Abstract
Fertilization triggers rapid changes in intracellular free calcium that serve to activate multiple signaling events critical to the initiation of successful development. Among the pathways downstream of the fertilization-induced calcium transient is the calcium-calmodulin dependent protein tyrosine kinase PTK2b or PYK2 kinase. PTK2b plays an important role in fertilization of the zebrafish oocyte and the objective of the present study was to establish whether PTK2b also functions in mammalian fertilization. PTK2b was activated during the first few hours after fertilization of the mouse oocyte during the period when anaphase resumption was underway and prior to the pronuclear stage. Suppression of PTK2b kinase activity in oocytes blocked sperm incorporation and egg activation although sperm-oocyte binding was not affected. Oocytes that failed to incorporate sperm after inhibitor treatment showed no evidence of a calcium transient and no evidence of anaphase resumption suggesting that egg activation did not occur. The results indicate that PTK2b functions during the sperm-egg fusion process or during the physical incorporation of sperm into the egg cytoplasm and is therefore critical for successful development.
Keywords: PTK2b, PYK2 kinase, oocyte, fertilization, egg activation
Introduction
Fertilization triggers a preprogrammed series of signal transduction events that rapidly establish a block to polyspermy, induce resumption of the meiotic cell cycle, stimulate oocyte metabolism, and initiate embryonic development. Many of these events are triggered by the fertilization-induced calcium transient which activates CaMKII and PKC[5], events required for development to proceed [25]. Recent work in the zebrafish system demonstrated that PTK2b kinase (PYK2) was also activated by the fertilization-induced calcium transient and was critical for successful fertilization in the zebrafish oocyte [21], however the situation in mammalian oocytes is unknown. PYK2 is expressed in mouse oocytes and microscopic studies revealed that the activated kinase became concentrated at the site of sperm-oocyte interaction [16], but it is not known whether fertilization activates PYK2 in mammalian oocytes or simply induces redistribution of the active enzyme near the oocyte cortex.
The Focal Adhesion Kinase (FAK) kinase family includes two cytoplasmic protein kinases PTK2 (FAK) and PTK2b (PYK2). These enzymes transduce cell surface receptor signaling events to cytoplasmic pathways through their unique capability as scaffolding proteins as well as through catalytic activity. FAK is activated by integrin clustering and plays a key role in transducing extracellular stimuli into cytoplasmic responses and controls assembly/disassembly of adherens junctions and voltage-sensitive calcium channels [9;10]. PYK2 is very similar in structure to FAK and responds to integrin signaling, as well as environmental stress and growth factor stimuli. It functions to integrate extracellular and intracellular signals to regulate Rho activity [18] and control actin cytoskeleton dynamics [22] during cell process formation [8] and phagocytosis [19;20]. PYK2 is also active in the regulation of ion channels and mitogenic and hypertrophic responses [12–14]. As a protein tyrosine kinase, PYK2 is unique in that it requires calcium-calmodulin for full activation. Both FAK and PYK2 contain several tyrosine phosphorylation sites that are involved in intramolecular interactions that control access to the catalytic site and the phosphorylation state of these sites is commonly used as an indication of kinase activity [4]. PYK2 activation involves an initial autophosphorylation at Tyr402 in the catalytic domain which occurs in response to cell surface stimuli, but dimerization and transphosphorylation of Tyr579 & 580 to achieve full kinase activity is calcium-dependent [26].
The objective of the present study was to establish whether PYK2 becomes activated in response to fertilization in a mammalian oocyte and determine the extent to which kinase activity is required for successful completion of the fertilization process. The approach involved kinase activity measurements via western blot analysis of a key PYK2 phosphorylation site, combined with functional analysis through pharmacological suppression of PYK2 activity. The results contribute valuable information relating to fertilization in mammals highlighting a previously unrecognized pathway component of fertilization.
