Abstract
Studies suggest T cells modulate arterial pressure. Since robust sex differences exist in the immune system and in hypertension, we investigated sex differences in T cell modulation of angiotensin II (Ang II)-induced increases in mean arterial pressure (MAP) in male (M) and female (F) wild type (WT) and recombination-activating-gene-1-deficient (Rag1−/−) mice. Sex-differences in peak MAP in WT were lost in Rag1−/− mice [mmHg: WT-F, 136±4.9 vs. WT-M, 153±1.7; P<0.02; Rag1−/−-F, 135±2.1 vs. Rag1−/−-M, 141±3.8]. Peak MAP was 13 mmHg higher after adoptive transfer of male (CD3M→Rag1−/−-M) vs. female (CD3F→Rag1−/−-M) T-cells. CD3M→Rag1−/−-M mice exhibited higher splenic frequencies of pro-inflammatory interleukin-17A (2.4-fold) and tumor-necrosis factor-α (2.2-fold)-producing T cells and lower plasma levels (13-fold) and renal mRNA expression (2.4-fold) of interleukin-10 while CD3F→Rag1−/−-M mice displayed a higher activation state in general and T-helper 1-biased renal inflammation. Greater T cell infiltration into perivascular adipose tissue and kidney associated with increased pressor responses to Ang II if the T cell donor was male but not female and these sex differences in T cell subset expansion and tissue infiltration were maintained for 7–8 weeks within the male host. Thus, the adaptive immune response and role of pro- and anti-inflammatory cytokine signaling in hypertension is distinct between the sexes and needs to be understood to improve therapeutics for hypertension-associated disease in both men and women.
Keywords: hypertension, angiotensin, T cells, Rag1−/−-M mice, sex differences
The onset of essential hypertension occurs earlier in men than in women. A similar sex difference is observed in most animal models of hypertension regardless of whether the models are genetic or induced1. Thus, elucidating the mechanisms that underlie this robust sex difference in susceptibility to hypertension could have profound implications for the prevention and treatment of hypertension and its morbid outcomes for both men and women
Hypertension is associated with the development of inflammation in numerous tissues including the vasculature, kidney and brain2. T cells are critical mediators of this inflammatory process and recent studies suggest T cells modulate arterial pressure. Male mice deficient in T and B cells (Rag1−/−-M) were shown to have lower MAP after Ang II infusion compared to male WT mice3. Furthermore, adoptive transfer of male T cells restored the magnitude of the Ang II-induced hypertension to WT-M levels while adoptive transfer of male B cells under the same conditions was without effect.
It is well known that women are far more prone to develop autoimmune disease than men4 and that they have higher circulating levels of CD4+ T cells5 and IgM and class-switched antibodies6. Recent studies have also shown that female T cells have a higher propensity to expand in response to antigenic stimulation than male T cells7. T helper (Th) cells from females also tend to produce higher levels of the Th1 cytokine interferon gamma (IFN-γ), while male T cell immune responses are more biased towards Th17 cytokine production7,8.
In this study, we investigated whether or not sex differences in T cell modulation of MAP could contribute to sex differences in susceptibility to Ang II-dependent hypertension. As we and others have found previously9,10, Ang II infusion increased MAP to a greater extent in male as compared to female WT mice. In contrast, we found no sex differences in the magnitude of Ang II-induced hypertension in Rag1−/− mice, suggesting that sex differences in T cell actions contribute to the higher MAP found in male compared to female mice after Ang II infusion. Furthermore, CD3+ T cells from male and female WT mice differed markedly in their ability to induce hypertension in the male Rag1−/− host after Ang II infusion; Rag1−/−-M mice after adoptive transfer of male T cells were more susceptible to Ang II-induced hypertension than after adoptive transfer of female T cells. The primary T cell sex difference that associated with susceptibility or resistance to Ang II-induced hypertension was that male splenic T cells exhibited a higher frequency of the pro-inflammatory cytokines interleukin (IL)-17A and tumor necrosis factor-α (TNF-α) compared to female splenic T cells. In addition, after two weeks of Ang II infusion, we observed lower levels of IL-10 in the circulation and lower expression of IL-10 mRNA in the kidneys of Rag1−/−-M mice that received male as compared to female T cells. These observations indicate that susceptibility or resistance to hypertension are at least in part determined by the sex of the T cell.
Materials and Methods
A detailed Methods section is provided in the online-only Data Supplement.
Results
Effect of T and B cell deficiency on sex differences in MAP and HR responses to Ang II
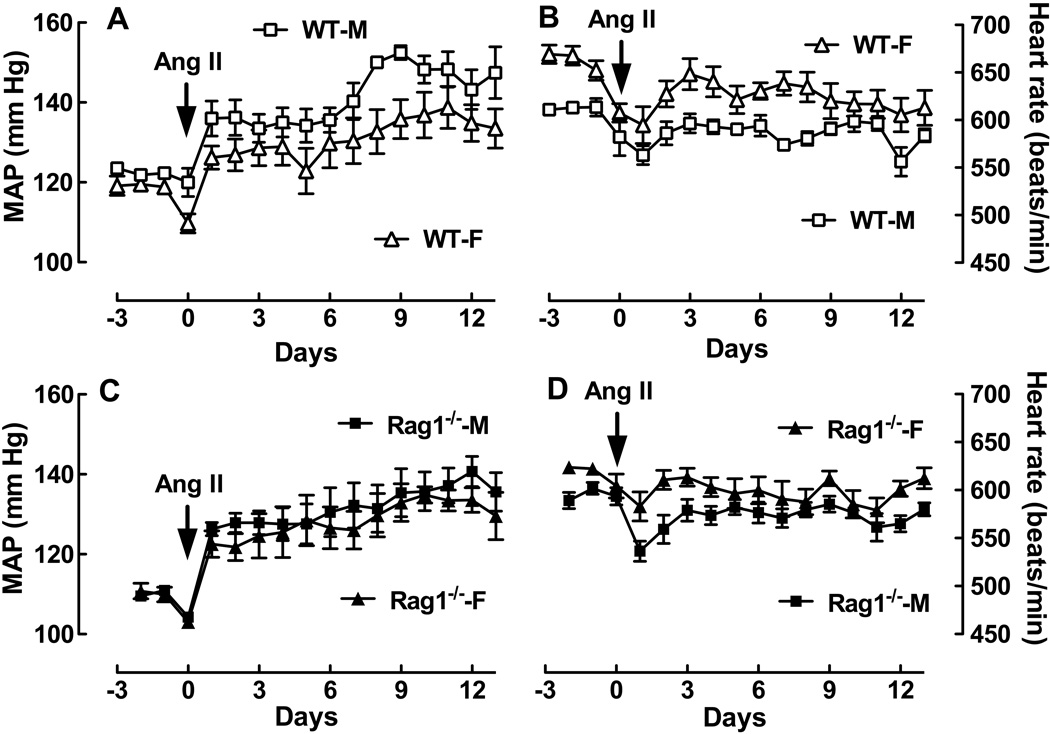
It is well known that males (M) have higher blood pressure (BP) than females (F) in many experimental models of hypertension including Ang II infusion1. Using radiotelemetry, we confirmed this finding in WT mice infused with Ang II at 490 ng/kg/min for two weeks. Ang II caused a larger increase in MAP in WT-M than WT-F mice (Figure 1A; P<0.0001 by two-way ANOVA). The biggest difference in MAP (17 mm Hg) occurred on day 9 (mm Hg: WT-M, 153 ± 1.7 vs. WT-F, 136 ± 4.9; P<0.02 by t-test). In contrast, there were no detectable differences in MAP between male and female Rag1−/− mice (Figure 1C). As we and others have shown previously9,10, significant differences in HR were observed between male and female WT mice (Figure 1B; P<0.0001 by two-way ANOVA). Basal HR was 50 bpm lower in male compared to female WT mice [HR (bpm): WT-M, 613 ± 4.7 vs. WT-F, 663 ± 8.7; P<0.001 by t-test] and there was a smaller drop in HR over the Ang II infusion period in male compared to female mice (Figure 1B). In contrast, the sex differences in basal HR and HR responses to Ang II infusion were less pronounced in male and female Rag1−/− mice (Figure 1D). While basal HR was 28 bpm lower in the male Rag1−/− mouse (P< 0.005 by t-test), the drop in HR due to Ang II was indistinguishable between the sexes at the end of the two week infusion period. These findings suggest T and/or B cells contribute to sex differences in hypertension since male-female differences in MAP and HR responses to Ang II are substantially attenuated or eliminated.
