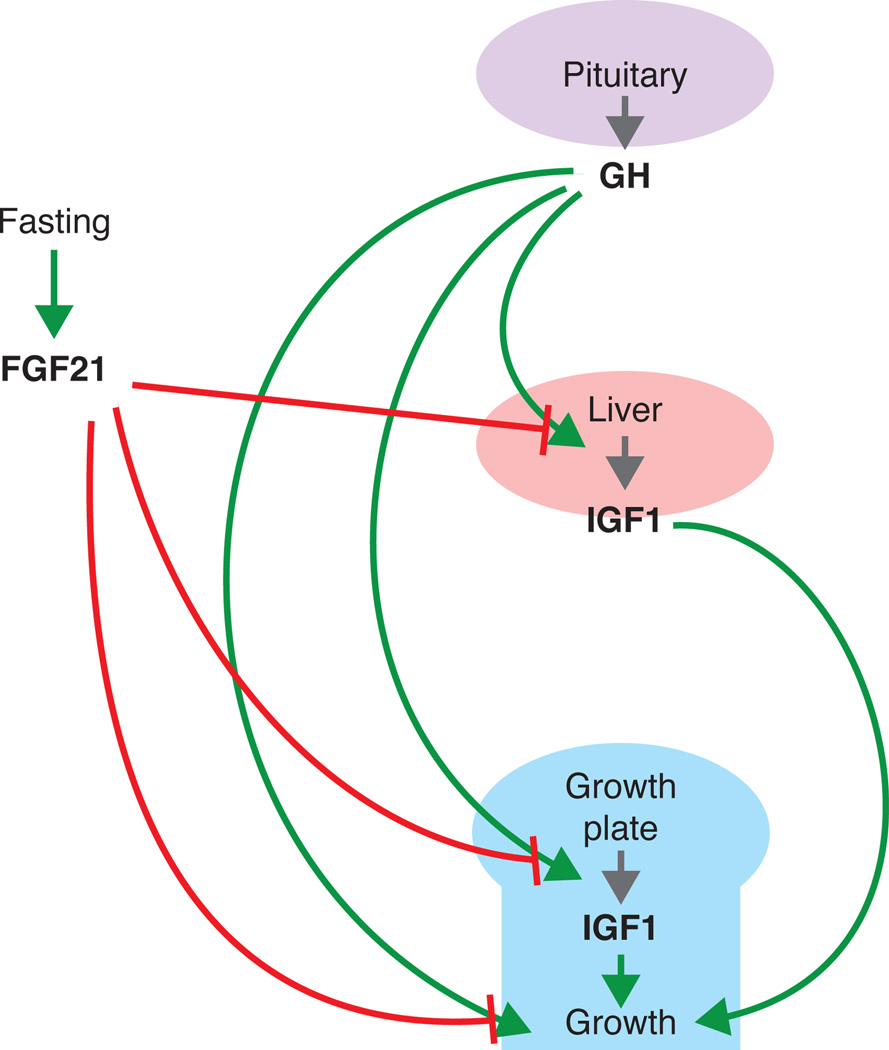
An interesting crosstalk between FGF signaling and GH-IGF-I signaling in the growth plate has recently been discovered that primarily involves FGF21 (Inagaki et al. 2008). FGF21 is a part of a subfamily of FGFs (other members include FGF15/19 and FGF23) that lack the FGF heparin-binding domain (Kharitonenkov et al. 2005), and therefore can act both locally in a paracrine fashion and diffuse from the tissue of synthesis to act as an endocrine factor. FGF21 can activate FGFR1 and FGFR3 (Suzuki et al. 2008), both of which elicit growth-inhibitory signaling as discussed earlier. Consistently, transgenic mice overexpressing Fgf21 exhibit reduced bone growth, and interestingly, hepatic GH insensitivity (Inagaki et al. 2008).
