Abstract
Immunity to viral infections in the model organism Drosophila melanogaster involves both RNA interference and additional induced responses. The latter include cellular mechanisms such as programmed cell death and autophagy, but also the induction of a large set of genes, some of which contribute to the control of viral replication and resistance to infection. This induced response to infection is complex and involves both virus-specific and cell-type specific mechanisms. We review here recent developments, from the sensing of viral infection to the induction of signaling pathways and production of antiviral effector molecules. Our current understanding, although still partial, validates the Drosophila model of antiviral induced immunity for insect pests and disease vectors, as well as for mammals.
Introduction
Viral infections represent a major burden for all organisms. Not only do they have an important impact on human health, as illustrated by epidemics such as HIV or flu, but they also represent a substantial economic burden, through their effects on crops and animals, including insects such as honeybees. Given that viruses replicate inside cells, the host discrimination between self and non-self presents particular challenges. In addition, the rapid evolution of viruses is manifest in viral mechanisms for suppressing host defenses. Investigating antiviral immunity in a wide range of organisms provides a broad view of the antiviral strategies adopted throughout evolution in different species and can reveal novel therapeutic targets.
An important facet of resistance to viral infections in insects is RNA interference (RNAi), which provides a sequence-specific intrinsic defense against viral infections [1]. In addition, viral infections can trigger cellular responses such as apoptosis or autophagy, and the induction of a range of anti-viral gene products. Whereas RNAi is triggered by double stranded (ds) RNA generated during viral replication, little is known about the receptors and mechanisms mediating viral sensing in insects. We therefore start this article by discussing the contribution of inducible responses to the control of viral infection in flies. The contribution of the NF-κB and STAT signaling pathways to antiviral responses and our current understanding of viral sensing in Drosophila is reviewed. Potential approaches for further research are identified.
Induced cell death and autophagy contribute to antiviral immunity
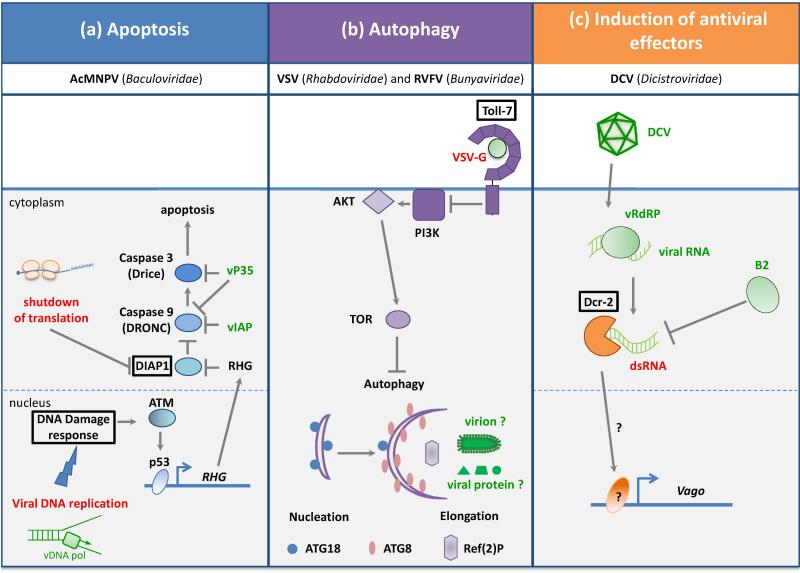
Two cellular mechanisms, apoptosis and autophagy, restrict viral replication and dissemination in insects (Fig. 1a, b). Apoptosis is triggered in lepidopteran and Drosophila cells in response to infection by the baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV), and this programmed cell death reduces viral production [2]. Apoptosis is also induced following infection by RNA viruses, such as the Flock House Virus (FHV), a RNA virus belong to the Nodaviridae family [3]. Caspases, the proteases that trigger apoptosis, are tightly regulated by the members of the IAP (inhibitor of apoptosis protein) family (e.g. DIAP1 in Drosophila) [4]. Four Drosophila genes clustered in a small region of the 3rd chromosome (RHG genes: reaper, hid, grim and sickle) encode antagonists of IAPs. Expression of these genes is induced in cells destined to die during development, or in response to genotoxic stress. Drosophila containing a deletion of the irradiation responsive enhancer region (IRER), which controls expression of the RHG genes, are deficient for apoptosis and are unable to restrain baculovirus or FHV infection in larvae or adults, respectively. Virus-induced apoptosis and control of viral load in infected flies is also impaired in mutants for the transcription factor p53, a key regulator of apoptosis [5••].