Figure 1. Effect of the sex of the animal on MAP and HR responses to Ang II in WT and Rag1−/− mice.
Shown is MAP (A, C) and HR (B, D) as a function of time in female (triangle) and male (square) WT (opened symbol) and Rag1−/− (closed symbol) mice after Ang II infusion (490 ng/kg/min). WT-M (n=6); WT-F (n=9);Rag1−/−-M (n=7);Rag1−/−-M-F (n=6). See text for statistical comparisons.
Effect of the sex of the T cell donor on MAP and HR responses to Ang II in the male Rag1−/− host
Previous studies have shown that adoptive transfer of male T cells into Rag1−/−-M mice increases the magnitude of Ang II-induced hypertension3. To determine if this T cell exacerbation of Ang II-dependent hypertension depends upon the sex of the T cell, we first conducted an Ang II dose response in WT-M and Rag1−/−-M mice (Figure S1) and then compared the magnitude of the hypertension induced by Ang II infusion at 490 ng/kg/min after adoptive transfer of cells from male and female WT mice into Rag1−/−-M mice.
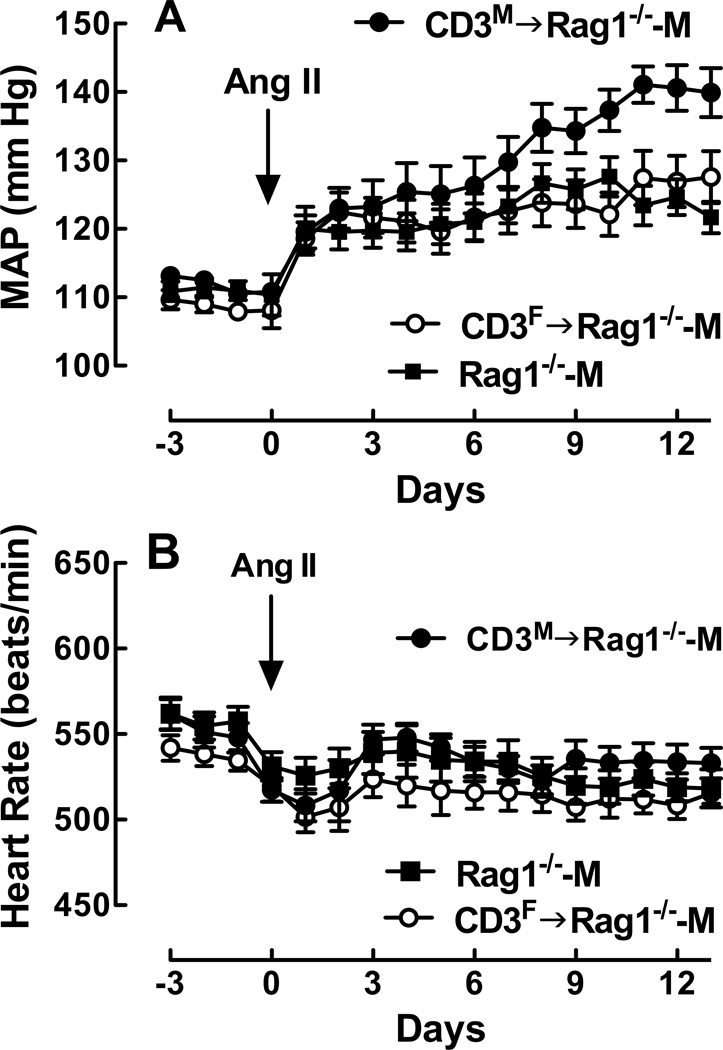
As observed previously3, adoptive transfer of CD3M ultimately restored the magnitude of the Ang II-induced increase in MAP to that observed in WT-M mice. No differences in the peak MAP response or in the MAP at the end of the Ang II infusion period were detected between CD3M→Rag1−/−-M and WT-M mice; however, the time course of the MAP response to Ang II was slower in the CD3M→Rag1−/−-M compared to the WT-M mice (Figs. 1) & 2A; P<0.0001 by two-way ANOVA).
While there was no effect of the sex of the T cell donor on basal MAP between CD3M→Rag1−/−-M and CD3F→Rag1−/−-M, adoptive transfer of CD3M cells resulted in a greater MAP response to Ang II compared to CD3F cells (Figure 2A). The MAP was 13 mm Hg higher at the peak [mmHg: CD3M→Rag1−/−-M, 141 ± 2.7 vs. CD3F→Rag1−/−-M, 128 ± 3.8; P<0.02 by t-test] and 12 mm Hg higher at the end of the infusion period [mmHg: CD3M→Rag1−/−-M, 140 ± 3.6 vs. CD3F→Rag1−/−-M, 128 ± 3.8; P<0.04 by t-test] in CD3M→Rag1−/−-M compared to CD3F→Rag1−/−-M mice. In fact, adoptive transfer of CD3F into the Rag1−/−-M mice did not increase the MAP response to Ang II; no differences in MAP were observed in the Ang II time course between the CD3F→Rag1−/−-M and the Rag1−/−-M mice. These differences in MAP were not due to differences in BW since no differences in BW were observed between these two groups [BW (g): CD3F→Rag1−/−-M, 30 ± 0.46 vs CD3M→Rag1−/−-M, 29 ± 0.43] and two weeks of Ang II infusion had no effect on BW (data now shown). Furthermore, no differences in plasma Ang II [(pg/ml): CD3F→Rag1−/−-M, 81 ± 20 vs CD3M→Rag1−/−-M, 76 ± 23] were detected two weeks after Ang II infusion.
Figure 2. Effect of the sex of the T cell donor on MAP and HR responses to Ang II in the male Rag1−/− host during Ang II infusion.
Shown is MAP (A) and HR (B) as a function of time in Rag1−/−-M (closed square; n=13/group) and in Rag1−/−-M after adoptive transfer of T cells isolated from female (open circle; CD3F→Rag1−/−-M; n=19/group) or male (closed diamond; CD3M→Rag1−/−-M; n=13/group) WT mouse spleens. See text for statistical comparisons.
Small but significant differences were detected in the time course of the HR response to Ang II between CD3M→Rag1−/−-M and CD3F→Rag1−/−-M mice (Figure 2B; P<0.05 by two way ANOVA); however, there were no detectable sex differences in the peak drop in HR or in HR at the end of the infusion period. These results indicate T cell modulation of Ang II-dependent hypertension in the hormonal milieu of the male Rag1−/−-M mouse depends upon the sex of the T cell donor; i.e., under these conditions, male T cells were pro-hypertensive while female T cells were not.
