Abstract
Here we review the examples of great longevity and potential immortality in the earliest animal types and contrast and compare these to humans and other higher animals. We start by discussing aging in single-celled organisms such as yeast and ciliates, and the idea of the immortal cell clone. Then we describe how these cell clones could become organized into colonies of different cell types that lead to multicellular animal life. We survey aging and longevity in all of the basal metazoan groups including ctenophores (comb jellies), sponges, placozoans, cnidarians (hydras, jellyfish, corals and sea anemones) and myxozoans. Then we move to the simplest bilaterian animals (with a head, three body cell layers, and bilateral symmetry), the two phyla of flatworms. A key determinant of longevity and immortality in most of these simple animals is the large numbers of pluripotent stem cells that underlie the remarkable abilities of these animals to regenerate and rejuvenate themselves. Finally, we discuss briefly the evolution of the higher bilaterians and how longevity was reduced and immortality lost due to attainment of greater body complexity and cell cycle strategies that protect these complex organisms from developing tumors. We also briefly consider how the evolution of multiple aging-related mechanisms/pathwayshinders our ability to understand and modify the aging process in higher organisms.
Keywords: Metazoa, Bilateria, Cnidaria, planaria, flatworms, hydra, sponge, regeneration, rejuvenation, stem cell, pluripotent, neoblast
1. Introduction
The human lifespan is fairly long compared to that of many other animals, but is nevertheless limited with most people living about 80 years and rare individuals reaching 100 years. Yet there are some animals, plants and fungi that can live for several hundred or even several thousand years, and often show negligible senescence. What are the mechanisms underlying great longevity and can we apply such knowledge to enhance the health and longevity of humans? The longest living animals are also among the simplest ones – the ones that are called basal metazoans, a group that includes sponges, corals, jellyfish, comb jellies, hydras, and sea anemones. All of the more advanced animals including humans are bilaterians, and the simplest of these are the flatworms (Table 1). Basal metazoans typically maintain many pluripotent stem cells that are capable of differentiating into all typesof cells in the body (Table 2); this gives these animals incredible abilities to grow, regress, regrow and regenerate their bodies as needed (Table 3; Rando, 2006; Pearson and Sánchez Alvarado, 2008; Tanaka and Reddien, 2011; Rink, 2013; Solana, 2013). They can become in some cases potentially immortal (Rando, 2006). However, during the evolution of more complex animal body forms, these abilities were reduced or lost, apparently in an effort to produce complex body structures for sophisticatedfunctions while still avoiding the production of destructive tumors (Rando, 2006; Pearson and Sánchez Alvarado, 2008; Popov, 2012). Nevertheless, there is no direct correlation of increasedbody complexity with reduced lifespan. For example, among the bilaterians, some vertebrates such as tortoises and whales can live more than 200 years and some clams live over 500 years, while the adult roundworm, Caenorhabditis elegans, although only a little more complex in design than flatworms, lacks somatic stem cells and lives only a couple of weeks (Rando, 2006; Pearson and Sánchez Alvarado, 2008; Popov, 2012, Petralia et al., 2014; Butler et al., 2013; Rink, 2013; Treaster et al., 2013; Aitlhadj and Stürzenbaum, 2014). The evolution of animals with greater complexity included the development of mechanisms forlimitinglifespan and senescence. In this review, we concentrate on aging and associated phenomena in the basal metazoans and flatworms. We will start by briefly reviewing basic mechanisms of aging. Next we will describe the one-celled organisms that evolved before multicellular animal life and look at what strategies for lifespan and aging were retainedin the first animals. Then we will examine these strategies in each of the groups of simple animals; and finally we will consider in general terms how all of this changed in the evolution of the higher Bilateria including those with long lifespans like humans as well as those with short lifespans like C. elegans.
Table 1.
List of major animals discussed in this review.
| Protozoa | Ciliates (e.g., Paramecium, Tetrahymena, suctorians) Choanoflagellates |
| Basal Metazoans | Porifera (sponges) Placozoa Ctenophora (comb jellies) Cnidaria (hydras, jellyfish, sea anemones, colonial hydroids, corals) Myxozoa |
| Bilateria | Acoelomorpha (acoel flatworms) Platyhelminthes (planaria and other free-living flatworms, flukes, tapeworms) Other groups included for comparison: Nematoda (Caenorhabditis elegans) Annelida (polychaete worms) Mollusca (snails, clams, oysters) Arthropoda (Crustacea, Drosophila) Chordata (sea squirts, vertebrates such as fish, frogs, mice, humans) |
Table 2.
Stem cell terminology.
| Stem cells | Undifferentiated cells that can differentiate into specialized cell types and can divide through mitosis to produce more stem cells, either for a limited number of divisions or potentially as an immortal cell clone. |
| Unipotent/oligopotent stem cells | Stem cells that can differentiate into only one/few cell types. For example, vertebrate muscle satellite cells are stem cells that produce myoblasts that form into muscle cells (see Fig. 8). |
| Multipotent stem cells | Stem cells that can differentiate into many cell types. An example would be the I-cells of hydras (see text). |
| Pluripotent somatic stem cells | Stem cells that can differentiate into all known somatic cell types of an animal. |
| Primordial germ cells | The initial cells of a developing germline, which is capable only of forming germ cells (gametogenesis). |
| Totipotent stem cells | Pluripotent stem cells that also are capable of gametogenesis. Examples would be mammalian zygotes and early embryos. Choanocytes and archeocytes of sponges and neoblasts of planaria may be totipotent or pluripotent, depending on various authors’ definitions and descriptions (see text). |
Table 3.
Mechanisms affecting aging and longevity in the simplest animals.
| Ciliate Protozoa | asexual clonal immortality asymmetric budding cycling between dedifferentiation and differentiation encystment regeneration |
| Porifera | asexual reproduction including gemmule formation, budding and stolon formation degeneration and regeneration dissociation and reorganization |
| Cnidaria | asexual budding clonal colonial growth continuous cellular renewal dedifferentiation of cells dissociation and reorganization regeneration by morphallaxis rejuvenation |
| Flatworms (Acoelomorpha and Platyhelminthes) | alternating regression and growth asexual reproduction via uneven fission controlled shrinkage during starvation regeneration from body fragments |
Some of these phenomena are associated with negligible senescence, and have been largely lost in the evolution of the higher Metazoa; they may be worth exploring in the search for solutions to extend the human life span and avoid the diseases of aging. See text for discussion.
2. Mechanisms of aging overview
In this review, we will discuss the extensive literature that reveals a fascinating feature of aging throughout animal evolution, that is, a gradual decline in the abundance of totipotent/pluripotent stem cells. Since these cells maintain the potential to give rise to any cell type that makes up the organism, their reduction or absence in more complex animals means that most fully differentiated adult cells in complex animals will age and die, eventually leading to the death of the animal, therefore preventing the possibility of immortality.
Aging of the fully differentiated adult cells results from a combination of mechanisms (Fig. 1; López-Otin et al., 2013). We will describe several of these mechanisms throughout this article. In this section, we briefly discuss five problems, which are known to play prominent roles in aging. We refer the reader to López-Otin et al., 2013 for a comprehensive review of aging mechanisms.
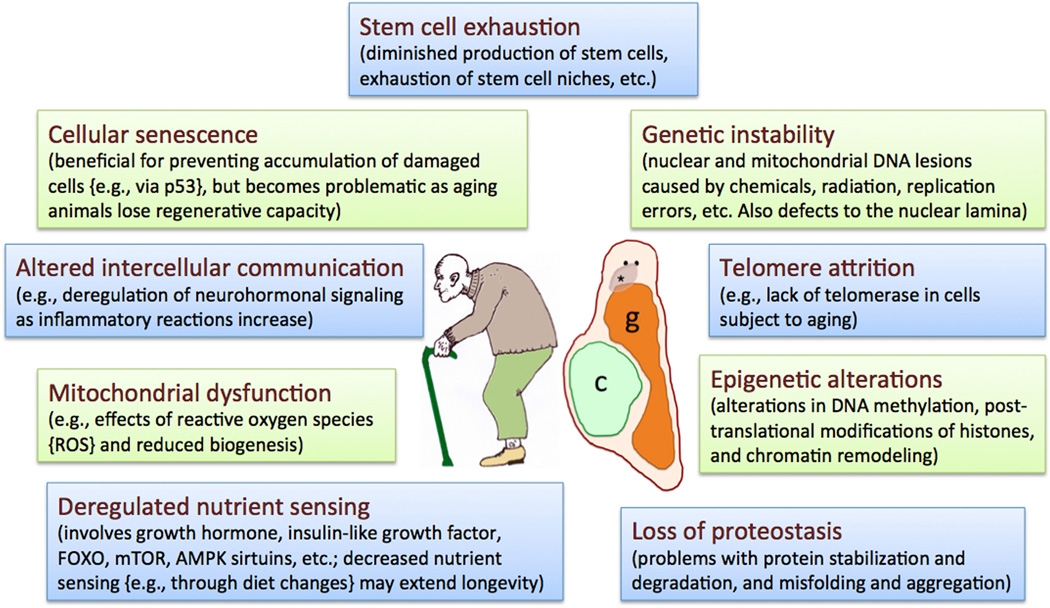
Figure 1.
Mechanisms of aging. The diagram illustrates the nine hallmarks of aging as described by López-Otin et al (2013); several of these mechanisms are described in section 2 of this review. Aging animals often display noticeable changes in external appearance, body shape and posture. The center shows a stereotypical silhouette of an aging person with balding head and stooped posture due to various degenerative diseases. Some aging flatworms can show comparable changes such as a surface head groove (asterisk) and a body deformed by cysts (c); note how the digestive tract or gut (g) is pushed to the side. The 2 black dots represent the eyes. The drawing is an original based on micrographs of Macrostomum lignano (Mouton et al., 2009; see section 7).
First, the genomic DNA and epigenetic problem. Throughout the lifespan, it is inevitable for a cell to encounter a range of challenges and insults from the surrounding environment and within the cell. These insults cause lesions to DNA molecules (Hoeijmakers 2009). The examples of such DNA lesions include point mutation, double-strand break, translocation, and telomere shortening (Hoeijmakers 2009; López-Otin et al., 2013). In order to fix these DNA lesions, cells are equipped with highly elaborate and dedicated DNA repair machineries (Lord and Ashworth, 2012; Yang et al., 2013). Extensive evidence has suggested that increased DNA lesions, and or, weakened DNA repair systems directly contribute to the aging process (Lord and Ashworth, 2012; Baker et al., 2013; López-Otin et al., 2013;Yang et al., 2014). In addition to DNA molecules themselves, epigenetic factors are also critical to aging processes. Among these epigenetic factors, histone modifications seem to play particularly important and widespread roles in aging (Han and Brunet, 2012; Kanfi et al., 2012; Kawahara et al., 2009; Schellenberg et al., 2011).For example, the reduction of NAD-dependent SIRT6 deacetylase (a member of the sirtuin family) significantly shortens the lifespan in mice; conversely, forced overexpression of this enzyme in mice enhances longevity (Kanfi et al., 2012; Kawahara et al., 2009). The roles of histone modifications in regeneration and stem cell biology have been studied in the planarian Schmidtea mediterranea (Robb and Sánchez Alvarado, 2014).
Second, the protein quality control problem. Once proteins are synthesized, they undergo careful folding, which is an uncompromising requirementfor functionally competent proteins and for healthy cells (Balch et al., 2008). Accordingly, cells ranging from unicellular organisms to humans all have developed tightly-regulated systems that execute the proper folding (Brodsky, 2014), and also promptly remove those proteins that are unfolded or misfolded (Jarozs et al., 2010). The maintenance or disruption of such proteostasis directly impacts cellular aging (Koga et al., 2011). The importance of proteostasis is further illustrated by the characteristic pathology - aggregation of misfolded proteins - found in several age-related diseases, such as Alzheimer’s disease and Parkinson’s disease (Fig. 2; Valastyan and Lindquist, 2014).
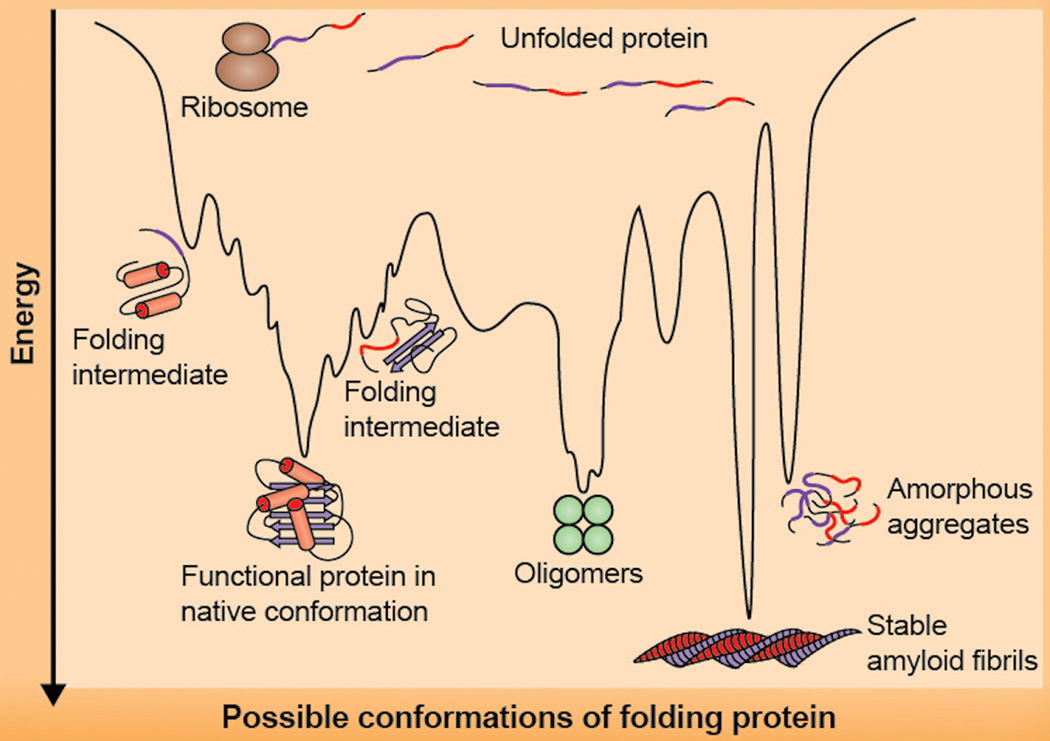
Figure 2.
Misfolding of proteins can lead to degenerative changes associated with aging. This diagram illustrates energy changes associated with protein folding, and shows how misfolding can favor protein aggregation involved in some diseases of aging. “Protein folding allows an amino acid chain to become a functional molecule. Multiple factors in this dynamic process can result in misfolding. A mutation can stabilize an intermediate in the folding process, causing this misfolded state to be favored over the properly folded protein. Furthermore, for many proteins, the functionally folded form is not the lowest energy state of the protein. Many proteins can form stable amyloid fibers or amorphous aggregates that can accumulate and disrupt cellular processes. Given the importance of proper folding to protein function, it is not surprising that each of these misfolding events can be associated with disease.” The figure and text are reprinted from part of a figure in the open-access review of Valastyan and Lindquist (2014).
