Abstract
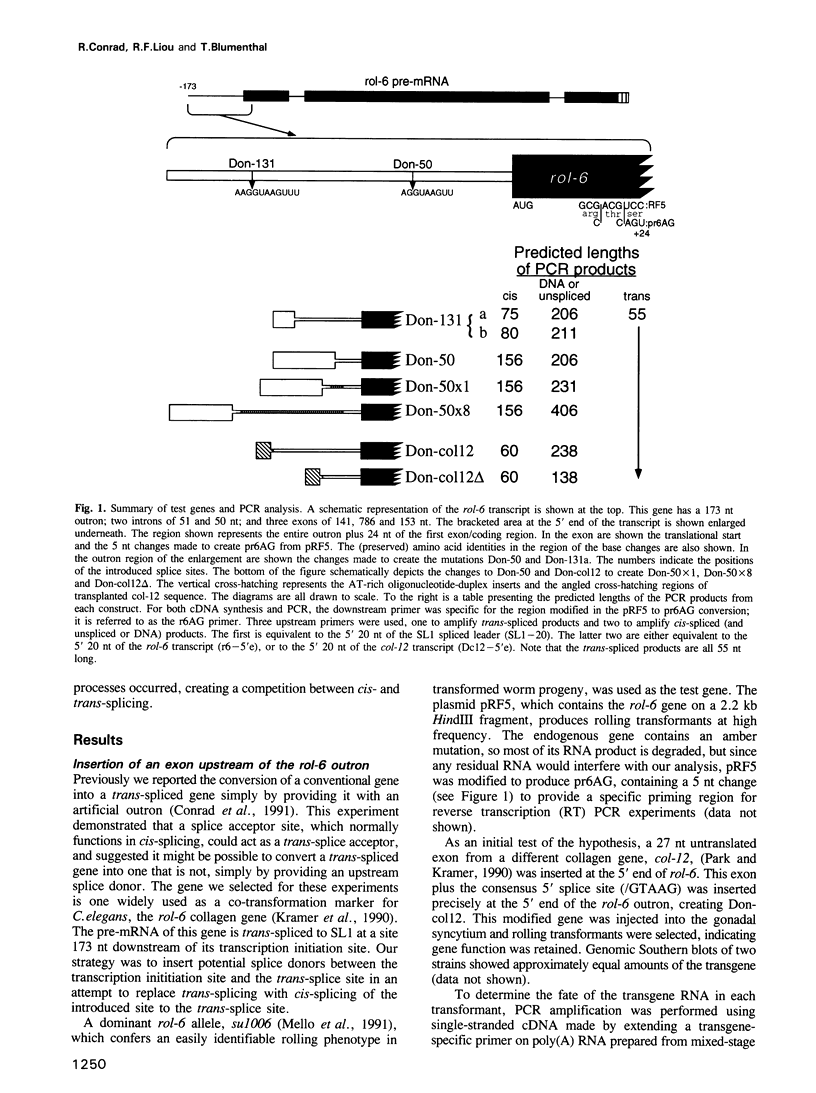
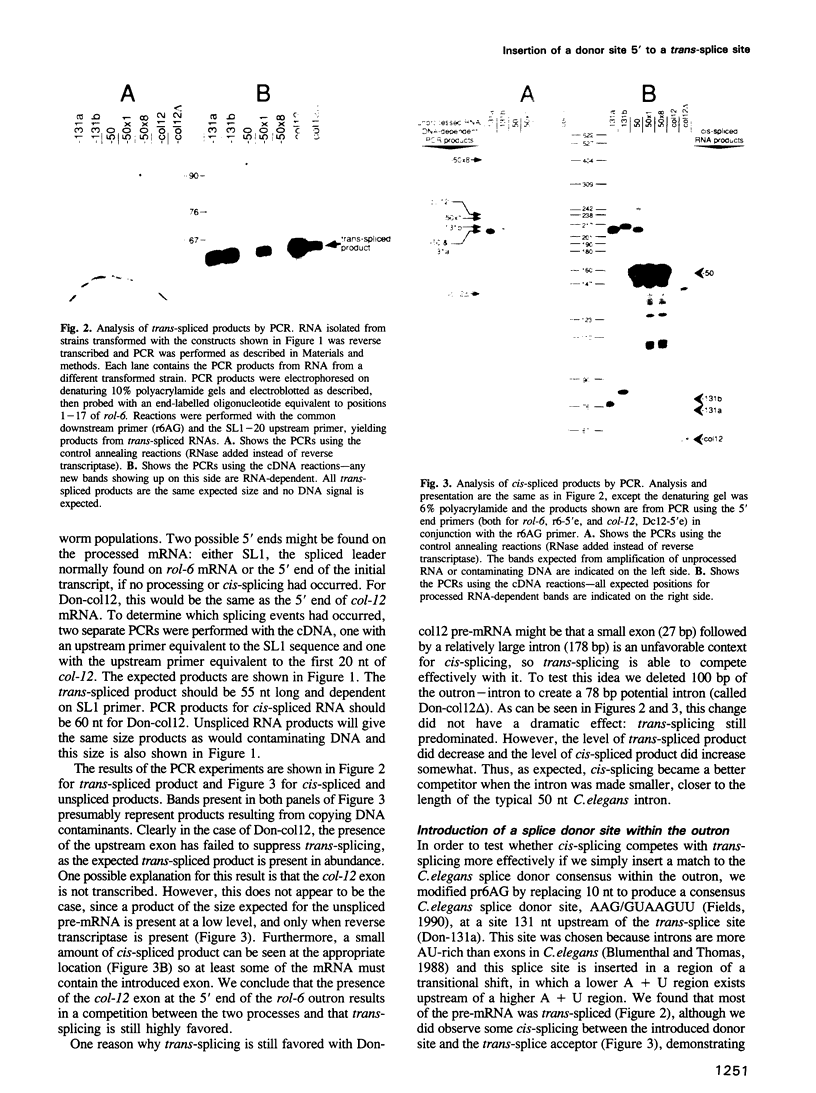
In Caenorhabditis elegans, pre-mRNAs that are trans-spliced are distinguished by the presence of an 'outron', intron-like RNA at the 5' end followed by a splice acceptor. We report that trans-splicing of the rol-6 gene can be completely suppressed simply by introducing a donor site into its 173 nt outron, at a site 50 nt upstream of the trans-splice site, thereby converting rol-6 into a conventional gene with a spliced intron near its 5' end. When the consensus donor site was inserted at sites further upstream it was less effective in replacing transplicing with cis-splicing. Surprisingly, the length of the intron was not the important variable, since lengthening of the 50 nt intron to 250 nt did not restore trans-splicing. Apparently the context into which the splice site was introduced determined the efficiency of its use. These results support the conclusion that the sole signal for trans-splicing is the presence of an outron. Clearly, cis- and trans-splice acceptor sites are interchangeable, allowing the possibility of competition between the two types of splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Bektesh S. L., Hirsh D. I. C. elegans mRNAs acquire a spliced leader through a trans-splicing mechanism. Nucleic Acids Res. 1988 Jun 24;16(12):5692–5692. doi: 10.1093/nar/16.12.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Thomas J. Cis and trans mRNA splicing in C. elegans. Trends Genet. 1988 Nov;4(11):305–308. doi: 10.1016/0168-9525(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Conrad R., Thomas J., Spieth J., Blumenthal T. Insertion of part of an intron into the 5' untranslated region of a Caenorhabditis elegans gene converts it into a trans-spliced gene. Mol Cell Biol. 1991 Apr;11(4):1921–1926. doi: 10.1128/mcb.11.4.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Laufer J. S., Kusch M., Edgar R. S. Genetic and Phenotypic Characterization of Roller Mutants of CAENORHABDITIS ELEGANS. Genetics. 1980 Jun;95(2):317–339. doi: 10.1093/genetics/95.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelson J. E., Zeng W. A comparison of trans-RNA splicing in trypanosomes and nematodes. Parasitol Today. 1990 Oct;6(10):327–334. doi: 10.1016/0169-4758(90)90177-6. [DOI] [PubMed] [Google Scholar]
