Abstract
Many tissues are sensitive to mechanical stimuli; however, the mechanotransduction mechanism used by cells remains unknown in many cases. The primary cilium is a solitary, immotile microtubule-based extension present on nearly every mammalian cell which extends from the basal body. The cilium is a mechanosensitive organelle and has been shown to transduce fluid flow-induced shear stress in tissues such as the kidney and bone. The majority of microtubules assemble from the mother centriole (basal body), contributing significantly to the anchoring of the primary cilium. Several studies have attempted to quantify the number of microtubules emanating from the basal body and the results vary depending on the cell type. It has also been shown that cellular response to shear stress depends on microtubular integrity. This study hypothesizes that changing the microtubule attachment of primary cilia in response to a mechanical stimulus could change primary cilia mechanics and, possibly, mechanosensitivity. Oscillatory fluid flow was applied to two different cell types and the microtubule attachment to the ciliary base was quantified. For the first time, an increase in microtubules around primary cilia both with time and shear rate in response to oscillatory fluid flow stimulation was demonstrated. Moreover, it is presented that the primary cilium is required for this loading-induced cellular response. This study has demonstrated a new role for the cilium in regulating alterations in the cytoplasmic microtubule network in response to mechanical stimulation, and therefore provides a new insight into how cilia may regulate its mechanics and thus the cells mechanosensitivity.
Keywords: mechanical stimulus, cytoskeleton, mechanotransduction, centriole, cell mechanosensitivity
1. Introduction
Cells are sensitive to their environments and can detect mechanical signals [Huang et al., 2004; Hughes-Fulford, 2004]. Mechanical stimuli have been shown to play important roles in cellular processes such as proliferation [Simons and Walz, 2006; Yamamoto et al., 2003], differentiation [Wong et al., 2003; Yamamoto et al., 2003] and pathology [Cheng et al., 2006; Low et al., 2006; Stokes et al., 2006] in tissues including bone, cartilage, endothelium and kidney. However, several of these cellular mechanosensors remain unknown in many of these tissues.
A primary cilium is a solitary, non-motile, microtubule-based cellular antenna that extends from the surface of nearly every cell in the human body [Davenport and Yoder, 2005], including bone cells [Hoey et al., 2011; Kwon et al., 2010] and kidney cells [Downs et al., 2012]. The primary cilium consists of a membrane bound axoneme containing nine circumferentially arranged doublet microtubules, which extend upwards from the mother centriole/basal body. Unlike motile cilium and flagellum, primary cilia lack two additional central microtubules. Moreover, the doublets of motile cilia and flagellum are interconnected via nexin links, which in combination with radial spokes, reinforce the axoneme resulting in an order of magnitude increase in the resistance to bending (flexural rigidity) in comparison to primary cilia [Downs et al., 2012; Hoey et al., 2012; Rikmenspoel and Sleigh, 1970; Schwartz et al., 1997]. This demonstrates that, at these scales, even a small change in molecular arrangement can generate a dramatic difference in organelle level mechanical behavior. The basal body functions as the template for ciliogenesis and represents the transition between the ciliary axoneme and the cytoplasmic compartments of the cell. Moreover, the basal body regulates protein entry and exit from the cilia compartment [Berbari et al., 2009].The basal body is connected to a collar of conical structures known as basal feet which extend laterally forming attaching points for cytoskeletal microtubules [Albrecht-Buehler and Bushnell, 1980; Hoey et al., 2012]. This means that microtubules significantly contribute to the structural integrity of basal body and to the anchoring of primary cilia.
Cilia are assembled by the conserved process of intraflagellar transport (IFT). IFT is a bidirectional microtubule-based motility process during which groups of large protein complexes (IFT particles) are transported along the axonemal doublet microtubules from the base of the cilium to its distal tip by kinesin-2-motors and then from the tip back to the cell body by cytoplasm dynein-2 [Pedersen and Rosenbaum, 2008]. IFT is thought to be the sole mechanism for transporting proteins required for cilia assembly, maintenance, and function to their location along the axoneme, and that mutations leading to defective IFT can result in the development of severe diseases (ciliopathies) and developmental defects [Davenport and Yoder, 2005].
In the past few decades several studies have revealed the cilium to play an important mechanosensory role in numerous tissues [Singla and Reiter, 2006], and, in particular, to be involved in fluid flow sensing [Hoey et al., 2012; Lee et al., 2010; Praetorius and Spring, 2003b; Singla and Reiter, 2006]. For example, fluid flow within the kidney results in the deflection of the primary cilium which in turn triggers an extracellular calcium dependent increase in intracellular calcium, a response that was lost with the removal of primary cilium [Praetorius and Spring, 2001; Praetorius and Spring, 2003a]. Nauli et al. first demonstrated that this calcium response is mediated by a mechanosensory complex located at the base of the cilium. This complex consists of two membrane bound proteins, Polycystein 1 (PC1) and a stretch activated cationic channel known as Polycystein 2 (PC2). Briefly, PC1, with its large extracellular domains, acts as a sensory molecule for fluid shear stress that transmits the signal from the extracellular fluid environment to PC2, which, in turn, produces sufficient Ca2+ influx to activate intracellular ryanodine receptors through Ca2+-induced Ca2+ release [Nauli et al., 2003]. In response to fluid flow stimulation, osteocytes display a rapid transient decrease in cAMP levels, a response that is necessary for the increase in COX- 2 expression. Furthermore, upon removal of the primary cilium through RNA interference, the decrease in cAMP in response to flow is lost, demonstrating that this response is cilia-mediated [Kwon et al., 2010].