Third, the organelle problem. Changes during aging are not restricted to DNA and proteins, but also affect, and are affected by cellular organelles. In the case of the nucleus, the lamina - one of the nuclear envelope components - has been implicated in the aging process. For example, mutations in genes encoding components of the nuclear lamina have been found to be the cause of several premature aging syndromes (Eriksson et al., 2003; Cabanillas et al., 2011). Studies of normal aging cells have demonstrated that lower levels of, or defective lamin A or B subtypes accelerate the aging process (Schffidi and Misteli, 2008; Shimi et al., 2011). For this reason, nuclear lamina has been used as a biomarker of aging (Ragnauth et al., 2010; Freund et al., 2012). A recent study of healthy centenarians has shown that nuclear accumulation of prelamin A contributes to longevity by preventing stress-induced DNA damage (Lattanzi et al., 2014), further supporting the critical role of nuclear lamina in maintaining healthy aging. Another class of organelle that is associated with cellular aging is mitochondria (Trifunovic and Larsson, 2008; Lanza and Nair, 2010). Mitochondrial DNA is particularly susceptible to age-dependent mutations, at least in part, due to the DNA repair systems that are less powerful than those used for genomic DNA (Linnane et al., 1989; Vermulst et al., 2008; Wallace, 2010). Moreover, since mitochondria provide a major level of regulation to a range of intracellular processes such as converting energy substrates into ATP, reactive oxygen species (ROS) metabolism, calcium signaling, and iron homeostasis (Mattson, 2008; Mallikarjun et al., 2014), it is likely that the dysfunction of mitochondria has major impacts on the molecular processes that contribute to normal aging and age-related diseases.
Fourth, the signaling pathway problem. A number of signaling pathways have been shown to play key roles in cellular aging. Prominent among such age-regulating pathways is the insulin/IGF-1 signaling pathway (Tatar et al., 2003; Guarente and Picard, 2005). The insulin/IGF-1 pathway is important because it regulates energy metabolism, food intake and stress responses (Mattson et al., 2004). The involvement of the insulin/IGF-I pathway in aging has been documented in a variety of organisms. In hydras, a protein that bears resemblance to mammalian insulin receptor is expressed, and cells expressing such receptors undergo active proliferation in response to mammalian insulin (Steele et al., 1996). In Drosophila, an insulin receptor substrate protein CHICO affects cell growth and the loss of this protein extends lifespan significantly (Clancy et al., 2001).In C. elegans, extensive evidence suggests that the insulin/IGF-1 signaling events directly influence longevity (for example, see Kenyon et al., 1993). And in mice, it has been found that proteins that transduce insulin/IGF-1-like signaling have direct roles in determining lifespan (Migliaccio et al., 1999; Holzenberger et al., 2003). Together, these studies suggest a remarkable conserved role for the insulin/IGF-1 pathway in longevity.
Last, the oxidative stress problem. Cells generate reactive oxygen species (ROS) during mitochondrial respiration and by the activity of various oxygenases; these include superoxide anion radical, hydrogen peroxide, hydroxyl radical, nitric oxide and peroxynitrite (Lambert and Brand, 2009). Such ROS can damage proteins, nucleic acids and cell membranes (lipid peroxidation). However, cells have evolved multiple mechanisms to prevent the formation of, or remove, ROS including the superoxide dismutases (SOD1 and SOD2), glutathione peroxidases, catalase, and glutathione. Such ROS-related mechanisms have been described in hydras and other cnidarians where they are involved in growth, development and reproduction (Blackstone, 2003; Habetha and Bosch, 2005); and ROS are involved in injury-induced cell death and activation of regeneration in Hydra, as well as in Drosophila and zebrafish (reviewed in Vriz et al., 2014). In essentially all species that age there occurs increased accumulation of oxidatively damage molecules, and reducing this oxidative damage can extend lifespan in experimental animals such as yeast and C. elegans (for review see Labuschagne and Brenkman, 2013; Merksamer et al., 2013).
Interestingly, however, when cells and organisms are subjected to mild intermittent oxidative stress, they become resistant to more severe stress as a result of adaptive responses that bolster their antioxidant defense systems (Arumugam et al., 2006). Such adaptive stress response mechanisms are highly conserved and may be largely responsible for the extension of lifespan by energy restriction and exercise (Mattson, 2012).
3. Aging in individual cells – general considerations
Aging studies usually involve research on clonal organisms at the population level, whether referring to a colony of single-celled organisms or an individual multicellular organism (groups of cells that arose asexually from a single cell). Aging can also be studied at the individual cell level, either single-celled organisms or individual cells in a multicellular organism. Single-celled organisms usually divide in a few hours or days. Each daughter cell can begin a potentially immortal cell clone. Even daughter cells of prokaryotes such as bacteria are believed to be potentially immortal (Ackermann et al., 2003); laboratory colonies have been maintained for over 25 years and more than 50,000 generations (Wiser et al., 2013).However, in some single-celled organisms, the division is uneven. The bacterium, Caulobacter crescentus, begins as a motile swarmer and then attaches to a substrate with a stalk (Fig. 3A; Ackermann et al., 2003). Cell division is asymmetric: the larger stalked “mother” producing a smaller, free-swimming daughter swarmer. Over time, the stalked mother cell shows an accelerating decrease in reproductive rate, indicating that it is undergoing senescence.
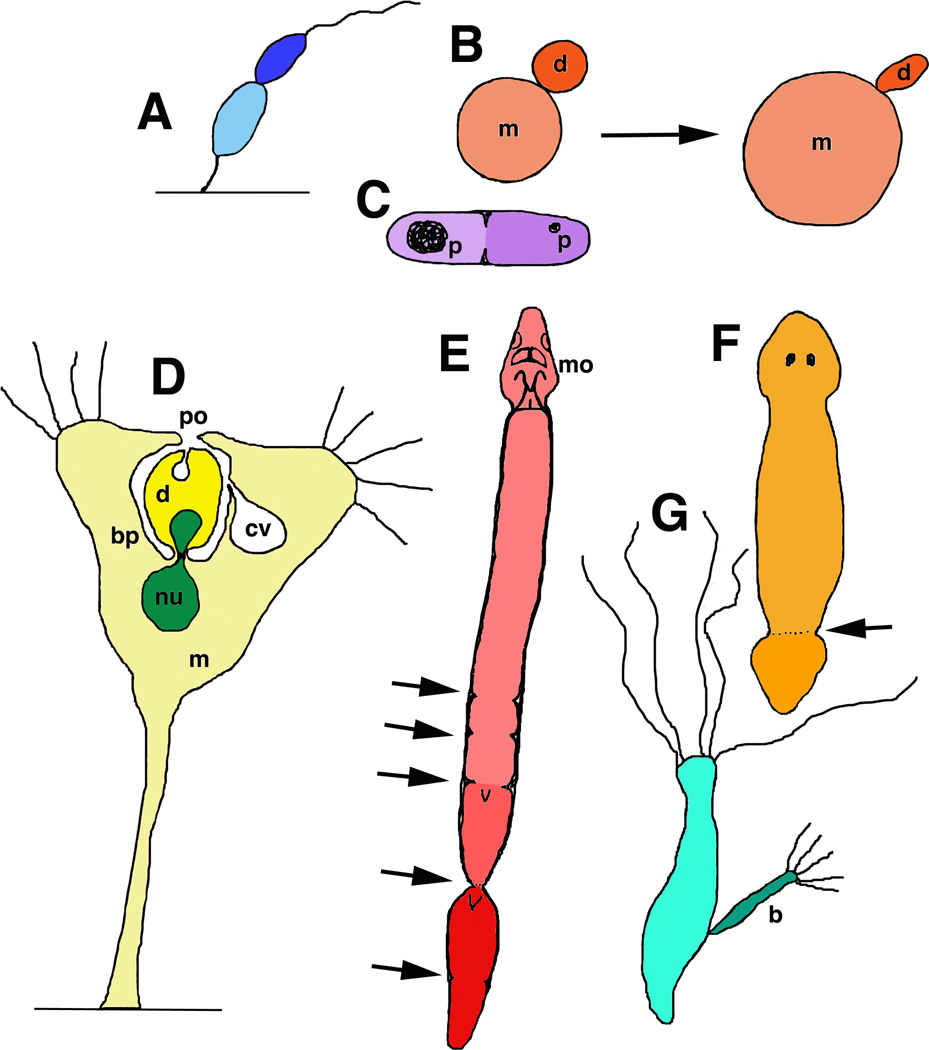
Figure 3.
Asymmetric asexual reproduction and senescence. All drawings are original and based on drawings and micrographs in the cited references. A–E. These are examples of asymmetric cell/body division where the “mother” shows signs of senescence (see text for details).A. The sessile, stalked bacterium, Caulobacter crescentus, produces motile swarmer cell progeny (Ackerman et al., 2003). B. Budding yeast (Saccharomyses cerevisiae) mother cells (m) get bigger as they age and produce more ellipsoidal daughters (d; Lee et al, 2012). C. In contrast, fission yeast, such as Schizosaccharomyces pombe, divide symmetrically under normal conditions. But under stress, there is asymmetric accumulation of protein aggregates (p) leading to aging of the cell that retains most of these aggregates. D. Stalked mother cells (m) of some sessile suctorian ciliates, such as Acineta tuberosa (Bardele, 1970) and Tokophrya infusionum (Rudzinska, 1961; Millecchia and Rudzinska, 1970; Karakashian et al., 1984), produce an offspring in a brood pouch (bp) that is born through a birth pore (po). The brood pouch is associated with the contractile vacuole/excretory system (cv; the smaller contractile vacuole of the offspring is shown also, but not labeled). Also note how a portion of the mother’s macronucleus (nu) pinches off into the offspring. E. The flatworm, Stenostomum incaudatum (Sonneborn, 1930; Nuttycombe and Waters, 1938; Newmark and Sánchez Alvarado, 2002), produces a caudal chain of developing offspring. Arrows show locations rostral to the heads of the forming juveniles (note the pinching in of the digestive tract), where they will separate, eventually, from the mother. mo, mouth. F,G. In contrast to the previous examples, asymmetric asexual reproduction in some planaria by fission (F; arrow; Saló, 2006)and hydras via budding (G; b; Bosch, 2009) may not lead to increased senescence of the mother. In these cases, rapid cell proliferation continuously replaces all cells of the body in a short time period, thus continuously renewing the “mother.”
The best-known example of asymmetric division associated with cell aging is found in budding yeast. In the common budding yeast, Saccharomyces cerevisiae, a mother cell produces a smaller daughter cell as a bud that separates from the mother (Fig. 3B); the mother cell can only go through a specific number of divisions, depending on the strain, and then dies (reviewed by Osiewacz, 2002; Gershon and Gershon, 2000). Typically, budding yeast have a cell cycle of ~2 hours and have 20–30 generations (of budding). Aging yeast cells show changes reminiscent of human aging: cell size increases and the cell’s surface wrinkles as loss of turgor develops. Moreover, bud scars increase; generation (cell cycle) time increases; vacuole size increases and its acidity decreases; protein synthesis, ribosome activity and polysome recruitment decrease; and nucleolar fragmentation appears (Jazwinski, 2001; Clay and Barral, 2013). In addition, there is increased formation of extrachromosomal ribosomal DNA circles, which are linked to genomic instability; these are preferentially retained in the mother cell (Clay and Barral, 2013). Mother cells also selectively retain those mitochondria with more oxidizing redox potential and higher superoxide levels; thus the mother retains the lower-functioning mitochondria that ultimately help cause age-associated decline in cellular fitness (Jazwinski, 2002; McFaline-Figueroa et al, 2011; although as the mother gets older, her daughters inherit progressively lower functioning mitochondria from her).
Stated simply, mother cells retain all the ‘bad stuff’ to assure that their daughters can have a fresh start in life. In contrast to budding yeast, fission yeast such as Schizosaccharomyces pombe divide symmetrically, with both daughter cells relatively equal so that each daughter can give rise to a potentially immortal cell clone (Coelho et al., 2013; Moseley, 2013). However, under stress induced by heat or oxidation, large protein aggregates form and the major aggregatesare segregated into one of the daughters during cell division (Fig. 3C). Like the mother cell of budding yeast, the cell with the big protein aggregates tends to get old and die (Coelho et al., 2013; Moseley, 2013).
To our knowledge, there are no cases reported of individual single-celled organisms living for years without dividing, althoughdocumenting this would be difficult. The exception though may be organisms that are revived after being in stasis for long periods. The ciliate protozoan, Euplotes neapolitanus, can survive encystment, induced by sudden starvation, for up to 6 months (Wichterman, 1964). Goodey (1915) was able to revive protozoa from soil samples that were nearly 50 years old. But the record is probably~34,000 years for bacteria (Schubert et al., 2010).Similarly, for cells within a multicellular organism, the record may be ~32,000 years for regeneration of fertile plants from seedsofSilene stenophylla found in fruit tissue buried in Siberian permafrost (Yashina et al., 2012). Viruses also have been revived from Siberian permafrost after ~30,000 years (Legendre et al., 2014).
In vertebrates, some somatic mitotic cells live and function in the G0 phase of the cell cyclefor many months. Such cells may exhibit a high quality of maintenance of proteome homeostasis (proteostasis), thus minimizing the accumulation of damaged or misfolded proteins (Vilchez et al., 2014; also Treaster et al., 2013). Postmitotic cells such as neurons can survive and function normally for the entire life of the animal, in some cases (e.g., fish, tortoises, whales) for over 200 years. Interestingly, the cellular niche can control the lifespan of these neurons; for example, embryonic neuron precursors from short-lived mice (~18 month life span) that are transplanted into rats (~30 month life span) live twice as long (Magrassi et al., 2013). The neurons arising from the transplanted neuronal precursors(Purkinje cells) remain smaller than the corresponding rat neurons but retain normal polarity and orientation with age; they also show a similar age-dependent loss of dendritic spines with age as seen in the rat neurons. While these long-lived neurons function much as they do in their youth, there are numerous changes that occur with normal aging, especially at the synapses between these neurons, a topic covered in a recent review(Petralia et al., 2014).
4. Aging in ciliate protozoa – clonal immortality, regeneration, and dedifferentiation/differentiation contribute to longevity
The protozoa are one-celled, eukaryotic organisms, usually heterotrophic, that often show active movements like metazoan animals. Generally, the term is not commonly usedin the recent literature, and instead, the organisms are defined only as belonging to particular phyla of protists (protists are eukaryotes that are not fungi, plants or animals). Here we are concerned mainly with aging in ciliates, which include many of the most well-knownand studied protozoa, such as Parameciumand Tetrahymena(we will discuss another group, the choanoflagellates, in the section on Porifera).We want to develop here the main premise of this review, as noted in the Introduction: that immortal clonal lines of protozoa evolved into the first metazoans (multicellular animals) where they continued as large populations of totipotent/pluripotent stem cells; this gave these basal animals both great regenerative powers and potentially very long lives. Subsequentevolution resulted in animals with greater complexity and fewer and less multipotent stem cells, and thus reduced regenerative powers and shortenedlifespan.