FGF21 expression does not seem to be required for normal development of the growth plate, as Fgf21 knockout mice showed no significant difference in body weight and body length as compared to wild type mice (Kubicky et al. 2012). However, mounting evidence suggest that FGF21 plays an important role in fasting-induced growth inhibition (Fig.2). It is well established that reduced caloric intake in mammals causes reduced skeletal growth and hepatic GH insensitivity, which is partly attributed to decreased GH receptor (GHR) expression in the liver (Bornfeldt et al. 1989; Straus & Takemoto 1990). Numerous studies have shown that FGF21 expression is induced by fasting (Galman et al. 2008). Interestingly, when wild type and Fgf21 knockout mice were placed under food restriction, Fgf21 knockout mice showed significantly improved linear growth and growth plate thickness as compared to wild type, suggesting the growth suppression by fasting is elicited by Fgf21 (Kubicky et al. 2012). Most importantly, many of the molecular changes induced by fasting, including decreased hepatic GH sensitivity and decreased GHR and IGF-I expression in the growth plate, were corrected by FGF21 deletion (Kubicky et al. 2012). More recently, in vitro studies using cultured growth plate chondrocytes suggested FGF21 may inhibit bone growth by directly suppressing chondrogenesis and GH action locally at the growth plate (Wu et al. 2012; Wu et al. 2013). Whether FGF21 mediates the effects of malnutrition on childhood growth in humans is less clear. Circulating FGF21 levels in humans appear to be less responsive to fasting than in the rodent and are actually elevated in obese humans (Woo et al. 2013).
Figure 2. Proposed role of FGF21 in fasting-induced growth inhibition.
Green arrows, stimulation; red blunt end arrows, inhibition; grey arrows, production. Evidence suggests that fasting-induced FGF21 inhibits GH-induced IGF-I production in the liver, as well as the local effects of GH (IGF-I-dependent and IGF-I-independent) at the growth plate.
Modulation of the GH/IGF-I axis by SOCS2
The importance of GH and IGF-I in stimulating longitudinal growth has long been established. GH excess caused by pituitary adenomas in childhood can lead to gigantism. Conversely, GH deficiency or GH insensitivity caused by mutations in the GH receptor or signaling pathways markedly impairs postnatal growth (Rosenfeld et al. 2007). Patients with untreated isolated GH deficiency have an average final height SDS of −4.7 (range: −6.1 to −3.9) (Wit et al. 1996). Interestingly, GH has no apparent role in fetal growth, despite the presence of its receptor (GHR) in embryos (Garcia-Aragon et al. 1992). Experimental ablation of the pituitary in animals, or mutations of GHR that affects GH actions in both mice and humans has no significant effect on prenatal growth (Laron et al. 1993; Lupu et al. 2001). In contrast, IGF-I is important for both fetal and postnatal growth, as suggested by the observations that mutations of IGF1 or IGF1R, the gene encoding its receptor, in humans lead to intrauterine (Abuzzahab et al. 2003; Fang et al. 2012) and postnatal (Baker et al. 1993) growth retardation.
GH affects the growth plate through several mechanisms. Some stimulatory effect is mediated through circulating IGF-I, as evidenced by the observation that combined deficiency in acid-labile subunit and liver-specific deficiency of IGF-1 modestly decreases longitudinal bone growth in mice (Yakar et al. 2002). However, Col2-driven ablation of IGF-I in also decreases linear growth suggesting a role for local skeletal IGF-I production in regulating growth plate function (Govoni et al. 2007), although not necessarily from chondrocytes (Parker et al. 2007). Furthermore, mice lacking both the GH receptor and IGF-I have shorter bones than mice lacking only IGF-I, suggesting that GH, at least at a super-physiologic circulating concentrations, has an IGF-I-independent effect on bone growth (Lupu et al. 2001).
The effect of GH on longitudinal growth can be mediated by stimulation of liver-derived/endocrine IGF-I, or its local effects on the growth plate, which can be further divided into the stimulation of local IGF-I production or its direct, IGF-independent effects. Much work has been devoted to distinguish between these effects of GH, and excellent reviews on this subject are available elsewhere (Wit & Camacho-Hubner 2011; Ahmed & Farquharson 2010) and therefore will not be discussed further. Instead, here we highlight some of the recent work that established SOCS2 as a key modulator of local GH action in the growth plate.