The primary cilium has also been implicated as a complex signaling center for the cell, regulating signaling pathways during development such as Hedgehog (Shh) and Wingless (Wnt). The cilium functions as a regulatory switch to control the balance between the canonical and non-canonical Wnt pathways. In the canonical pathway, a Wnt ligand binds to the co-receptors Frizzled and LRP. This inhibits the activity of the β-catenin destruction complex possibly through the Dishevelled (Dvl) protein and leads to stabilization of β-catenin, which accumulates in the nucleus and in combination with lymphocyte enhancer factor and T cell factor activates target genes. In the noncanonical pathway, Wnt binds to a frizzled receptor, independent of LRP. This activates a membrane form of Dvl which regulates downstream targets. The non-canonical Wnt signal activates Inversin, which resides in multiple locations in the cell, including the cilium or at the base of the cilium. Inversin induces the degradation of cytoplasmic but not the membrane form of Dvl. In a ciliated cell, both the canonical and non-canonical pathways are operative and the strength of the canonical pathway is thought to be influenced by the non-canonical Wnt pathway. In the absence of cilia, the model would suggest that the non-canonical pathway is unable to efficiently antagonize the activity of the canonical pathway [Berbari et al., 2009]. Wnt signaling regulates the dynamics and organization of microtubules resulting in profound effects on cell behavior. Many components of this pathway have been associated with the cytoskeleton. For example, Dvl has been shown to increase microtubule stability and the formation of looped microtubules. Also, canonical Wnt signalling has been shown to activate Gsk3, and this process is associated with the capture of microtubules at the cell cortex [Salinas, 2007].
The structural integrity of the primary cilium is crucial to its function as defects in this structure have been linked to numerous pathologies such as osteoporosis, polycystic kidney disease, obesity and cancer [Adams et al., 2008; Badano et al., 2006; Hildebrandt et al., 2011; Veland et al., 2009]. Changes in the structural mechanics of primary cilia can greatly affect the molecular mechanics of mechanosensing. For example, it has been demonstrated that upon mechanical perturbation the primary cilium decreases its length, fine tuning its sensitivity to the extracellular environment [Besschetnova et al., 2010]. Therefore, alterations in primary cilia length, and possibly other mechanical properties and features, might be a central mechanism for regulating cellular mechanosensitivity.
Several studies have attempted to count the number of microtubules emanating from the basal body and the results vary from 10 to 100 depending on the cell type [Alieva et al., 1992]. Moreover, it has been discovered that the mean number and length changed depending on cell attachment, spreading and migration where long microtubules were more prominent in spread out, fully adhered cells [Gudima et al., 1983a; Gudima et al., 1983b]. In a fully adhered, polarized cell, the cilium would extend perpendicular into the extracellular environment. Interestingly, a decrease in the shear response marker KLF2 to an applied fluid flow induced force was found after disrupting the microtubule network in epithelial cells while maintaining the primary cilium. A similar decrease was seen in cells from which cilia were removed keeping the microtubule network [Hierck et al., 2008]. This demonstrates that the cilium mediated cell response to shear stress depends on microtubular integrity and a direct connection between the cilium and the microtubule network. Therefore, this interface may prove to be critical in terms of signal transduction.
The aim of this study is to investigate the effects of a mechanical stimulation (oscillatory fluid flow induced shear stress) on the microtubule based primary cilium and cytoplasmic microtubule network in kidney and bone cells. It is proposed here that the primary cilium-mediated flow-induced mechanotransduction mechanism in these cell types is dependent upon cytoplasmic microtubules. We hypothesized that oscillatory fluid flow is an important stimulus to both cell types, would induce significant changes in microtubule network morphology and in the number of microtubules that exist at the base of primary cilia, and that these changes depend on the presence of the primary cilium.
2. Results
2.1. Effect of flow: microtubule network
Microtubule networks have a more buckled and dense structure after being subjected to oscillatory fluid flow (Figure 1). This was quantified by plotting the distribution of intensity of the three pictures together (Figure 2). For both cell types, it is possible to see that as the duration of flow increases more pixels have higher intensity values. Also, there are a greater number of lower intensity values in the no flow pictures.
Figure 1.
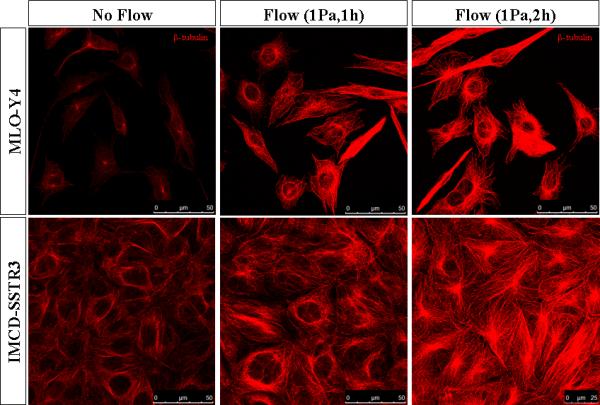
Microtubules networks imaged with confocal microscope. Microtubules stained with β-tubulin (red) in MLO-Y4 and IMCD-SSTR3 cells. Images for each cell type were captured with the same intensity.