Aging variesamong protozoa both in the lifespan of individual cells and in that of clonal cultures. Most protozoa reproduce asexually by dividing into two equivalentdaughter cells, but some reproduce by asymmetric budding. As described above for budding yeast, budding protozoa are mother cells that produce a juvenile daughter cell. Some, such as the sessile suctorian ciliates, Acineta tuberosa (Bardele, 1970) and Tokophrya infusionum (Rudzinska, 1961; Millecchia and Rudzinska, 1970; Karakashian et al., 1984),have endogenous budding, a process that lookssuperficially like vertebrate live birth (Fig. 3D).The mother cell carries the embryo in a brood sac thatmay be derived from the excretory vacuolar system of the mother cell. She gives birth by forcibly extruding her daughter through the birth pore; the daughter later goes through a metamorphosis, changing from the motile juvenile form to the sessile adult form. Mother cellsof Tokophrya infusionumlive for about 10 days (up to 37 days) and show distinctive signs of progressive senescence. In addition, daughters produced by older mothers tend to show greater variability in life span and in the number of daughters that they produce. Old mother cells display numerous signs of senescence including: enlarged size and sometimes an irregular outline; fewer feeding tentacles that also are less efficient at capturing prey; slower feeding; lower reproduction rate and finally a loss of reproduction; fewer and more peripheral mitochondria; accumulation of lipid-like pigment granules; large oval or circular areas filled with large, dense particles (possibly accumulating solid waste products; Rudzinska, 1961). Diet also affects the lifespan of the mother: those fed little food with intermittent starvation live twice as long as those fed regularly (Rudzinska, 1952). Very overfed individuals can turn into giants (180 µm versus 35 µm) that stop reproducing and die young (Rudzinska, 1951).
Typically, protozoans divide into two daughter cells of the same size. The daughter cells initially have a juvenile structure while they are reorganizing. In the large, complex ciliate Bursaria, dedifferentiation of the gullet occurs prior to normal cell division and later the two daughter cells differentiate, reforming adult structures (Lund, 1917). Interestingly, a similar sequence occurs if the organism is cut into two pieces with a scalpel: dedifferentiation precedes differentiation; the process of encystment is also similar (Lund, 1917). Thus, the morphogenetic processes of asexual reproduction, regeneration, and encystment in protozoa may be various manifestations of the same event. In the same sense, the somatic mechanisms of aging and regeneration in simple multicellular animals (basal metazoans) may be related to each other (Sánchez Alvarado, 2000). As mentioned above, discussions of aging in most protozoa, where cell division is symmetrical, concerns the aging of the clonal cultures and not the individual organisms, since cells typically divide every few hours or days (e.g., Hungate, 1942; Wichterman, 1964). We can draw direct analogies between this clonal aging in protozoa and the aging process in multicellular animals (Nanney, 1974). We will consider clonal aging in protozoa in the same way that we consider its involvement in the immortality of totipotent/pluripotent somatic stem cells; we will discuss the implications of this for aging in animalsin later sections of this article.
Seemingly immortal lines of cultured protozoans are well known. Woodruff (1943) noted that a culture of Paramecium aurelia that was started in 1907 was still viable after 36 years and ~21,800 generations. Similarly, a culture of an amicronucleate strain of Tetrahymena pyriformis started in 1923, was still viable 51 years later (although there were some inconsistencies in the strains reported in the literature; Nanney, 1974). Note that ciliate protozoa typically have both large polyploid macronuclei for somatic (vegetative) growth and small diploid micronuclei that maintain the germline for sexual reproduction (and there can be several different sexes). But generally, these immortal lines of protozoa lose most or all of their ability to produce sexual offspring after a certain number of asexual cell divisions (they may fail to complete sexual encounters or if they do, the offspring may die; Nanney, 1974; Nanney and Simon, 1999). In some protozoa this clonal aging may produce structural abnormalities; in Euplotes minuta, the number of dorsal ciliary rows decreases gradually after about 500 fissions (i.e., beginning with an individual formed from a sexual encounter, called conjugation), and the clonal culture terminates at ~700 fissions; Nanney, 1974).
In summary, protozoa can possess several characteristics relevantto aging, including asexual clonal immortality, regeneration, and the ability to cycle between dedifferentiation and differentiation; thesefeatures likely predisposed some of them to evolve into the first multicellular animals, as we shall discuss in the following sections.
5. Aging in multicellular organisms – general comments
As noted above, most aging studies look at clonal collections of cells derived from a single cell; this includes multicellular organisms typically derived from a single (usually fertilized) egg cell. In many cases, these cell clones appear to be immortal, showing no signs of aging over many years, as we will discuss in more detail in subsequent sections. We will concentrate in this review on aging in multicellular animals with a simple body design, especially looking at the role of totipotent cells and the nature of immortality, and compare this to aging in humans as representatives of more complex animal body plans.
But other multicellular organisms also show this propensity for immortality. In the multicellular fungi, there are some cases of senescence occurring after a short lifetime of about 25 days, but most multicellular fungi appear to be immortal, continuously growing by tip elongation of their filamentous cells, called hyphae (Osiewacz, 2002). These can form into a largely underground structure called a mycelium (a thallus) that can cover many acres, and individual organisms may live for thousands of years (>1,500 years for an individual of Armillaria bulbosa; Smith et al., 1992; Casselman, 2007). Plants grow by cell division of stem cell areas called meristems so that even though, periodically, parts of the plant such as the leaves may show senescence and death, the plant as a whole can live on for many years (Heyman et al., 2014). In several cases, a single clonal organism, in which an extensive group of above ground trees originates from a single mass of underground roots or rhizomes, has been shown to be living for many thousands of years (Wherry, 1972; Munné-Bosch, 2008). For example, Wherry (1972) describes a Box-huckleberry that may be 13,000 years old, and other studies have claimed even greater ages, although some of these claims may be excessive (Mock et al., 2008). Nevertheless, even single individual trees can reach great ages – about 5,000 years for the bristlecone pine (Munné-Bosch, 2008).
6. Aging in basal metazoans – among three lesser groups of basal metazoans, tissue reorganization and regeneration affect longevity
Five main groups constitute the simplest multicellular animals or metazoans, including the Placozoa, Porifera (sponges),Ctenophora, Cnidaria, and Myxozoa; the simplest metazoans evolved from groups of single-celled protozoans (Evans et al., 2010; Schnitzler et al., 2012; Ryan et al., 2013). The simplicity of their multicellularity can be demonstrated readily by breaking them up into individual cells in the laboratory; when this has been done to some placozoans, sponges and cnidarians, the group of cells often can reorganize themselves back into the whole animal, as will be discussed below. Many also can rejuvenate their bodies, as we shall discuss. Placozoans and Porifera (sponges) are the simplest; they show definite differentiation into various cell types with specialized functions, but lack a definitive nervous system. Ctenophores (comb jellies) and Cnidaria (hydras, corals, jellyfish) are a little more complex, having a basic nervous system, and for many cnidarians, simple eyes. Placozoa and Cnidaria may have evolved from ancestors that evolved into the Bilateria - more complex animals with bilateral symmetry, including insects, clams, humans, etc. Bilateria are triploblastic animals, the embryo showing endoderm, mesoderm and ectoderm, and show a basic bilateral symmetry (lost to some extent in adult echinoderms). The simplest major groupswithin the Bilateria are the flatworms (Platyhelminthes and Acoelomorpha) and they are the first known kinds of animals to evolve a true brain.
Little is known about aging in three of these groups – ctenophores, placozoans, and myxozoans, and they will be covered here only briefly. Myxozoans are small parasites of fish and aquatic invertebrates. They originally were considered protozoa but then phylogenetic analyses showed that they are metazoans, either related to Cnidaria or more likely, to Bilateria (Evans et al., 2010). Typically, host animals are infected by spores containing one or more sporoblast cells and one or more polar capsules. The polar capsulescontain a filament that anchors the spore to the host tissue, and resemble the nematocysts (stinging cells) of cnidarians. The spore-producing stage may be a multicellular, often ameboid plasmodium, containing generative cells within the multinucleate plasmodium, although some kinds form into a closed sac or a worm-like organism (Lom and Dyková, 2006). The latter form (Buddenbrockia) has four longitudinal muscle bands reminiscent of nematodes, and appears to be a triploblastic animal like a bilaterian (Okamura et al, 2002). Little is written about aging of Myxozoa, probably because they are all parasitic and tied to the life cycle of their hosts. Typical infections of fish develop over several months. This may be preceded by an initial infection in an invertebrate such as the tubifex worm, and that also can last several months (El-Matbouli et al., 1992).
Ctenophores are an enigmatic group; they may have evolved from ancestors prior to the ones that evolved into Porifera and Placozoa, and may have a nervous system unrelated to that of cnidarians and Bilateria (Schnitzler et al., 2012; Pennisi, 2013; Ryan et al., 2013). The ctenophore, Pleurobrachia pileus, has been reared from eggs and maintained in culture for up to 250 days (Greve, 1970). Ctenophores often have very delicate bodies but can regenerate parts that are damaged (Greve, 1970). P. pileus tentacles undergo a continuous regeneration process since they are injured or destroyed easily during feeding. Stem cells are found in restricted areas within the tentacle roots as well as in other main parts of the body and contain genes involved in germline determination and maintenance such as Piwi and Vasa, as described in subsequent sections (Alié et al., 2011).
Placozoans are simple plate-like animals up to 1–2 mm in diameter with anupper and lower epithelium and some cells in between. They typically divide by binary fission every 2–3 days, but also can reproduce asexually by other means, producing smaller daughter animals via floating swarmers that bud off of the dorsal surface, as well as by non-floating spheres or by extension of a long stolon from the dorsal surface (sexual reproduction also occurs; Thiemann and Ruthman, 1990, 1991). Most cell types in the placozoans retain the ability to divide, and totipotent cells may occur along the margins of the upper and lower epithelia (Thiemann and Ruthman, 1991; Jakob et al., 2004). The body remains very malleable; impaled on a pin, these animals simply flow around the obstruction (Thiemann and Ruthman, 1991).
Animals that are disaggregated experimentally will reaggregate and in some cases can reform into a viable animal again (we will discuss this more for sponges and cnidarians; Ruthman and Terwelp, 1979). There is little discussion in the literature about aging in placozoans. However, placozoans from old cultures may go through a degenerative phase where they can form into nearly spherical moribund structures (Grell and Benwitz, 1971; reviewed in Thiemann and Ruthman, 1990).
6a. Aging in sponges –their totipotent/pluripotent somatic stem cells, perhaps representing an evolution from choanoflagellate ancestral protozoa, control their great longevity
Sponges (Porifera) may represent the simplest stage of metazoan evolution, and show very close relationships to certain protozoans, especially the choanoflagellates, which appear to be a sister group to all metazoans (Paps et al., 2013). Some choanoflagellates form colonies that resemble clusters of the main filtering cell of sponges, the collar cells or choanocytes (Fig. 4). Structural complexity may be an obstacle to cell-line immortality, and so one might expect the simplest animals to be longest living also. We already have discussed the idea that protozoa and other single-celled organisms can form into potentially immortal cell clones, but it is technically difficult to find evidence of great age in such cell clones. In contrast, sponges can be aged fairly accurately based on the structure of their skeletal elements called spicules, and these studies indeed have indicated that sponges are the longest-living animals. Thus, Jochum et al. (2012) provide substantial evidence that an individual of the deep-sea sponge, Monorhaphis chuni, that forms giant spicules up to 3 m long, is about 11,000 years old. Other studies have estimated that various sponges could be hundreds or thousands of years old.
Figure 4.

Evolution of choanoflagellate colony to basal metazoans with choanocytes and archeocytes (as found in modern sponges).Single-celled choanoflagellates (A) evolved into colonial forms made of clusters or spheres of cells (B), as still living today. Note the characteristic single flagellum (f) surrounded by a collar (c) made of microvilli. The ancestral metazoan may have evolved from a similar colony. Initially, cell proliferation may have become restricted to a subset of the cells. As the metazoan evolved, cells became specialized for a variety of functions (i.e., division of labor for bodily functions; C). Continued replacement of these various cell types became the task of the ancestral somatic stem cells; in the case of sponges, these were probably choanocytes - cells that retained the basic morphology of the ancestral choanoflagellates (asterisks). As these metazoans evolved into more complex forms with both surface and interior cell types of various kinds (e.g., skeleton-forming cells such as sponge sclerocytes; s), an additional population of stem cells evolved that could migrate inside the body (asterisks; these would be the archeocytes in sponges). This combination of exterior and interior pluripotent stem cells could form an immortal cell clone, e.g., thus allowing some sponges and other basal metazoans to live for thousands of years (see text for details). Based on Funayama, 2010.
Sponges then offer a clear connection between potentially ageless lines of single-celled organisms and the earliest and simplest multicellular animals that are equally ageless. This evolutionary sequence would begin with solitary choanoflagellate protozoa (Carr et al., 2008; King et al., 2008; Dayel et al., 2011; Funayama, 2013). As discussed above for other protozoa, during cell division, choanoflagellates regress to a simpler morphology. In this case, they lose their flagellum just prior to cell division and the daughter cells show other juvenile features (Karpov and Leadbetter, 1997; Leadbetter, 2008a). Next in evolution would be the colonial choanoflagellates (Leadbetter, 2008a; Dayel et al., 2011; Maharbiz, 2012). One colonial choanoflagellate, Salpingoeca rosetta, can differentiate into at least 5 different cell types including 2 that form into different kinds of colonies; cells in colonies are connected by fine intercellular bridges, bear filopodia, and sit in a shared extracellular matrix (Dayel et al., 2011). In comparison, sponges have 5–10 different cell types organized into a multicellular animal (Funayama, 2013). The evolutionary transition from colonial choanoflagellates to sponges presumably involved intermediate forms that started to form different cell types to specialize in different functions (Fig. 4). Early on there would be a stage where some of the cells retained their ability to proliferate while others became nonproliferative. The proliferative cells in the sponges and their ancestors then would represent the immortal cell clones that evolved from similar immortal cell clones of protozoa (Funayama, 2013). Sponges contain two kinds of proliferative cells that are considered totipotent/pluripotent somatic stem cells, i.e., cells from which all other cell types may be derived by differentiation and division. These include the choanocytes, which look like choanoflagellate protozoans, and are the main filter-feeding cell of the sponge, and archeocytes, which are ameboid cells that migrate through the central matrix of the sponge, called the mesohyl (Funayama, 2013). Archeocytes are the best-studied stem cells, but in some sponges, choanocytes can dedifferentiate and become wandering ameboid cells that are probably archeocytes, and choanocytes may be the main type of totipotent/pluripotent stem cell in some kinds of sponges. Both types expressgenes involved in germline determination and maintenance such as Piwi and others (Funayama et al., 2010; Funayama, 2013). There are no separate germ cells in sponges and it is these 2 cell types that form gametes for sexual reproduction. They also are involved in the various forms of asexual reproduction seen in sponges, such as gemmule formation, budding, and stolon formation. Also, under environmental stress, some sponges can degenerate, withdrawing their cells into a central mass of ameboid cells that are mainly archeocytes; the sponge then regenerates under more favorable conditions (Bisbee et al., 1989; Shostak, 2008). In addition, like placozoans and some cnidarians, sponges can be dissociated into cells that then can aggregate and reconstitute themselves into a whole sponge again (reviewed in Funayama, 2013).
So what might explain both the great longevity and seeming immortality of sponges (and other basal metazoans as described later here) as well as their great regenerative powers? Based on a great deal of evidence, as summarized briefly above, we can speculate that an ancestor similar to the living colonial choanoflagellates evolved into true multicellular animals with an organized structure made up of several different cell types with different functions, and supported by a siliceous or calcareous skeleton (interestingly, some living choanoflagellates produce siliceous “house” structures called lorica; King, 2004; King et al., 2008; Leadbetter, 2008b; Dayel et al., 2011; Funayama, 2013). The choanoflagellates would become the choanocytes of these animals, and would essentially remain as continuous ageless clones; but instead of the daughter cells living on their own, they become cells of the growing sponge ancestor. These choanocyte stem cells would produce all the other types of somatic cells of the sponge ancestor. But as these animals evolved into larger species, some choanocytes would dedifferentiate to form wandering stem cells, i.e., the archeocytes, that could disperse readily through the animal and be more available for replacement of the various somatic cell types throughout the body (Funayama et al., 2010; Funayama, 2013). The living sponge thusretains its agelessness through the continuous proliferation of its stem cells that are the equivalent of a colony of ageless protozoans.
