Abstract
AIM
The long-term effects of the ketogenic diet, a high fat diet for treating intractable epilepsy, on resting energy expenditure (REE) are unknown. The aim of this study was to evaluate the impact of 15 months of ketogenic diet treatment on growth and REE in children with intractable epilepsy.
METHOD
Growth, body composition and REE were assessed at baseline, 3 and 15 months in 24 children (14 males, 10 females; mean age 5y 6mo (SD 26mo), range 7mo–6y 5mo), 10 with cerebral palsy [CP]). Fifteen were identified as ketogenic diet responders at 3 months and continued on the ketogenic diet until 15 months. These were compared to 75 healthy children (43 males, 32 females; mean age 6y 3mo [SD 21mo] age range 2–9y). REE was expressed as percentage predicted, growth as height (HAz) and weight (WAz) z-scores, and body composition as fat and fat free mass (FFM).
RESULTS
HAz declined −0.2 and −0.6 from baseline to 3 and 15 months, respectively (p=0.001), while WAz was unchanged. In ketogenic diet responders, FFM, age and CP diagnosis predicted REE (overall R2=0.76, p<0.001) and REE did not change. REE adjusted for FFM was lower (p<0.01) in children with CP at baseline (mean [standard error], −143[51] kcals/d) and 15 months (−198[53] kcals/d) compared to the healthy children.
INTERPRETATION
After 15 months of the ketogenic diet, linear growth status declined while weight status and REE were unchanged. REE remained reduced in children with CP.
Half of the people with epilepsy are children and up to 30% become refractory to medications and develop intractable epilepsy.1 Children with intractable epilepsy are exposed to many treatments and concomitant side effects2,3 and have suboptimal growth and diets compared to healthy children.4,5 The ketogenic diet is a treatment for intractable epilepsy and typically a third of the patients will experience a greater than 90% reduction in seizure frequency.6 The ketogenic diet is often discontinued after three years, but in children whose seizures return during a gradual taper, the ketogenic diet can be extended for more than 10 years.7 Ketogenic diet treatment can result in height velocity deceleration and growth failure.8–13 We previously demonstrated that the growth failure seen in children with intractable epilepsy was not associated with an increase in resting energy expenditure (REE).14 However, children with intractable epilepsy and CP had reduced REE compared to those without CP and to healthy children.14 The long-term effect of ketogenic diet treatment on REE has not been evaluated in children with intractable epilepsy, nor in the subgroup of children with CP who have a greater risk for growth and body composition abnormalities.15,16 The purpose of this study was to determine if ketogenic diet treatment results in altered REE or growth in children with intractable epilepsy with and without CP over 15 months. A better understanding of the long-term effects on growth and energy requirement may guide changes in the ketogenic diet protocol to optimize growth, nutrition and health status in these children.
METHOD
Participants
This study of the longitudinal REE response to the ketogenic diet was part of a prospective trial where children with intractable epilepsy were randomized to one of two initiation protocols, a fasting compared to a gradual initiation of ketogenic diet.17 The ketogenic diet provided a caloric composition of about 90% fat, 7% protein and 3% carbohydrate. During the study, all participants received a sugar-free, one-a-day type multivitamin and mineral children’s supplement with additional calcium and phosphorus. The study was conducted at the Children’s Hospital of Philadelphia, PA, and approved by the institutional review board. Informed consent was obtained from a parent or guardian and assent was given by children who were cognitively able to participate in the process. A cohort of 48 children age 1 to 14 years with a current history of one or more seizures per 28 days and with a diagnosis of intractable epilepsy were included in the trial. We restricted this analysis to pre-pubertal children (ages 2 to 9y) with long-term follow-up to avoid the complication of variable maturity rates through puberty. REE was assessed at baseline before initiation of the ketogenic diet and after 3 months of treatment. Participants were then identified as ketogenic diet responders at the 3-month assessment if they experienced a decrease in seizure activity of 50% or more. Ketogenic diet responders continued the ketogenic diet and were evaluated at a 15-month protocol visit. A sample of historical and contemporary healthy children with no known conditions affecting nutrition or growth status served as the comparison group. They were assessed once for growth, body composition, and REE and served as the comparison group for both baseline and 15-month time points.