The Suppressor of Cytokine Signaling (SOCS) family contains eight members, SOCS1-7 and cytokine inducible SH2-containing protein (CIS). SOCS proteins are upregulated in response to cytokine stimulation, and can subsequently bind through their SH2 domain to phosphorylated tyrosines in the cytokine receptor-JAK complex to inhibit further cytokine receptor activation. As such, SOCS proteins form part of a classical negative feedback circuit (Krebs & Hilton 2001). The role of SOCS2 in postnatal growth was demonstrated by the overgrowth phenotype of Socs2 knockout mice (Metcalf et al. 2000), Socs2−/− mice showed increased body length and body weight, and increased GH/IGF-I signaling with wider proliferative and hypertrophic zones in the growth plate (Metcalf et al. 2000; MacRae et al. 2009). Recent evidence suggests that SOCS2 acts locally at the growth plate to modulate GH signaling. Chondrocytes isolated from Socs2−/− mice showed increased STATs phosphorylation upon incubation with GH (Pass et al. 2012), while cells overexpressing SOCS2 did not. Similarly, GH was able to stimulate growth in fetal metatarsals isolated from Socs2−/− mice, but not that from wild type mice (Pass et al. 2012), suggesting local GH action at the growth plate is negatively regulated by SOCS2. Some evidence suggests such local modulation of GH action is IGF-independent, since the GH-induced Socs2−/− metatarsal bone growth is not accompanied by increase in Igf1 or Igfbp3 transcript levels, and occurred in the presence of an IGF-I receptor inhibitor (NVP-AEW541) (Dobie et al. 2013, unpublished). A role of SOCS2 in human growth is suggested by the identification of SOCS2 in a locus associated with human height variation by GWAS (Lui et al. 2012; Weedon et al. 2008; Lango et al. 2010). Interestingly, a missense mutation in SOCS2 has been reported (in meeting abstract form) to cause gigantism (Suda et al. 2011).
Summary and future prospective
The understanding of the paracrine regulation of longitudinal bone growth at the growth plate has advanced substantially in recent years. In this brief review we have focused on some of the recent advances that have been possible due to microdissection, microarray analysis, inducible and tissue-specific gene targeting in mice, genome-wide association studies, and genetic studies of rare disease. These studies have not only described important biological mechanisms and processes, but also identified many new genes and pointed to a promising potential treatment for achondroplasia that is currently being evaluated in human studies. However, many important questions remain to be elucidated. For example, information on how the endocrine system interacts with the paracrine signals to regulate growth plate chondrogenesis is mostly lacking, as well as molecular mechanisms for the orientation of proliferative chondrocytes into columns and mechanisms that causes the proliferation rate and growth rate to slow with age and thus limits the overall size of the skeleton and thus the organism. Continued methodological advancements promise to accelerate progress in our understanding of skeletal development, skeletal growth, and the disorders affecting these processes and will likely yield new therapeutic targets and approaches.
Acknowledgements
JCL and JB were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), US National Institutes of Health (NIH). ON was supported by an ESPE Research Fellowship Grant and grants from the Swedish Research Council (K2012-99X-21998-01-3), the Swedish Society of Medicine, Her Royal Highness Crown Princess Lovisa’s Foundation for Pediatric Care, Wera Ekstrom’s Foundation for Pediatric Research, Märta och Gunnar V Philipson’s Foundation, Sällskapet Barnavård, Stiftelsen Frimurare Barnhuset i Stockholm, and Karolinska Institutet.