Materials and Methods
In vitro fertilization and pharmacological treatment
CF1 female and B6D2F1 males (Harlan Labs, Indianapolis, IN, USA) were housed in a temperature/light-controlled room and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. (Institute of Laboratory Animal Resources (U.S.) Committee on Care and Use of Laboratory Animals 1996; National Research Council (U.S.) 2011). Experimental procedures were approved by the University of Kansas Medical Center IACUC committee. Fak-null oocytes were produced by crossing the Fak flox/flox -CAT-eGFP line [1] with the C57BL/6-TgN(Zp3-Cre)93Knw (Zp3cre) line [11] obtained from Jackson laboratories (Bar Harbor, ME) producing Fakflox/wt Zp3cre+ mice which were re-crossed to produce Fakflox/flox Zp3cre+ animals. For production of Fak-null oocytes for experimental use, Fakflox/flox Zp3cre+ males were crossed with Fakflox/flox females and the resulting Fakflox/flox Zp3cre+ females were used for oocyte or embryo collection. Genotyping of mice was conducted according to a published protocol [1] in addition to the Jackson Laboratory protocol (http://jaxmice.jax.org/strain/003651.html). Oocytes were collected, stripped of cumulus cells and zona-pellucida, then fertilized in vitro under sperm-limiting conditions as previously described [16]; [7]. Incubation of oocytes with the PYK2 inhibitor PF04594755 (Pfizer Corporation, Groton CT), or the SRC-family kinase inhibitor SKI 606 (Bosutinib, Wyeth, Pearl River, NY) was carried out by addition of the inhibitor to medium after a 90 minute recovery from the zona-removal process. Oocytes were incubated with the indicated inhibitor for 30 minutes, and washed twice prior to addition of sperm. After a 2hr incubation with sperm, oocytes were transferred to a fixative containing 2% formaldehyde and 1% saturated picric acid for 2 hrs then permeabilized with 0.1% triton X100 in PBS. Oocytes were then labeled with 10uM DRAQ5 (Cell Signaling, Danvers, MA) to label DNA and 25nM phalloidin alexa fluor 488 (Invitrogen Carlsbad, CA) to label filamentous actin. The number of bound sperm (those confirmed to be outside of the cortical actin layer) were counted by confocal fluorescence using a 40X objective. Fertilization was established by the presence of sperm heads or expanded pronuclei that were clearly within the cortical actin layer. Oocyte activation was determined by resumption of anaphase as indicated by separation of the meiotic chromosomes.
Western blot analysis
Samples of 10–20 oocytes were resolved on a 10% SDS-polyacrylamide gel with a 4% stacking gel cast with a micro-scale comb to produce wells 1mm in width. Proteins were electro-transferred to immobilon-P (Millipore Corp. Billeria, MA), blocked with 5% BSA in tris-buffered saline containing 0.1% Tween 20 (Fisher scientific, Pittsburgh, PA), then probed with anti-GAPDH (EMD Millipore, Billerica, MA) and anti-PYK2 PY579 (Invitrogen Carlsbad, CA). Other antibodies used included antibodies to activated FAK (anti-FAK PY861 (Invitrogen, Carlsbad, CA)) and anti-activated SRC (clone 28), (Biosource International, Camarillo, CA). Chemiluminescence detection was done using the Femto-Kit (Thermo scientific, Rockford, IL, USA). Statistical analysis of band intensity within different experimental groups was performed by t test or by Mann Whitney rank sum analysis using Sigmastat software (Systat Software Inc. Chicago, IL).
Fluorescence Microscopy
Oocytes were fixed and prepared for labeling with Draq 5 to detect DNA and phalloidin alexa fluor 488 (Invitrogen Carlsbad, CA) to detect filamentous actin as previously described [16] and imaged with a Nikon TE2000 confocal microscope using either the confocal system or the conventional UV illumination. Total PYK2 PY579 content per cell was measured by slight modification of a published method [2]. Oocytes were grouped in a glass bottom microwell (Delta TBG dish, Fisher Scientific, St. Louis, MO) and imaged in a single frame by fluorescence microscopy at low magnification to keep all oocytes in the same focal plane. The fluorescence intensity of each oocyte was measured with Metafluor software (Meta Imaging Devices, Downington, PA, USA).
Ca2+ imaging in live oocytes
Zona-free oocytes were incubated in mKSOM with 1μM fura-2 AM/0.02% pluronic F-127 (Invitrogen, Carlsbad, CA) prior to addition of 10μM PF04594755 or 0.1% DMSO as a control. After a 15 minute fertilization period, the fertilization droplets were monitored at 37°C on a Nikon TE2000-U microscope equipped with a Lambda 10-2 Optical Filter Changer (Sutter Instruments, Novato, CA, USA). Fura-2 was excited at 340 and 380 nm and emissions at 530 nm were collected at 15 second intervals for 45 minutes. The fluorescence excitation ratio at 340/380nm was monitored and quantified by Metafluor software (Meta Imaging Devices, Downington, PA, USA).