Anthropometric and dietary measures
At each visit, height, weight, upper arm circumference and triceps, biceps, sub-scapular and supra-iliac skinfold thickness were measured with standard research techniques as described previously.14 Height (HAz), weight (WAz) and BMI (BMIz) z-scores were computed.20 Total upper arm muscle and fat areas were calculated and z-scores computed.21,22 Using age and sex-specific equations, fat free mass (FFM) and fat mass in kilograms were calculated from the four skinfolds.23–26 For children with intractable epilepsy, dietary intake was assessed at each visit using 3-day (including one weekend day) weighed food records. Families were provided with a calibrated food scale (CS-200; Ohaus Corporation, Pinebrook, NJ, USA), accurate to 0.1g, and instructed in detail by a research dietitian to weigh and report everything that the child consumed. Dietary intake data were collected and analyzed (Nutrition Data System, version 2005; Minneapolis, MN, USA). Energy intake (kcals/d) was assessed and expressed as a percentage of the estimated energy requirement (%EER) for children with a low active physical activity level.27
Resting energy expenditure
REE (kcals/d) and respiratory quotient were measured by open-circuit, indirect calorimetry during an overnight research admission at baseline, 3 and 15 months. Participants fasted for 12 hours before REE measurement and were brought by wheelchair for the assessment. A computerized metabolic cart (Sensor medic 2900 Z; Sensor Medics, Yorba Linda, CA, USA) was used to measure REE for 60 minutes in a quiet, thermal neutral room. Participants rested awake in a supine position under a large, clear, ventilated hood. Expiratory gases were sampled and analyzed every 20 seconds, and 1-minute averages were recorded. The first 10 minutes of the measurement period were devoted to environmental acclimatization of the child and were not used in the analysis. Additionally, periods of significant movement were documented, and if associated with changes in REE were excluded from the analysis. The remaining minute-by-minute data were averaged and REE was calculated from oxygen consumption and carbon dioxide production by the equations of Weir.28 For this analysis, the participants were restricted to those who were resting and awake during the REE measurement, because of known alteration of REE between awake and sleep states. REE is presented as kcals/d and as a percentage of predicted value using the Schofield equation29 which is based on age, sex, weight, and height and often used in clinical care.
Statistical analysis
Data analysis occurred in two phases. In the first phase, descriptive statistics were performed, and group differences between children with intractable epilepsy with and without CP were assessed on the entire sample. All variables were tested for normality, and nonparametric tests were used as appropriate. Differences between children with intractable epilepsy with and without CP at baseline in continuous variables were determined using Student’s independent samples (unpaired) t-tests for the normally distributed variables and Mann–Whitney U tests for skewed data while Fisher’s exact tests were used for categorical variables. Change from baseline to 3 months was assessed for growth status, body composition, REE and respiratory quotient in children with and without CP using Student’s dependent samples (paired) t-tests for the normally distributed variables and Wilcoxon matched pairs single rank tests for skewed variables. Differences in the change variables for each outcome time point between groups were assessed with unpaired t-tests for the normally distributed variables and Mann–Whitney U tests for skewed data. Spearman’s correlations were performed to assess the association between seizure frequency and REE variables. The second phase of analysis focused on ketogenic diet responders and included the healthy comparison children. Multiple regression analyses were used to assess the difference in REE (kcals/d) adjusted for FFM among ketogenic diet responders with CP, without CP, and healthy comparison children at baseline and 15 months. Lastly, longitudinal mixed effects (LME) models were used to assess change in REE over time and significant predictors of REE over the 15 months in the ketogenic diet responders only. Potential predictors of REE including FFM, fat mass, age, sex, time and randomization group (fasting vs gradual initiation of ketogenic diet) were tested in these models. All statistical analyses were performed with STATA 12.0 (STATA Corp., College Station, TX, USA). The results were considered significant at p<0.05 and data are presented as mean (SD; normal distribution) or median and range (skewed distribution).
RESULTS
There were 24 participants with intractable epilepsy who had baseline and 3-month visits and 15 participants who were ketogenic diet responders with baseline, 3- and 15-month visits. Baseline characteristics of the 24 children with intractable epilepsy are presented in Table I for the whole sample and separately for those with (n=10) and without (n=14) CP. Children with and without CP did not differ in age at onset of seizures, duration of seizures, or seizure type. Each group had a similar history of status epilepticus, abnormal magnetic resonance image result, and microcephaly. Moderate or severe learning disability was more common in children with CP (p=0.005) and these children were less likely to be ambulatory (p<0.005). Children with intractable epilepsy had suboptimal energy intake, with 65% below the recommended %EER for low active children, and energy intake did not change at 3 and 15 months.
Table I.