Reference List
- Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 2002;143:1851–1857. doi: 10.1210/endo.143.5.8776. [DOI] [PubMed] [Google Scholar]
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfaffle R, Raile K, Seidel B, Smith RJ, Chernausek SD. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N.Engl.J.Med. 2003;349:2211–2222. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC.Dev.Biol. 2007;7:18. doi: 10.1186/1471-213X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SF, Farquharson C. The effect of GH and IGF1 on linear growth and skeletal development and their modulation by SOCS proteins. J Endocrinol. 2010;206:249–259. doi: 10.1677/JOE-10-0045. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, Kant S, Cole T, Morton J, Cormier-Daire V, Faivre L, Lees M, Kirk J, Mortier GR, Leroy J, Zabel B, Kim CA, Crow Y, Braverman NE, van den Akker F, Warman ML. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am.J Hum.Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt KE, Arnqvist HJ, Enberg B, Mathews LS, Norstedt G. Regulation of insulin-like growth factor-I and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol. 1989;122:651–656. doi: 10.1677/joe.0.1220651. [DOI] [PubMed] [Google Scholar]
- Chau M, Forcinito P, Andrade AC, Hegde A, Ahn S, Lui JC, Baron J, Nilsson O. Organization of the Indian hedgehog--parathyroid hormone-related protein system in the postnatal growth plate. J Mol.Endocrinol. 2011;47:99–107. doi: 10.1530/JME-10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Adar R, Yang X, Monsonego EO, Li C, Hauschka PV, Yayon A, Deng CX. Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. J Clin.Invest. 1999;104:1517–1525. doi: 10.1172/JCI6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Deng CX. Roles of FGF signaling in skeletal development and human genetic diseases. Front Biosci. 2005;10:1961–1976. doi: 10.2741/1671. [DOI] [PubMed] [Google Scholar]
- Christiansen J, Kolte AM, Hansen T, Nielsen FC. IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J Mol.Endocrinol. 2009;43:187–195. doi: 10.1677/JME-09-0016. [DOI] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc.Natl.Acad.Sci.U.S.A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca F, Barnes KM, Uyeda JA, De-Levi S, Abad V, Palese T, Mericq V, Baron J. Regulation of growth plate chondrogenesis by bone morphogenetic protein-2. Endocrinology. 2001;142:430–436. doi: 10.1210/endo.142.1.7901. [DOI] [PubMed] [Google Scholar]
- Emons J, Chagin AS, Hultenby K, Zhivotovsky B, Wit JM, Karperien M, Savendahl L. Epiphyseal fusion in the human growth plate does not involve classical apoptosis. Pediatr Res. 2009;66:654–659. doi: 10.1203/PDR.0b013e3181beaa8c. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Schlessinger J. Skeletal overgrowth is mediated by deficiency in a specific isoform of fibroblast growth factor receptor 3. Proc.Natl.Acad.Sci.U.S.A. 2007;104:3937–3942. doi: 10.1073/pnas.0700012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Hi CY, Derr MA, Rosenfeld RG, Hwa V, Cowell CT. Severe Short Stature Caused by Novel Compound Heterozygous Mutations of the Insulin-Like Growth Factor 1 Receptor (IGF1R) J.Clin.Endocrinol.Metab. 2012;97:E243–E247. doi: 10.1210/jc.2011-2142. [DOI] [PubMed] [Google Scholar]
- Foldynova-Trantirkova S, Wilcox WR, Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum.Mutat. 2012;33:29–41. doi: 10.1002/humu.21636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Garcia S, Dirat B, Tognacci T, Rochet N, Mouska X, Bonnafous S, Patouraux S, Tran A, Gual P, Le Marchand-Brustel Y, Gennero I, Gouze E. Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Sci.Transl.Med. 2013;5:203ra124. doi: 10.1126/scitranslmed.3006247. [DOI] [PubMed] [Google Scholar]