Figure 2.
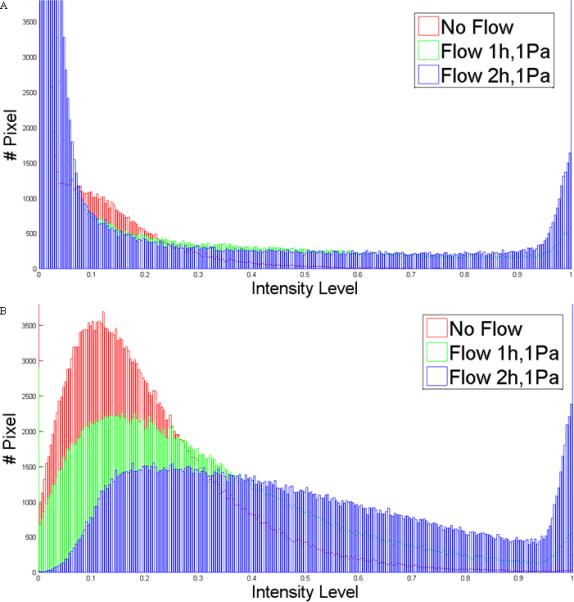
Distribution of intensity in images of three different conditions: no flow, flow 1h,1Pa and flow 2h,2Pa for MLO-Y4 (A) and IMCD-SSTR3 (B). A higher intensity value means a higher amount of polymerized β-tubulin which corresponds to a denser microtubule network.
The number of buckling regions was computed using a chord-to-point distance accumulation (CPDA) method [Awrangjeb et al., 2009] implemented in Matlab. The CPDA method is a discrete curvature estimation technique to detect salient features of an image: corner points or buckling points [Awrangjeb and Lu, 2008]. This algorithm is based on the chord-to-point distance accumulation for the discrete curvature estimation. Chord-length denotes the arc-length of the interior curve-segment. A buckling point refers to when a beam becomes unstable and deforms under a load. This method has been applied previously to quantify buckling points of microtubules within osteocytes under hydraulic pressure [Liu et al., 2010]. For each experimental group, 6 areas of 120x120 pixels were chosen randomly to quantify the buckling points. An example of the CPDA analysis for each experimental group is shown in Figure 3A. The number of detected corners, which corresponds to the number of buckling points in microtubules, found for each case is in Figure 3B. As can be observed there is an increase in the number of buckling points with the presence of flow for both MLO-Y4 and IMCD-SSTR3 cells.
Figure 3.
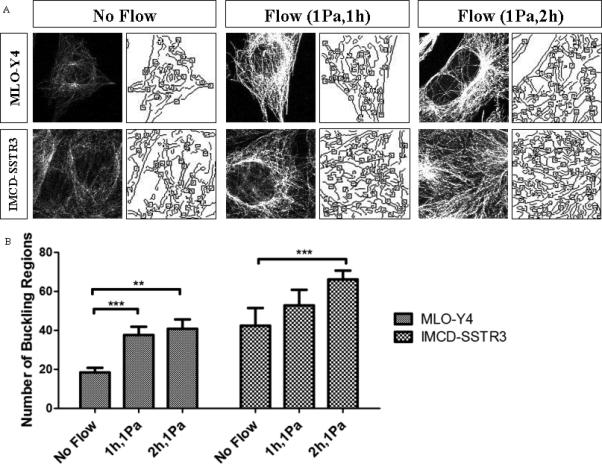
Number of buckling regions. A: An example of CPDA analysis for each experimental group illustrating the analyzed region (left) and CPDA analysis of buckling regions (right). B: Number of buckling regions found for each experimental group: MLO-Y4 and IMCD-SSTR3 cells. * indicates values are significantly different (**p<0.01; ***p<0.05; N=6).
2.2. Effect of flow on microtubule attachment at the base of cilium
1Pa oscillatory flow was applied for 1 hour to determine the effect of shear stress on microtubule reinforcement at the base of the cilium. We observed a significant increase in the number of basal microtubules in response to flow (Figure 4).
Figure 4.
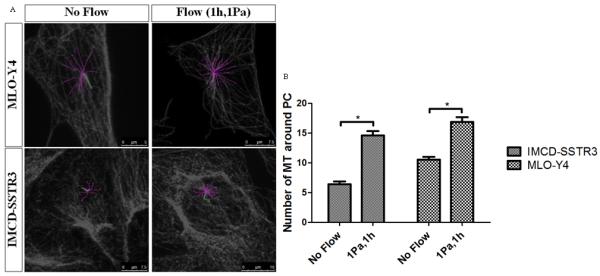
Effects of flow in microtubules at the base of primary cilia. A: Examples of the quantification of microtubules around primary cilia in both cell types for two different conditions: no flow and 1Pa flow for 1h (green – primary cilium; pink – microtubules). B: Effect of flow in the number of microtubules around primary cilia for different cell types: IMCD-SSTR3 and MLO-Y4. * indicates values are significantly different (*p<0.001, N≥10).