- Fields C. Information content of Caenorhabditis elegans splice site sequences varies with intron length. Nucleic Acids Res. 1990 Mar 25;18(6):1509–1512. doi: 10.1093/nar/18.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A. Integrative transformation of Caenorhabditis elegans. EMBO J. 1986 Oct;5(10):2673–2680. doi: 10.1002/j.1460-2075.1986.tb04550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A., Waterston R. H. Proper expression of myosin genes in transgenic nematodes. EMBO J. 1989 Nov;8(11):3419–3428. doi: 10.1002/j.1460-2075.1989.tb08506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Nilsen T. W. U small nuclear ribonucleoprotein requirements for nematode cis- and trans-splicing in vitro. J Biol Chem. 1991 Dec 5;266(34):22792–22795. [PubMed] [Google Scholar]
- Huang X. Y., Hirsh D. RNA trans-splicing. Genet Eng (N Y) 1992;14:211–229. doi: 10.1007/978-1-4615-3424-2_12. [DOI] [PubMed] [Google Scholar]
- Keller M., Tessier L. H., Chan R. L., Weil J. H., Imbault P. In Euglena, spliced-leader RNA (SL-RNA) and 5S rRNA genes are tandemly repeated. Nucleic Acids Res. 1992 Apr 11;20(7):1711–1715. doi: 10.1093/nar/20.7.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990 May;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W. Trans-splicing in nematodes. Exp Parasitol. 1989 Nov;69(4):413–416. doi: 10.1016/0014-4894(89)90191-4. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Meyer B. J. Early aspects of Caenorhabditis elegans sex determination and dosage compensation are regulated by a zinc-finger protein. Nature. 1991 May 2;351(6321):65–68. doi: 10.1038/351065a0. [DOI] [PubMed] [Google Scholar]
- Park Y. S., Kramer J. M. Tandemly duplicated Caenorhabditis elegans collagen genes differ in their modes of splicing. J Mol Biol. 1990 Jan 20;211(2):395–406. doi: 10.1016/0022-2836(90)90360-X. [DOI] [PubMed] [Google Scholar]
- Rajkovic A., Davis R. E., Simonsen J. N., Rottman F. M. A spliced leader is present on a subset of mRNAs from the human parasite Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8879–8883. doi: 10.1073/pnas.87.22.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Abelson J. Pre-mRNA splicing in yeast. Trends Genet. 1991 Mar;7(3):79–85. doi: 10.1016/0168-9525(91)90276-V. [DOI] [PubMed] [Google Scholar]
- Seraphin B., Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989 Oct 20;59(2):349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. D., Conrad R. C., Blumenthal T. The C. elegans trans-spliced leader RNA is bound to Sm and has a trimethylguanosine cap. Cell. 1988 Aug 12;54(4):533–539. doi: 10.1016/0092-8674(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Van Doren K., Hirsh D. Trans-spliced leader RNA exists as small nuclear ribonucleoprotein particles in Caenorhabditis elegans. Nature. 1988 Oct 6;335(6190):556–559. doi: 10.1038/335556a0. [DOI] [PubMed] [Google Scholar]