Baseline characteristics of children with intractable epilepsy (IE) with and without cerebral palsy (CP)
| Characteristics | Participants with IE | IE with CPa | IE without CP |
|---|---|---|---|
| Number, n | 24 | 10 | 14 |
| Age, y:mo, mean (SD) | 5:6 (26mo) | 4:8 (25mo) | 6:0 (26mo) |
| Males/females, n | 14/10 | 4/6 | 10/4 |
| Age at first seizure, median [range], y | 2.2 [0–6.4] | 0.8 [0–6.4] | 3.0 [0.4–6.0] |
| Duration of seizures, median [range], y | 2.2 [0.5–7.6] | 2.4 [1.– 6.8] | 1.6 [0.5–7.6] |
| No. of seizures per week, median [range] | 3.4 [0.2–4116] | 8.8 [0.2–196] | 3.1 [0.5–4116] |
| Seizure type, n | |||
| Generalized seizures | 11 | 6 | 5 |
| Partial seizures | 13 | 4 | 9 |
| History of status epilepticus | 11 | 6 | 5 |
| Intellectual disabilityb (moderate or severe), n | 16 | 10 | 6b |
| Microcephaly, n | 4 | 1 | 3 |
| Ambulation, n | 17 | 4 | 13b |
| Energy intake, kcals/d, mean (SD) | 1345 (471) | 1133 (410) | 1497 (466) |
| %EER, mean (SD) | 96 (28) | 90 (28) | 100 (28) |
Of the 10 children with CP, 8 had spastic and 2 hypotonic CP; there were 4 with hemiplegia, 1 with diplegia and 5 with quadriplegia.
Significant difference between children with IE and CP and children with IE without CP (Fisher’s exact test): p<0.01. Estimated energy requirement (EER), energy intake as % EER for children with a low physical activity level.
Table II presents growth, body composition, REE and respiratory quotient results at baseline with changes observed over 3 months and 15 months in children with intractable epilepsy, comparing those with and without CP. HAz decreased (−0.2, p<0.001) and WAz was unchanged over 3 months. Fat mass increased (0.4 kg, p=0.01) while FFM was unchanged at 3 months. Children with intractable epilepsy with and without CP were equally distributed in the ketogenic diet fasting vs. gradual initiation randomization groups (50% fasting for those with CP, and 57% for those without CP). REE was unchanged over 3 months regardless of CP status. Baseline characteristics for the subset of 15 ketogenic diet responders were comparable to the total sample of 24 participants presented in Table I. In ketogenic diet responders, WAz was unchanged and HAz decreased (−0.6, p=0.001) over 15 months. At 15 months, a decline in HAz was observed in 12 of 15 participants. Looking at children with and without CP separately, only those with CP had a significant decrease in HAz at 15 months (p=0.005). Fat mass and FFM increased (+1.1 kg [p<0.001] and +1.2 kg [p<0.001] respectively) in the group of ketogenic diet responders at 15 months.
Table II.
Changes in growth status, body composition, resting energy expenditure (REE) and respiratory quotient (RQ) from baseline to 3 and 15 months, mean (SD)