6b. Aging in cnidarians – their abilities to reorganize, rejuvenate and regenerate can produce great longevity and potential immortality
Among the basal metazoans, the Cnidaria and Ctenophora are the most advanced, possessing a simple nervous system, sense organs (eyes in Cnidaria and statocysts in both), and muscles; in contrast, definitive examples of these structures are lacking in the simplest metazoans, the Porifera and Placozoa. Thus, cnidarians and ctenophores represent an intermediate stage of body complexity between poriferans and placozoans, and the Bilateria, which includes all of the other major phyla of animals. Interestingly, they are also intermediate in theirmaximum lifespans. As described above, there is good evidence that some individual sponges live for more than 10,000 years. In contrast, some individuals in the major groups of Bilateria have lifespans up to about 500 years, and often with negligible senescence (e.g., >500 years for some marine clams, 50–100 years for lobsters, and over 200 years for some vertebrates; Finch, 2009; Petralia et al., 2014; Treaster et al., 2013).Among the Cnidaria, individual living corals, which are formed by a colony of polyps (an elongate structure with a mouth surrounded by tentacles at the top) that are joined together by a continuous sheet of living tissue (the coenosarc), can be more than 4,000 years old (Roark et al, 2009). Perhaps this indicates a reciprocal relationship between maximum ages and body complexity in the evolution of animal aging. Of course it is difficult to study changes in aging of a long-lived creature in the laboratory; but among cnidarians, some sea anemones have been observed to live in captivity for more than 80 years, without obvious signs of aging, budding continuously; apparently, they died of neglect, so they probably would have lived longer if cared for properly (reviewed in Comfort, 1956). Ashworth and Annandale (1904) described these latter sea anemones when they were about 50 years old, and noted that they were a little more sensitive to cold and dark compared to young mature sea anemones, and their sexual reproductive fertility seemed to have decreased; however, it was not clear if these differences were due to different rearing conditions. Nevertheless, senescence and death from old age do occur in some Cnidaria. In particular, the individual polyps in corals or hydrozoans typically have a short life. For example, polyps of the colonial hydroid, Campanularia flexuosa live for 4–8 days at 25°C; ATP content decreases during their lifespan, yet they seem to catch and eat food normally until they die (Strehler and Crowell, 1961). Acropora cytherea corals show growth anomalies in the older, central portions of large colonies; these anomalies are associated with reduced productivity and dysfunctional gametogenesis (Irikawa et al., 2011). However, in both of these cases, it is the older polyps within a clonal colony that are dying, and the colony, which is really a single organism with continuous cytoplasm, lives on. But the entire colony of the branching coral, Stylophora pistillata, dies after a few years, and shows decreases in reproduction and growth about 6 months earlier (Rinkevich and Loya, 1986).
Above we discussed the idea of agelessness or immortality in protozoa and sponges. Similarly many cnidarians are likely immortal. This was best illustrated in a study by Martínez (1998) in which individuals of the common polyp-type cnidarian, Hydra vulgaris, were followed for 4 years; during the study period, animals showed no signs of age-related mortality and there were no definitive changes in reproductive rates for either asexual (budding) or sexual reproduction (animals grew testes or eggs normally throughout the study, but were kept isolated). Analyses of reproduction and mortality rates led to predictions that these animals indeed were immortal. One mortality trajectory of Hydra magnipapillata, based on a laboratory study lasting several months (Schaible et al., 2011), estimated that 5% of adults would be alive after 1400 years (Jones et al., 2014).Hydras have a long trunk with a foot or basal disc at one end and a head with mouth surrounded by tentacles at the other end (Fig. 3G). The cells of the main body column divide continuously and these replace all of the non-dividing, differentiated cells in the hydra within 20 days; thus the hydra’s body is renewed continuously - at least 60 times over the 4 years of the Martínez study (Martínez, 1998; Watanabe et al., 2009; Tanaka and Reddien, 2011; Gold and Jacobs, 2013). Hydras typically maintain a steady adult size; most excess cells are formed into buds that develop into daughter hydras that separate from their mother (Fig. 3G); the remaining excess cells are sloughed off of the ends of the hydra. Cell divisions involve 3 cell types; epithelial cells of the 2 body wall layers (ectoderm and endoderm) divide about every 3–4 days, while small interstitial cells (I-cells) from the ectodermal layer divide about once a day to form several specialized cell types: neurons, nematocysts (stinging cells), secretory cells, and gametes. These I-cells then are considered the multipotent stem cells of the hydra. The rejuvenating (regenerative) powers of hydras are amazing. Dissociated hydra cells can reaggregate and form a new hydra (Gierer et al., 1972; Bosch, 2007), as noted also in the previous sections for some sponges and placozoans. Shimizu et al. (1993) showed that a tiny piece of tissue cut from the trunk of a hydra (Hydra magnipapillata) will reorganize into a 0.2 mm sphere (the animal is about 7 mm long) with only about 270–300 cells, and then finally into a complete mini-hydra less than a mm long. Many factors are probably involved that help guide the cells to reorganize into the hydra shape. For example, Wnt signaling, important in axial patterning in the vertebrate embryo, induces head formation in the hydra (Lengfeld et al., 2009; Watanabe et al., 2009). Regeneration of hydra into a new animal is mainly due to a reorganization of existing cells rather than via cellular proliferation; this is called morphallaxis (Bosch, 2007. Thus, the hydra may attain immortality; essentially it keeps itself young by having a highly malleable and renewable body and by replacing its cells continuously. But not all hydras may be immortal. Yoshida et al. (2006) examined aging in 3 species of hydra kept at 10°C to induce and maintain the sexual phase. They found that one species, Hydra magnipapillata, that still reproduced asexually by budding (apparently along with sexual reproduction) at this temperature did not seem to age during the experimental period. But the other two hydra species (mainly Hydra oligactis) showed signs of aging and died off in a few months. The aging hydra showed declines in food capture, contractile movements, and reproduction; tentacles became shorter progressively and there was general atrophy of the body after 60 days. This possible correlation of senescence with sexual reproduction has been noted for some other cnidarians (Gold and Jacobs, 2013) and we already have mentioned this phenomenon briefly for protozoa above. Yoshida et al. (2006) noted that the I-cells of H. oligactis started producing mainly germ cells for sexual reproduction, and decreased their production of nerve cells, nematocytes, and gland cells; this explains the decline in food capture as the animals got older. The authors also found changes in gene expression during degeneration and found genes in these hydras that are associated with the aging process of other animals, such as members of the insulin/IGF-I signaling pathway and sir2.
In contrast to the hydra, which is among the simplest of cnidarians, most other kinds of cnidarians have complex body plans and life cycles, with thus many variations in aging and regeneration phenomena. We can only cover some highlights of this variety in this review. Hydras require 3 kinds of cells to continuously regrow their bodies, but in contrast, the marine colonial hydroid, Hydractinia echinata, has I-cells that are true totipotent/pluripotent migratory stem cells, similar to the arrangement that we described for the sponges (Müller et al., 2004; Künzel et al., 2010). In Hydractinia echinata, Polynem (Pln), a POU domain transcription factor gene, is a key regulator of these stem cells, as are similar POU genes in mammalian embryos (Millane et al., 2011; Gold and Jacobs, 2013). These stem cells also have various known cnidarian stem cell markersincluding germline markers, Nanos, Vasa and Piwi, similar to what we described for sponge stem cells. The presence of true stem cells are not definitive in many other cnidarians; in some cases, interstitial cells or amebocytes have been identified as stem cells, but more study of their function is needed (Gold and Jacobs, 2013).Isolated striated muscle cells from the medusa (jellyfish) stage of Podocoryne can dedifferentiate into a stem cell in the laboratory, and when combined with endoderm cells, can make all of the cell types and form a new, partial medusa structure that can feed, grow, produce gametes, and live for months (Schmid et al., 1982; Gold and Jacobs, 2013). Thus, cnidarians may retain a broad ability to create stem cells from various cell types, to regenerate and rejuvenate their bodies; this is similar to what we described above for the stem cell functions of choanocytes and archeocytes in sponges.
The most amazing case of body rejuvenation among cnidaria and possibly among all animals is seen in the life cycle of Turritopsis nutricula (Fig. 5; Piraino et al., 1996). Like many cnidarians, this animal goes through a complex metamorphosis; sexually mature medusae produce mobile larvae (planula stage) that eventually settle to form a colonial hydroid (polyp) stage, which grows and produces new medusae asexually. In many other Cnidaria with this life style, medusae eventually die after reproducing sexually one or more times. But in Turritopsis nutricula, sexually mature medusae, if stressed in the laboratory, will reverse metamorphose back to the colonial polyp stage that can feed and bud off new medusae. This is analogous to a butterfly changing back into a caterpillar!Here then may be an example of an animal that can achieve immortality by regularly cycling between a complex mature adult stage and a simpler larval stage.
Figure 5.
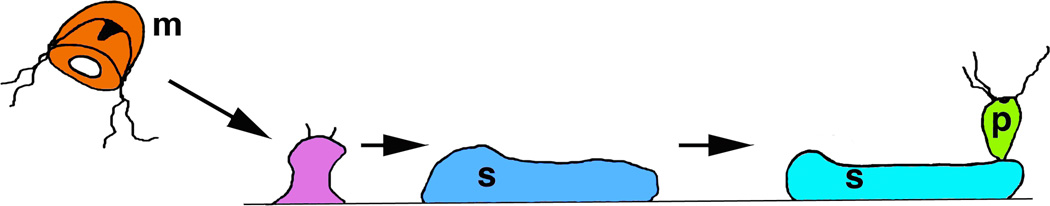
An “immortal” jellyfish? In the hydrozoan, Turritopsis nutricula (Piraino, 1996), asexual reproduction by budding of hydroid polyps (p) from a stolon (s) follows rejuvenation from the sexual medusoid (jellyfish) stage (m) in a unique mechanism of reverse metamorphosis. In theory, these animals could continue this forever, hydroid colonies producing sexual medusae that eventually return to the hydroid stage and then repeat the cycle, leading to the popular name in news accounts of “The Immortal Jellyfish.”
7. Aging in flatworms – longevity depends on asexual fission, controlled shrinkage during starvation, and regeneration and involves abundant totipotent/pluripotent neoblast stem cells
Planaria and other flatworms are very important model animals for studying stem cells and regeneration (e.g., Liu et al., 2013; Sikes and Newmark, 2013; Umesono et al., 2013). Flatworms are probably the most primitive of the Bilateria; all are bilaterally symmetrical, but unlike most higher Bilateria (earthworms, clams, insects, humans, etc.), they lack a body cavity (acoelomate). They make up 2 phyla including:1) Platyhelminthes, which includes planaria and other free-living flatworms of marine, freshwater, and land environments, as well as two large groups of parasites, the trematodes or flukes, and the tapeworms; and 2) the Acoelomorpha (a phylum or subphylum recently separated from Platyhelminthes)that includes the acoel flatworms, which have no gut tract. The acoel flatworms may be close to the basal group of the Bilateria, representing the transition between the basal metazoans such as placozoans, sponges and cnidarians, and the more advanced Bilateria such as planaria, insects, humans, etc.
Aging in planaria and other flatworms is not well understood. Lifetimes of both individuals and clonal colonies in the laboratory have been variable, probably due both to species and culture differences; a number of studies indicate that many kinds of flatwormscan live for at least several years, maybe in the range of mice and rats (Balázs and Burg, 1962; Haranghy and Balázs, 1964; Mouton et al., 2010).
Individuals of Dugesia tigrina, collected in the wild, were still alive in captivity after 7 years and produced egg capsules annually (Goldsmith, 1942). Parasitic flatworms such as flukes and tapeworms live in a protected environment in their hosts, and may have even longer lifespans; records of 25 to more than 35 years have been noted (reviewed in Comfort, 1956). Christopherson (1924) reports that a scientist infected with Schistosoma flukes passed live eggs in his urine for at least 28 years (the life cycle requires an intermediate host), after which he stopped monitoring their passage; eggs were absent when checked after a total of 42 years.
Mouton et al. (2010) found that the free-living flatworm, Macrostomum lignano, ages gradually with an average lifespan of 205 days (maximum is 2.4 years). Signs of old age in these animals include body deformities, grooves in the head region, and liquid-filled cysts, as well as disintegration of gonads in some animals (Fig. 1). The size of cysts in this animal does not increase with age; they seem to form as aging animals lose their ability for efficient wound healing, so cyst size may be more dependent on the size of the initial wound rather than animal age (Crucke, 2010). In contrast to M. lignano, Schmidtea polychroa does not show signs of metabolic aging, at least not over a three-year experimental period (Mouton et al., 2011). After an initial growth phase of five months (sexual maturation by two months), S. polychroa undergoes alternating phases of regressionand growth. Those few animals that did die during the experiment first showed intense regressionand sometimes deformities, prior to death. In addition to these examples, asexual strains have been maintained from several species, including Stenostomum tenuicaudatum, Schmidtea mediterraneaandspecies of Dugesia(Nuttycombe and Waters, 1938; Baguñá and Romero, 1981, Newmark and Sánchez Alvarado, 2002; Isaeva et al., 2005 [colony maintained for 40 years]; Ishizuka et al., 2007; Rink, 2013), and these are considered to be immortal lines (Newmark and Sánchez Alvarado, 2002, Saló, 2006, Nuttycombe and Waters, 1938; Tan et al., 2012), as we have describedin previous sections for examples of exclusive asexual reproduction in Protozoa and Cnidaria. Furthermore, individual flatworms may be potentially immortal, as described above for S. polychroa (Mouton et al., 2011) and also for S. mediterranea (Tan et al., 2012). Interestingly, the asexual and sexual strains of S. mediterranea differ genetically by a chromosome translocation: animals with the translocation reproduce only asexually by transverse fission and those without it reproduce only sexually (as hermaphrodites; Baguñá et al., 1999; Newmark and Sánchez Alvarado, 2002, Zayas et al., 2005, Saló, 2006).In the wild, there may be different populations of sexual and asexual planarians (Baguñá et al., 1999).
Generally, flatworms can employ three mechanismsto avoid aging: asexual reproduction via fission, controlled shrinkage during starvation, and regeneration from body fragments (Newmark and Sánchez Alvarado, 2002, Saló, 2006, Mouton et al., 2011); all of these are believed to involve a rejuvenation of the animal, as we have discussed in previous sections for other organisms. During fission, the posterior, tail end splits off somewhere in the last two-thirds of the body and both parts regenerate into whole animals (Fig. 3F). Probably the most elaborate fission mechanism is found in Stenostomum incaudatum (Sonneborn, 1930; Nuttycombe and Waters, 1938; Newmark and Sánchez Alvarado, 2002). The posterior end of the body starts to differentiate into a miniature worm (called a zooid) and its head and brain start to become distinctive prior to separation from the anterior, typically longer end (Fig. 3E). Other zooids may start to grow before the end zooid separates and chains as long as nine zooids may occur. In a four month study, 100% of the anterior ends died, while only 8.5% of the posterior ends died; this phenomenon of differential aging in uneven divisions has been discussed in previous sections for budding yeast and for the protozoan, Tokophrya infusionum; basically the larger “mother” division of the organism grows old and dies eventually, after producing a number of offspring. Dying Stenostomum incaudatum worms stop growing and decrease in size and often show various abnormalities. The most elaborate fission methods are found in Convolutriloba acoels, which in different species produce small juveniles from the posterior end by: 1) transverse fission; 2) transverse fission followed by longitudinal fission of the juvenile into two individuals; or 3) reversed-polarity budding where the two juveniles bud from the posterior end of the mother, developing their heads pointed away from the mother (Sikes and Bely, 2008).