RESULTS
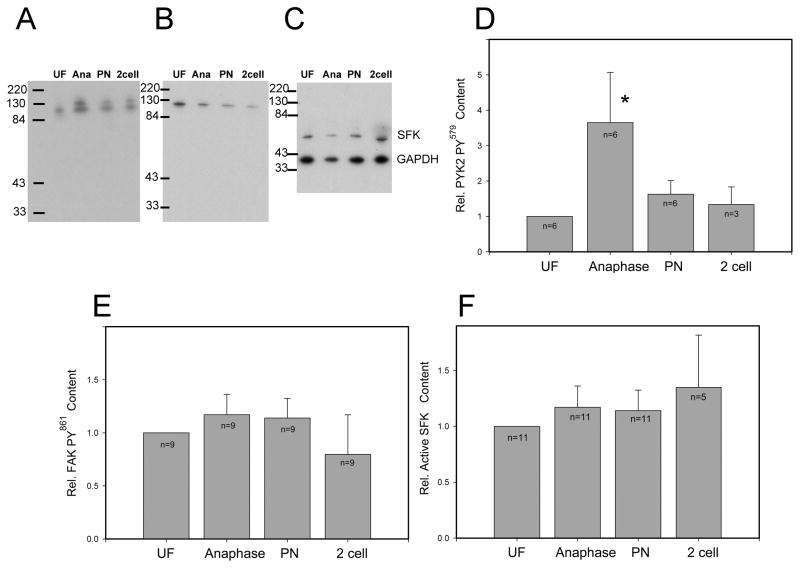
Recent work in the zebrafish oocyte demonstrated that the fertilization-induced calcium transient triggered activation of PYK2 kinase which was required for successful egg activation [21]. In order to determine whether a similar pathway occurred in mammalian oocytes, the impact of fertilization on PYK2 activity was tested in the mouse oocyte system. PKY2 kinase activity in oocytes was estimated by measurement of the relative content of phosphorylated PYK2 by western blot analysis using an antibody to the phosphorylated form of Y579 and anti-GAPDH as a loading control. Groups of 10–20 zona-free oocytes were fertilized under sperm-limiting conditions [7] designed to produce a relatively synchronous population of monospermic fertilization events. Samples were collected during anaphase of the second meiotic division (45–75 minutes post insemination), during the pronuclear stage (5–8 hrs post insemination) and at the 2-cell stage (24–28 hrs post insemination). As seen in Figure 1, tyrosine phosphorylated PYK2 appeared as a doublet which probably represents electrophoretic mobility shifts resulting from phosphorylation at multiple sites. Fertilization was followed by a significant increase in total oocyte PYK2 PY579 content (in the doublet) relative to GAPDH content, with activity declining during the pronuclear and 2 cell stages. In comparison, the activity of FAK kinase (panel B) and SRC-family kinases (panel C) did not change significantly after fertilization.
Figure 1. Effect of fertilization on PYK2 activation.
Groups of 20–30 zona-free oocytes were fertilized in vitro then washed free of unbound sperm and solubilized for western blot analysis as described in “Materials and Methods”. Blots were probed with anti PYK2 PY579 (panel A, D), anti-FAK PY861 (panel B, E), or anti-active SRC (panel C, F). Bound antibody was detected by chemiluminescence and films were scanned to quantify band intensity which was corrected for loading errors by GAPDH content and expressed relative to the band intensity in unfertilized oocytes (Panels D–F). (n) indicates the number of oocyte groups that were analyzed. (*) indicates the value was significantly different from that in unfertilized oocytes (P<0.05).