| All participants with IE | IE with CP | IE without CP | ||||
|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Baseline | Change | |
| All IE participant Baseline to 3mo |
n=24 | n=10 | n=14 | |||
|
| ||||||
| Height, cm | 109.1 (14.0) | 0.7 (1.1)b | 101.8 (10.4) | 1.1 (1.2)a | 113.9 (14.3)† | 0.5 (1.0) |
| HAz | −0.3 (1.1) | −0.2 (0.3)c | −0.6 (1.3) | −0.2 (0.3) | −0.1 (0.9) | −0.3 (0.3)b |
| Weight, kg | 18.2 (5.3) | 0.3 (1.1) | 15.6 (3.7) | 0.7 (0.9)a | 20.1 (5.6)† | 0.05 (1.16) |
| WAz | −0.8 (1.4) | −0.1 (0.5) | −1.3 (1.6) | 0.1 (0.4) | −0.4 (1.2) | −0.2 (0.5) |
| BMI | 14.9 (1.7) | 0.4 (1.4) | 14.3 (1.9) | 0.9 (1.7) | 15.3 (1.5) | −0.01 (0.98) |
| BMIz | −0.9 (1.7) | 0.3 (1.4) | −1.5 (2.0) | 0.9 (1.8) | −0.5 (1.3) | −0.04 (1.00) |
| FFM, kg | 15.3 (4.3) | −0.1 (0.7) | 13.4 (3.2) | 0.1 (0.5) | 16.8 (4.5) | −0.2 (0.7) |
| FM, kg | 2.9 (1.5) | 0.4 (0.7)a | 2.2 (1.2) | 0.6 (0.7)a | 3.4 (1.5) | 0.2 (0.6) |
| % body fat | 15.5 (5.1) | 1.8 (3.2)a | 14.2 (6.1) | 2.7 (4.2) | 16.4 (4.3) | 1.2 (2.4) |
| REE, % | 103 (14) | −2.0 (13.1) | 98 (12) | 0.4 (15.2) | 107 (14) | −3.8 (11.7) |
| RQ | 0.81 (0.05) | −0.08 (0.05)c | 0.80 (0.04) | −0.09 (0.04)c | 0.81 (0.06) | −0.07 (0.05)c |
|
| ||||||
| IE responders Baseline to 15mo |
n=15 | n=7 | n=8 | |||
|
| ||||||
| Height, cm | 107.5 (14.3) | 3.9 (1.9)c | 101.9 (11.9) | 4.6 (1.7)c | 112.3 (15.2) | 3.4 (2.0)b |
| HAz | −0.6 (0.9) | −0.6 (0.6)b | −0.9 (1.0) | −0.7 (0.5)b | −0.3 (0.7) | −0.5 (0.7) |
| Weight, kg | 17.7 (5.2) | 2.3 (1.1)c | 15.5 (4.5) | 2.6 (1.2)b | 19.5 (5.2) | 2.0 (1.1)b |
| WAz | −0.9 (1.4) | 0.04 (0.50) | −1.4 (1.5) | 0.2 (0.5) | −0.4 (1.1) | −0.1 (0.5) |
| BMI | 15.0 (1.7) | 0.9 (1.0)b | 14.6 (1.7) | 1.1 (1.0)a | 15.4 (1.7) | 0.7 (0.9) |
| BMIz | −0.8 (1.6) | 0.8 (1.1)a | −1.2 (1.8) | 1.1 (1.2) | −0.5 (1.4) | 0.6 (1.0) |
| FFM, kg | 14.9 (4.3) | 1.2 (0.6)c | 13.2 (3.8) | 1.4 (0.7)b | 16.3 (4.5) | 1.1 (0.6)b |
| FM, kg | 2.8 (1.3) | 1.1 (0.8)c | 2.3 (1.3) | 1.2 (0.8)b | 3.2 (1.2) | 0.9 (0.8)a |
| % body fat | 15.7 (5.0) | 3.8 (3.6)b | 14.7 (5.9) | 4.4 (3.8)a | 16.6 (4.4) | 3.2 (3.6)a |
| REE, % | 103 (13) | −0.0 (16) | 95 (12) | −7 (17) | 110 (8)d | 6 (14) |
| RQ | 0.81 (0.06) | −0.09 (0.07)c | 0.81 (0.05) | −0.09 (0.06)a | 0.81 (0.07) | −0.1 (0.1)b |
Change over time significant at p<0.05;
p<0.01;
p<0.001;
Baseline values between children with cerebral palsy (CP) and without CP (non-CP) significant at p<0.05.
IE, intractable epilepsy; HAz, height z-score; WAz, weight z-score; BMI, body mass index; BMIz, body mass index z-score; FFM, fat free mass; FM, fat mass; REE, resting energy expenditure, kcals/d; RQ, respiratory quotient.