Many flatworms respond to starvation by decreasing in size significantly, while generally maintaining body proportions and integrity (Baguñá and Romero, 1981; Newmark and Sánchez Alvarado, 2002, Saló, 2006). In a few months, the worm can decrease its length 5–10x or more. Its cell number decreases proportionally; for example, a 7 mm Dugesia tigrina with about 520,000 cells can change into a miniature individual, 2 mm long with 60,000 cells, yet proportions and distribution of the different cell types show little change overall, and are similar to those of a juvenile individual. Thus, the starving worm appears to return to its juvenile state (Baguñá and Romero, 1981).Many flatworms adjust their size by periods of growth and shrinkage to adapt to food availability and other environmental factors (Baguñá and Romero, 1981; Rink, 2013).
Planarians are famous for their capacity to regenerate. For example, Montgomery and Coward (1974) found that a fragment as small as 0.08 mm3 of an asexual Dugesia dorotocephala (7–12 mm)can regenerate completely. Thisfantastic ability to regenerate depends on the presence of large numbers of pluripotent stem cells called neoblasts (Reddien and Sánchez Alvarado, 2004; Peter et al., 2004; Shibata et al., 2010; Tanaka and Reddien, 2011; Rink. 2013). These stem cells are probably present in all kinds of flatworms, including acoels (Fig. 6; De Mulder et al., 2009a, b), tapeworms (Toledo et al., 1997; Brehm, 2010) and trematodes (Collins et al., 2013).These are small, round cells with little cytoplasm, spread through the mesenchyme around the organs and making up about ¼ of planarian cells (Baguñá and Romero, 1981;Reddien and Sánchez Alvarado, 2004).Neoblasts form all of the somatic cells and continuously replace them so that the entire planarian body turns over in a few weeks (Rink, 2013; similar to hydras as discussed in the previous section). This is particularly evident in a study by Wagner et al. (2011), who lethally irradiated planaria of a sexual strain of Schmidtea mediterranea(because the sexual strain is more tolerant of radiation than asexual ones) and then implanted a single neoblast derived from an asexual strain. Some of these planaria survived and regenerated lost parts and fed, and ultimately were expanded into entire asexual strains. This indicates that the single neoblast transformed the recipient (sexual strain) into an asexually reproducing animal with the donor genotype. More importantly, it shows that an individual neoblast is truly pluripotent, capable of producing all kinds of somatic cells. Imagine if a very old and sick person could inject a single stem cell, frozen away since youth, under his/her skin and rejuvenate their body – youthful and disease-free!
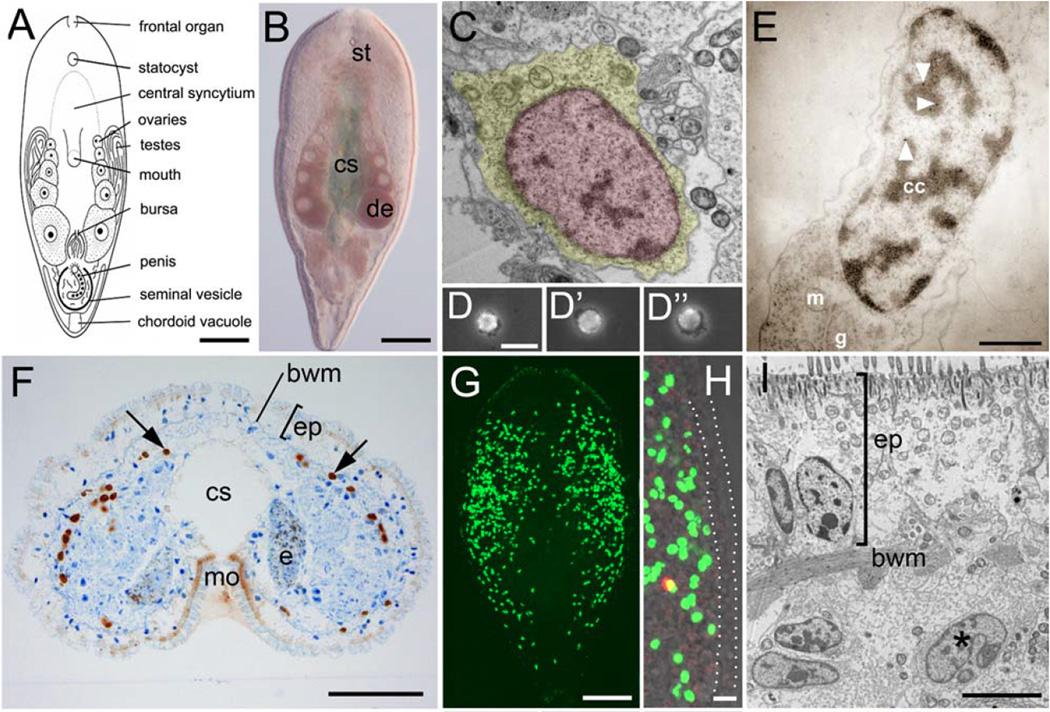
Figure 6.
“The stem cell system of Isodiametra pulchra (A,B). Morphology (C–E) and distribution (F–I) of neoblasts. (A) Schematic drawing. (B) Differential interference contrast image. (C) Typical neoblast with nucleus (red) and thin rim of cytoplasm (yellow). (D-D”) Macerated BrdU labeled cells show typical neoblast-like morphology (E) BrdU labeled neoblast, as shown by immunogold staining after a 30 min BrdU pulse; arrowheads point to gold particles. (F) Histological cross section; brown spots are BrdU labeled S-phase cells. (G,H) Confocal projection overview (G) and detail of lateral body margin (H) after 30 min BrdU pulse; the red spot in (H) is a mitotic figure. Note that S-phase cells [green fluorescence] were lacking in the epidermis (between dotted lines). (I) Electron microscopic image of a posterior-lateral body margin.” “bwm, body wall musculature; cc, condensed chromatin; cs, central syncytium; e, egg; de, developing eggs; ep, epidermis; g, Golgi; m, mitochondria; mo, mouth opening; st, statocyst. Scale bars (A,B,G) 100 µm; (C,E) 1 µm; (D,H) 10 µm; (F) 25 µm; (I) 5 µm.” Figure and legend are reprinted (with some terms excluded from the quoted term list) from Fig. 1A–I of the open-access report of De Mulder et al. (2009a).
The molecular genetics of neoblasts and planarian regeneration has been studied widely and we can only discuss it briefly in this review. In addition to the somatic pluripotent neoblasts that we have been discussing, flatworms also have some germline neoblasts; these may cycle at a slower rate than somatic neoblasts and contain the gene nanos, associated with gonad development and germ celldevelopment and regeneration (Wang et al., 2007; Rink, 2013).Interestingly, both sexual and asexual strains have germ cells expressing nanos, so that inhibition of development of mature reproductive organs in asexual strains must occur downstream of nanos function. Generally for animals, viability of populations of germline stem cells is maintained through many cell divisions by overcoming the end-replication problems of chromosomes via telomere elongation, which occurs during embryogenesis. So how do asexual planaria strains maintain the viability of their somatic neoblasts? These cells can produce higher levels of telomerase via alternative splicing of active telomerase so that telomere length is maintained during the cell replication events that occur during fission or regeneration (Tan et al., 2012).Thus, this might explain how asexual planaria achieve potential immortality. These neoblasts also express several Piwi homologues, more typically associated with germline stem cells (see discussion in earlier sections of this review); these homologues help to maintain the neoblast genome integrity (Rink, 2013). Neoblasts also have chromatoid bodies and their numerous associated proteins, classically linked to germ cell function; this isprobably indicative of their close link in evolution. Related to this, we described above how stem cells of sponges and Cnidaria and other groups are involved in both somatic and germ cell lineages (Rink, 2013).Most likely, in flatworms of both kinds, acoels and Platyhelminthes, germ cells and pluripotent somatic neoblasts are both derived from totipotent neoblasts that self-renew (Bely and Sikes, 2010; Solana, 2013).
8. How does aging in higher animals differ from that of simple animals?
Aging strategies among animals fall roughly into two or three groups, related to cell and tissue maintenance and regeneration, and to aging of the individual cells; proposed mechanisms vary according to the author (Shostak, 2008; Iaseva, 2011; Tanaka and Reddien, 2011; Solana, 2013; Vilchez et al.,2014). Solana (2013) examined the relative amount of totipotency found in some cell populations among all animal groups, using this to define a population of primordial stem cells (PriSCs; Fig. 7) that can form all germ and somatic cell types. In early metazoan evolution, these unlimited PriSCs continue into the adult; they include especially the archeocytes of sponges, and the I-cells of many or most cnidaria and neoblasts of flatworms; i.e., probably all of the groups that we have discussed in detail in this review. These PriSCs allow many members of this first group to reproduce by asexual reproduction (fission, budding, etc.), often imparting potential immortality as has been discussed in previous sections; but the PriSCs also can give rise to germ cells for sexual reproduction (however, germ cells also can form earlier in development and be retained into the adult). The second group has restricted PriSCs and includes some annelids (segmented worms) and some molluscs (snails and clams, etc.). In these animals, an early embryonic cell, commonly the 4d blastomere, divides to form primordial germ cells that form adult germ cells, but this blastomere also produces somatic stem cells that continue into the adult (notably as the MPGZ [mesodermal posterior growth zone] of some polychaete annelids), allowing limited pluripotency in the adult animal. The third group has rudimentary PriSCs. Solana (2013) includes in this group the oyster, Crassostrea gigas (a mollusk), as well as C. elegans(a nematode), Drosophila (an arthropod), and Xenopus (a frog) and other vertebrates. In these organisms, some early cell stages in embryogenesis gives rise to primordial germ cells and thus adult germ cells (Extavour and Akam, 2003; Solana, 2013), as in the second group, but only some initial somatic cells; thus there are no adult pluripotent somatic stem cells (Solana, 2013). In fact, numerous studies in fish and amphibians and other vertebrates support the idea that vertebrates normally lack adult pluripotent somatic stem cells (Tanaka and Reddien, 2011).Interestingly, mammalian embryonic stem cells have the same basic molecular determinants of pluripotency that are found in planarian neoblasts, suggesting that the mechanism is evolutionarily conserved (Önal et al., 2012).
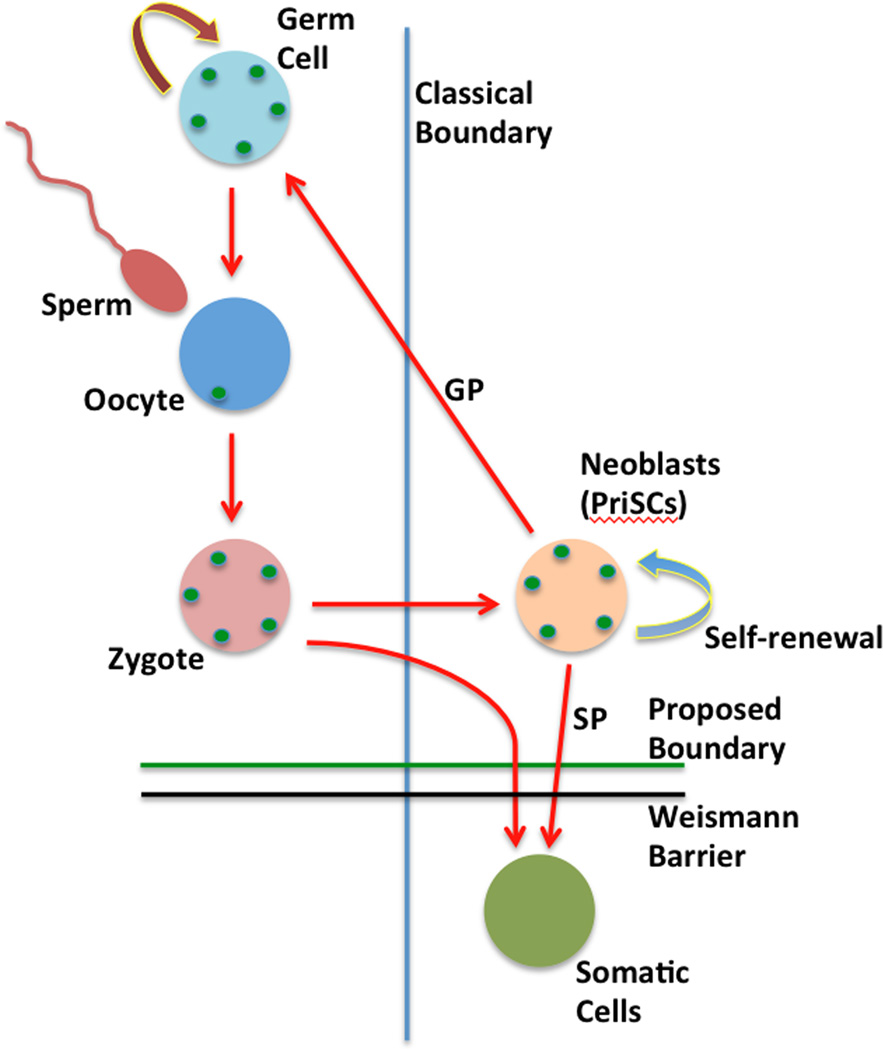
Figure 7.
“The germline cycle in freshwater planarians. Freshwater planarians possess a population of stem cells, the so-called neoblasts, which represents the primordial stem cells in these organisms (PriSC). Neoblasts are able to give rise to the germ cells and to somatic cells. Germ cells give rise to oocytes and sperm, which jointly give rise to the zygote. The zygote gives rise to both somatic cells and the PriSCs. The planarian PriSCs have unlimited self-renewal and both germ potential (GP) and somatic potential (SP). Green dots represent the presence of nuage granules and germ plasm components. The vertical blue line represents the position of the germ-to-soma boundary, as classically understood. The horizontal green line represents the proposed position of the germ-to-soma boundary as postulated in the Primordial Stem Cells hypothesis (of Solana, 2013); this coincides with the Weismann barrier (solid black line) in freshwater planarians.” This figure is an original simplified drawing, based on Fig. 2 of Solana (2013; open access; see that paper and this review text for details; abbreviations and some label designations were changed in the quoted legend). Note that the Weismann barrier essentially prevents somatic cell mutations from affecting the germline and future generations. The PriSCs can allow some planaria to renew all of the cells of their bodies continuously, thus potentially avoiding senescence.
Thus, there was a trend of reduced incidence of asexual reproduction as more complex metazoans evolved. Within the third group described above, we can recognize two different strategies in the evolution of more complex animal forms from the simple metazoans that show continuous rejuvenation (and potential immortality) in the adult (Pearson and Sánchez Alvarado, 2008; Tanaka and Reddien, 2011; Rink, 2013; Solana, 2013). First, for vertebrates and some other higher metazoans, there is maintenance of some limited rejuvenation abilities in the adult resulting in a moderately long lifespan. In contrast, for C. elegans and some other animals, rejuvenation is restricted to sexual reproduction and the embryo, resulting in a short lifespan and an increase in generations per unit time.