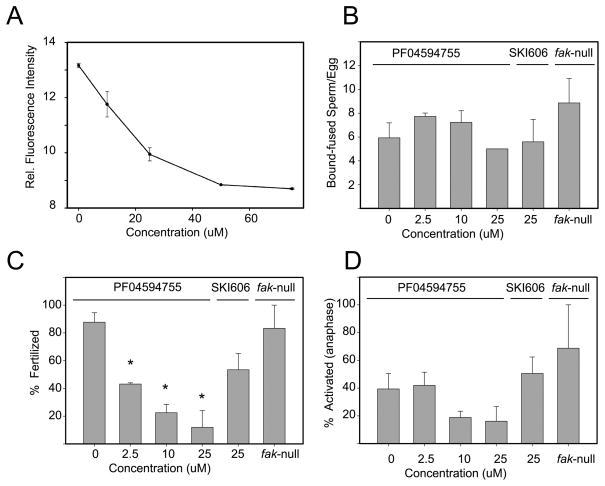
In order to determine whether PYK2 activity was required for fertilization and subsequent development, the highly specific PYK2 inhibitor PF04594755 [2] was used to suppress PYK2 activity in intact oocytes [21] during a 30 minute pre-incubation, after which oocytes were washed free of inhibitor and fertilized in vitro. The success of fertilization was monitored by confocal fluorescence microscopy which allowed us to determine whether sperm heads had penetrated the oocyte plasma membrane and begun to expand into male pronuclei. For example, the number of bound sperm per oocyte was determined by counting sperm heads that were attached to each oocyte as seen in Figure 2A which depicts a control DMSO-treated oocyte with bound sperm (arrows). Sperm were considered to have fertilized the oocyte if the sperm head was clearly inside the ooplasm as seen in Figure 2B which represents a control oocyte with two pronuclei (arrows) indicating that at least one sperm had fertilized the oocyte. The success of oocyte activation was determined by assessing whether the meiotic spindle had begun anaphase resumption as indicated by movement of chromosomes toward the spindle poles. Figure 2C demonstrates a PF04594755 treated oocyte with bound sperm (arrows) that did not incorporate any sperm heads and failed to enter anaphase indicating that it was not activated. In contrast, Figure 2D shows a PF04594755 treated oocyte that was parthenogenetically activated by 5mM Sr2+ and exhibits chromosomes that have migrated toward the spindle poles (arrows) indicating that the inhibitor did not prevent artificially induced oocyte activation. The effective concentration range of PF04594755 during the limited, 30 minute pre-incubation protocol was determined by measuring the autophosphorylation of PYK2 at Y579 via the quantitative immunofluorescence-based assay described above. The results (Figure 3A) indicated that PF04594755 was effective at suppressing PYK2 phosphorylation between 10 and 50μM. Oocytes treated with PF04594755 within this range retained the ability to respond to parthenogenetic activation by 5mM SrCl2 as demonstrated in Figure 2D.
Figure 2. Effect of PYK2 inhibition on sperm incorporation.
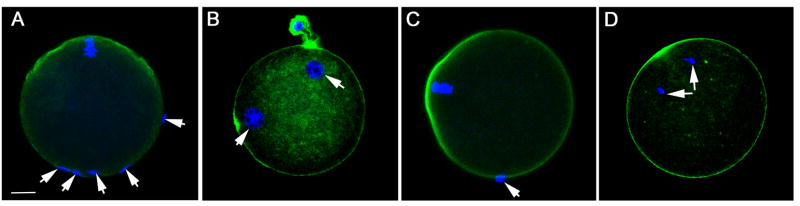
Zona free oocytes treated with different concentrations of PF04594755, (panel A,C) or DMSO as a solvent control (panel B) prior to insemination in vitro were fixed and stained with Draq5 (blue) to stain DNA and phalloidin-alexafluor 488 (green) to stain the cortical actin layer in order to define whether sperm had actually penetrated the oocyte plasma membrane and gained access to the ooplasm. Panel A shows a PF04594755 (25μM) treated oocyte with attached sperm (arrows) classified as bound. Panel B shows a control (DMSO treated) oocyte with two pronuclei (arrows) indicating that the oocyte was fertilized with at least one sperm incorporated. The meiotic spindle was also inspected to determine whether oocytes were activated triggering meiosis resumption. Panel C shows a PF04594755 treated oocyte with bound sperm (arrow) and a meiotic spindle that remains arrested at MII indicating that oocyte activation had not begun. Panel D represents a PF04594755 treated oocyte activated by exposure to SrCl2 (5mm) that has undergone oocyte activation and completed anaphase. Magnification is indicated by the bar which represents 10μm.
Figure 3. Effect of PYK2 inhibition on sperm binding, fertilization, and oocyte activation.