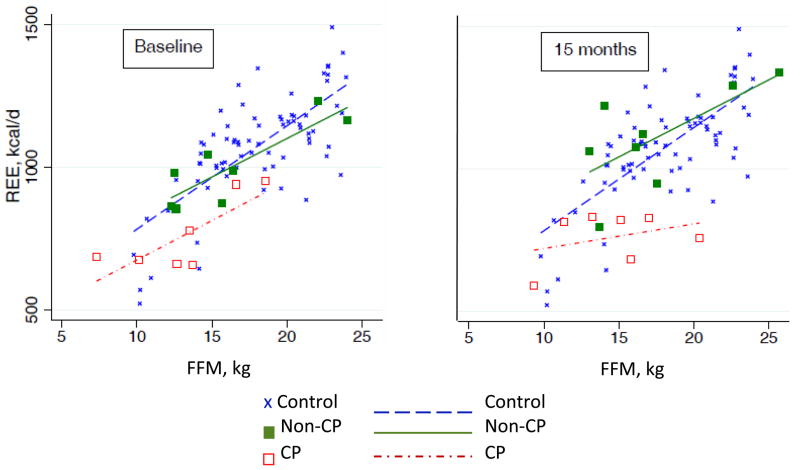
REE at baseline was 103 (13) % predicted in the ketogenic diet responders but was lower in children with CP than in those without CP (95 [12] versus 110 [8] % predicted, p=0.01). REE did not change over 15 months regardless of CP status. As expected, respiratory quotient significantly decreased (p<0.01) after 3 and 15 months of ketogenic diet treatment in children with intractable epilepsy with and without CP reflecting increased fat intake and fat oxidation on the ketogenic diet. The 75 healthy children in the comparison group (43 males, 32 females, mean age 6y 3mo [SD 21mo] age range 2–9y) had one REE measurement with a result of 111 (13) %. The relationship between REE and FFM at baseline and 15 months for the ketogenic diet responders with and without CP is presented in Figure 1, compared to the healthy comparison group. Results from regression analyses showed that REE adjusted for FFM was lower in children with intractable epilepsy and CP than in healthy children at both baseline (mean [SE], −143 [51] kcals/d, adj. R2 =0.61, p<0.01) and 15 months (−198 [53] kcals/d, adj. R2=0.57, p<0.001). Children with intractable epilepsy without CP did not differ from healthy children at either time point. Among children with intractable epilepsy who responded to the ketogenic diet (Table III), the LME models showed that FFM, age and diagnosis of CP were significant predictors of REE over the 15 months. Neither randomization group nor time of ketogenic diet treatment significantly predicted REE.
Figure 1.
Relationship between resting energy expenditure (REE) and fat free mass (FFM) at baseline and 15 months in the ketogenic diet responders. Children with intractable epilepsy and cerebral palsy (CP; n=7) and children with IE without CP (non-CP, n=8) compared with the healthy comparison children (n=75).
Table III.
Predictors of resting energy expenditure (kcals/d); for the ketogenic diet responders (n=15) at 3 and 15 months
| Coefficient | Standard error | p | Overall R | Overall p-value | |
|---|---|---|---|---|---|
| FFM, kg | 45.3 | 11.3 | <0.001 | 0.76 | <0.001 |
| FM, kg | −13.7 | 17.4 | 0.430 | ||
| Age, y | −40.4 | 16.8 | 0.016 | ||
| Cerebral palsy | −177.7 | 33.3 | <0.001 | ||
| Time at 3mo | 24.3 | 38.0 | 0.522 | ||
| Time at 15mo | 58.4 | 41.5 | 0.160 | ||
| Constant | 200.1 | 71.2 | 0.005 |
FFM, fat free mass; FM, fat mass.
There was no difference in seizure frequency between children with and without CP at any point in time. The median seizures’ frequency (seizures per week) at baseline was 8.8 (0.2, 196) in children with CP and 3.1 (0.5, 4116) in children without CP. The seizures’ frequency decreased in both groups at 3 months (CP: −7.6 [−133, 0], p<0.01; non-CP: −1.5 [−4114, 7.3], p=0.01) and 15 months (CP: −1.5 [−196, −0.2], p=0.02; non-CP:−12.4 [−4116, 0], p=0.01). Using Spearman’s rank correlations, there was no significant association between seizures frequency and REE or respiratory quotient.