Effect of the sex of the T cell donor on T cell reconstitution in the male Rag1−/− host after adoptive transfer followed by two weeks of Ang II infusion
To investigate the mechanisms by which the sex of the T cell donor impacts Ang II-dependent hypertension within the male host, we compared the extent of T cell reconstitution in both the spleen and peripheral blood of CD3F→Rag1−/−-M and CD3M→Rag1−/−-M mice after two weeks of Ang II infusion. We detected a higher number of mononuclear cells in the Rag1−/−-M spleen after adoptive transfer of female compared to male T cells [# of splenocytes: CD3F→Rag1−/−-M, 4.64 ± 0.80 × 106 vs CD3M→Rag1−/−-M, 3.34 ± 0.38 × 106; P<0.05 by t-test; n=15]; however, there were no detectable sex differences in the splenocyte population in terms of the T cell frequencies of CD4+ [% of splenocytes: CD3F→Rag1−/−-M, 32.1 ± 2.6 vs CD3M→Rag1−/−-M, 31.9 ± 2.1; n=15] or CD8+ [% of splenocytes: CD3F→Rag1−/−-M, 25.8 ± 2.1 vs CD3M→Rag1−/−-M, 30.9 ± 2.3; n=15]. Furthermore, sex differences were also not detected in splenocytes from the donor WT mice with respect to the T cell frequency of CD4+ [% of splenocytes: WT-F, 14.2 ± 0.30 vs WT-M, 14.6 ± 0.53; n=6] or CD8+ [% of splenocytes: WT-F, 11.3 ± 0.38 vs WT-M, 12.0 ± 0.30; n=6], nor was there any effect of Ang II infusion on the ratio of CD4:CD8 in male and female mouse spleens (data not shown).
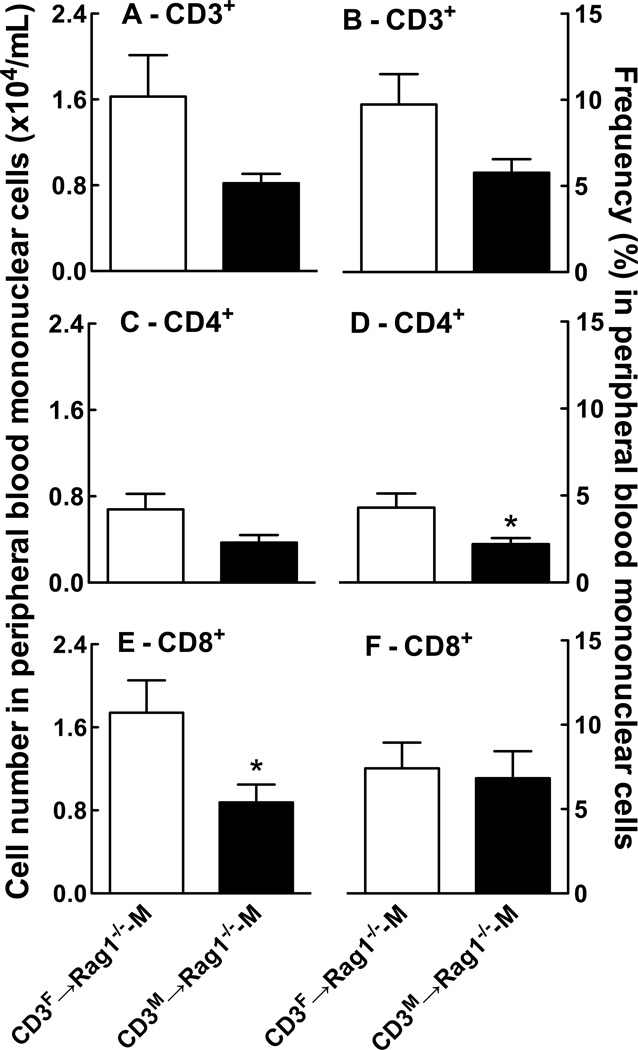
Similar to findings in the spleen, we detected a higher number of CD8+ cells and a trend towards a higher number of CD3+ and CD4+ cells in the peripheral blood of Rag1−/−-M mice after adoptive transfer of CD3F compared with CD3M T cells (Figure 3 A, C & E). CD4+ T cells were also detected at a higher frequency in peripheral blood of CD3F→Rag1−/−-M compared to CD3M→Rag1−/−-M mice (Figure 3D). Together, these findings suggest that female T cells are more efficient than male T cells at seeding the lymphopenic environment of the male Rag1−/− host and that they are trafficking more actively than male T cells. These findings also point out that Ang II-induced hypertension in the male Rag1−/− host does not merely correlate with increased T cell expansion or mobilization.
Figure 3. Effect of the sex of the T cell donor on the number and frequency of T cell populations in PBMC isolated from the male Rag1−/− host after adoptive transfer and Ang II infusion.
Shown are the number and frequency of CD3+ (A & B), CD4+ (C & D) and CD8+ (E & F) T cells in PBMC from Rag1−/−-M mice after adoptive transfer of CD3+ T cells isolated from female (white bar) or male (black bar) WT mouse spleens followed by two weeks of Ang II infusion. The data were analyzed by t-test; *P<0.05 vs. CD3F→Rag1−/−-M; n=7/group.
Effect of the sex of the T cell donor on the frequency of T cell subsets in the spleen of the male Rag1−/− host after adoptive transfer followed by two weeks of Ang II infusion
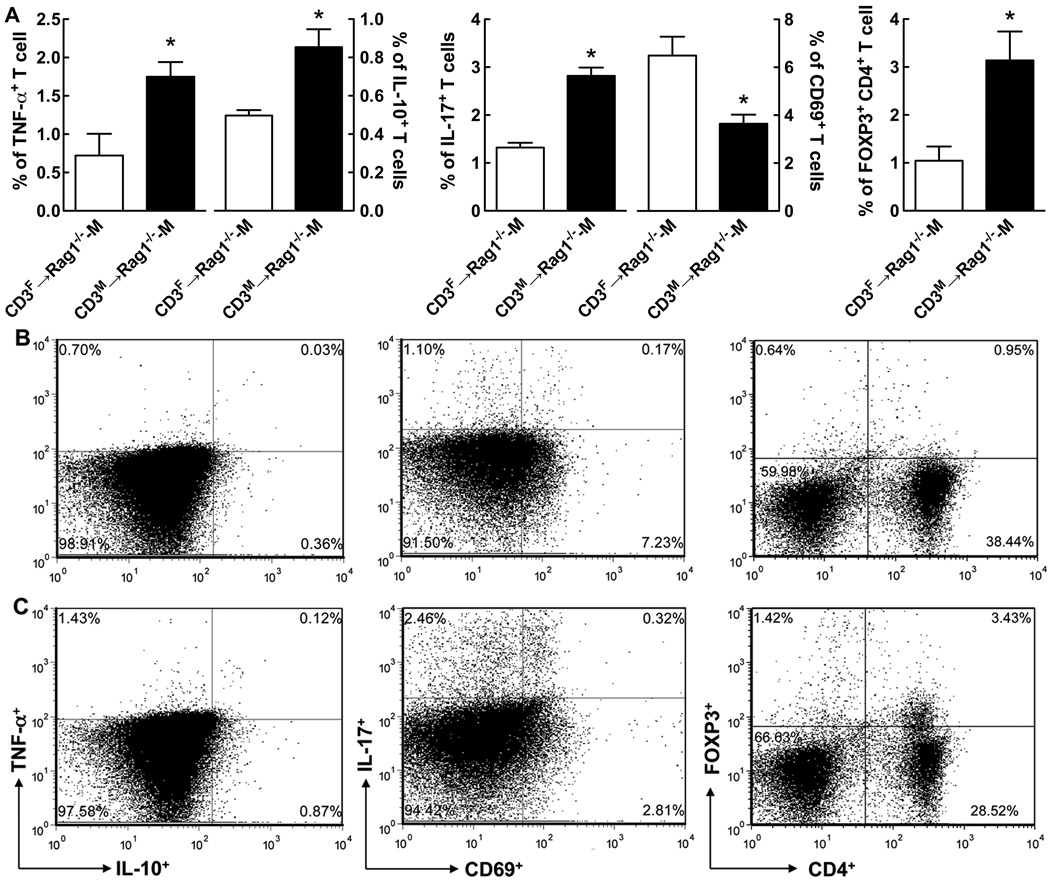
To further investigate the activation status of T cells, we investigated the proportions of pro-inflammatory (TNF- and IL-17-producing)11 and anti-inflammatory (IL-10-producing and CD4+FOXP3+ Treg)12 T cell subsets that have been previously implicated in modulating Ang II-induced hypertension. Coinciding with the greater development of Ang II-induced hypertension in CD3M→Rag1−/−-M compared to CD3F→Rag1−/−-M mice, CD3M→Rag1−/−-M exhibited a higher frequency of T cells producing the pro-inflammatory cytokines TNF (2.4-fold) and IL-17 (2.2-fold) (Figure 4).