To determine whether this loading-induced increase in MTs at the base of the cilium is time-dependent 1Pa oscillatory flow was applied for 15minutes and 2 hours in addition to the 1hr presented previously. Figure 5A shows examples of the quantification made for these two different conditions for both cell types. Significant increases in the number of microtubules with the duration of flow were observed for both cell types (Figure 5B).
Figure 5.
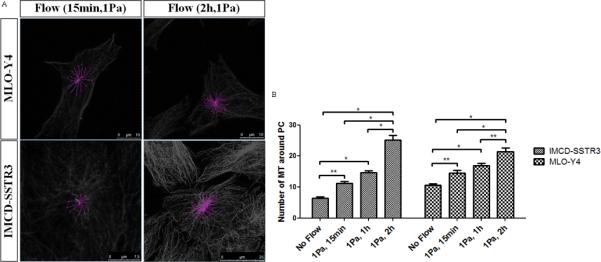
Effects of time of flow in microtubules at the base of primary cilia. A: Examples of the quantification of microtubules around primary cilia in both cell types for two different conditions: 1Pa flow for 15min and 1Pa flow for 2h (green – primary cilium; pink – microtubules). B Effect of time in the number of microtubules around primary cilia for different cell types: IMCD-SSTR3 and MLO-Y4. * indicates values are significantly different (*p<0.001, **p<0.01; N≥10).
To determine whether this loading-induced increase in microtubules at the base of the cilium is magnitude-dependent, oscillatory fluid flow was applied for 1hr but with 2Pa as shear rate in addition to the 1Pa presented previously. Examples of the quantification made for the 2Pa flow situation is shown in Figure 6A. The results obtained are present in Figure 6B. It is clear that the number of microtubules rise with the increase of the shear from oscillatory fluid flow.
Figure 6.
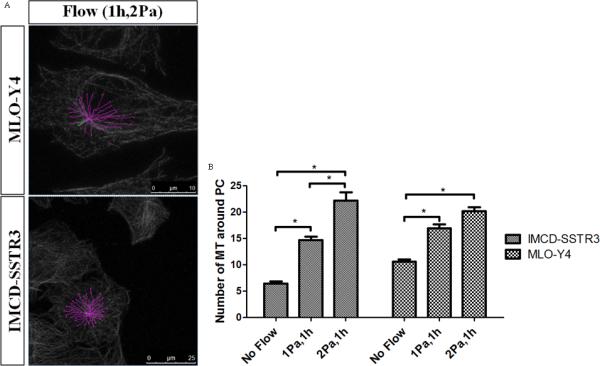
Effects of shear rate of flow in microtubules at the base of primary cilia. A: Examples of the quantification of microtubules around primary cilia in both cell types for 2Pa flow for 1h (green – primary cilium; pink – microtubules). B: Effect of shear in the number of microtubules around primary cilia for different cell types: IMCD-SSTR3 and MLO-Y4. * indicates values are significantly different (*p<0.001; N≥10).
2.3. Ciliary control of loading-induced microtubule dynamics
In order to investigate the role of primary cilia in this mechanically-mediated response, the formation of primary cilia was inhibited by small interfering RNA (siRNA)-mediated depletion of IFT88 [Taulman et al., 2001]. The successful knockdown of IFT88 mRNA was verified using qRT-PCR and the results are shown in Figure 7.
Figure 7.
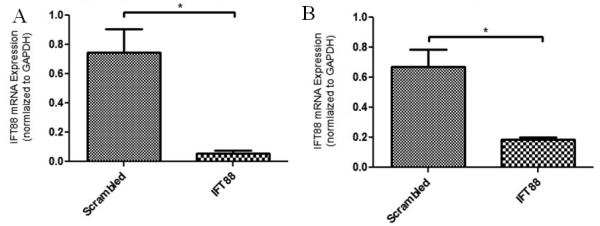
IFT88 mRNA levels of expression in cells transfected with scrambled siRNA or IFT88 siRNA for both cell types. A: IMCD-SSTR3; B: MLO-Y4. * indicates values are significantly different (p<0.001).
After inhibiting the formation of cilia, 1Pa oscillatory fluid flow was applied for 1h. Examples of the quantification made for both bells after IFT88 siRNA transfection are shown in Figure 8A. For both cell types the increase in the number of microtubules with flow does not occur without primary cilia (Figure 8B and Figure 8C).
Figure 8.
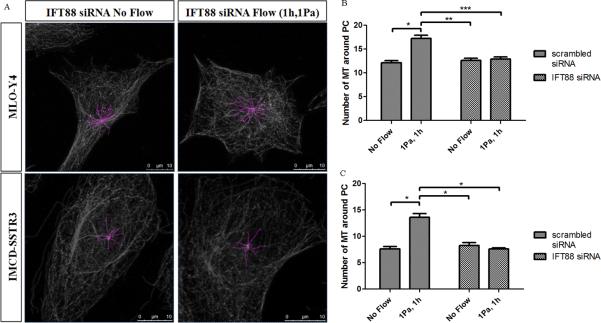
Effects of flow in microtubules at the base of primary cilia without treatment and transfected with IFT88 siRNA. A: Examples of the quantification of microtubules around primary cilia in both cell types after IFT88 siRNA transfection for two different conditions: no flow and 1Pa flow for 1h (pink – microtubules). B,C: Effect of flow in the number of microtubules around primary cilia in IMCD-SSTR3 cells (B) and MLO-Y4 cells (C) without any treatment and transfected with IFT88 siRNA. * indicates values are significantly different (*p<0.001; **p<0.01; ***p<0.05; N≥10).