- Garcia-Aragon J, Lobie PE, Muscat GE, Gobius KS, Norstedt G, Waters MJ. Prenatal expression of the growth hormone (GH) receptor/binding protein in the rat: role for GH in embryonic and fetal development? Development. 1992;114:869–876. doi: 10.1242/dev.114.4.869. [DOI] [PubMed] [Google Scholar]
- Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007;30:354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Sakaguchi H, Itakura M, Yoshimoto T, Furuya M, Tanaka S, Hirose S. Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J Biol.Chem. 1994;269:10729–10733. [PubMed] [Google Scholar]
- Hannema SE, van Duyvenvoorde HA, Premsler T, Yang RB, Mueller TD, Gassner B, Oberwinkler H, Roelfsema F, Santen GW, Prickett T, Kant SG, Verkerk AJ, Uitterlinden AG, Espiner E, Ruivenkamp CA, Oostdijk W, Pereira AM, Losekoot M, Kuhn M, Wit JM. An activating mutation in the kinase homology domain of the natriuretic peptide receptor-2 causes extremely tall stature without skeletal deformities. J Clin.Endocrinol.Metab. 2013;98:E1988–E1998. doi: 10.1210/jc.2013-2358. [DOI] [PubMed] [Google Scholar]
- Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, Christiansen J, Nielsen FC. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol.Cell Biol. 2004;24:4448–4464. doi: 10.1128/MCB.24.10.4448-4464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Chagin AS, Kobayashi T, Mackem S, Kronenberg HM. Parathyroid hormone/parathyroid hormone-related protein receptor signaling is required for maintenance of the growth plate in postnatal life. Proc.Natl.Acad.Sci.U.S.A. 2011;108:191–196. doi: 10.1073/pnas.1005011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IH, Yu K, Lavine KJ, Ornitz DM. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev.Biol. 2007;307:300–313. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob AL, Smith C, Partanen J, Ornitz DM. Fibroblast growth factor receptor 1 signaling in the osteo-chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev.Biol. 2006;296:315–328. doi: 10.1016/j.ydbio.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin.Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc.Natl.Acad.Sci.U.S.A. 2005;102:18023–18027. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel L, Wuelling M, Schneider S, Vortkamp A. Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development. 2005;132:5249–5260. doi: 10.1242/dev.02097. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- Krejci P, Krakow D, Mekikian PB, Wilcox WR. Fibroblast growth factors 1, 2, 17, and 19 are the predominant FGF ligands expressed in human fetal growth plate cartilage. Pediatr Res. 2007;61:267–272. doi: 10.1203/pdr.0b013e318030d157. [DOI] [PubMed] [Google Scholar]
- Krejci P, Salazar L, Goodridge HS, Kashiwada TA, Schibler MJ, Jelinkova P, Thompson LM, Wilcox WR. STAT1 and STAT3 do not participate in FGF-mediated growth arrest in chondrocytes. J Cell Sci. 2008;121:272–281. doi: 10.1242/jcs.017160. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kubicky RA, Wu S, Kharitonenkov A, De LF. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology. 2012;153:2287–2295. doi: 10.1210/en.2011-1909. [DOI] [PubMed] [Google Scholar]
- Lango AH, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, Willer CJ, Jackson AU, Vedantam S, Raychaudhuri S, Ferreira T, Wood AR, Weyant RJ, Segre AV, Speliotes EK, Wheeler E, Soranzo N, Park JH, Yang J, Gudbjartsson D, Heard-Costa NL, Randall JC, Qi L, Vernon SA, Magi R, Pastinen T, Liang L, Heid IM, Luan J, Thorleifsson G, Winkler TW, Goddard ME, Sin LK, Palmer C, Workalemahu T, Aulchenko YS, Johansson A, Zillikens MC, Feitosa MF, Esko T, Johnson T, Ketkar S, Kraft P, Mangino M, Prokopenko I, Absher D, Albrecht E, Ernst F, Glazer NL, Hayward C, Hottenga JJ, Jacobs KB, Knowles JW, Kutalik