Figure 1. Induced antiviral responses in Drosophila.
Induction of specific antiviral pathways in Drosophila melanogaster triggered by different viruses. (a) Apoptosis is induced during the infection by the DNA virus AcMNPV. (b) Infection by VSV and RVFV, two negative sense single-stranded (ss) RNA viruses, trigger antiviral autophagy program. The inhibition of PI3K by Toll-7 and the contribution of Ref(2)P remain poorly characterized. (c) Induction of antiviral effectors during the infection of the positive ssRNA DCV is mediated by DExD/H box helicase Dicer-2, which senses dsRNA produced by the viral RNA-dependent RNA polymerase (vRdRP). The triggers are indicated in red, the sensors are boxed and the viral components are in green. See the text for details.
Autophagy is a conserved process that targets cytoplasmic contents to lysosomes for digestion. This process involves formation of a cup-shaped isolation membrane termed the phagophore, which sequesters a portion of the cytosol. During this process, adaptor proteins like Sequestosome 1 couple the autophagic cargo to the phagophore membrane [6•]. One hallmark of autophagy is the proteolytic processing and recruitment of the protein Atg8/LC3, to dot-like structures in the phagophore. In flies, autophagy participates in the control of two different arthropod-borne viruses (arboviruses), Vesicular Stomatitis virus (VSV) and Rift valley fever virus (RVFV) [7,8]. Interestingly, the gene ref(2)P, which encodes the fly homologue of Sequestosome-1, is an important restriction factor for the natural fly pathogen Sigma virus (SIGMAV), a member of the Rhabdoviridae, like VSV [9••]. This suggests that Drosophila Sequestosome-1 may interact with SIGMAV components, thus triggering autophagy. However, replication of SIGMAV is more efficient in flies homozygous for the sensitive allele of ref(2)P than in null mutant flies, indicating that ref(2)P can have a proviral, rather than antiviral, role [10].
Virus induced genes and the control of viral infection
Several genome-wide microarray analyses [11–16•] or cells [17–19] indicate that viral infections trigger a transcriptional response. Some overlap exists between the genes induced by viruses and bacteria or fungi. For example, antimicrobial peptides (AMPs) are upregulated following viral infection [20,21], although not as strongly as in the case of bacterial infections [22].
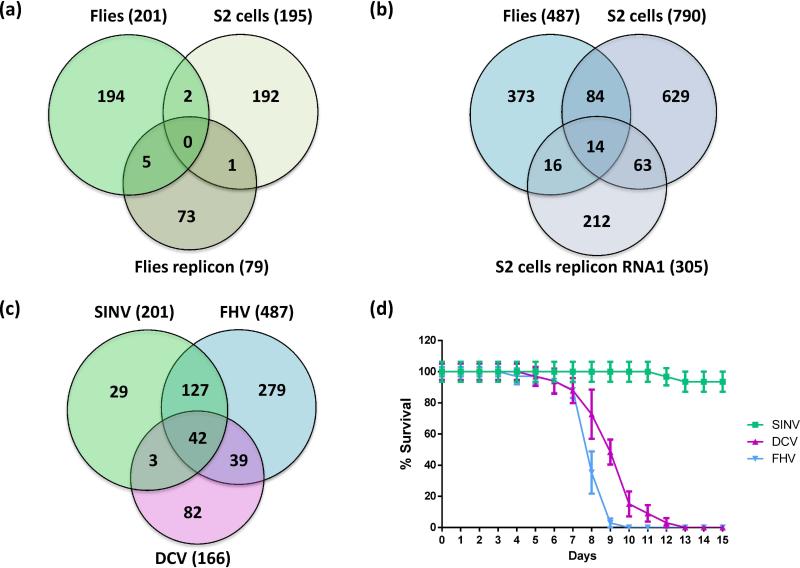
Understanding the induced response to viral infection is complicated by the poor reproducibility of the transcriptomic data, as shown for two viruses, SINV and FHV. Three independent studies analyzed gene expression in SINV infected wild-type flies [16•], in transgenic flies expressing a SINV-GFP replicon [15], and in infected tissue culture S2 cells [18]. As shown in Fig. 2a, there is little overlap between the induced genes reported in these studies. Besides methodological differences in RNA quantification and data analysis, these discrepancies probably reflect the response of the whole organism vs a more homogenous population of tissue culture cells, and differences between a bona fide infection and expression of a replicon in transgenic flies, which bypasses essential steps of the viral cycle. It is interesting to note however that four of the five genes induced by both the replicon and SINV infection in vivo (Vago, Frost, CG11671, CG10912) are induced by other viruses (DCV, FHV, SIGMAV), and that one of them (Vago) encodes a protein associated with antiviral activity (see below). In the case of FHV as well, differences between datasets monitoring host gene expression following infection in flies or tissue culture cells are apparent (Fig. 2b).