It should be noted that, when imaged, the majority of the cells that were transfected with IFT88 siRNA did not show primary cilia (Figure 8A) and very few presented shortened cilia. All the networks quantified without any siRNA treatment for all different flows presented a primary cilium. On the other hand, from all the networks recorded post-IFT88 siRNA treatment (30 for both no flow and flow situations for each cell type) only one for each cell type presented a short cilium. This means that only approximately 3.3% of cells presented a primary cilium after being subjected to an IFT88 siRNA transfection while 100% of non-treated cells showed a cilium.
3. Discussion
Many tissues are sensitive to mechanical stimuli; however, the mechanotransduction mechanisms are understood in remarkably few cases. This mechanosensitivity is essential for physiological functioning; therefore determining the processes that allow a cell to convert a physical signal into a biochemical response represents a critical gap in current knowledge. The results of this study demonstrate that the application of oscillatory fluid flow alters the cellular microtubule network in a magnitude and time dependent manner. We found that oscillatory fluid flow induce microtubule networks to have a more dense, yet buckled structure. Dynamic flow also led to an increase in the number of microtubules attached to the base of the primary cilium. Moreover, these increases did not occur after primary cilia removal. Our findings prove the importance of fluid flow in the mechanotransduction process in these cell types. Additionally, the results demonstrate that the primary cilium mediated flow-induced mechanism depends on the microtubule network at the base of the cilium which provides stabilization to the cilium. Therefore this study gives new insights into a new role for primary cilia in the mechanotransduction process.
We demonstrated that oscillatory fluid flow increased the amount of microtubule protein (polymerized β-tubulin) and induced microtubule networks to be more dense and buckled which might be related to the fact that mechanotransduction mechanism is governed by the overall structural status of the entire cytoskeleton [Alenghat et al., 2004]. Another important element is that the cilium is regarded as a specialized extension of the cytoskeleton and, therefore, many ciliary proteins are also beginning to emerge as key players in cellular processes that involve the cytoskeleton. These include cell cycle progression and vesicular transport which are heavily dependent on the configuration of the microtubule architecture within a cell [May-Simera and Kelley, 2012]. Moreover, these results may be due to the support role that microtubules have in order to provide cell rigidity which have been shown to be a part of these cytoskeletal structures [Hutcheson and Griffith, 1996]. Furthermore, some studies have provided evidence to suggest that microtubules act as internal support struts, stabilizing the cytoplasm and nucleus against lateral compression induced by external shear and internal cytoskeleton contraction [Ingber, 1997]. Additionally, it has been proposed that cytoskeletal microtubules work together with integrins in extracellular matrix adhesions and cadherins in cell-cell junctions to resist the pull of tensile forces generated within the actin cytoskeleton, and thereby establish a mechanical force balance that stabilizes the shape of the entire cell [Ingber, 1997; Ingber, 2003; Wang et al., 2001]. This might be a possible explanation for our results, that is, the enhanced microtubule networks verified after application of fluid flow may be a cellular response in order to resist the mechanical stimulus and stabilize the cell. Different microtubule networks may represent a different rigidity for the whole cell and therefore may alter its mechanical properties.
We verified for all cell types that the number of microtubules at the base of the cilium increase with both duration and shear rate of the fluid flow. Downs et al. [Downs et al., 2012] investigated the mechanical properties of primary cilia by acquiring 3D images of primary cilia under flow and developed a coupled fluid-structure interaction model. The authors observed on average a 10° rotation at the base of the cilium. These different rotations might be related to different ciliary anchorage provided by microtubules which would in turn alter primary cilium mechanics and potentially the mechanosensitivity of this antenna. The mechanotransduction process in response to oscillatory fluid flow may include the stabilization of primary cilium by the microtubule network which would enable the cilium to extend into the extracellular space ideally positioning the cilium for sensing. Furthermore, as microtubules are highly abundant in the region of the basal body, their association and interaction with ciliary proteins are essential for ciliary function. We demonstrated that the ciliary anchorage is adaptive and changes in response to load. This anchorage is likely a place of high cytoskeletal loads due to the high bending moments that would be present there. Also, the ciliary anchorage is known to be a center of biochemical signaling, suggesting that it may be a link for the integration of physical and biochemical signaling.
Interestingly, we found that the increase in the number of microtubules surrounding the cilium observed for both cell types did not occur after the removal of primary cilia which leads to the conclusion that this dynamic cytoskeletal process is mediated by primary cilia. Two possible explanations can be proposed for these observations. On one hand, the mechanism by which the cell senses the flow and increases the microtubules anchoring primary cilia depends on IFT88 and primary cilia presence in the cell. On the other hand, without the primary cilium no bending is transmitted to the basal body and without that force the reorganization of the microtubule network does not occur.