Another way of dividing metazoan reproductive/aging strategies is given by Shostak (2008). Shostak argues that basal metazoans have determinativedevelopmentwith narrow potencies, but also, as we have noted, with continuous proliferation of cells followed by asexual reproduction to get rid of the excess cells. Simple metazoans get rid of excess cells, due to proliferation, by asexual reproduction to reduce body size. The best example of this is the hydra as described in a previous section; hydras utilize three kinds of stem cells to proliferate and replace all of the cells of their body in a short time, with excess cells either used to make daughter buds or simply sloughed off at both ends of the body. In this case at least, two of the three kinds of stem cells, i.e., the ones producing the two epithelial cell layers, appear to have a limited potency. Other relatively simple metazoans such as the nematode C. elegans, also have determinative growth but without cell proliferation in the adults (except for the germline and gametogenesis), and with a consequently short lifespan (Extavour and Akam, 2003; Solana, 2013). This probably is not possible in more complex animalsthat are thus in danger of developing cancer (see also Pearson and Sánchez Alvarado, 2008; López-Otin et al., 2013). Onesolution is to constrain proliferation using a more regulative type of development: 1) adult stem cells have asymmetric distribution of DNA strands to constrain steady state populations (Fig. 8; Conboy et al., 2007); 2) a subset of cells can have a count-down timer, possible involving telomere length (capping ends of chromosomes), so that after receiving only freshly-replicated DNA strands for the adult stem cells, precursor cells revert to symmetric division and for a limited number of divisions. In addition, López-Otin et al. (2013) note that some mechanisms promote cellular senescence, which “…protects the organism from cancer but which, in excess, can promote aging.” (Fig. 1).However, strategies to regulate cell and tissue development as a strategy to prevent tumors are not limited to higher metazoans; even planaria can get tumors (as noted in a previous section) and thus have developed molecular mechanisms to avoid them (Oviedo et al., 2008; Pearson and Sánchez Alvarado, 2008).
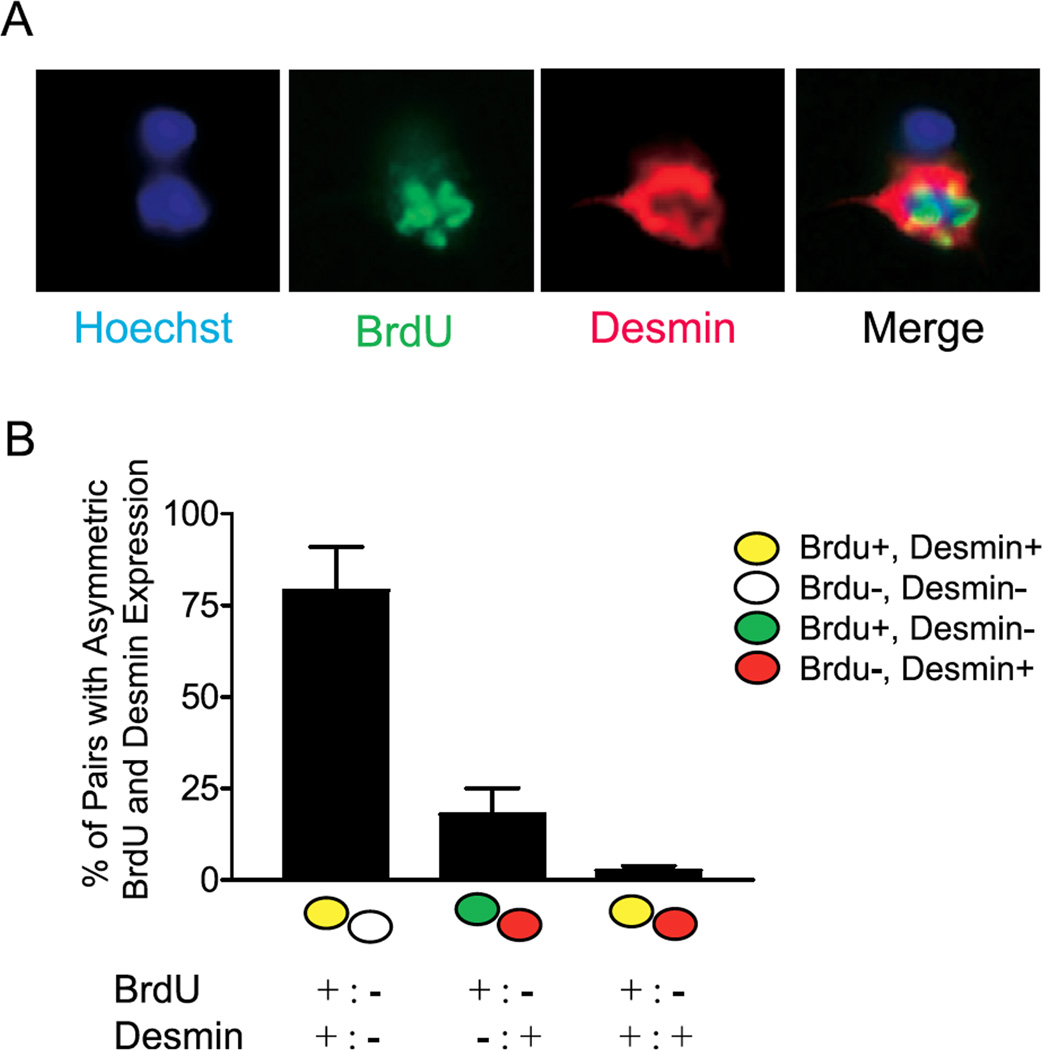
Figure 8.
“Divergent cell fates associated with asymmetric segregation of template strands. (A) Pairs of cells were co-immunostained for BrdU and the myoblast marker Desmin; representative images are shown. (B) The data from experiments as in (A) were quantified. Pairs with Desmin expression and asymmetric BrdU labeling were distinguished based on the pattern of Desmin expression (asymmetric and coincident with BrdU, asymmetric and mutually exclusive with BRdU, or symmetric). The legend for individual cells is shown to the right, and the legends for the cell pairs are shown below. Data represent mean +/− SEM (n=4).” Figure and legend reprinted from Fig. 3 of the open-access review of Conboy et al. (2007; doi:10.1371/journal.pbio.0050102.g003.). In this study of dividing mouse muscle stem cells (satellite cells) we see that the daughter cells inheriting the older templates (no BrdU) usually retain the more immature phenotype, thus apparently remaining in the stem cell population, while the daughter cells inheriting the younger template (BrdU positive) show that they are forming the differentiated cell type, in this case, the Desmin-containing myoblast. This kind of asymmetric division is different from the examples shown in figure 3 of the present review. The strategy of asymmetric segregation of template strands may ensure that the stem cells remain as an immortal cell clone, while the fate of the differentiated cell is only eventual senescence.
Within the complex bodies of vertebrates and other higher Metazoa, we can see that different stem cell populations showing unipotency or oligopotency utilize one of two strategies to maintain their cell populations (Vilchez et al., 2014). Either the stem cells have a high quality of proteostasis (maintenance of proteome homeostasis as noted above) and produce differentiated cells with a high turnover/short lifespan (e.g., blood and epithelial cells), or the stem cells have a lower quality of proteostasis but produce differentiated cells with a low or no turnover/long lifespan that show a corresponding higher quality of maintenance of proteostasis (e.g., neurons, cardiomyocytes; see also Rando, 2006).
Stem cell maintenance in hydras depends on the transcription factor, FoxO, and may account for the potential immortality of hydras (Boehm et al., 2012, 2013). The FoxOgene is expressed at high levels in all threekinds of stems cells of the hydra. FoxO may promote longevity in hydras both by maintaining the stem cells and by supporting innate immune pathways that defend the animal against microbe invasion. FoxO also has been found in other cnidarians, as well as in sponges and placozoans (Boehm et al., 2012, 2013), so it probably is a key longevity factor in basal metazoans. FoxO also has been associated with aging regulation in bilaterians, including C. elegans, Drosophila and humans. Interestingly, in humans, more polymorphisms in the FOXO3A gene may be associated with people in their 100s compared to those in their 90s or younger (Flachsbart et al., 2009). So why does FoxO not promote immortality in humans and other higher bilaterians? As animal life evolved into more complex forms, FoxO seems to have taken on several other functions and has become coupled to pathways involvedmainly with the maintenance of post-mitotic cells as the animal ages (Schaible and Sussman, 2013). Thus, Schaible and Sussman (2013) suggest that these other pathways distract FoxO from its original function of stem cell renewal; some of these pathways may even lead to inhibition of FoxO. Ultimately, in higher bilaterians, FoxO may help maintain the aging body at the expense of lost immortality.
Thus, the groups of basal metazoans and simple flatworms that we have emphasized in this review, and as we have noted previously, have a simple structure that allows them to retain adult pluripotent or totipotent stem cells and to reproduce using both asexual and sexual mechanisms. Adult pluripotency and asexual reproduction seem to work together as a single strategy that is successful mainly for the simplest metazoans, and both are lost in most of the higher metazoans. However, there are exceptions: Isaeva (2011) reviews studies of pluripotent gametogenic stem cells of colonial parasitic rhizocephalan crustaceans (phylum Arthropoda, i.e., the phylum that includes Drosophila) and in colonial ascidians (types of tunicates or sea squirts, i.e., invertebrates in the phylum, Chordata, that includes vertebrates).
9. Conclusions
The evolutionary goal of any species presumably is the continuance of that species into the future. This can be accomplishedby extending the life of the individual organism or by maximizing reproduction of juveniles, i.e., the next generation. Protozoans opt for a short individual cell life and a continuation of the identical cell clone or a modification of that cell clone through sexual reproduction. This mechanism became more complicated with the evolution of multicellular animals. Now there were many different interdependent cell clones all living together as one organism. The earliest strategy was to maintain the total organism by developing pluripotent stem cells that could replace all cell types as needed, for regeneration and rejuvenation of the individual organism. But sexual reproduction has been present from the beginning of multicellularity also, and so we see a great variety of combinations of sexually reproducing individuals that sometimes have short lives, and asexually reproducing ones that are potentially immortal. Then, as we move up the evolutionary treeto more complex animals including humans, we see more and more losses in longevity and a complete loss in potential immortality. People have long hoped forlifespan extension, and even the potential immortality of simple animals. There are many aging and rejuvenation mechanisms as we have discussed briefly, and all of these may have to be considered in this quest for “immortality.”
Ultimately, we may be able to derive benefits to humans from research on phenomena such as dedifferentiation and regeneration in the simplest animals (Table 3); these studies may provide valuable information useful to overcome human diseases and other problems of aging. Stem cell research already has shown many advances, especially in mouse models of human diseases, and including the formation of various specialized cell types and even the generation of entire organs in vitro(reviewed in Sánchez Alvarado and Yamanaka, 2014; Tanabe et al., 2014) and possible genetic rejuvenation of aging muscles in mice in vivo (Sousa-Victor et al., 2014).Also, recent studies have put more emphasis on the influence of the stem cell niche, i.e., the physical microenvironment where stem cells reside in the organism, studied so far mainly in Drosophila and vertebrates (reviewed by Scadden, 2014), but noted also for sponges (Müller, 2006) and flatworms (Mouton et al., 2009). This will be an important consideration in future studies that utilize simple animals as models of stem cell function in human aging and disease. Thus, the knowledge gained from studying the simplest animals may someday provide keys for the extension of healthy human life to ages far beyond what is possible today.
Highlights.
Basal metazoans like sponges, jellyfish, corals and hydras often show great longevity and potential immortality.
These animals can have many pluripotent stem cells that maintain somatic cell lineages central to regeneration and rejuvenation.
These stem cell clones represent a holdover from immortal cell clones of single-celled organisms.
Early bilaterians such as flatworms often retain this system of stem cell-based immortality.
Higher bilaterians including humans have traded immortality for greater complexity and safeguards against unregulated cell growth.