Figure 2. Inducible transcriptional responses to viral infection in Drosophila.
(a) Venn diagram of genes induced during an infection by SINV in adult flies [16], in S2 cells [18] and adult flies expressing a SINV replicon [15]. (b) Venn diagram of genes induced during an infection by FHV in adult flies [16], in S2 cells and S2 cells expressing a RNA1 FHV replicon [17]. (c) Venn diagram of genes induced during an infection by SINV, FHV and DCV in wild type Oregon-R adult flies [16]. (d) Survival of wild type flies Oregon-R following infection by the indicated viruses. For panels a-c, published data were compared using GeneVenn (http://genevenn.sourceforge.net/). The total of genes induced in each condition is indicated in parenthesis. See supplementary Table 1 for details.
These limitations notwithstanding, a comparative genome wide microarray analysis revealed that three distinct RNA viruses, DCV, FHV and SINV trigger overlapping but different responses in flies [16•] (Fig. 2c). This study identified 42 genes that are upregulated by all three viruses, but also revealed different patterns of genes induced by DCV on one hand, and FHV and SINV on the other (Fig. 2c, d). These altered expression patterns may reflect either differences in viral replication strategies (e.g. IRES-dependent translation for the picorna-like virus DCV) and tissue tropism, or co-evolution of DCV with its natural host. Interestingly, the existence of virus-specific induced responses is supported by genetic evidence (see below).
Antiviral effectors
One hallmark of the Drosophila response to bacterial/fungal infections is the secretion in the hemolymph of a cocktail of AMPs [23]. Two AMP coding genes, Diptericin (Dpt)B and Attacin (Att)C, are upregulated in transgenic flies expressing a SINV replicon. Silencing their expression led to a modest but significant increase in SINV replication, suggesting that DptB and AttC have non-redundant antiviral functions [15]. Another Drosophila study however reported that the single overexpression of any of the seven canonical AMPs is not sufficient to protect flies against infection by Drosophila X virus (DXV) [20]. In Aedes aegypti, a member of the Cecropin family was induced in the salivary glands following Dengue virus (DENV) infection. Biochemical experiments revealed that this Cecropin, in addition to antibacterial activity, also has antiviral activity against both DENV and Chikungunya virus [24].
Besides AMPs, the analysis of the transcriptome of virus-infected insects or cells provides an as yet underexploited list of other antiviral effector candidates. In Aedes mosquitoes, microarray analysis led to the identification of two Jak/STAT-regulated genes encoding proteins with antiviral activity, DENV restriction factors (DVRF) 1 and 2 [25]. Another virus induced factor, the thiol-ester containing protein TEPII, has been proposed to downregulate SINV infection in A. albopictus and Drosophila cell lines, through its effects on the processing of the non structural polyprotein [18]. Finally, in flies, one gene associated with antiviral activity is Vago [26]. Identified among the genes induced by DCV and SINV infection, Vago encodes a 18-kilodalton cysteine-rich polypeptide participating in the control of viral infection in the fat body. Interestingly, orthologues of Vago are induced by viral infection in Culex quinquefasciatus and Aedes albopictus cell lines. Furthermore, the Culex Vago orthologue opposes replication of the arbovirus West-Nile virus (WNV) in a cell line [27••,28]. In this ex vivo cellular model, CxVago, secreted upon WNV infection, appears to activate the Jak/STAT pathway, suggesting that CxVago is an antiviral cytokine.