Z, Monda KL, Polasek O, Preuss M, Rayner NW, Robertson NR, Steinthorsdottir V, Tyrer JP, Voight BF, Wiklund F, Xu J, Zhao JH, Nyholt DR, Pellikka N, Perola M, Perry JR, Surakka I, Tammesoo ML, Altmaier EL, Amin N, Aspelund T, Bhangale T, Boucher G, Chasman DI, Chen C, Coin L, Cooper MN, Dixon AL, Gibson Q, Grundberg E, Hao K, Juhani JM, Kaplan LM, Kettunen J, Konig IR, Kwan T, Lawrence RW, Levinson DF, Lorentzon M, McKnight B, Morris AP, Muller M, Suh NJ, Purcell S, Rafelt S, Salem RM, Salvi E, Sanna S, Shi J, Sovio U, Thompson JR, Turchin MC, Vandenput L, Verlaan DJ, Vitart V, White CC, Ziegler A, Almgren P, Balmforth AJ, Campbell H, Citterio L, De GA, Dominiczak A, Duan J, Elliott P, Elosua R, Eriksson JG, Freimer NB, Geus EJ, Glorioso N, Haiqing S, Hartikainen AL, Havulinna AS, Hicks AA, Hui J, Igl W, Illig T, Jula A, Kajantie E, Kilpelainen TO, Koiranen M, Kolcic I, Koskinen S, Kovacs P, Laitinen J, Liu J, Lokki ML, Marusic A, Maschio A, Meitinger T, Mulas A, Pare G, Parker AN, Peden JF, Petersmann A, Pichler I, Pietilainen KH, Pouta A, Ridderstrale M, Rotter JI, Sambrook JG, Sanders AR, Schmidt CO, Sinisalo J, Smit JH, Stringham HM, Bragi WG, Widen E, Wild SH, Willemsen G, Zagato L, Zgaga L, Zitting P, Alavere H, Farrall M, McArdle WL, Nelis M, Peters MJ, Ripatti S, van Meurs JB, Aben KK, Ardlie KG, Beckmann JS, Beilby JP, Bergman RN, Bergmann S, Collins FS, Cusi D, den HM, Eiriksdottir G, Gejman PV, Hall AS, Hamsten A, Huikuri HV, Iribarren C, Kahonen M, Kaprio J, Kathiresan S, Kiemeney L, Kocher T, Launer LJ, Lehtimaki T, Melander O, Mosley TH, Jr, Musk AW, Nieminen MS, O'Donnell CJ, Ohlsson C, Oostra B, Palmer LJ, Raitakari O, Ridker PM, Rioux JD, Rissanen A, Rivolta C, Schunkert H, Shuldiner AR, Siscovick DS, Stumvoll M, Tonjes A, Tuomilehto J, van Ommen GJ, Viikari J, Heath AC, Martin NG, Montgomery GW, Province MA, Kayser M, Arnold AM, Atwood LD, Boerwinkle E, Chanock SJ, Deloukas P, Gieger C, Gronberg H, Hall P, Hattersley AT, Hengstenberg C, Hoffman W, Lathrop GM, Salomaa V, Schreiber S, Uda M, Waterworth D, Wright AF, Assimes TL, Barroso I, Hofman A, Mohlke KL, Boomsma DI, Caulfield MJ, Cupples LA, Erdmann J, Fox CS, Gudnason V, Gyllensten U, Harris TB, Hayes RB, Jarvelin MR, Mooser V, Munroe PB, Ouwehand WH. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z, Lilos P, Klinger B. Growth curves for Laron syndrome. Arch.Dis.Child. 1993;68:768–770. doi: 10.1136/adc.68.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JE, Hegde A, Andrade AC, Nilsson O, Baron J. Fibroblast growth factor expression in the postnatal growth plate. Bone. 2007;40:577–586. doi: 10.1016/j.bone.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Li C, Chen L, Iwata T, Kitagawa M, Fu XY, Deng CX. A Lys644Glu substitution in fibroblast growth factor receptor 3 (FGFR3) causes dwarfism in mice by activation of STATs and ink4 cell cycle inhibitors. Hum.Mol.Genet. 1999;8:35–44. doi: 10.1093/hmg/8.1.35. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, Wendt DJ, Bell SM, Bullens S, Bunting S, Tsuruda LS, O'Neill CA, Di RF, Munnich A, Legeai-Mallet L. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am.J Hum.Genet. 2012;91:1108–1114. doi: 10.1016/j.ajhg.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JC, Andrade AC, Forcinito P, Hegde A, Chen W, Baron J, Nilsson O. Spatial and temporal regulation of gene expression in the mammalian growth plate. Bone. 2010;46:1380–1390. doi: 10.1016/j.bone.2010.01.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JC, Nilsson O, Chan Y, Palmer CD, Andrade AC, Hirschhorn JN, Baron J. Synthesizing genome-wide association studies and expression microarray reveals novel genes that act in the human growth plate to modulate height. Hum.Mol.Genet. 2012;21:5193–5201. doi: 10.1093/hmg/dds347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev.Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- MacRae VE, Horvat S, Pells SC, Dale H, Collinson RS, Pitsillides AA, Ahmed SF, Farquharson C. Increased bone mass, altered trabecular architecture and modified growth plate organization in the growing skeleton of SOCS2 deficient mice. J Cell Physiol. 2009;218:276–284. doi: 10.1002/jcp.21593. [DOI] [PubMed] [Google Scholar]