The effectiveness of PF04594755 during a 30minute pre-treatment incubation was tested by incubating zona free oocytes with concentrations of PF04594755 between 10 and 75μM, then assessing total oocyte PYK2 PY579 content (Panel A (relative fluorescence intensity)) as described in “Materials and Methods”. The effect of different concentrations of PF04594755 or SKI606 on sperm binding (Panel B), fertilization (Panel C), and oocyte activation (Panel D), was tested as described in “Materials and Methods”. After 2 hrs incubation with capacitated sperm, oocytes were fixed and prepared for confocal fluorescence to assess sperm binding, sperm penetration, and anaphase resumption (egg activation). In some experiments, oocytes recovered from flox-Fak-CAT-eGFP homozygous females carrying the Zp3-cre transgene (Fak-null oocytes) were used for fertilization. The number of bound sperm per oocyte (Panel B), number of fertilized eggs containing incorporated sperm heads in the ooplasm (Panel C), or number of oocytes showing evidence of anaphase resumption (activated oocytes) (Panel D). Values represent the mean of at least 5 experiments +/− SEM. (*) indicates a value significantly different from that of unfertilized oocytes (P<0.05).
Figure 3 demonstrates the effect of PYK2 suppression on sperm-oocyte binding, fertilization, and oocyte activation at different concentrations of PF04594755. As a control for possible non-specific effects of the inhibitor, a different compound, SKI606 (a SRC-family inhibitor [3]) was used at 25 μM to treat additional groups of oocytes. To control for possible effects of PF04594755 on FAK kinase, some experiments were performed on Fak-null oocytes recovered from flox-Fak-CAT-eGFP homozygous females carrying the Zp3-cre transgene which do not express detectable levels of FAK protein (supplementary material). As seen in Figure 3B, PF04594755 incubation at concentrations of 2.5 to 25μM had no effect on the ability of sperm to bind or attach to the oocyte surface. However, these same concentrations of PF04594755 caused a significant reduction in the frequency of oocytes that had been penetrated by sperm and had successfully incorporated the sperm nucleus indicating that they were fertilized (Figure 3C). In addition, the frequency of egg activation shown by anaphase resumption (Figure 3D) was lower in oocytes exposed to 10 and 25μM PF04594755 which is consistent with a reduced fertilization rate. The effects induced by PF04594755 were not caused by treatment with the SRC-family kinase inhibitor SKI-606 at concentrations previously shown to induce pronuclear arrest [15] indicating that the PF04594755 effects were not non-specific effects on sperm-oocyte interactions. The Fak-null oocytes were fertilized at normal rates indicating that possible inhibition of FAK by PF04594755 did not cause the failure of oocytes to become fertilized.
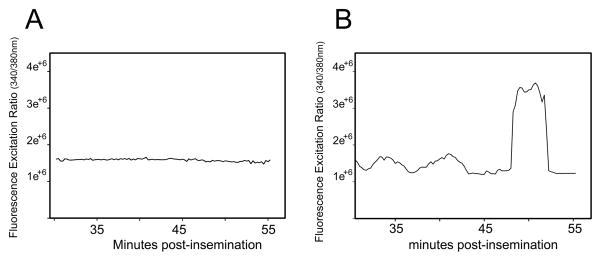
The above results demonstrate that PYK2 suppression caused a failure of sperm incorporation, fertilization, and egg activation. To test whether the oocytes that failed to fertilize showed any evidence of a calcium transient, a separate series of experiments was performed to detect changes in intracellular [Ca2+]i among the PF04594755 treated oocytes. Zona-free oocytes were preloaded with the calcium-sensitive dye fura-2am prior to treatment with PF04594755 (10μM) then fertilized as in Figure 2 and imaged by fluorescence ratiometric imaging to detect changes in [Ca2+]i. Successful fertilization was assessed by the appearance of pronuclei. Pre-treatment of oocytes with PF04594755 blocked the fertilization-induced calcium transient in the majority of (11 out of 14) oocytes from two different experiments (Figure 4A). The non-responsive oocytes in the treatment group did not form polar bodies or pronuclei indicating that they were not fertilized. The oocytes that did exhibit a calcium transient (Figure 4B) went on to form pronuclei indicating that they were fertilized.
Figure 4. Effect of PYK2 inhibition on the fertilization-induced calcium transient.