DISCUSSION
In our 15-month study of ketogenic diet treatment, height status declined while weight status was unchanged. REE did not change regardless of CP status. REE was significantly lower at both baseline and 15 months in participants with intractable epilepsy and CP, compared to healthy children. Bergqvist et al.14 previously demonstrated that children with intractable epilepsy before ketogenic diet treatment had a lower percentage predicted REE compared to healthy children and children with intractable epilepsy and CP had an even lower FFM-adjusted REE. Recently, Tagliabue et al.30 examined the impact of the ketogenic diet on REE in 18 children (mean age 12y 5mo [SD 5y 7mo]) with intractable epilepsy without CP and showed that six months of ketogenic diet treatment increased fat oxidation and decreased the respiratory quotient as expected, without significant changes in REE. Our results confirm these findings of unchanged REE in the short term and extend the observation to 15 months in participants with and without CP. Having intractable epilepsy alone or being treated with ketogenic diet did not alter REE but CP status is known to have an impact on energy expenditure. Children with CP are known to have lower energy expenditure and energy requirements.31–34 The nutrition-related growth failure and abnormal pattern of REE was likely to be related in part to inadequate energy intake.34 We also documented a reduced REE in children with CP and intractable epilepsy, and that the predictors of REE were FFM, age and the diagnosis of CP. Energy intake was not a significant predictor in the longitudinal model of REE with ketogenic diet treatment, although it was a significant predictor of REE in our previous study of children with intractable epilepsy at baseline, before ketogenic diet treatment.14
While REE did not change, linear growth status declined over 15 months of ketogenic diet treatment. HAz decreased overall from baseline to 3 and 15 months indicating height velocity deceleration and this was particularly true for children with CP. However, weight velocity was maintained, as indicated by unchanged HAz over 3 and 15 months in both groups. Numerous studies have evaluated the impact of ketogenic diet treatment on growth. Three retrospective studies reported that one year of ketogenic diet resulted in reduced height and weight gain of children with intractable epilepsy.8–10 Kim et al.13 retrospectively studied 40 children with intractable epilepsy and documented a decrease in height and weight status over two years of treatment with a catch-up growth in height and weight after a year of ketogenic diet discontinuation. In a shorter-term study, Tagliabue et al.30 prospectively assessed 18 children with intractable epilepsy and reported that height and weight status were unchanged over six months of ketogenic diet. Duration of treatment exposure may be important in linear growth evaluation because of a relatively slow rate of growth compared to measurement error. It is possible that a 6-month interval was too brief to show the changes observed in the longer studies. Groesbeck et al.7 retrospectively studied 28 children treated with the ketogenic diet for more than 6 years. While less than half were below the 10th centile for height and weight at the introduction of the ketogenic diet, after at least 6 years of ketogenic diet most fell below the 10th centile. In their sample, 15 children had CP and the percentage below the 10th centile for height doubled over 6 years. Vining et al.11 conducted a cohort study that included 76 children treated with ketogenic diet for at least 2 years. They reported a decline in height status overall, and a decline in weight status also, which was more pronounced in children with good weight status before ketogenic diet initiation. In the subgroup analysis of children with CP, non-ambulatory and non-communicating children had a greater decline in WAz.
The ketogenic diet-related alteration in the neuroendocrine axis may contribute to the growth failure and change in body composition, with increased fat mass, FFM, reduced height velocity and unchanged WAz that we observed. Insulin-like growth factor I (IGF-I) regulatory system is essential for skeletal growth.35 Spulber et al.36 prospectively studied 22 children treated for a year with the ketogenic diet and showed a decrease in weight and height status. This was observed mainly in children with pronounced ketosis, indicated by elevated serum beta-hydroxybutyric acid. They reported a correlation between linear growth velocity and serum IGF-I and both decreased after the initiation of ketogenic diet. The strength of the significant association between IGF-I and growth was lower during the treatment phase than before initiation of ketogenic diet, and may be secondary to a change in IGF-I bioavailability. Fraser et al.37 studied the ketogenic diet as a treatment for adults with rheumatoid arthritis and demonstrated a 46% reduction in IGF-I after only seven days while providing adequate dietary calories and protein. The increase in fat mass that we observed has not been reported in humans before but has previously been demonstrated in animals treated with a ketogenic diet.38 We know that children with low IGF-1 have an increase in %fat mass as seen with Laron syndrome.39 The declining IGF-1 may, therefore, affect height velocity, bone acquisition and the increase %fat mass that we observed.
Further studies of the relationship between growth and the IGF-I axis during ketogenic diet treatment may provide new insight into the mechanisms of linear growth faltering and body composition changes.
CONCLUSION
The longer-term ketogenic diet treatment resulted in a decline in linear growth status in children with intractable epilepsy, with no associated alteration in weight status or REE over time. The linear growth deceleration in the settings of stable weight and REE supports the hypothesis that ketogenic diet may decrease IGF-I without caloric deprivation. Furthermore, REE was reduced in children with CP at baseline and remained reduced. This study emphasizes the fact that children with intractable epilepsy on ketogenic diet treatment require careful monitoring of growth, and this is particularly true for children with intractable epilepsy and CP.
What this paper adds.
Linear growth declines in children with intractable epilepsy treated with the ketogenic diet for 15 months.
REE is not affected by long-term ketogenic diet treatment.
REE in children with intractable epilepsy and CP is reduced before and after ketogenic diet treatment.