- Marchini A, Hacker B, Marttila T, Hesse V, Emons J, Weiss B, Karperien M, Rappold G. BNP is a transcriptional target of the short stature homeobox gene SHOX. Hum.Mol.Genet. 2007;16:3081–3087. doi: 10.1093/hmg/ddm266. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Kitoh H, Ohkawara B, Mishima K, Kaneko H, Ito M, Masuda A, Ishiguro N, Ohno K. Meclozine Facilitates Proliferation and Differentiation of Chondrocytes by Attenuating Abnormally Activated FGFR3 Signaling in Achondroplasia. PLoS One. 2013;8:e81569. doi: 10.1371/journal.pone.0081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Wilcox WR, Chan YY, Kawanami A, Bukulmez H, Balmes G, Krejci P, Mekikian PB, Otani K, Yamaura I, Warman ML, Givol D, Murakami S. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum.Mol.Genet. 2009;18:227–240. doi: 10.1093/hmg/ddn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mericq V, Uyeda JA, Barnes KM, De LF, Baron J. Regulation of fetal rat bone growth by C-type natriuretic peptide and cGMP. Pediatr Res. 2000;47:189–193. doi: 10.1203/00006450-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, Hilton DJ, Alexander WS. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- Miura K, Namba N, Fujiwara M, Ohata Y, Ishida H, Kitaoka T, Kubota T, Hirai H, Higuchi C, Tsumaki N, Yoshikawa H, Sakai N, Michigami T, Ozono K. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS One. 2012;7:e42180. doi: 10.1371/journal.pone.0042180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Parker EA, Hegde A, Chau M, Barnes KM, Baron J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J Endocrinol. 2007;193:75–84. doi: 10.1677/joe.1.07099. [DOI] [PubMed] [Google Scholar]
- Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin.Endocrinol.Metab. 2006;91:1229–1232. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- Ozasa A, Komatsu Y, Yasoda A, Miura M, Sakuma Y, Nakatsuru Y, Arai H, Itoh N, Nakao K. Complementary antagonistic actions between C-type natriuretic peptide and the MAPK pathway through FGFR-3 in ATDC5 cells. Bone. 2005;36:1056–1064. doi: 10.1016/j.bone.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Parker EA, Hegde A, Buckley M, Barnes KM, Baron J, Nilsson O. Spatial and temporal regulation of GH-IGF-related gene expression in growth plate cartilage. J Endocrinol. 2007;194:31–40. doi: 10.1677/JOE-07-0012. [DOI] [PubMed] [Google Scholar]
- Pass C, MacRae VE, Huesa C, Faisal AS, Farquharson C. SOCS2 is the critical regulator of GH action in murine growth plate chondrogenesis. J Bone Miner.Res. 2012;27:1055–1066. doi: 10.1002/jbmr.1544. [DOI] [PubMed] [Google Scholar]
- Pejchalova K, Krejci P, Wilcox WR. C-natriuretic peptide: an important regulator of cartilage. Mol.Genet.Metab. 2007;92:210–215. doi: 10.1016/j.ymgme.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr.Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Priore R, Dailey L, Basilico C. Downregulation of Akt activity contributes to the growth arrest induced by FGF in chondrocytes. J Cell Physiol. 2006;207:800–808. doi: 10.1002/jcp.20620. [DOI] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld RG, Belgorosky A, Camacho-Hubner C, Savage MO, Wit JM, Hwa V. Defects in growth hormone receptor signaling. Trends Endocrinol.Metab. 2007;18:134–141. doi: 10.1016/j.tem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le MM, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Shu B, Zhang M, Xie R, Wang M, Jin H, Hou W, Tang D, Harris SE, Mishina Y, O'Keefe RJ, Hilton MJ, Wang Y, Chen D. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DS, Takemoto CD. Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Mol.Endocrinol. 1990;4:91–100. doi: 10.1210/mend-4-1-91. [DOI] [PubMed] [Google Scholar]