Virus-specific contribution of NF-κB and STAT signaling pathways in antiviral defense
In Drosophila, two pathways, Toll and IMD, regulate transcription factors of the NF-κB family and production of AMPs during bacterial and fungal infections [29]. These pathways may also be involved in antiviral immunity. For example, flies mutant for some genes of the Toll pathway fail to control infection by DENV [30] or DXV [20], although they show normal resistance to SINV infection [21]. The Toll pathway is also involved in the control of DENV infection in the disease-vector mosquito, Aedes aegypti [31]. The IMD pathway may participate in the control of two other RNA viruses, Cricket paralysis virus (CrPV) and SINV [21,32].
An unbiased analysis of the promoters of the genes induced by DCV pointed to the involvement of another evolutionarily conserved innate immunity signaling pathway, namely Jak-STAT. Indeed, many DCV-induced genes contain STAT binding sites in their proximal promoters, and flies mutant for the gene hopscotch, which encodes the Drosophila Jak kinase, are more sensitive to DCV infection than wild-types [13]. Interestingly, resistance of hopscotch mutant flies to FHV and SINV, which induce a different transcriptional response than DCV (Fig. 2c) is not affected [16•]. These flies however have increased susceptibility to infection by another virus of the Dicistroviridae family, CrPV, indicating that the Jak/STAT pathway is activated upon sensing of a feature specific to picorna-like viruses. Indeed, expression of the cytokines Unpaired (Upd) 2 and 3, which activate the receptor Domeless and the Jak/STAT pathway, is strongly induced following infection by either virus [16•]. The Jak/STAT pathway also participates in the control of viral infection in Aedes and Culex mosquitoes [25,27••].
Sensing viral molecules by the innate immune system in insects
Innate immunity is activated by “microbial associated molecular patterns”, which are recognized by pattern recognition receptors (PRRs). A molecular pattern typical of viral infections is dsRNA generated during replication. The dsRNA binding protein B2 encoded by FHV prevents induction of the gene Vago [26], suggesting that this response to viral infection is triggered by dsRNA. Indeed, induction of Vago depends on Dicer-2, which triggers both RNAi and an inducible response upon sensing viral RNAs (Fig. 1c) [26,27••]. Interestingly, the N-terminal DExD/H box helicase domain of Dicer-2 is phylogenetically related to that of the vertebrate RIG-I-like receptors, implying that the involvement of this domain in antiviral innate immunity is an ancient evolutionarily conserved feature [26]. A key issue that is still not resolved is how Dicer-2 triggers gene expression after sensing viral RNAs. In Culex, Dicer-2 triggers a pathway including a TRAF protein and the NF-κB protein REL2 [28].
In mammals, some viral proteins are sensed by a different family of PRRs, the transmembrane Toll-like receptors (TLRs) [33]. In Drosophila, four Toll receptors function as cytokine receptors [34–36]. In particular, Toll-6, -7 and -8 function as neurotrophin receptors in the central nervous system [35,36]. In addition, Toll-7 has been proposed to recognize the glycoprotein G from VSV, or an as yet unidentified component of the RVFV virion to trigger autophagy (Fig. 1b) [8,37]. It is not yet known if other components of viral particles such as lipids or sugar moieties can be sensed by the innate immune system of the fly.
Sensing stress or altered cell metabolism in virus infected flies
Besides molecular patterns, alteration of cellular functions or danger signals can also activate innate immunity. In the case of baculovirus infection, two mechanisms account for the activation of caspases in infected cells: (i) inhibition of IAP synthesis, resulting from virus-induced shutdown of translation [3,38] and (ii) induction of IAP antagonist proteins (Fig. 1a) [5••]. Baculoviruses and other large DNA viruses trigger a DNA damage response (DDR), possibly as a result of the detection of short-lived stretches of single stranded DNA or dsDNA ends formed in the course of viral DNA replication. This leads to activation of the transcription factor p53 [39,40]. In turn, p53 induces expression of RHG proteins [5••]. The recent discovery that viral DNA forms are generated in FHV infected cells suggests that the DDR may contribute to the response to this RNA virus [41••].