Acknowledgments
We are grateful to the children and their caregivers for their participation in this research study. We also thank the Children’s Hospital of Philadelphia Ketogenic Diet Team and Clinical and Translational Research Center team for their support and commitment. This project was supported by the NIH (RR-K23-16074), Clinical and Translational Research Center (UL1RR024134) and Nutrition Center at the Children’s Hospital of Philadelphia, and Ste-Justine University Hospital Center, Montreal, Canada.
ABBREVIATIONS
- %EER
Percentage of estimated energy requirement for healthy children
- BMI
Body mass index
- BMIz
Body mass index z-score
- FFM
Fat free mass, kg
- HAz
Height z-score
- LME
Longitudinal mixed effect
- REE
Resting energy expenditure
- WAz
Weight z-score
Footnotes
The authors have stated they had no interests that might be perceived as posing a conflict or bias.
References
- 1.Sillanpää M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain. 2006;129:617–24. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 2.Hanai T. Quality of life in children with epilepsy. Epilepsia. 1996;37:28–32. doi: 10.1111/j.1528-1157.1996.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 3.McEwan MJ, Espie CA, Metcalfe J, Brodie MJ, Wilson MT. A systematic review of the contribution of qualitative research to the study of quality of life in children and adolescents with epilepsy. Seizure. 2004;13:3–14. doi: 10.1016/s1059-1311(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 4.Volpe SL, Schall JI, Gallagher PR, Stallings VA, Bergqvist AG. Nutrient intake of children with intractable epilepsy compared with healthy children. J Am Diet Assoc. 2007;107:1014–8. doi: 10.1016/j.jada.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Bertoli S, Cardinali S, Veggiotti P, Trentani C, Testolin G, Tagliabue A. Evaluation of nutritional status in children with refractory epilepsy. Nutr J. 2006;5:1–9. doi: 10.1186/1475-2891-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006;21:193–8. doi: 10.2310/7010.2006.00044. [DOI] [PubMed] [Google Scholar]
- 7.Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48:978–81. doi: 10.1017/S0012162206002143. [DOI] [PubMed] [Google Scholar]
- 8.Neal EG, Chaffe HM, Edwards N, Lawson MS, Schwartz RH, Cross JH. Growth of children on classical and medium-chain triglyceride ketogenic diets. Pediatrics. 2008;122:e334–40. doi: 10.1542/peds.2007-2410. [DOI] [PubMed] [Google Scholar]
- 9.Williams S, Basualdo-Hammond C, Curtis R, Schuller R. Growth retardation in children with epilepsy on the ketogenic diet: a retrospective chart review. J Am Diet Assoc. 2002;102:405–7. doi: 10.1016/s0002-8223(02)90093-3. [DOI] [PubMed] [Google Scholar]
- 10.Peterson SJ, Tangney CC, Pimentel-Zablah EM, Hjelmgren B, Booth G, Berry-Kravis E. Changes in growth and seizure reduction in children on the ketogenic diet as a treatment for intractable epilepsy. J Am Diet Assoc. 2005;105:718–25. doi: 10.1016/j.jada.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Vining EP, Pyzik P, McGrogan J, et al. Growth of children on the ketogenic diet. Dev Med Child Neurol. 2002;44:796–802. doi: 10.1017/s0012162201002961. [DOI] [PubMed] [Google Scholar]
- 12.Bergqvist AG, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88:1678–84. doi: 10.3945/ajcn.2008.26099. [DOI] [PubMed] [Google Scholar]
- 13.Kim JT, Kang H-C, Song J-E, et al. Catch-up growth after long-term implementation and weaning from ketogenic diet in pediatric epileptic patients. Clin Nut. 2013;32:98–103. doi: 10.1016/j.clnu.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Bergqvist AG, Trabulsi J, Schall JI, Stallings VA. Growth failure in children with intractable epilepsy is not due to increased resting energy expenditure. Dev Med Child Neurol. 2008;50:439–44. doi: 10.1111/j.1469-8749.2008.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stallings VA, Charney EB, Davies JC, Cronk CE. Nutritional status and growth of children with diplegic or hemiplegic cerebral palsy. Dev Med Child Neurol. 1993;35:997–1006. doi: 10.1111/j.1469-8749.1993.tb11582.x. [DOI] [PubMed] [Google Scholar]
- 16.Stallings VA, Charney EB, Davies JC, Cronk CE. Nutrition-related growth failure of children with quadriplegic cerebral palsy. Dev Med Child Neurol. 1993;35:126–38. doi: 10.1111/j.1469-8749.1993.tb11614.x. [DOI] [PubMed] [Google Scholar]