- Suda K, Iguchi G, Yamamoto M, Handayaningsih AE, Nishizawa H, Takahashi M, Okimura Y, Kaji H, Chihara Y, Takahashi Y. A Case of Gigantism Associated with a Missense Mutation in the SOCS2 Gene. Endocrine Society Annual Meeting. 2011 OR36-1. 2011. Ref Type: Conference Proceeding. [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol.Endocrinol. 2008;22:1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol.Chem. 2005;280:14288–14292. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- Ulici V, James CG, Hoenselaar KD, Beier F. Regulation of gene expression by PI3K in mouse growth plate chondrocytes. PLoS One. 2010;5:e8866. doi: 10.1371/journal.pone.0008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasques GA, Amano N, Docko AJ, Funari MF, Quedas EP, Nishi MY, Arnhold IJ, Hasegawa T, Jorge AA. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) gene as a cause of short stature in patients initially classified as idiopathic short stature. J Clin.Endocrinol.Metab. 2013;98:E1636–E1644. doi: 10.1210/jc.2013-2142. [DOI] [PubMed] [Google Scholar]
- Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, LeMerrer M, Mortier G, Mundlos S, Nishimura G, Rimoin DL, Robertson S, Savarirayan R, Sillence D, Spranger J, Unger S, Zabel B, Superti-Furga A. Nosology and classification of genetic skeletal disorders: 2010 revision. Am.J Med.Genet.A. 2011;155A:943–968. doi: 10.1002/ajmg.a.33909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, Freathy RM, Perry JR, Stevens S, Hall AS, Samani NJ, Shields B, Prokopenko I, Farrall M, Dominiczak A, Johnson T, Bergmann S, Beckmann JS, Vollenweider P, Waterworth DM, Mooser V, Palmer CN, Morris AD, Ouwehand WH, Zhao JH, Li S, Loos RJ, Barroso I, Deloukas P, Sandhu MS, Wheeler E, Soranzo N, Inouye M, Wareham NJ, Caulfield M, Munroe PB, Hattersley AT, McCarthy MI, Frayling TM. Genome-wide association analysis identifies 20 loci that influence adult height. Nat.Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit JM, Camacho-Hubner C. Endocrine regulation of longitudinal bone growth. Endocr.Dev. 2011;21:30–41. doi: 10.1159/000328119. [DOI] [PubMed] [Google Scholar]
- Wit JM, Kamp GA, Rikken B. Spontaneous growth and response to growth hormone treatment in children with growth hormone deficiency and idiopathic short stature. Pediatr.Res. 1996;39:295–302. doi: 10.1203/00006450-199602000-00018. [DOI] [PubMed] [Google Scholar]
- Woo YC, Xu A, Wang Y, Lam KS. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin.Endocrinol.(Oxf) 2013;78:489–496. doi: 10.1111/cen.12095. [DOI] [PubMed] [Google Scholar]
- Wu S, Grunwald T, Kharitonenkov A, Dam J, Jockers R, De LF. Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing leptin receptor overlapping transcript (LEPROT) and leptin receptor overlapping transcript-like 1 (LEPROTL1) expression. J Biol.Chem. 2013;288:27375–27383. doi: 10.1074/jbc.M113.462218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu S, Levenson A, Kharitonenkov A, De LF. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol.Chem. 2012;287:26060–26067. doi: 10.1074/jbc.M112.343707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin.Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat.Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006;133:4667–4678. doi: 10.1242/dev.02680. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]