Zona free oocytes were preloaded with fura2-am prior to treatment with 10μM PF04594755 or 0.1% DMSO, then inseminated in vitro as in Figure 3. The fertilization droplets were imaged by fluorescence microscopy to measure changes in [Ca2+ ]i. Panel A shows the ratio of fluorescence emission at excitation wavelengths of 340 and 380nm (Y axis) in a PF04594755 treated oocyte that failed to become fertilized as shown by absence of a second polar body or pronuclei. Panel B shows the calcium response of a PF04594755 treated oocyte that did exhibit a 2nd polar body and pronuclei indicating that it was fertilized.
Discussion
The present study has demonstrated that fertilization triggers activation of the PYK2 kinase in mouse oocytes fertilized in vitro. Kinase activation occurs during anaphase resumption, a result similar to that observed in the zebrafish oocyte. The increased PYK2 activity was evident between 45 and 75 minutes after the addition of capacitated sperm and was not likely to reflect increased protein synthesis which does not begin until much later in the egg activation process. The closely related FAK kinase and the SRC-family kinases did not exhibit signification increases in activation state even though our previous studies by confocal immunofluorescence demonstrated that the activated forms of both PYK2 and FAK became concentrated in the oocyte cortex during anaphase resumption [16]. It thus appears that fertilization triggers the activation of PYK2 as well as concentration of PYK2 and FAK in the oocyte cortex. The specific role of the calcium transient in PYK2 activation was not tested in this study, but the fact that PYK2 activity did not remain elevated during the pronuclear stages (when calcium oscillations normally cease) suggests that [Ca2+]i may play a role in the control of PYK2 activity during fertilization and further experiments are now underway to test that possibility.
The role of PYK2 kinase activity in fertilization was tested with the PF04594755 compound which was shown to be highly specific for PYK2 even when compared to the structurally similar FAK [2]. In the present study, PYK2 suppression accomplished by preincubation of oocytes prior to addition of sperm, significantly reduced the ability of oocytes to incorporate sperm and proceed into anaphase, although sperm attachment was not affected. While the methods used here did not have sufficient resolution to differentiate sperm-egg binding from the early stages of sperm-egg fusion, the fact that PYK2 inhibition reduced the frequency of sperm incorporation into the ooplasm indicates that PYK2 plays a role in either sperm-egg fusion or sperm engulfment and incorporation into the cytoplasm. The latter possibility seems more likely since PYK2 is concentrated in the microvilli covering most of the oocyte surface [16] and morphological analysis of sperm incorporation in mammalian oocytes indicates an active role of oocyte cell processes which appear to engulf the sperm head [24]. Work in other systems has shown that PYK2 is required for phagocytosis [20] and actin-mediated cell surface motility [8;17], so a role in sperm incorporation might occur through PYK2 regulation of the cytoskeletal elements involved in the elongation or movement of oocyte cell surface processes which surround the sperm head and incorporate it into the ooplasm. Alternatively, PYK2 suppression by PF04594755 might mimic the membrane-block to polyspermy [6] by altering the phosphorylation state of critical cytoskeletal components needed for gamete fusion and thereby prevent subsequent sperm incorporation. The fact that PYK2 activation is required for the early stages of fertilization does not preclude additional functions later in the egg activation process. During normal fertilization, sperm-egg fusion allows diffusion of the sperm-borne PLCζ into the ooplasm [23;27] which triggers the fertilization-induced calcium oscillations and initiates resumption of the second meiotic cell cycle. However, the observation that PF04594755 treated oocytes could still undergo meiosis resumption in response to the parthenogenetic Sr2+ ion demonstrated that loss of PYK2 activity did not impair the ability of the cell cycle machinery to respond to an artificially induced calcium transient.
In summary, fertilization results in a significant increase in the level of activated PYK2 in mouse oocytes which plays an important role in successful sperm incorporation. PYK2, and downstream elements responsible for the cytoskeletal movements involved in sperm incorporation may therefore represent possible targets for contraceptive development or parameters which, if mis-regulated, could reduce fertilizing capacity of oocytes and reduce fertility.
Supplementary Material
Highlights.
PTK2b is expressed in oocytes and is activated following fertilization.
PTK2b suppression in oocytes prevents fertilization, but not parthenogenetic activation.
PTK2b suppression prevents the oocyte from fusing with or incorporating bound sperm.