Figure 4. Effect of the sex of the T cell donor on the frequency of T cell subsets in splenocytes isolated from Rag1−/−-M after adoptive transfer and Ang II infusion.
Shown is the mean ± SEM of the frequency of TNF-α-, IL-10-, IL-17- producing and CD69- and Treg- expressing T cell subsets in Rag1−/−-M splenocytes after adoptive transfer of female (white bar) or male (black bar) T cells and two weeks of Ang II infusion (A). Representative images from flow cytometry are shown for CD3F-Rag1-M (B) and CD3M-Rag1-F (C) mice for T cell subsets quantitated in A. The data were analyzed by t-test; *P<0.05 vs. CD3F→Rag1−/−-M; n=7/group.
Though WT-F mice exhibited a 1.6-fold higher frequency of Treg in the spleen than WT-M mice (Table S1), after adoptive transfer into the Rag1−/−-M host, the splenocyte frequency of Treg (Figure 4) was surprisingly 3.0-fold higher when the donor T cells were male rather than female. Moreover, while no detectable sex differences in IL-10-producing cells were observed in WT mouse spleens (Table S1), IL-10 producing cells were 1.7-fold higher after adoptive transfer of male compared to female T cells (Figure 4). Interestingly, the T cell subset pattern in the peripheral blood differed from the pattern in the spleen. We found a strong trend for a higher frequency of IL-10+ cells in PBMC from CD3F→Rag1−/−-M compared to CD3M→Rag1−/−-M mice [(% IL-10): Female, 5.83 ± 1.5 vs. Male, 4.3 ± 0.98; n=6]. Furthermore, the plasma levels of IL-10 were 12.7-fold higher after adoptive transfer of female compared to male T cells [(pg/ml): CD3F→Rag1−/−-M, 52.3 ± 23 vs CD3M→Rag1−/−-M, 4.1 ± 1.4; P<0.05 by t-test; n=6]. These findings suggest that the ability of female T cells to resist Ang II-induced increases in arterial pressure within the male host is due to an enhanced mobilization of anti-inflammatory T cells including IL-10-producing cells.
We also examined the proportion of recently-activated (CD69+) and memory (CD44+) T cells in the Rag1−/−-M spleen after adoptive transfer and Ang II infusion. Consistent with our finding of a higher reconstitution of CD3F cells than CD3M (Figure 3), we detected an 1.8-fold higher frequency of T cells that expressed CD69 in CD3F→Rag1−/−-M compared to CD3M→Rag1−/−-M (Figure 4), whereas after adoptive transfer of male T cells, we detected a small but significantly higher (1.1-fold) proportion of memory CD44+ T cells [% of splenocytes: CD3M→Rag1−/−-M, 68.6±2.0 vs. CD3F→Rag1−/−-M, 61.2±1.3; P<0.02 by t-test; n=6]. With the exception of higher CD69 and CD44 expression in female T cells, we did not observe evidence of similar differences in these T cell subsets in the spleens of male and female WT mice (Table S1).
Effect of the sex of the donor T cell on in vitro activated T cell cytokine responses to Ang II
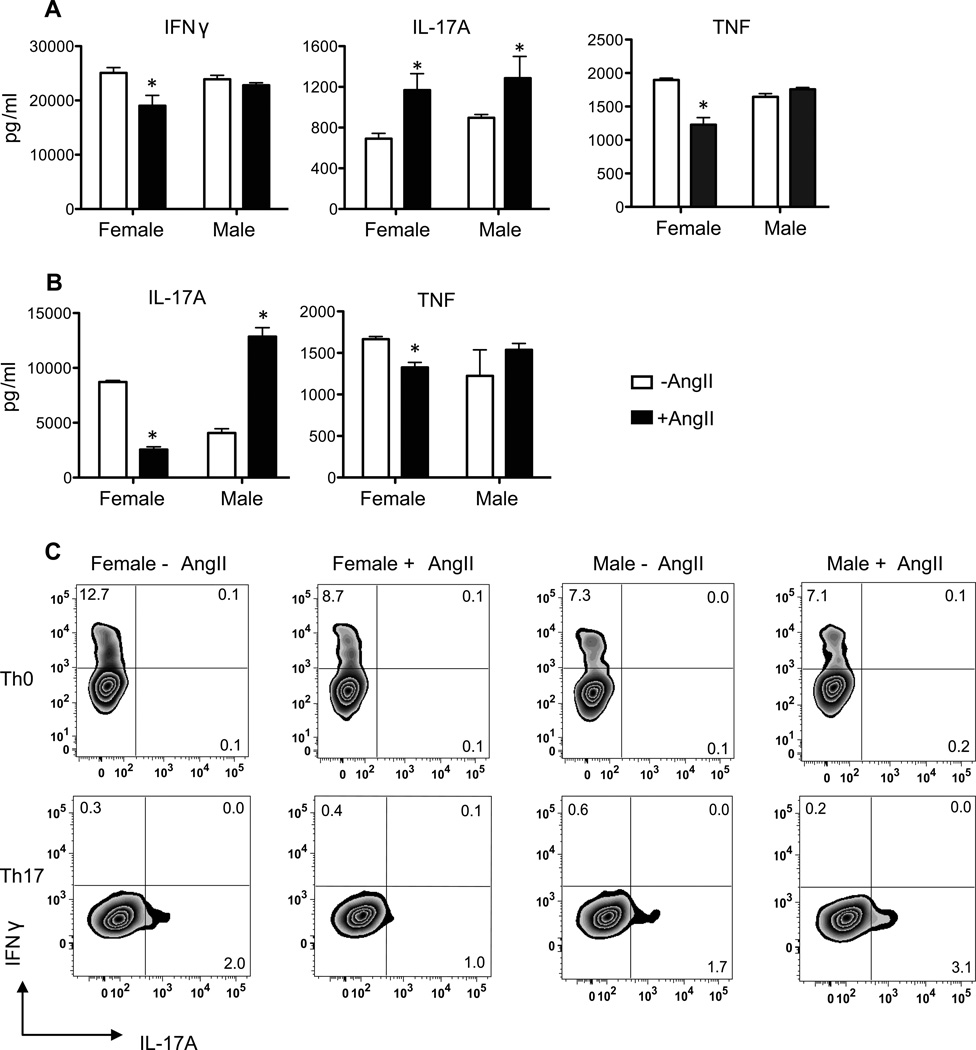
Our finding that male T cells produced more IL-17A and TNF after adoptive transfer than female T cells suggested that Ang II infusion induced a sex-specific expansion of pro-inflammatory T cells in the male Rag1−/− host. This expanded T cell subset could be T helper 17 (Th17) since IL-17A is the cytokine that defines Th17 and these CD4+ cells produce a number of other pro-inflammatory cytokines including TNF13. However, TNF is also a cytokine product of T helper 1 (Th1) cells. To assess these two possibilities, we activated lymph node cells from WT male and female mice in vitro with anti-CD3 and anti-CD28 (Th0 conditions) in the absence or presence of Ang II (100 nM) and then measured the production of TNF, IL-17A and IFNγ by responding T cells both by ELISA and by intracellular cytokine analysis (Figure 5 A & C). We chose this concentration of Ang II since it is nearly 100 times higher than the affinity of the angiotensin type 1 receptor (AT1R) for Ang II and because this concentration was shown to increase apoptotic Treg cells in cultured splenic T cells in an AT1R-dependent manner14. We also examined the effects of Ang II on T cell cytokine production under conditions that promote Th17 differentiation (i.e., high IL-6 and TGF-β, and in the presence of antibodies that neutralize IFNγ and IL-4) (Figure 5 B & D).