Kinesins are not only required for trafficking of structural or signaling components to and along the cilium, but are also required for stabilization and regulation of the cytoskeletal infrastructure and trafficking of components within the cytoplasm [May-Simera and Kelley, 2012]. The ability of kinesins to control microtubule dynamics enables these molecules to exert regulatory function. Such regulatory mechanisms are coupled to various signaling pathways, which could, in turn, control their associations with microtubules or regulate microtubules and/or microtubule dynamics directly. As kinesins can be both signaling targets and signaling intermediates, kinesin control over microtubule dynamics is complex. MCAK, a kinesin, has been shown to be critical for microtubule dynamics and organization. MCAK has also been shown to associate with the microtubule-associated protein EB1 via its C-terminus at growing ends of microtubules [Lee et al., 2008]. When associated with EB1, MCAK appears to be in an inactive conformation. This could provide a mechanism to facilitate fast switches between growth and depolymerization of microtubules [May-Simera and Kelley, 2012]. These factors might be behind the increase in the number of microtubules we see with the application of flow.
It has been demonstrated that disrupting cytoplasmic microtubules eliminates fluid shear-induced increases in intracellular calcium. As this increase was also shown to be dependent on the cilium localized calcium channel PC-2, this would indicate that this primary cilium based mechanotransduction mechanism is regulated by the overall structural status of the entire cytoskeleton [Alenghat et al., 2004]. Our findings may corroborate the findings of these authors and may indicate a reciprocate regulation.
Integral Wnt signaling is crucial in modulating the cytoskeleton and, reciprocally, Wnt signaling requires an intact cytoskeleton. Downstream effects of the canonical Wnt signaling associate heavily with microtubule and cytoskeletal components [May-Simera and Kelley, 2012]. Cdc42, a Rho family GTPase, is a key player in Wnt dependent cytoskeletal rearrangements and a key regulator of cell polarity. Direct interaction of Cdc42 with Par6 leads to activation of aPKC and subsequent accumulation of APC at the plus end tips of microtubules promoting polarization of the microtubule cytoskeleton. The requirement of Dvl, Axin, and the Wnt5a ligand in this process implicates the Cdc42/Par6 complex in establishing microtubule polarity via transcription-independent non-canonical Wnt signaling [Schlessinger et al., 2007]. Moreover, it has been shown that flow upregulates Wnt5a which is necessary for mechanically induced osteogenic lineage differentiation [Arnsdorf et al., 2009]. This may explain the flow induced increase in the number of microtubules and the absence of this increase after removal of primary cilia.
Several of the Bardet-Biedl syndrome proteins have shown to participate in microtubule nucleation and growth [May-Simera and Kelley, 2012]. Some of these proteins localize to the cilia and this might also explain the absence of polymerization of microtubules resulting in an increase of microtubules after removal of primary cilia.
Even though the shear range used in this study is suitable for bone cells [Weinbaum et al., 1994] it may represent a limitation when analyzing kidney cells which are exposed to lower levels of shear [Alenghat et al., 2004]. Despite this limitation we were interested in investigating if this mechanism was shared across cell types. Studies have shown that cilia in kidney cells are mechanoresponsive at physiological shear levels [Nauli et al., 2003]. Moreover, it has been shown for physiological loads that altering cytoplasmic force balance by interfering with microtubule polymerization inhibits the calcium spike in response to fluid stress [Alenghat et al., 2004]. This may suggest that our findings may translate to lower magnitude flows.
Furthermore, although this study demonstrates a trend of increased fluid shear-induced microtubule formation at the base of the primary cilium over three time-points (15min, 1hr, and 2hr), a detailed evolution of this change over time could not be elucidated through the techniques utilized in this study. It is therefore unclear how fast this response occurs or indeed how long an optimal adaptation requires. Interestingly, we have demonstrated a 2-fold increase in microtubule attachment at the ciliary base after only fifteen minutes of flow which we have shown previously to dramatically affect ciliary bending profiles [Hoey et al., 2012]. This immediate change in cell mechanics after such a short period generates significant interest in the potential alteration in cell behavior at later time-point such as 2hrs where we have documented a 4-fold increase in microtubule number. Further study utilizing fluorescently labeled microtubules would allow the real-time monitoring of these changes over time.
This study demonstrates a novel effect of oscillatory fluid flow on the microtubule network and, in particular, on the microtubules at the base of primary cilia. We demonstrated that the number of microtubules at the base of primary cilia increase with the presence of oscillatory fluid flow. Moreover, our results showed that both time and shear rate of the stimulus influence that response showing that ciliary anchorage is adaptive and might be a site of mechanotransduction. The cytoplasmic pool of microtubules, through their role of stabilizing the ciliary axoneme, affects the primary cilia mediated flow induced mechanotransduction mechanism in these cell types. Furthermore, evidence that the primary cilium plays a role in mechanotransduction are presented providing a novel mechanism by which a mechanical stimulus is translated into a change in the microtubule network conformation. This study demonstrates a role for the primary cilium in controlling mechanically mediated increases in the number of microtubules at the base of primary cilia. Our findings bridge a gap in the knowledge of the complex mechanisms behind mechanotransduction.