Acknowledgments
Supported by the Intramural Research Programs of NIDCD/NIH and NIA/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. doi: 10.1126/science.1083532. [DOI] [PubMed] [Google Scholar]
- Aitlhadj L, Sturzenbaum SR. Caenorhabditis elegans in regenerative medicine: a simple model for a complex discipline. Drug discovery today. 2014 doi: 10.1016/j.drudis.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Alié A, Leclere L, Jager M, Dayraud C, Chang P, Le Guyader H, Queinnec E, Manuel M. Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: ancient association of “germline genes” with stemness. Developmental biology. 2011;350:183–197. doi: 10.1016/j.ydbio.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing research reviews. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Ashworth JH, Annandale N. Observations on some aged specimens of Sagartia troglodytes and on the duration of life in Coelenterates. Proc Roy Soc Edin. 1904;25:295–308. [Google Scholar]
- Baguñá J, Carranza S, Pala M, Ribera C, Giribet G, A AM, Ribas M, Riutort M. From morphology and karyology to molecules. New methods for taxonomical identification of asexual populations of freshwater planarians. A tribute to Professor Mario Benazzi. Ital J Zool. 1999;66:207–214. [Google Scholar]
- Baguñá J, Romero R. Quantitative anaysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia. 1981;84:181–194. [Google Scholar]
- Baker DJ, Dawlaty MM, Wijshake T, Jeganathan KB, Malureanu L, van Ree JH, Crespo-Diaz R, Reyes S, Seaburg L, Shapiro V, Behfar A, Terzic A, van de Sluis B, van Deursen JM. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nature cell biology. 2013;15:96–102. doi: 10.1038/ncb2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs A, Burg M. Span of life and senescence of Dugesia lugubris. Gerentologia. 1962;6:227–236. doi: 10.1159/000211125. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Bardele CF. Budding and metamorphosis in Acineta tuberosa. An electron microscopic study on morphogenesis in Suctoria. J Protozool. 1970;17:51–70. [Google Scholar]
- Bely AE, Sikes JM. Acoel and platyhelminth models for stem-cell research. Journal of biology. 2010;9:14. doi: 10.1186/jbiol223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbee JW, Francis JC, Harrison FW. Cytological examination of freshwater sponge regeneration from reduction bodies. Trans Amer Micros Soc. 1989;108:299–303. [Google Scholar]
- Blackstone NW. Redox signaling in the growth and development of colonial hydroids. The Journal of experimental biology. 2003;206:651–658. doi: 10.1242/jeb.00138. [DOI] [PubMed] [Google Scholar]
- Boehm AM, Khalturin K, Anton-Erxleben F, Hemmrich G, Klostermeier UC, Lopez-Quintero JA, Oberg HH, Puchert M, Rosenstiel P, Wittlieb J, Bosch TC. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19697–19702. doi: 10.1073/pnas.1209714109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm AM, Rosenstiel P, Bosch TC. Stem cells and aging from a quasi-immortal point of view. BioEssays : news and reviews in molecular, cellular and developmental biology. 2013;35:994–1003. doi: 10.1002/bies.201300075. [DOI] [PubMed] [Google Scholar]
- Bosch TC. Why polyps regenerate and we don’t: towards a cellular and molecular framework for Hydra regeneration. Developmental biology. 2007;303:421–433. doi: 10.1016/j.ydbio.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Bosch TCG. Hydra and the evolution of stem cells. BioEssays : news and reviews in molecular, cellular and developmental biology. 2009;31:478–486. doi: 10.1002/bies.200800183. [DOI] [PubMed] [Google Scholar]
- Brehm K. Echinococcus multilocularis as an experimental model in stem cell research and molecular host-parasite interaction. Parasitology. 2010;137:537–555. doi: 10.1017/S0031182009991727. [DOI] [PubMed] [Google Scholar]
- Brodsky JL. The threads that tie protein-folding diseases. Disease models & mechanisms. 2014;7:3–4. doi: 10.1242/dmm.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PG, Wanamaker AD, Jr., Scourse JD, Richardson CA, Reynolds DJ. Variability of marine climate on the North Icelandic Shelf in a 1357-year proxy archive based on growth increments in the bivalve Arctica islandica. Palaeogeography, Paleoclimatology, Pelaeoecology. 2013;373:141–151. [Google Scholar]
- Cabanillas R, Cadinanos J, Villameytide JA, Perez M, Longo J, Richard JM, Alvarez R, Duran NS, Illan R, Gonzalez DJ, Lopez-Otin C. Nestor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. American journal of medical genetics. 2011;155A:2617–2625. doi: 10.1002/ajmg.a.34249. Part A. [DOI] [PubMed] [Google Scholar]
- Carr M, Leadbeater BS, Hassan R, Nelson M, Baldauf SL. Molecular phylogeny of choanoflagellates, the sister group to Metazoa. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16641–16646. doi: 10.1073/pnas.0801667105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr M, Nelson M, Leadbeater BS, Baldauf SL. Three families of LTR retrotransposons are present in the genome of the choanoflagellate Monosiga brevicollis. Protist. 2008;159:579–590. doi: 10.1016/j.protis.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Casselman A. Strange but true: the largest organism on earth is a fungus. Sci Amer. 2007 [Google Scholar]
- Christopherson JB. Longevity of parasitic worms; The term of living existence of Schistosoma haematobium in the human body. The Lancet. 1924:742–743. [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Clay L, Barral Y. New approaches to an age-old problem. Current opinion in biotechnology. 2013;24:784–789. doi: 10.1016/j.copbio.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Coelho M, Dereli A, Haese A, Kuhn S, Malinovska L, DeSantis ME, Shorter J, Alberti S, Gross T, Tolic-Norrelykke IM. Fission yeast does not age under favorable conditions, but does so after stress. Current biology : CB. 2013;23:1844–1852. doi: 10.1016/j.cub.2013.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, III, Wang B, Lambrus BG, Tharp ME, Iyer H, Newmark PA. Adult somatic stem cells in the human parasite Schistosoma mansoni. Nature. 2013;494:476–479. doi: 10.1038/nature11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort A. The Biology of Senescence. New York: Rinehart; 1956. [Google Scholar]
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS biology. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucke J. Characterization of the Macrostomum lignano ageing phenotype, Faculty of Sciences -Nematology Section. Ghent, Belgium: Universiteit Gent; 2010. p. 65. [Google Scholar]
- Dayel MJ, Alegado RA, Fairclough SR, Levin TC, Nichols SA, McDonald K, King N. Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Developmental biology. 2011;357:73–82. doi: 10.1016/j.ydbio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mulder K, Kuales G, Pfister D, Willems M, Egger B, Salvenmoser W, Thaler M, Gorny A-K, Hrouda M, Borgonie G, Ladurner P. Characterization of the stem cell system of the acoel Isodiametra pulchra. BMC Dev Biol. 2009;9:1–17. doi: 10.1186/1471-213X-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mulder K, Pfister D, Kuales G, Egger B, Salvenmoser W, Willems M, Steger J, Fauster K, Micura R, Borgonie G, Ladurner P. Stem cells are differentially regulated during development, regeneration and homeostasis in flatworms. Developmental biology. 2009;334:198–212. doi: 10.1016/j.ydbio.2009.07.019. [DOI] [PubMed] [Google Scholar]
- El-Matbouli M, Fischer-Scherl T, Hoffmann RW. Present knowledge on the life cycle, taxonomy, pathology, and therapy of some Myxosporea spp. important to freshwater fish. Ann Rev Fish Diseases. 1992:367–402. [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NM, Holder MT, Barbeitos MS, Okamura B, Cartwright P. The phylogenetic position of Myxozoa: exploring conflicting signals in phylogenomic and ribosomal data sets. Molecular biology and evolution. 2010;27:2733–2746. doi: 10.1093/molbev/msq159. [DOI] [PubMed] [Google Scholar]
- Extavour CG, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130:5869–5884. doi: 10.1242/dev.00804. [DOI] [PubMed] [Google Scholar]
- Finch CE. Update on slow aging and negligible senescence - a mini-review. Gerontology. 2009;55:307–313. doi: 10.1159/000215589. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Molecular biology of the cell. 2012;23:2066–2075. doi: 10.1091/mbc.E11-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N. The stem cell system in demosponges: suggested involvement of two types of cells: archeocytes (active stem cells) and choanocytes (food-entrapping flagellated cells) Development genes and evolution. 2013;223:23–38. doi: 10.1007/s00427-012-0417-5. [DOI] [PubMed] [Google Scholar]
- Funayama N, Nakatsukasa M, Mohri K, Masuda Y, Agata K. Piwi expression in archeocytes and choanocytes in demosponges: insights into the stem cell system in demosponges. Evolution & development. 2010;12:275–287. doi: 10.1111/j.1525-142X.2010.00413.x. [DOI] [PubMed] [Google Scholar]
- Gershon H, Gershon D. The budding yeast, Saccharomyces cerevisiae, as a model for aging research: a critical review. Mechanisms of ageing and development. 2000;120:1–22. doi: 10.1016/s0047-6374(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Gierer A, Berking S, Bode H, David CN, Flick K, Hansmann G, Schaller H, Trenkner E. Regeneration of hydra from reaggregated cells. Nature: New biology. 1972;239:98–101. doi: 10.1038/newbio239098a0. [DOI] [PubMed] [Google Scholar]
- Gold DA, Jacobs DK. Stem cell dynamics in Cnidaria: are there unifying principles? Development genes and evolution. 2013;223:53–66. doi: 10.1007/s00427-012-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith ED. Sexuality of Dugesia tigrina (syn. Planaria maculata) Nature. 1942;150:351. [Google Scholar]
- Goodey T. Note on the remarkable retention of vitality by protozoa from old stored soils. Ann Appl Biol. 1915;1:395–399. [Google Scholar]
- Grell KG, Benwitz G. Die ultrastuktur von Trichoplax adhaerens F.E. Schulze. Cytobiologie. 1971;4:216–240. [Google Scholar]
- Greve W. Cultivation experiments on North Sea ctenophores. Helgolander wiss. Meeresunters. 1970;20:304–317. [Google Scholar]
- Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Habetha M, Bosch TC. Symbiotic Hydra express a plant-like peroxidase gene during oogenesis. The Journal of experimental biology. 2005;208:2157–2165. doi: 10.1242/jeb.01571. [DOI] [PubMed] [Google Scholar]
- Han S, Brunet A. Histone methylation makes its mark on longevity. Trends in cell biology. 2012;22:42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haranghy L, Balázs A. Ageing and rejuvenation in planarians. Experimental gerontology. 1964;1:77–91. [Google Scholar]
- Heyman J, Kumpf RP, De Veylder L. A quiescent path to plant longevity. Trends in cell biology. 2014 doi: 10.1016/j.tcb.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. The New England journal of medicine. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hungate RE. The culture of Eudiplodinium neglectum, with experiments of the digestion of cellulose. Biol Bull. 1942;83:303–319. [Google Scholar]
- Irikawa A, Casareto BE, Suzuki Y, Agostini S, Hidaka M. Growth anomalies on Acropora cytherea corals. Marine Pollut Bull. 2011;62:1702–1707. doi: 10.1016/j.marpolbul.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Isaeva V, Alexandrova Y, Reunov A. Interaction between chromatoid bodies and mitochondria in neoblasts and gonial cells of the asexual and spontaneously sexualized planarian, Girardia (Dugesia) tigrina. Invert Reprod Devel. 2005;48:119–128. [Google Scholar]
- Isaeva VV. Pluripotent gametogenic stem cells of asexually reproducing invertebrates. In: Kallos M, editor. Embryonic Stem Cells - Basic Biology to Bioengineering. Rijeka, Croatia: Intech; 2011. pp. 449–478. [Google Scholar]
- Ishizuka H, Maezawa T, Kawauchi J, Nodono H, Hirao Y, Nishimura O, Nakagawa H, Sekii K, Tasaka K, Tarui H, Agata K, Hoshi M, Kobayashi K, Sakakibara Y, Matsumoto M. The Dugesia ryukyuensis database as a molecular resource for studying switching of the reproductive system. Zool Sci. 2007;24:31–37. doi: 10.2108/zsj.24.31. [DOI] [PubMed] [Google Scholar]
- Jakob W, Sagasser S, Dellaporta S, Holland P, Kuhn K, Schierwater B. The Trox-2 Hox/ParaHox gene of Trichoplax (Placozoa) marks an epithelial boundary. Development genes and evolution. 2004;214:170–175. doi: 10.1007/s00427-004-0390-8. [DOI] [PubMed] [Google Scholar]
- Jarosz DF, Taipale M, Lindquist S. Protein homeostasis and the phenotypic manifestation of genetic diversity: principles and mechanisms. Annual review of genetics. 2010;44:189–216. doi: 10.1146/annurev.genet.40.110405.090412. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. New clues to old yeast. Mechanisms of ageing and development. 2001;122:865–882. doi: 10.1016/s0047-6374(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Growing old: metabolic control and yeast aging. Annual review of microbiology. 2002;56:769–792. doi: 10.1146/annurev.micro.56.012302.160830. [DOI] [PubMed] [Google Scholar]
- Jochum KP, Wang X, Vennemann TW, Sinha B, Müller WEG. Silieous deep-sea sponge Monorhaphis chuni: A potential paleoclimate archive in ancient animals. Chem Geol. 2010;300–301:143–151. [Google Scholar]
- Jones OR, Scheuerlein A, Salguero-Gomez R, Camarda CG, Schaible R, Casper BB, Dahlgren JP, Ehrlen J, Garcia MB, Menges ES, Quintana-Ascencio PF, Caswell H, Baudisch A, Vaupel JW. Diversity of ageing across the tree of life. Nature. 2014;505:169–173. doi: 10.1038/nature12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Karakashian SJ, Lanners HN, Rudzinska MA. Cellular and clonal aging in the suctorian protozoan Tokophrya infusionum. Mechanisms of ageing and development. 1984;26:217–229. doi: 10.1016/0047-6374(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Karpov SA, Leadbeater BS. Cell and nuclear division in a freshwater choanoflagellate, Monosiga ovata Kent. Europ J Protistol. 1997;33:323–334. [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- King N. The unicellular ancestry of animal development. Devel Cell. 2004;7:313–325. doi: 10.1016/j.devcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing research reviews. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel T, Heiermann R, Frank U, Müller W, Tilmann W, Bause M, Nonn A, Helling M, Schwartz RS, Plickert G. Migration and differentiation potential of stem cells in the cnidarian Hydractinia analysed in eGFP-transgenic animals and chimeras. Developmental biology. 2010;348:120–129. doi: 10.1016/j.ydbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Labuschagne CF, Brenkman AB. Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing research reviews. 2013;12:918–930. doi: 10.1016/j.arr.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods in molecular biology. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Archiv : European journal of physiology. 2010;459:277–289. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi G, Ortolani M, Columbaro M, Prencipe S, Mattioli E, Lanzarini C, Maraldi NM, Cenni V, Garagnani P, Salvioli S, Storci G, Bonafe M, Capanni C, Franceschi C. Lamins are rapamycin targets that impact human longevity: a study in centenarians. Journal of cell science. 2014;127:147–157. doi: 10.1242/jcs.133983. [DOI] [PubMed] [Google Scholar]
- Leadbeater BS. Choanoflagellate lorica construction and assembly: the nudiform condition. I. Savillea species. Protist. 2008;159:259–268. doi: 10.1016/j.protis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Leadbetter BSC. Choanoflagellate evolution: the morphological perspective. Protistology. 2008;5:256–267. [Google Scholar]
- Lee SS, Vizcarra IA, Huberts DHEW, Lee LP, Heinemann M. Whole lifespan microscopic observation of budding yeast aging through a microfluidic dissection platform. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4916–4920. doi: 10.1073/pnas.1113505109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre M, Bartoli J, Shmakova L, Jeudy S, Labadie K, Adrait A, Lescot M, Poirot O, Bertaux L, Bruley C, Coute Y, Rivkina E, Abergel C, Claverie JM. Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:4274–4279. doi: 10.1073/pnas.1320670111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld T, Watanabe H, Simakov O, Lindgens D, Gee L, Law L, Schmidt HA, Ozbek S, Bode H, Holstein TW. Multiple Wnts are involved in Hydra organizer formation and regeneration. Developmental biology. 2009;330:186–199. doi: 10.1016/j.ydbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1:642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Liu SY, Selck C, Friedrich B, Lutz R, Vila-Farre M, Dahl A, Brandl H, Lakshmanaperumal N, Henry I, Rink JC. Reactivating head regrowth in a regeneration-deficient planarian species. Nature. 2013;500:81–84. doi: 10.1038/nature12414. [DOI] [PubMed] [Google Scholar]
- Lom J, Dykova I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia parasitologica. 2006;53:1–36. [PubMed] [Google Scholar]
- López-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- Lund EJ. Reversibility of morphogenetic processes in Bursaria. J Exp Zool. 1917;24:1–33. [Google Scholar]