Figure 5. Effect of the sex of the T cell on Th17 polarizing T cell responses to Ang II.
Lymph node mononuclear cells were harvested from male and female WT mice (N=5/group) and stimulated with anti-CD3 and anti-CD28 under Th0 (no added cytokines) (A&C) or under Th17-polarization conditions (treatment with IL-6, TGFβ, anti-IL-4 and anti-IFNγ) (B&D) in the presence of vehicle (water) or Ang II (100 nM). Cytokine production by lymph node cells was measured using cytokine ELISA kits (A&B) and intracellular cytokine analyses (C&D). Cytokine levels are expressed as means ± SEM in triplicate cultures (A&B) or as the frequencies of cytokine producing cells in the live CD4+ gate in pooled cells from triplicate cultures (C&D); Cytokine gates were set using fluorescence minus one controls. Cytokine data were analyzed by two-way ANOVA to identify sex by treatment interactions. This was followed by one-way ANOVA and Tukey post-hoc test to determine individual differences between groups. *P≤0.05 vs. vehicle, same sex.
In the absence of Ang II, there was a tendency for increased frequencies of IFNγ-producing cells (Figure 5C) and lower production of IL-17A by CD4+ T cells (Figure 5A) in female compared to male T cells after antigen receptor and CD28 co-stimulation (i.e., under Th0 conditions). This finding is consistent with our previous reports demonstrating that female T cells exhibit a Th1-bias in cytokine production compared to male T cells7,8. Treatment of these cells with Ang II under Th0 conditions promoted T cell production of IL-17A in both sexes but decreased T cell production of IFNγ and TNF only in females (Figure 5A). Interestingly, under Th17 polarizing conditions, we observed a significant sex by Ang II interaction (two-way ANOVA, sex by treatment eta2 =83%; P<0.0001). Ang II selectively enhanced IL-17A levels in male T cells, but decreased the levels of this cytokine in female T cells (Figure 5B & D). Under these conditions, there was also a trend for a similar effect on TNF (Figure 5B). Two weeks of Ang II infusion in WT mice did not result in sex differences in splenic T cell cytokine mRNA expression (Table S2). Therefore, the enhanced IL-17A and TNF production detected in spleens of CD3M→Rag1−/−-M compared to CD3M→Rag1−/−-M post-Ang II treatment is likely due to sex-specific effects of Ang II on T cells post adoptive transfer.
Effect of the sex of the T cell donor on T cell infiltration into PVAT and kidney in the male Rag1−/− host after adoptive transfer followed by two weeks of Ang II infusion
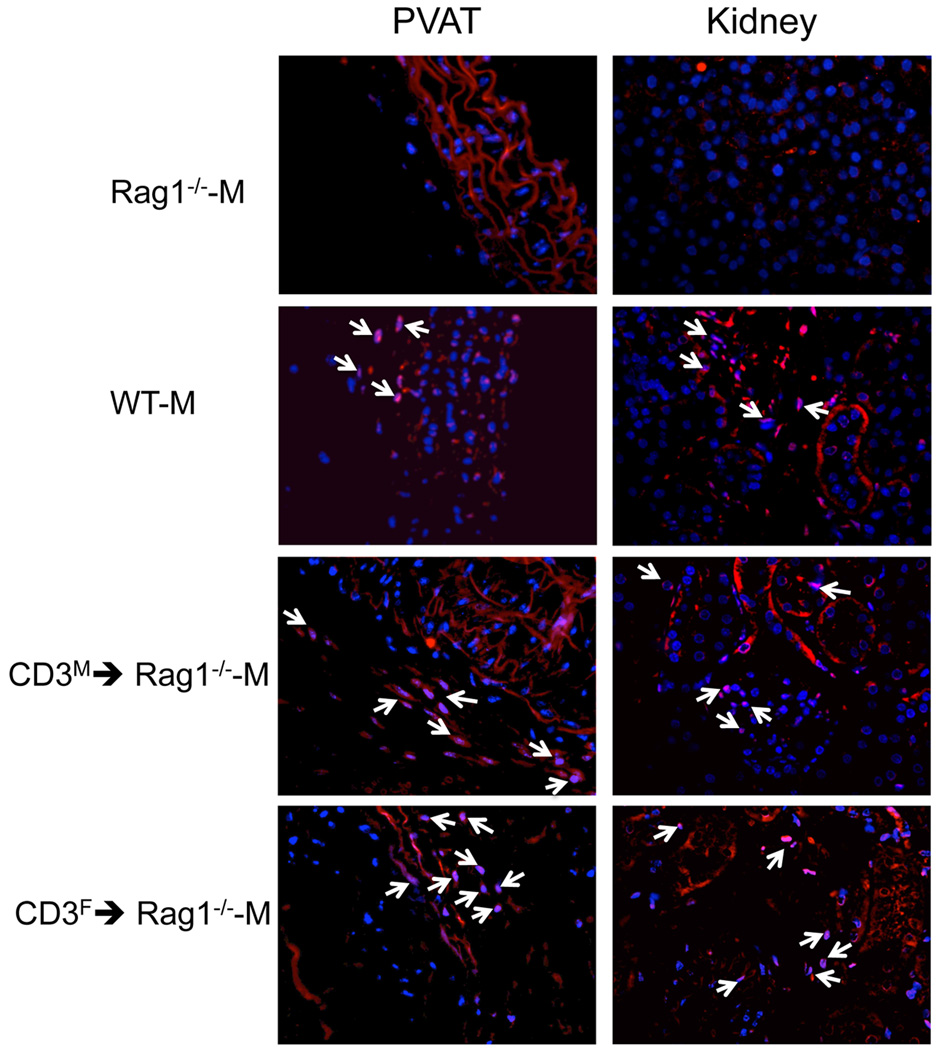
To determine if the sex of the T cell donor also impacts T cell infiltration into Ang II target tissues, we assessed the infiltration of CD3+, CD4+, CD8+ T cells, and CD4+FOXP3+ Treg into kidney and perivascular adipose tissue (PVAT) after adoptive transfer of male and female T cells into Rag1−/−-M mice followed by two weeks of Ang II infusion. Consistent with our finding of more T cells in the periphery after adoptive transfer of female compared to male T cells, more CD4+ T cells infiltrated the PVAT in CD3F→Rag1−/−-M compared to CD3M→Rag1−/−-M mice and there was a trend for more infiltration of CD8+ T cells as well; however, we did not find a sex difference in the frequency of CD4+FOXP3+ T cells in PVAT under these conditions and no sex differences in the infiltration of CD4+, CD8+, or Treg were observed in the kidney (Table 1, Figure 6). Thus, the pro-hypertensive potential of male T cells was not associated with either a lower number or with a reduced frequency of Treg cells in two tissues thought to be critically involved in the development of hypertension. These findings suggest that sex differences in Treg frequencies do not underlie the sex-disparate effects of T cells on Ang II-dependent hypertension in the male host; however, these results do not rule out a role for sex differences in other immune regulatory populations in these key Ang II target tissues.
Table 1.