4. Materials and Methods
4.1. Cell culture
Two different cell lines were used in this study: the MLO-Y4 cell line (gift from Lynda Bonewald, University of Missouri–Kansas City, Kansas City, MO) which has characteristics similar to osteocytes; and the IMCD-SSTR3 cell line (gift from Prof. Bradley Yoder, University of Alabama at Birmingham [Berbari et al., 2008]), which are inner medullary collecting duct cells, transfected with a somatostatin receptor-3 green florescent protein (GFP) fusion protein that specifically targets the primary cilium. MLO-Y4 cells were maintained in α-MEM supplemented with 5% fetal bovine serum (FBS), 5% calf serum (CS) and 1% penicillin-streptomycin (P/S). IMCD-SSTR3 cells were maintained with DMEM/F12 supplemented with 10% FBS, 1% P/S and 200µg/ml geneticin G4-18 antibiotic.
For each experiment, MLO-Y4 and IMCD-SSTR3 cells were seeded at 4000cells/cm2 on type-1 collagen (0.15mg/ml, BD) coated microscope slides. 24h after seeding, media was changed to serum-free media for 48h to synchronize the cell cycle and promote ciliogenesis [Ruhfus et al., 1998] before the application of fluid flow.
4.2. Oscillatory Fluid Flow
Oscillatory fluid flow was applied to cells using a parallel plate flow chamber which has been described previously [Jacobs et al., 1998]. Briefly, oscillatory fluid flow was driven by Hamilton glass syringes in series with rigid walled tubing and a parallel plate flow chamber. The syringe was mounted in and driven by a previously described custom-built mechanical loading device [Jacobs et al., 1998]. The flow rate was selected to yield a peak shear stress of either 1Pa or 2Pa, with a frequency of oscillation of 1Hz. Cells were exposed to this type of mechanical stimulus for different time periods (15min, 1h and 2h). No flow control slides were similarly loaded into flow chambers, but fluid flow was not applied.
4.3. Immunocytochemistry
All cells were fixed immediately after oscillatory fluid flow with 10% formalin solution for 10 minutes. Fixed cells were permeabilized with 0.1% triton X-100 for 4 minutes. Cells were treated in 1% bovine serum albumin (BSA) for 1 hour to reduce the non-specific binding. Cells were then incubated in monoclonal mouse anti-β-tubulin antibody (T5201, Sigma-Aldrich) to detect microtubules and polyclonal rabbit anti-CEP135 antibody (abcam, ab75005) to detect the centrioles/basal body, both diluted 1:1000 in PBS containing 1% BSA, for 2h at room temperature. This was followed by AlexaFluor (Invitrogen, A11004) 568 anti-mouse IgG and AlexaFluor (Invitrogen, A11008) 488 anti-rabbit IgG, both diluted 1:200 in PBS containing 1% BSA, for 1 hour at room temperature.
4.4. Confocal Imaging and Image Processing
Cells were imaged with a Leica TCS SP5 laser scanning confocal microscope equipped with a ×100, 1.46 NA oil immersion objective. Primary cilia were identified as linear structures consisting of a dense collection of microtubules emanating from a centriole. Once the cilium was identified, a z-stack with approximately 0.08μm between each slice was acquired through length of the ciliary axoneme, the basal body and surrounding microtubule network. This represented roughly 40 to 50 slices for each cell which was captured in approximately 1 minute. At least 10 z-stacks were captured for each experimental group.
Image processing was accomplished by using the open-source platform Fiji and the plugin: Simple Neurite Tracer. Fiji is a distribution of the popular open-source software ImageJ focused on biological-image analysis [Schindelin et al., 2012]. The plugin Simple Neurite Tracer is designed for images from light microscopy techniques such as confocal and it allows semi-automatic tracing of neurons or other tube-like structures [Longair et al., 2011]. The image processing procedure used in this study is captured in Figure 9. The stack is first opened in Fiji where the primary cilium, the basal body and the network of microtubules are identified (Figure 9A). Images are then converted into black and white pictures (Figure 9B). This step is followed by the segmentation where paths are found between two different points. The first structure identified is the primary cilium and a path is found between a point on the tip and a point on the base. After that the segmentation of microtubules starts and it is the same for each microtubule. Each microtubule originates from the base of the cilium (Figure 9C).
Figure 9.
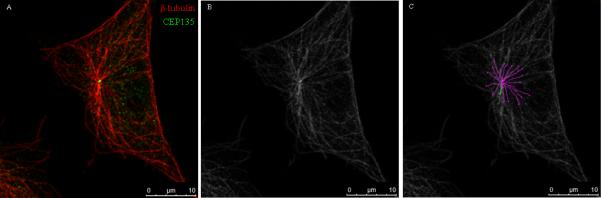
Image processing applied to count microtubules (MLO-Y4 cells). A: Original image obtained with confocal microscope (microtubules stained with β-tubulin (red) and basal body stained with CEP135 (green)); B: original image in black and white; C: segmentation of the microtubules network (pink - different microtubules; green - primary cilium).