PTK2b suppressed oocytes that fail to fertilize do not exhibit calcium oscillations.
Acknowledgments
We are indebted to Lily Zhang and Ling Chen for technical assistance. This work was supported by NICHD HD062860 to W.H.K.
Abbreviations
- IVF
In vitro fertilization
- PTK
protein tyrosine kinase
- MII
metaphase-II
- FAK
focal adhesion kinase
- PYK2
protein tyrosine kinase-2b
- SFK
Src-Family kinase
- mKSOMAA
modified potassium simplex-optimized medium with amino acids
- BSA
bovine serum albumin
- CaMKII
calcium-calmodulin dependent kinase II
- PKC
protein kinase C
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnette PC, Robinson BS, Silva JC, Stokes MP, Brosius AD, Baumann A, Buckbinder L. Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. J Proteomics. 2010;73:1306–1320. doi: 10.1016/j.jprot.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Boschelli DH, Wu B, Ye F, Wang Y, Golas JM, Lucas J, Boschelli F. Synthesis and Src kinase inhibitory activity of a series of 4-[(2,4-dichloro-5-methoxyphenyl)amino]-7-furyl-3-quinolinecarbonitriles. J Med Chem. 2006;49:7868–7876. doi: 10.1021/jm061031t. [DOI] [PubMed] [Google Scholar]
- 4.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 5.Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadella BM, Evans JP. Membrane fusions during mammalian fertilization. Adv Exp Med Biol. 2011;713:65–80. doi: 10.1007/978-94-007-0763-4_5. [DOI] [PubMed] [Google Scholar]
- 7.Gardner AJ, Williams CJ, Evans JP. Establishment of the mammalian membrane block to polyspermy: evidence for calcium-dependent and -independent regulation. Reproduction. 2007;133:383–393. doi: 10.1530/REP-06-0304. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. J Cell Biol. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui P, Chao JT, Wu X, Yang Y, Davis GE, Davis MJ. Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling. Adv Exp Med Biol. 2010;674:69–79. doi: 10.1007/978-1-4419-6066-5_7. [DOI] [PubMed] [Google Scholar]
- 10.Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281:14015–14025. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- 11.Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo MM, de Vries WN, Knowles BB, Solter D. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 12.Lakkakorpi PT, Nakamura I, Nagy RM, Parsons JT, Rodan GA, Duong LT. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 13.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–8924. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev. 2010;22:966–976. doi: 10.1071/RD09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinnis LK, Luo J, Kinsey WH. Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption. Mol Reprod Dev. 2013;80:260–272. doi: 10.1002/mrd.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitaka T, Shindoh M, Mochizuki Y, Sasaki H, Ishino M, Matsuya M, Ninomiya T, Sasaki T. Restricted expression of cell adhesion kinase-beta in rat tissues. Am J Pathol. 1997;150:267–281. [PMC free article] [PubMed] [Google Scholar]
- 18.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen KA, Thomas KS, Bouton AH. The differential expression of Yersinia pseudotuberculosis adhesins determines the requirement for FAK and/or Pyk2 during bacterial phagocytosis by macrophages. Cell Microbiol. 2007;9:596–609. doi: 10.1111/j.1462-5822.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharma D, Kinsey WH. PYK2: a calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Dev Biol. 2013;373:130–140. doi: 10.1016/j.ydbio.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CK, Ng KT, Lim ZX, Cheng Q, Lo CM, Poon RT, Man K, Wong N, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes cell motility of hepatocellular carcinoma through induction of epithelial to mesenchymal transition. PLoS One. 2011;6:e18878. doi: 10.1371/journal.pone.0018878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Tengowski MW. Microscopic techniques for studying sperm-oocyte interaction during fertilization and early embryonic development. Methods Mol Biol. 2004;253:165–199. doi: 10.1385/1-59259-744-0:165. [DOI] [PubMed] [Google Scholar]
- 25.Wakai T, Vanderheyden V, Fissore RA. Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations. Cold Spring Harb Perspect Biol. 2011;3:a006767. doi: 10.1101/cshperspect.a006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu SS, Jacamo RO, Vong SK, Rozengurt E. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal. 2006;18:1932–1940. doi: 10.1016/j.cellsig.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCzeta causes Ca(2+) oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P(2) Mol Biol Cell. 2012;23:371–380. doi: 10.1091/mbc.E11-08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.