Effect of the sex of the T cell donor on the number of CD4+, CD4+FOXP3+ Treg and CD8+ infiltrating T cells in PVAT and kidney tissue sections from male ag1−/−-M mice after adoptive transfer and Ang II infusion
| T cell Subset | WT-M + Veh (n=5) |
WT-M + Ang II (n=6) |
CD3M→Rag1−/−-M + Ang II (n=7) |
CD3F →Rag1−/−-M + Ang II (n=7) |
|---|---|---|---|---|
| PVAT | ||||
| CD4+ | 2.5 ± 0.4 | 20 ± 4† | 6.7 ± 2 | 14 ± 2* |
| CD4+FOXP3+ | 3.5 ± 0.4 | 11 ± 1† | 10 ± 1 | 9.0 ± 1 |
| CD8+ | 2.8 ± 0.8 | 22 ± 4† | 6.6 ± 2 | 9.3 ± 1* |
| Kidney | ||||
| CD4+ | 5.3 ± 1 | 14 ± 1† | 6.9 ± 1 | 6.4 ± 0.9 |
| CD4+FOXP3+ | 4.0 ± 1 | 12 ± 1 | 7.7 ± 1 | 7.4 ± 1 |
| CD8+ | 7.0 ± 1 | 19 ± 3† | 9.2 ± 0.4 | 11 ± 1 |
The data were analyzed by t-test;
p<0.05 compared to CD3M→Rag1−/−-M + Ang II, same tissue and genotype;
p<0.05 compared to vehicle (Veh), same tissue and genotype
Figure 6. Effect of the sex of the T cell donor on CD4+ T cell infiltration into PVAT and kidneys of Rag1−/−-M mice after adoptive transfer and Ang II infusion.
Shown are representative images of CD4+ immunofluorescence in PVAT (left panel) or kidney (right panel) sections from Rag1−/−-M male mice after adoptive transfer of T cells isolated from male or female WT mouse spleens and two weeks of Ang II infusion.
Effect of the sex of the T cell donor on renal cytokine mRNA expression in the male Rag1−/− host after adoptive transfer followed by two weeks of Ang II infusion
We measured the mRNA expression of various T cell-produced cytokines (IFNγ, IL-2, IL-17A, IL-10), tissue chemokines (MCP-1, CCL5), and Treg markers (FOXP3 and IL-10) in the kidney of Rag1−/−-M after adoptive transfer and two weeks of Ang II infusion (Table 2) to further investigate sex-specific T cell differences in Ang II target tissues. As compared to CD3M→Rag1−/−-M, CD3F→Rag1−/−-M mice displayed enhanced expression of most genes examined including those with both pro- (IFNγ, CCL5, IL-2) and antiinflammatory (FOXP3, IL-10, AT2R) functions; however, no sex differences in renal AT1R mRNA expression were observed (Table 2) or in splenic AT1R radioligand binding [Bmax (fmol/mg protein): CD3F→Rag1−/−-M, 54.7±11.7 vs CD3M→Rag1−/−-M, 45.4±9.6; ns by t-test; n=4]. Taken together, these studies suggest the major correlate of the prohypertensive properties of male T cells was that they were more likely to polarize towards pro-inflammatory Th17 cells while anti-inflammatory IL-10-producing T cells appeared to be retained in the spleen instead of being mobilized to the blood and Ang II target organs.
Table 2.
Effect of the sex of the T donor on renal mRNA expression in the male Rag1−/− host after adoptive transfer and Ang II infusion
| Renal mRNA | CD3M →Rag1−/−-M + Ang II Mean ± SE |
CD3F→Rag1−/−-M + Ang II Mean ± SE |
|---|---|---|
| FOXP3 | 0.99 ± 0.08 | 1.4 ± 0.1** |
| CCL5 | 1.5 ± 0.2 | 2.5 ± 0.4* |
| IFN-γ | 1.3 ± 0.3 | 3.1 ± 0.6* |
| MCP-1 | 0.83 ± 0.2 | 1.6 ± 0.3* |
| IL-2 | 1.6 ± 0.2 | 2.8 ± 0.3** |
| IL-6 | 0.64 ± 0.08 | 1.0 ± 0.1* |
| IL-10 | 0.59 ± 0.1 | 1.44 ± 0.2** |
| IL-17A | 0.95 ± 0.2 | 1.9 ± 0.4 |
| AT1R | 0.93 ± 0.06 | 1.1 ± 0.06 |
| AT2R | 0.50 ± 0.1 | 0.93 ± 0.1* |
The data were analyzed by t-test;
P<0.05,
P<0.01 compared to CD3M→Rag1−/−-M; n = 6/group.
Discussion
It has long been known that the immune system influences arterial pressure15. Hypertension is associated with inflammatory processes in key target tissues including the vasculature, kidney and brain and immunosuppression was shown to lower arterial pressure in the spontaneously hypertensive rat16 and in induced models of hypertension including a model of partial renal infarction17. In 2007, Guzik et al.3 demonstrated a key role for the adaptive immune system in susceptibility to Ang II-induced hypertension in male mice by showing that Rag1−/−-M mice exhibited a blunted hypertensive response compared to WT-M mice following infusion of Ang II at 490 ng/kg/min. Adoptive transfer of T, but not B, cells from donor WT-M mice restored the Ang II-induced hypertension in these Rag1−/−-M mice to levels observed in WT mice. Our study both confirms and extends these findings by demonstrating that the magnitude of this protection from Ang II-induced hypertension, due to T cell deficiency, varies with the dose of Ang II administered (Supplement Figure S1). The difference in BP between Rag1−/−-M and WT-M mice is even more exaggerated at a lower dose of Ang II (200 ng/kg/min), while at a higher dose (1000 ng/kg/min), Rag1−/−-M mice were no longer protected from Ang II-induced hypertension when compared to WT-M mice. Our dose response study indicates the immune system plays a critical role in the slow pressor response to Ang II. Given that the gradual increase in blood pressure at initial subpressor Ang II doses is thought to be due to hypertrophy of the systemic resistance vasculature18,19 and mediated by Ang II-induced oxidative stress20, our data suggest that the immune system affects these processes in vessel walls.
It is well known that hypertension occurs earlier in men than women and arterial pressure is higher in males than females in the vast majority of animal models of hypertension1. Less well understood is how male and female immune systems differentially contribute to blood pressure dysregulation. Here, we show that Rag1−/− mice no longer exhibit sex differences in the magnitude of Ang II-induced hypertension (Figure 1A & C). Under these same conditions, sex differences in HR responses between WT males and females were also diminished in Rag1−/− mice (Figure 1B & D). These findings provide evidence that sex differences in the immune system play a key role in the mechanisms explaining why males are more susceptible to hypertension than ovarian intact females since known sex differences in vascular and renal function and in sympathetic activity failed to maintain expected blood pressure differences between male and female Rag1−/− mice after Ang II infusion. These findings also suggest there is an increase in baroreflex sensitivity in the Rag1−/− mouse compared to WT mice and that this altered HR reflex is similar in male and female Rag1−/− mice. We previously showed that the Ang II-induced baroreflex bradycardia response was significantly less than that induced by phenylephrine while this blunted Ang II response was absent in intact females21. Thus taken together, these findings suggest T cells modulate baroreflex regulation of HR.
Our major finding from this study is that the magnitude of hypertension induced by a subpressor dose of Ang II was far greater if adoptive transfer of splenic T cells into Rag1−/−-M mice came from a male compared to a female WT host (Figure 2). Adoptive transfer of male and female donor T cells enabled the sex of the T cell to be isolated from sex differences inherent in innate immune cell types, as well as the vasculature, kidney and brain. Thus, the sex differences in MAP after Ang II infusion were due solely to the sex of the T cell donor. Since reconstitution of Rag1−/−-M mice with male T cells rescued the male-bias in development of hypertension in response to Ang II, we concluded that male T cells have a more pro-hypertensive phenotype than female T cells. It is also possible, however, that female T cells are not as effective in inducing hypertension because a subset of these T cells have a protective function against hypertension development.