4.5. Abrogation of Primary cilia
The formation of functional primary cilia was inhibited by small interfering RNA (siRNA)-mediated depletion of IFT88 in both MLO-Y4 and IMCD cells. IFT88 is an intraflagellar transport protein (IFT) which has been shown to localize to cilium and basal body [Taulman et al., 2001]. Moreover, IFT88 has been shown to be required for primary cilia biogenesis and function [Yoder et al., 2002]. Cells were transfected either with 0.15 μM siRNA targeting IFT88 (sequence 50-AAUAGCAUCUGAAUACUGACCAGCC-30-) or with 0.15 μM scrambled control siRNA for 6 hours using Lipofectamine 2000 (Invitrogen). Cells were maintained in growth media for a further 65 hours before the application of a mechanical stimulus. Normal cellular morphology and microtubular architecture was maintained both for IFT88 siRNA and scrambled siRNA. Ciliogenesis was not affected in cells transfected with scrambled siRNA. The successful knockdown of IFT88 mRNA was confirmed using qRT-PCR (see below).
4.6. Quantitative real-time PCR
Total RNA was extracted with TriReagent according to the manufacturer’s instructions. The 260/280 absorbance ratio was measured for verification of the purity and concentration of RNA and reverse transcribed (Applied Biosystems, 4368813). The resulting cDNA was subjected to real-time PCR using the standard curve method (ABI Prism 7900 sequence detection system). GAPDH was used as an endogenous control in all experiments. All sample and standards were run in triplicate.
4.7. Statistical analysis
All data are expressed as means ± SEM. Two-way ANOVA was used for analysis of variance with Bonferroni post-tests to compare between groups. For two sample comparisons, a Student's t test was used.
Supplementary Material
Acknowledgments
Funding provided by FCT in Portugal through the PhD scholarship SFRH / BD / 66521 / 2009 and Project PTDC/EME-PME/104498/2008, NIH grant AR62177, European Research Council Starting Grant (No 336882) and New York State Stem Cell grant (N089-210).
Footnotes
Conflict of Interest
There is not any financial and personal relationship with other people or organizations that could inappropriately influence this work.
References
- Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet. 2008;45:257–67. doi: 10.1136/jmg.2007.054999. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G, Bushnell A. The ultrastructure of primary cilia in quiescent 3T3 cells. Exp Cell Res. 1980;126:427–37. doi: 10.1016/0014-4827(80)90282-7. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301:23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Alieva IB, Nadezhdina ES, Vaisberg EA, Vorobjev IA. Microtubule and intermediate filament patterns around the centrosome in interphase cells. The Centrosome. 1992;15:103–129. [Google Scholar]
- Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awrangjeb M, Lu G. Robust Image Corner Detection Based on the Chord-to-Point Distance Accumulation Technique. IEEE Transactions on Multimedia. 2008;10:1059–1072. [Google Scholar]
- Awrangjeb M, Lu G, Fraser CS, Ravanbakhsh M. Digital Image Computing: Techniques and Applications. Melbourne, VIC: 2009. A Fast Corner Detector Based on the Chord-to-Point Distance Accumulation Technique. [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–7. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol. 2010;20:182–7. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–69. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Downs ME, Nguyen AM, Herzog FA, Hoey DA, Jacobs CR. An experimental and computational analysis of primary cilia deflection under fluid flow. Comput Methods Biomech Biomed Engin. 2012 doi: 10.1080/10255842.2011.653784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudima GO, Vorob'ev IA, Chentsov Iu S. Behavior of the cell center in the distribution of fibroblasts. Nauchnye Doki Vyss Shkoly Biol Nauki. 1983a:45–50. [PubMed] [Google Scholar]
- Gudima GO, Vorob'ev IA, Chentsov Iu S. Structural change in the cell center during fibroblast polarization and movement. Tsitologiia. 1983b;25:883–8. [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–35. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45:17–26. doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412:182–7. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol. 2004;287:C1–11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;2004:RE12. doi: 10.1126/stke.2492004re12. [DOI] [PubMed] [Google Scholar]
- Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow-but not agonist-induced EDRF release. Br J Pharmacol. 1996;118:720–6. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–73. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–76. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. Faseb J. 2010;24:2859–68. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Hoey D, Jacobs CR. Primary Cilia-Mediated Mechanotransduction in Bone. Clinical Reviews in Bone and Mineral Metabolism. 2010;8:201–212. [Google Scholar]
- Lee T, Langford KJ, Askham JM, Bruning-Richardson A, Morrison EE. MCAK associates with EB1. Oncogene. 2008;27:2494–500. doi: 10.1038/sj.onc.1210867. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao Y, Cheung WY, Gandhi R, Wang L, You L. Effects of cyclic hydraulic pressure on osteocytes. Bone. 2010;46:1449–56. doi: 10.1016/j.bone.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–4. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia 1. 2012 doi: 10.1186/2046-2530-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–9. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003a;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003b;12:517–20. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R, Sleigh MA. Bending moments and elastic constants in cilia. J Theor Biol. 1970;28:81–100. doi: 10.1016/0022-5193(70)90065-2. [DOI] [PubMed] [Google Scholar]
- Ruhfus B, Bauernschmitt HG, Kinne RK. Properties of a polarized primary culture from rat renal inner medullary collecting duct (IMCD) cells. In Vitro Cell Dev Biol Anim. 1998;34:227–31. doi: 10.1007/s11626-998-0128-4. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 2007;17:333–42. doi: 10.1016/j.tcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–61. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–8. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–64. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res. 2006;24:1327–34. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–99. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–70. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–93. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081–8. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr., Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–52. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