- Magrassi L, Leto K, Rossi F. Lifespan of neurons is uncoupled from organismal lifespan. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4374–4379. doi: 10.1073/pnas.1217505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharbiz MM. Synthetic multicellularity. Trends in cell biology. 2012;22:617–623. doi: 10.1016/j.tcb.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Mallikarjun V, Sriram A, Scialo F, Sanz A. The interplay between mitochondrial protein and iron homeostasis and its possible role in aging. Experimental gerontology. 2014 doi: 10.1016/j.exger.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Martínez DE. Mortality patterns suggest lack of senescence in Hydra. Experimental gerontology. 1998;33:217–225. doi: 10.1016/s0531-5565(97)00113-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Annals of the New York Academy of Sciences. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell metabolism. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing research reviews. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, Pon LA. Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging cell. 2011;10:885–895. doi: 10.1111/j.1474-9726.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging. 2013;5:144–150. doi: 10.18632/aging.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- Millane RC, Kanska J, Duffy DJ, Seoighe C, Cunningham S, Plickert G, Frank U. Induced stem cell neoplasia in a cnidarian by ectopic expression of a POU domain transcription factor. Development. 2011;138:2429–2439. doi: 10.1242/dev.064931. [DOI] [PubMed] [Google Scholar]
- Millecchia LL, Rudzinska MA. The ultrastructure of brood pouch formation in Tokophrya infusionum. J Protozool. 1970;17:574–583. [Google Scholar]
- Mock KE, Rowe CA, Hooten MB, Dewoody J, Hipkins VD. Clonal dynamics in western North American aspen (Populus tremuloides) Molecular ecology. 2008;17:4827–4844. doi: 10.1111/j.1365-294X.2008.03963.x. [DOI] [PubMed] [Google Scholar]
- Montgomery JR, Coward SJ. On the minimal size of a planarian capable of regeneration. Trans Amer Micros Soc. 1974;93:386–391. [PubMed] [Google Scholar]
- Moseley JB. Cellular aging: symmetry evades senescence. Current biology : CB. 2013;23:R871–R873. doi: 10.1016/j.cub.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton S, Verdoodt F, Willems M, Dhondt I, Crucke J, Braeckman BP, Houthoofd W. Establishing a flatworm ageing model. Belg J Zool. 2010;140:195–197. [Google Scholar]
- Mouton S, Willems M, Braeckman BP, Egger B, Ladurner P, Scharer L, Borgonie G. The free-living flatworm Macrostomum lignano: a new model organism for ageing research. Experimental gerontology. 2009;44:243–249. doi: 10.1016/j.exger.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Mouton S, Willems M, Houthoofd W, Bert W, Braeckman BP. Lack of metabolic ageing in the long-lived flatworm Schmidtea polychroa. Experimental gerontology. 2011;46:755–761. doi: 10.1016/j.exger.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Müller WA, Teo R, Frank U. Totipotent migratory stem cells in a hydroid. Developmental biology. 2004;275:215–224. doi: 10.1016/j.ydbio.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Müller WE. The stem cell concept in sponges (Porifera): Metazoan traits. Seminars in cell & developmental biology. 2006;17:481–491. doi: 10.1016/j.semcdb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. Do perennials really senesce? Trends in plant science. 2008;13:216–220. doi: 10.1016/j.tplants.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Nanney DL. Aging and long-term temporal regulation in ciliated protozoa. A critical review. Mechanisms of ageing and development. 1974;3:81–105. doi: 10.1016/0047-6374(74)90008-6. [DOI] [PubMed] [Google Scholar]
- Nanney DL, Simon EM. Laboratory and evolutionary history of Tetrahymena thermophila. Meth Cell Biol. 1999;62:3–25. doi: 10.1016/s0091-679x(08)61527-7. [DOI] [PubMed] [Google Scholar]
- Newmark PA, Sánchez Alvarado A. Not your father’s planarian: a classic model enters the era of functional genomics. Nature reviews. Genetics. 2002;3:210–219. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- Nuttycombe JW, Waters AJ. The American species of the genus Stenostomum. Proc Amer Phil Soc. 1938:79. [Google Scholar]
- Okamura B, Curry A, Wood TS, Canning EU. Ultrastructure of Buddenbrockia identifies it as a myxozoan and verifies the bilaterian origin of the Myxozoa. Parasitology. 2002;124:215–223. doi: 10.1017/s0031182001001184. [DOI] [PubMed] [Google Scholar]
- Onal P, Grun D, Adamidi C, Rybak A, Solana J, Mastrobuoni G, Wang Y, Rahn H-P, Chen W, Kempa S, Ziebold U, Rajewsky N. Gene expression of pluripotency determinants is conserved between mammalian and planarian stem cells. EMBO J. 2012;31:2755–2769. doi: 10.1038/emboj.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiewacz HD. Genes, mitochondria and aging in filamentous fungi. Ageing research reviews. 2002;1:425–442. doi: 10.1016/s1568-1637(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Paps J, Medina-Chacón LA, Marshall W, Suga H, Ruiz-Trillo I. Molecular phylogeny of unikonts: New insights into the position of apusomonads and ancyromonads and the internal relationship of opisthokonts. Protist. 2013;164:2–12. doi: 10.1016/j.protis.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BJ, Sánchez Alvarado A. Regeneration, stem cells, and the evolution of tumor suppression. Cold Spring Harb Symp Quant Biol. 2008;73:565–572. doi: 10.1101/sqb.2008.73.045. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Nervous system may have evolved twice. Science. 2013;339:391. doi: 10.1126/science.339.6118.391-a. [DOI] [PubMed] [Google Scholar]
- Peter R, Gschwentner R, Schürmann W, Rieger RM, Ladurner P. The significance of stem cells in free-living flatworms: one common source for all cells in the adult. J Appl Med. 2004;2:21–35. [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ. Communication breakdown: The impact of ageing on synapse structure. Ageing research reviews. 2014;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraino S, Boero F, Aeschbach B, Schmid V. Reversing the life cycle: Medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa) Biol Bull. 1996;190:302–312. doi: 10.2307/1543022. [DOI] [PubMed] [Google Scholar]
- Popov IY. Distribution of various aging patterns in the system of the animal world. Adv Gerontol. 2012;2:1–9. [Google Scholar]
- Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sánchez Alvarado A. Fundamentals of planarian regeneration. Annual review of cell and developmental biology. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Rink JC. Stem cell systems and regeneration in planaria. Development genes and evolution. 2013;223:67–84. doi: 10.1007/s00427-012-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich B, Loya Y. Senescence and dying signals in a reef building coral. Experentia. 1986;42:320–322. [Google Scholar]
- Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA. Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5204–5208. doi: 10.1073/pnas.0810875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb SM, Sanchez Alvarado A. Histone modifications and regeneration in the planarian Schmidtea mediterranea. Current topics in developmental biology. 2014;108:71–93. doi: 10.1016/B978-0-12-391498-9.00004-8. [DOI] [PubMed] [Google Scholar]
- Rudzinska MA. The influence of amount of food on the reproduction rate and longevity of a suctorian (Tokophrya infusionum) Science. 1951;113:10–11. doi: 10.1126/science.113.2923.10. [DOI] [PubMed] [Google Scholar]
- Rudzinska MA. Overfeeding and life span in Tokophrya infusionum. Journal of gerontology. 1952;7:544–548. doi: 10.1093/geronj/7.4.544. [DOI] [PubMed] [Google Scholar]
- Rudzinska MA. The use of a protozoan for studies on aging. I. Differences between young and old organisms of Tokophyra infusionum as revealed by light and electron microscopy. Journal of gerontology. 1961;16:213–224. doi: 10.1093/geronj/16.3.213. [DOI] [PubMed] [Google Scholar]
- Ruthman A, Terwelp U. Disaggregation and reaggregation of cells of the primitive metazoon Trichoplax adhaerens. Differentiation. 1979;13:185–198. [Google Scholar]
- Ryan JF, Pang K, Schnitzler CE, Nguyen A-D, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, Program NCS, Smith SA, Putnam NH, Haddock SHD, Dunn CW, Wolfsberg TG, Mullikin JC, Martindale MQ, Baxevanis AD. The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science. 2013;342:1336. doi: 10.1126/science.1242592. 1242592 (1242591–1242598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saló E. The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes) BioEssays : news and reviews in molecular, cellular and developmental biology. 2006;28:546–559. doi: 10.1002/bies.20416. [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A. Regeneration in the metazoans: why does it happen? BioEssays : news and reviews in molecular, cellular and developmental biology 22. 2000;6:578–590. doi: 10.1002/(SICI)1521-1878(200006)22:6<578::AID-BIES11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Yamanaka S. Rethinking Differentiation: Stem Cells, Regeneration, and Plasticity. Cell. 2014;157:110–119. doi: 10.1016/j.cell.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. Nice Neighborhood: Emerging Concepts of the Stem Cell Niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nature cell biology. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible R, Ringelhan F, Kramer BH, Miethe T. Environmental challenges improve resource utilization for asexual reproduction and maintenance in hydra. Experimental gerontology. 2011;46:794–802. doi: 10.1016/j.exger.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Schaible R, Sussman M. FOXO in aging: did evolutionary diversification of FOXO function distract it from prolonging life? BioEssays : news and reviews in molecular, cellular and developmental biology. 2013;35:1101–1110. doi: 10.1002/bies.201300078. [DOI] [PubMed] [Google Scholar]
- Schellenberg A, Lin Q, Schuler H, Koch CM, Joussen S, Denecke B, Walenda G, Pallua N, Suschek CV, Zenke M, Wagner W. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging. 2011;3:873–888. doi: 10.18632/aging.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid V, Wydler M, Alder H. Transdifferentiation and regeneration in vitro. Developmental biology. 1982;92:476–488. doi: 10.1016/0012-1606(82)90193-2. [DOI] [PubMed] [Google Scholar]
- Schnitzler CE, Pang K, Powers ML, Reitzel AM, Ryan JF, Simmons D, Tada T, Park M, Gupta J, Brooks SY, Blakesley RW, Yokoyama S, Haddock SH, Martindale MQ, Baxevanis AD. Genomic organization, evolution, and expression of photoprotein and opsin genes in Mnemiopsis leidyi: a new view of ctenophore photocytes. BMC biology. 2012;10:107. doi: 10.1186/1741-7007-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert BA, Lowenstein TK, Timofeeff MN, Parker MA. Halophilic Archaea cultured from ancient halite, Death Valley, California. Environmental microbiology. 2010;12:440–454. doi: 10.1111/j.1462-2920.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Shibata N, Rouhana L, Agata K. Cellular and molecular dissection of pluripotent adult somatic stem cells in planarians. Devel Growth Differ. 2010;52:27–41. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes & development. 2011;25:2579–2593. doi: 10.1101/gad.179515.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Sawada Y, Sugiyama T. Minimum tissue size required for hydra regeneration. Developmental biology. 1993;155:287–296. doi: 10.1006/dbio.1993.1028. [DOI] [PubMed] [Google Scholar]
- Shostak S. Speculation on the evolution of stem cells. Breast Dis. 2008;29:3–13. doi: 10.3233/bd-2008-29102. [DOI] [PubMed] [Google Scholar]
- Sikes JM, Bely AE. Radical modification of the A-P axis and the evolution of asexual reproduction in Convolutriloba acoels. Evolution & development. 2008;10:619–631. doi: 10.1111/j.1525-142X.2008.00276.x. [DOI] [PubMed] [Google Scholar]
- Sikes JM, Newmark PA. Restoration of anterior regeneration in a planarian with limited regenerative ability. Nature. 2013;500:77–80. doi: 10.1038/nature12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Bruhn JN, Anderson JB. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature. 1992;356:428–431. [Google Scholar]
- Solana J. Closing the circle of germline and stem cells: the primordial stem cell hypothesis. EvoDevo. 2013;4:1–16. doi: 10.1186/2041-9139-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn TM. Genetic studies on Stenostomum incaudatum (nov. spec.) I. The nature and origin of differences among individuals formed during vegetative reproduction. J Exp Zool. 1930;57:57–108. [Google Scholar]
- Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- Steele RE, Lieu P, Mai NH, Shenk MA, Sarras MP., Jr. Response to insulin and the expression pattern of a gene encoding an insulin receptor homologue suggest a role for an insulinlike molecule in regulating growth and patterning in Hydra. Development genes and evolution. 1996;206:247–259. doi: 10.1007/s004270050050. [DOI] [PubMed] [Google Scholar]
- Strehler BL, Crowell S. Studies on comparative physiology of aging. I. Function vs. age of Campanularia flexuosa. Gerentologia. 1961;5:1–8. [Google Scholar]
- Tan TC, Rahman R, Jaber-Hijazi F, Felix DA, Chen C, Louis EJ, Aboobaker A. Telomere maintenance and telomerase activity are differentially regulated in asexual and sexual worms. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4209–4214. doi: 10.1073/pnas.1118885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Takahashi K, Yamanaka S. Induction of pluripotency by defined factors. Proceedings of the Japan Academy. Series B, Physical and biological sciences. 2014;90:83–96. doi: 10.2183/pjab.90.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. 2011;21:172–185. doi: 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Thiemann M, Ruthman A. Spherical forms of Trichoplax adhaerens (Placozoa) Zoomorphology. 1990;110:37–45. [Google Scholar]
- Thiemann M, Ruthman A. Alternative modes of asexual reproduction in Trichoplax adhaerens (Placozoa) Zoomorphology. 1991;110:165–174. [Google Scholar]
- Toledo A, Cruz C, Fragoso G, Laciette JP, Merchant MT, Hernandez M, Sciutto E. In vitro culture of Taenia crassiceps larval cells and cyst regeneration after injection into mice. J Parasitol. 1997;83:189–193. [PubMed] [Google Scholar]
- Treaster SB, Ridgway ID, Richardson CA, Gaspar MB, Chaudhuri AR, Austad SN. Superior proteome stability in the longest lived animal. Age. 2013 doi: 10.1007/s11357-013-9597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. Journal of internal medicine. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Agata K. Evolution and regeneration of the planarian central nervous system. Development, growth & differentiation. 2009;51:185–195. doi: 10.1111/j.1440-169X.2009.01099.x. [DOI] [PubMed] [Google Scholar]
- Umesono Y, Tasaki J, Nishimura Y, Hrouda M, Kawaguchi E, Yazawa S, Nishimura O, Hosoda K, Inoue T, Agata K. The molecular logic for planarian regeneration along the anterior-posterior axis. Nature. 2013;500:73–76. doi: 10.1038/nature12359. [DOI] [PubMed] [Google Scholar]
- Valastyan JS, Lindquist S. Mechanisms of protein-folding diseases at a glance. Disease models & mechanisms. 2014;7:9–14. doi: 10.1242/dmm.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nature genetics. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends in cell biology. 2014;24:161–170. doi: 10.1016/j.tcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Vriz S, Reiter S, Galliot B. Cell death: a program to regenerate. Current topics in developmental biology. 2014;108:121–151. doi: 10.1016/B978-0-12-391498-9.00002-4. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332:811–816. doi: 10.1126/science.1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environmental and molecular mutagenesis. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zayas RM, Guo T, Newmark PA. nanos function is essential for development and regeneration of planarian germ cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5901–5906. doi: 10.1073/pnas.0609708104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Hoang VT, Mattner R, Holstein TW. Immortality and the base of multicellular life: Lessons from cnidarian stem cells. Sem Cell Devel Biol. 2009;20:1114–1125. doi: 10.1016/j.semcdb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Wherry ET. Box-huckleberry as the oldest living protoplasm. Castanea. 1972;37:94–95. [Google Scholar]
- Wichterman R. Description and life cycle of Euplotes neapolitanus sp. nov. (Protozoa, Ciliophora, Hypotrichida) from the Gulf of Naples. Trans Amer Micros Soc. 1964;83:362–370. [Google Scholar]
- Wiser MJ, Ribeck N, Lenski RE. Long-term dynamics of adaptation in asexual populations. Science. 2013;342:1364–1367. doi: 10.1126/science.1243357. [DOI] [PubMed] [Google Scholar]
- Woodruff LL. The pedigreed culture of Paramecium aurelia at Yale University. Proceedings of the National Academy of Sciences of the United States of America. 1943;29:135–136. doi: 10.1073/pnas.29.5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and Exercise Enhance Neuronal DNA Repair by Stimulating CREB-Mediated Production of Apurinic/Apyrimidinic Endonuclease 1. Neuromolecular medicine. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashina S, Gubin S, Maksimovich S, Yashina A, Gakhova E, Gilichinsky D. Regeneration of whole fertile plants from 30,000-y-old fruit tissue buried in Siberian permafrost. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4008–4013. doi: 10.1073/pnas.1118386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Fujisawa T, Hwang JS, Ikeo K, Gojobori T. Degeneration after sexual differentiation in hydra and its relevance to the evolution of aging. Gene. 2006;385:64–70. doi: 10.1016/j.gene.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Zayas RM, Hernández A, Habermann B, Wang Y, Stary JM, Newmark PA. The planarian Schmidtea mediterranea as a model for epigenetic germ cell specification: Analysis of ESTs from the hermaphroditic strain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18491–18496. doi: 10.1073/pnas.0509507102. [DOI] [PMC free article] [PubMed] [Google Scholar]