- 17.Bergqvist AG, Schall JI, Gallagher PR, Cnaan A, Stallings VA. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia. 2005;46:1810–9. doi: 10.1111/j.1528-1167.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 1980;33:2372–4. doi: 10.1093/ajcn/33.11.2372. [DOI] [PubMed] [Google Scholar]
- 19.Goran MI, Kaskoun M, Johnson R. Determinants of resting energy expenditure in young children. J Pediatr. 1994;125:362–7. doi: 10.1016/s0022-3476(05)83277-9. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarksy RJ, Ogden CL, Grummer-Strawn LM, et al. Center for Disease Control and Development (CDC) growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 21.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–5. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 22.Frisancho A. Anthropometric Standards for Assessment of Growth and Nutritional Status. Ann Arbor, MI: University of Michigan Press; 1990. [Google Scholar]
- 23.Brook CG. Determination of body composition of children from skinfold measurements. Arch Dis Child. 1971;46:182–4. doi: 10.1136/adc.46.246.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ittenbach RF, Buison AM, Stallings VA, Zemel BS. Statistical validation of air-displacement plethysmography for body composition assessment in children. Ann Hum Biol. 2006;33:187–201. doi: 10.1080/03014460500519925. [DOI] [PubMed] [Google Scholar]
- 25.Buison AM, Ittenbach RF, Stallings VA, Zemel BS. Methodological agreement between two-compartment body-composition methods in children. Am J Hum Biol. 2006;18:470–80. doi: 10.1002/ajhb.20515. [DOI] [PubMed] [Google Scholar]
- 26.Liu LF, Roberts R, Moyer-Mileur L, Samson-Fang L. Determination of body composition in children with cerebral palsy: bioelectrical impedance analysis and anthropometry vs dual-energy x-ray absorptiometry. J Am Diet Assoc. 2005;105:794–7. doi: 10.1016/j.jada.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine. Dietary Reference Intake for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington DC: Institute of Medicine, National Academy Press; 2002. [DOI] [PubMed] [Google Scholar]
- 28.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39:5–41. [PubMed] [Google Scholar]
- 30.Tagliabue A, Bertoli S, Trentani C, Borrelli P, Veggiotti P. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A 6-month prospective observational study. Clin Nut. 2012;31:246–9. doi: 10.1016/j.clnu.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Walker JL, Bell KL, Boyd RN, Davies PS. Energy requirements in preschool-age children with cerebral palsy. Am J Clin Nut. 2012;96:1309–15. doi: 10.3945/ajcn.112.043430. [DOI] [PubMed] [Google Scholar]
- 32.Bell KL, Davies PS. Energy expenditure and physical activity of ambulatory children with cerebral palsy and of typically developing children. Am J Clin Nutr. 2010;92:313–9. doi: 10.3945/ajcn.2010.29388. [DOI] [PubMed] [Google Scholar]
- 33.Arrowsmith FE, Allen JR, Gaskin KJ, et al. Nutritional rehabilitation increases the resting energy expenditure of malnourished children with severe cerebral palsy. Dev Med Child Neurol. 2012;54:170–5. doi: 10.1111/j.1469-8749.2011.04166.x. [DOI] [PubMed] [Google Scholar]
- 34.Stallings VA, Zemel BS, Davies JC, Cronk CE, Charney EB. Energy expenditure of children and adolescents with severe disabilities: a cerebral palsy model. Am J Clin Nutr. 1996;64:627–34. doi: 10.1093/ajcn/64.4.627. [DOI] [PubMed] [Google Scholar]
- 35.Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41:323–33. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spulber G, Spulber S, Hagenäs L, Amark P, Dahlin M. Growth dependence on insulin-like growth factor-1 during the ketogenic diet. Epilepsia. 2009;50:297–303. doi: 10.1111/j.1528-1167.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- 37.Fraser DA, Thoen J, Bondhus S, et al. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:209–14. [PubMed] [Google Scholar]
- 38.Bielohuby M, Menhofer D, Kirchner H, et al. Induction of ketosis in rat fed low-carbohydrate, high fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol and Metabol. 2011;300:E65–76. doi: 10.1152/ajpendo.00478.2010. [DOI] [PubMed] [Google Scholar]
- 39.Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Body composition in untreated adult patients with Laron Syndrome. Clin Endocrinol. 2006;65:114–7. doi: 10.1111/j.1365-2265.2006.02558.x. [DOI] [PubMed] [Google Scholar]