Activation of autophagy by VSV involves the repression of the PI3 kinase/AKT/TOR pathway, which inhibits autophagy under normal conditions [7]. This pathway also plays a role in the control of SINV infection in insect cells [42]. In this case however, SINV activates the pathway, which leads to the phosphorylation of 4E-BP1 and increased translation of viral proteins. Hence, activation of the PI3K/AKT/TOR pathway favors replication of two different viruses in flies, although by distinct mechanisms: repression of autophagy for VSV vs activation of cap-dependent translation for SINV. Of note, a major physiological regulator of PI3K/AKT/TOR pathway is insulin, which also activates the ERK pathway. This pathway was recently shown to be activated by viral infection, and to repress replication of DCV, SINV and VSV by an unknown mechanism [43••]. These findings highlight the complex interconnection between pathways regulating cellular metabolism and antiviral immunity in Drosophila.
Finally, indirect evidence suggests that tissue damage and release of cell debris can induce an antiviral immune response. Indeed, the DCV and CrPV-induced cytokines Upd2 and -3 [16•], which activate the Jak/STAT pathway, are regulated by stress and tissue damage [44]. The biochemical nature of the stimulus activating expression of Upd cytokines in virus-infected flies remains unknown.
Conclusion and perspectives
Research on antiviral immunity in Drosophila over the past ten years has identified a broad range of responses besides RNA interference. In addition, tissue-specific regulation of the cellular environment [45], virus-specific restriction factors [9••,46–48] and endosymbiont bacteria such as Wolbachia [30] also have an important impact on the outcome of viral infections in flies. Recent findings indicate that the inducible antiviral responses in Drosophila are conserved in mosquito vectors of human disease. In addition, the involvement of an evolutionarily conserved DExD/H box helicase domain in sensing viral RNAs together with the roles of NF-κB and STAT signaling pathways imply that this conservation extends to mammalian systems, thus highlighting the potential of the Drosophila viral response model.
Recent progress has provided a number of assays (e.g. processing of caspases or Atg8, induction of gene expression) to monitor activation of antiviral pathways, and characterize the receptors sensing viral infection in flies. Strategies to identify these molecules include a combination of biochemical and genetic approaches, similar to those used for the identification of the PRRs detecting bacterial and fungal infections in insects [29]. For example, purification of proteins binding RNAs with characteristic viral features (e.g. dsRNA, 5’ triphosphate or 2’O-unmethylated extremities), may identify viral sensors, as recently reported in mammals [49]. Gain of function approaches, such as those used recently in mammalian cells to functionally characterize the dozens of interferon stimulated genes (ISGs), may also reveal new receptors (e.g. [50]). Finally, genetics is the greatest asset of Drosophila. The major limitation of this approach has been the time consuming positional cloning of mutant genes, but advances in sequencing technology have facilitated the identification of mutations. In consequence, genetic screens to identify mutants impaired in the control of viral infections holds great promise to identify novel host antiviral genes, and, in particular, to identify the receptor molecules that sense viral infection.
Supplementary Material
Acknowledgement
We thank Carine Meignin, Akira Tajima-Goto, Dominique Ferrandon and David Gubb for critical reading of the manuscript, and NIH (PO1 AI070167), ANR (DARVIS) and CNRS for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Nayak A, Tassetto M, Kunitomi M, Andino R. RNA interference-mediated intrinsic antiviral immunity in invertebrates. Curr. Top. Microbiol. Immunol. 2013;371:183–200. doi: 10.1007/978-3-642-37765-5_7. [DOI] [PubMed] [Google Scholar]
- 2.Schultz KLW, Friesen PD. Baculovirus DNA replication-specific expression factors trigger apoptosis and shutoff of host protein synthesis during infection. J. Virol. 2009;83:11123–11132. doi: 10.1128/JVI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Settles EW, Friesen PD. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. J. Virol. 2008;82:1378–1388. doi: 10.1128/JVI.01941-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orme M, Meier P. Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death. Apoptosis. 2009;14:950–960. doi: 10.1007/s10495-009-0358-2. [DOI] [PubMed] [Google Scholar]