There were a number of major sex differences in the T cell phenotype that we observed post-adoptive transfer of T cells in the Rag1−/−-M after Ang II infusion. First, female T cells showed signs of having a higher activation state in general, as compared to male T cells. We observed a higher number of CD4+ T cells in the spleens, peripheral blood, and PVAT of CD3F→Rag1−/−-M as compared to CD3M→Rag1−/−-M mice, indicating an enhanced expansion of female T cells within the lymphopenic space in the Rag1−/−-M mouse (Table 1, Figure 3). Further evidence of this was that a higher frequency of female than male T cells expressed the “recent-activation” marker CD69 in the spleens of Rag1−/−-M recipients (Figure 4). Finally, CD3F→Rag1−/−-M mice showed higher cytokine mRNA expression in the kidney and PVAT as compared to CD3M→Rag1−/−-M mice (Table 2). These sex differences in renal expression of FOXP3, CCL5, IFN-γ, MCP-1, IL-2 and IL-10 mRNAs were induced by Ang II, since WT male and female T cells did not exhibit these differences under basal conditions (Table S2).
In addition to the sex difference in activation state, we also observed evidence of a differently-Th1-biased inflammatory response in the CD3F→Rag1−/−-M and CD3M→Rag1−/−-M mice. CD3F→Rag1−/−-M mice displayed a gene signature that was consistent with the development of Th1-inflammation in the kidney, while males showed evidence of higher Th17-cytokine production in the spleen (Tables 1 and 2). A Th1 bias in cytokine production was also observed in WT female T cells when they were activated ex vivo with anti-CD3 and anti-CD28 (Figure 5). These findings of a differential production of Th1 and Th17 cytokines by male and female T cells confirm our previous report that female T cells have a higher propensity for Th1 cytokine production while male T cells tend to produce higher levels of IL-177.
These sex differences in T cell biology in our model could be due to several factors. First, it is known that the gonadal hormone milieu is important in specifying T cell phenotype in male and female T cells4. For example, androgens reduce the potential of male T cells to proliferate and produce Th1 cytokines7,8. Taken in this light, our finding that T cell phenotype sex differences were retained even 7–8 weeks after transfer into male Rag1−/− mice suggests that gonadal hormone exposure during development evokes changes in gene expression or epigenetic modifications that are stable and resistant to change after exposure to the alternative sex hormone milieu. An alternative explanation for the higher activation potential of female T cells is that a subset of these female T cells may have been activated by male Y-encoded antigens within Rag1−/−-M mice. While male T cells would have been educated in the thymus by exposure to Y encoded antigens during development and would be tolerant to them, female T cells would not be. Thus, female T cells may have become activated by Y encoded antigens after adoptive transfer of female T cells into the male host22. Finally, reports that the process of X-chromosome inactivation may be defective in T cells during inflammation23 raises the possibility that sex differences in the sex chromosome complement and differential expression of X or Y encoded genes contribute to sex differences in the hypertension phenotype between CD3F→Rag1−/−-M and CD3M→Rag1−/−-M. Indeed, we have previously shown sex chromosome effects contribute to sex differences in susceptibility to hypertension10.
Only a few of the immune features that differed between CD3M→Rag1−/−-M and CD3F→Rag1−/−-M mice correlated with hypertension development. First, male T cells exhibited a higher frequency of pro-inflammatory-producing T cell subsets including IL-17A and TNF-α in the spleen after adoptive transfer followed by two weeks of Ang II infusion (Figure 4). Furthermore, sex differences were observed in Ang II stimulated production of IL-17 by antigen-activated T cells in vitro (Figure 5). In the presence of cytokines (IL-6 and TGFβ) known to induce Th17 polarization, Ang II promoted IL-17A production in males but suppressed production of this cytokine in females. Together, our data support previous studies that IL-17 protein expression is increased in the aorta during hypertension and that Ang II-induced hypertension is not sustained in IL17A−/− knockout mice24. These latter studies also demonstrated that T cell infiltration is reduced in aortae from IL-17−/− mice after Ang II infusion24. The mechanisms by which IL-17 modulates BP are not completely understood though some studies suggest IL-17 mediates Ang II-dependent hypertension by activating the RhoA/Rho-kinase25 and prostaglandin I226 pathways resulting in superoxide production11. Other studies implicate involvement of TNF production by Th17 cells and TNF-dependent signaling via p38/MAP kinase27. It is unclear why we did not observe evidence of an enhanced Th17-inflammatory response in the kidney or PVAT in CD3M→Rag1−/−-M mice (Figure 6). One possibility is Th17 inflammation in these tissues occurred earlier during the two week course of Ang II infusion. Alternatively, Th17 inflammation may have occurred at another site that we did not investigate in our study, such as the brain.
The literature on TNF contributions to the development of hypertension is more complex. Higher circulating levels of TNF have been reported in patients with hypertension and reducing both systolic and diastolic blood pressure in these patients with the AT1R antagonist valsartan was shown to significantly reduce serum levels of TNF28. Furthermore, TNF has been implicated as a pathogenic cytokine in animal models of hypertension. Ang II-induced hypertension in Rag1−/−-M mice after adoptive transfer of male T cells increased T lymphocyte production of TNF while treatment with the TNF antagonist etanercept prevented the development of hypertension in these mice3.
Some other studies, however, do not support a role for TNF in hypertension. While the AT1R antagonist losartan significantly reduced arterial pressure in a study of patients with uncomplicated mild hypertension, there were no detectable effects on plasma levels of TNF29. Furthermore, TNF inhibition reduced renal injury in DOCA-salt hypertensive rats without any effect on blood pressure30 and no differences in circulating TNF were detected between the spontaneously hypertensive male rat and its Wistar Kyoto control31. Thus, the contribution of TNF to hypertension may depend upon the stage or state of hypertension and could be masked due to the presence of other contributing or co-linked factors such as IL-17 production by a TNF-producing Th17 cell.
The other immune feature that correlated with the pro-hypertensive male T cell was lower IL-10 mRNA expression in the kidneys and lower plasma levels of IL-10 in CD3M→Rag1−/−-M mice compared to CD3F→Rag1−/−-M mice after T cell adoptive transfer and two weeks Ang II infusion. IL-10 is produced by both Treg and other regulatory T cell and immune cell populations and has a well-established protective role against hypertension development in males. For instance, co-infusion of the anti-inflammatory cytokine IL-10 with Ang II significantly reduced the Ang II-induced increase in systolic BP in WT-M mice32. Moreover, systemic overexpression of IL-10 reduced BP in the stroke-prone spontaneously hypertensive male rat33 and the male Dahl salt-sensitive rat34 and overexpression of IL-10 specifically in the paraventricular nucleus of the male mouse, attenuated hypertension induced by chronic Ang II infusion35.
Reduced IL-10 expression in the kidney is likely not due to differences in Tregs, since neither the frequency of FOXP3+ T cells or FOXP3 mRNA expression were lower in this tissue in CD3M→Rag1−/−-M mice compared to CD3F→Rag1−/−-M mice (Table 2). IL-10 is produced by M2-type (anti-inflammatory) macrophages36 and also by other T cell types, including Tr1 cells, which is a type of T regulatory cell that only transiently expresses FOXP3, but produces high levels of IL-10, TGF-β and lytic molecules such as granzyme B and perforin37. In addition, T helper cells (Th1, Th17, and Th2 cells) all have the potential to produce IL-10 at late stages of the immune response. The production of IL-10 by these cells is thought to be a mechanism of resolving inflammation and limiting tissue damage. Therefore, our finding of lower IL-10 expression in the kidney after adoptive transfer of male compared to female T cells in the Rag1−/−-M may reflect less-effective “damage-control” mechanisms in the male compared to the female T cell population.
In conclusion, we have shown striking sex-specific differences in the T cell immune response within the male hormonal milieu that correlate with sex-specific T cell modulation of Ang II-dependent hypertension. Adoptive transfer of female T cells had no effect on Ang II-induced hypertension in the male Rag1−/− host. Thus, the male T cell population amplifies blood pressure responses to Ang II under conditions in which the female T cell population does not contribute to arterial pressure elevation.
Perspectives
These studies demonstrate that immune mechanisms, which contribute to hypertension in the male are not readily extrapolated to the female. Therefore, the role of one's sex needs to be considered in studies investigating immune mechanisms of hypertension to fully understand contributions of the adaptive immune response and the balance between pro- and anti-inflammatory cytokine signaling in hypertension if we are to exploit this knowledge for optimizing therapy in both men and women. These findings also underscore the need for investigators who study basic science mechanisms in male animals to acknowledge the caveat that mechanisms determined in male animal models of disease may not be relevant to females and thus, basic research into the physiology and pathophysiology of disease needs to be carried out in both sexes38.
Supplementary Material
Novelty and Significance.
What Is New?
Using a classic well-studied model of hypertension (i.e., Ang II infusion), the sex of the T cell was shown to be the determining biological factor in susceptibility to hypertension. That is, we show for the first time that the sex of the T cell can be the sole difference between a hypertensive and normotensive mouse. This is the first report demonstrating that CD3+ T cell augmentation of Ang II-induced hypertension is dependent upon the sex of the T cell. Female CD3+ T cells did not augment Ang II-induced hypertension under conditions in which male CD3+ T cells caused a robust increase in mean arterial pressure. Furthermore, we showed that the T cell inflammatory phenotype is sex-specific. Male T cells exhibited a greater T-helper (Th)-17-biased inflammatory profile than female T cells while female T cells displayed a greater Th-1-biased inflammatory profile compared to male T cells.
What Is Relevant?
This study demonstrates that the immune mechanisms reported in the literature to contribute to Ang II-dependent hypertension are, at least in part, sex-specific. Therefore, the role of one's sex needs to be considered in studies investigating immune mechanisms of hypertension to fully understand contributions of the adaptive immune response and the balance between pro- and anti-inflammatory cytokine signaling in hypertension if we are to exploit this knowledge for optimizing therapy in both sexes.
Summary
This study indicates that the sex of the T cell is a major biological factor in determining susceptibility to hypertension. Greater T cell infiltration into perivascular adipose tissue and the kidneys associated with increased pressor responses to Ang II if the T cell donor was male, but not female, and sex differences in T cell subset expansion and tissue infiltration were maintained for several weeks within the male host. Thus, the role of the immune system in blood pressure regulation markedly differs between the sexes. These findings provide an explanation for why females are protected from the onset of hypertension compared to men. This study also illustrates how basic science mechanisms conducted in males are not necessarily extrapolatable to the female. Thus, the adaptive immune response and role of pro- and anti-inflammatory cytokine signaling in hypertension is distinct between the sexes and needs to be understood to improve therapeutics for hypertension-associated disease in both men and women.
Acknowledgements
We thank Andrea Linares, Elizabeth Goldberg and Arline Joachim for assistance with the radioligand binding study.
Sources of Funding
This work was supported by NIH grants to HJ (AG-037832), KS (AG/HL-19291, AG-039779 & AG-16902) and RCS (Pilot Award from the Translational Technologies Component of the Georgetown, Howard Universities Center for Clinical and Translational Science (UL1TR000101) and a MedStar Health Research Institute-Georgetown University partnership grant to JGU & KS.
Footnotes
Disclosures
None
References
- 1.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differences. 2012;3:1–21. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DG, Guzik TG, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, Immunity and Hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 5.Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA, Clementi M, Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth M, McClellan B, Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–1225. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Nat Acad Sci USA. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)α expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- 13.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 14.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mian MO, Paradis P, Schiffrin EL. Innate immunity in hypertension. Curr Hypertens Rep. 2014;16(2):1–9. doi: 10.1007/s11906-013-0413-9. [DOI] [PubMed] [Google Scholar]
- 16.Dzielak DJ. Immune mechanisms in experimental and essential hypertension. Am J Physiol Regul Integr Comp Physiol. 1991;260:R459–R467. doi: 10.1152/ajpregu.1991.260.3.R459. [DOI] [PubMed] [Google Scholar]
- 17.White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron. 1964;1:93–102. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 18.Lever AF. Slow pressor mechanisms in hypertension: a role for hypertrophy of resistance vessels? J Hypertens. 1986;4:515–524. doi: 10.1097/00004872-198610000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Edgley A, Kett M, Anderson W. 'Slow pressor' hypertension from low-dose chronic angiotensin II infusion. Clin Exp Pharmacol Physiol. 2001;28:1035–1039. doi: 10.1046/j.1440-1681.2001.03590.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 21.Pamidimukkala J, Taylor JA, Welshons WV, Lubahn DB, Hay M. Estrogen modulation of baroreflex function in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R983–R989. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- 22.von Boehmer H, Turton K, Haas W. The role of the left end of the H-2b haplotype in the male-specific cytotoxic T cell response. Eur J Immunol. 1979;9:913–915. doi: 10.1002/eji.1830091115. [DOI] [PubMed] [Google Scholar]
- 23.Lu Q, Wu A, Tesmer L, Ray D, Yousif N, Richardson B. Demethylation of CD40LG on the inactive X in T cells from women with lupus. J Immunol. 2007;179:6352–6358. doi: 10.4049/jimmunol.179.9.6352. [DOI] [PubMed] [Google Scholar]
- 24.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704. doi: 10.1093/cvr/cvs422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Dowell DR, Huckabee MM, Newcomb DC, Boswell MG, Goleniewska K, Lotz MT, Toki S, Yin H, Yao S, Natarajan C, Wu P, Sriram S, Breyer RM, Fitzgerald GA, Peebles RS., Jr Prostaglandin I2 signaling drives Th17 differentiation and exacerbates experimental autoimmune encephalomyelitis. PLoS ONE. 2012;7(5):1–12. doi: 10.1371/journal.pone.0033518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber J, Tiriveedhi V, Takenaka M, Lu W, Hachem R, Trulock E, Patterson GA, Mohanakumar T. Inhibition of renin angiotensin aldosterone system causes abrogation of obliterative airways disease through inhibition of tumor necrosis factor-α-dependant interleukin-17. J Heart Lung Transpl. 2012;31:419–426. doi: 10.1016/j.healun.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe S, Okura T, Watanabe S, Fukuoka T, Higaki J. Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol. 2005;46:735–739. doi: 10.1097/01.fjc.0000185783.00391.60. [DOI] [PubMed] [Google Scholar]
- 29.Sardo MA, Castaldo M, Cinquegrani M, Bonaiuto M, Fontana L, Campo S, Campo GM, Altavilla D, Saitta A. Effects of AT1 receptor antagonist losartan on sICAM-1 and TNF-α levels in uncomplicated hypertensive patients. Angiology. 2004;55:195–203. doi: 10.1177/000331970405500212. [DOI] [PubMed] [Google Scholar]
- 30.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz-Rosa D, Oubina MP, Cediel E, de Las Heras N, Vegazo O, Jimenez J, Lahera V, Cachofeiro V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-κB/IκB system. Am J Physiol Heart Circ Physiol. 2005;288:H111–H115. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- 32.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, Takeuchi K, Katsura KI, Mizukami H, Kume A, Ookawara S, Ikeda U, Katayama Y, Ozawa K. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther. 2009;16:383–391. doi: 10.1038/gt.2008.151. [DOI] [PubMed] [Google Scholar]
- 34.Nonaka-Sarukawa M, Okada T, Ito T, Yamamoto K, Yoshioka T, Nomoto T, Hojo Y, Shimpo M, Urabe M, Mizukami H, Kume A, Ikeda U, Shimada K, Ozawa K. Adeno-associated virus vector-mediated systemic interleukin-10 expression ameliorates hypertensive organ damage in Dahl salt-sensitive rats. J Gene Med. 2008;10:368–374. doi: 10.1002/jgm.1166. [DOI] [PubMed] [Google Scholar]
- 35.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol. 2012;3(30):1–12. doi: 10.3389/fimmu.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandberg K, Verbalis JG. Sex and the basic scientist: is it time to embrace Title IX? Biol Sex Differences. 2013;4:1–4. doi: 10.1186/2042-6410-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.