- 5••.Liu B, Behura SK, Clem RJ, Schneemann A, Becnel J, Severson DW, Zhou L. P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster. PLoS Pathog. 2013;9:e1003137. doi: 10.1371/journal.ppat.1003137. An elegant in vivo study demonstrating that apoptosis contributes to the control of viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. A thorough review on the complex role of autophagy in host-pathogen interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano M-V, Yagi Y, Fitzgerald KA, Stanger BZ, Soldan SS, et al. Antiviral Autophagy Restricts Rift Valley Fever Virus Infection and Is Conserved from Flies to Mammals. Immunity. 2013 doi: 10.1016/j.immuni.2013.10.020. doi:10.1016/j.immuni.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Magwire MM, Fabian DK, Schweyen H, Cao C, Longdon B, Bayer F, Jiggins FM. Genome-wide association studies reveal a simple genetic basis of resistance to naturally coevolving viruses in Drosophila melanogaster. PLoS Genet. 2012;8:e1003057. doi: 10.1371/journal.pgen.1003057. This elegant genetic analysis pinpoints novel restriction factors for three Drosophila RNA viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carré-Mlouka A, Gaumer S, Gay P, Petitjean AM, Coulondre C, Dru P, Bras F, Dezélée S, Contamine D. Control of sigma virus multiplication by the ref(2)P gene of Drosophila melanogaster: an in vivo study of the PB1 domain of Ref(2)P. Genetics. 2007;176:409–419. doi: 10.1534/genetics.106.063826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordes EJ, Licking-Murray KD, Carlson KA. Differential gene expression related to Nora virus infection of Drosophila melanogaster. Virus Res. 2013;175:95–100. doi: 10.1016/j.virusres.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter J, Hutter S, Baines JF, Roller J, Saminadin-Peter SS, Parsch J, Jiggins FM. The transcriptional response of Drosophila melanogaster to infection with the sigma virus (Rhabdoviridae). PLoS ONE. 2009;4:e6838. doi: 10.1371/journal.pone.0006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler J-L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 14.Durdevic Z, Hanna K, Gold B, Pollex T, Cherry S, Lyko F, Schaefer M. Efficient RNA virus control in Drosophila requires the RNA methyltransferase Dnmt2. EMBO Rep. 2013;14:269–275. doi: 10.1038/embor.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Kingsolver MB, Avadhanula V, Hardy RW. An antiviral role for antimicrobial peptides during the arthropod response to alphavirus replication. J. Virol. 2013;87:4272–4280. doi: 10.1128/JVI.03360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, et al. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013;190:650–658. doi: 10.4049/jimmunol.1102486. A comparative analysis of the response of Drosophila to a panel of RNA and DNA viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castorena KM, Stapleford KA, Miller DJ. Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication. BMC Genomics. 2010;11:183. doi: 10.1186/1471-2164-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mudiganti U, Hernandez R, Brown DT. Insect response to alphavirus infection--establishment of alphavirus persistence in insect cells involves inhibition of viral polyprotein cleavage. Virus Res. 2010;150:73–84. doi: 10.1016/j.virusres.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Grant G, Sabin LR, Gordesky-Gold B, Yasunaga A, Tudor M, Cherry S. Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell Host Microbe. 2012;12:531–543. doi: 10.1016/j.chom.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatier L, Jouanguy E, Dostert C, Zachary D, Dimarcq J-L, Bulet P, Imler J-L. Pherokine-2 and -3. Eur. J. Biochem. 2003;270:3398–3407. doi: 10.1046/j.1432-1033.2003.03725.x. [DOI] [PubMed] [Google Scholar]
- 23.Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luplertlop N, Surasombatpattana P, Patramool S, Dumas E, Wasinpiyamongkol L, Saune L, Hamel R, Bernard E, Sereno D, Thomas F, et al. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following Infection with Dengue Virus. PLoS Pathog. 2011;7:e1001252. doi: 10.1371/journal.ppat.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler J-L. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat. Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 27••.Paradkar PN, Trinidad L, Voysey R, Duchemin J-B, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. This article demonstrates that the antiviral gene Vago is conserved and encodes a cytokine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paradkar PN, Duchemin J-B, Voysey R, Walker PJ. Dicer-2-Dependent Activation of Culex Vago Occurs via the TRAF-Rel2 Signaling Pathway. PLoS Negl Trop Dis. 2014;8:e2823. doi: 10.1371/journal.pntd.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imler J-L. Overview of Drosophila immunity: a historical perspective. Dev. Comp. Immunol. 2014;42:3–15. doi: 10.1016/j.dci.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Rancès E, Johnson TK, Popovici J, Iturbe-Ormaetxe I, Zakir T, Warr CG, O'Neill SL. The toll and Imd pathways are not required for wolbachia-mediated dengue virus interference. J. Virol. 2013;87:11945–11949. doi: 10.1128/JVI.01522-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MBA, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 34.Weber ANR, Tauszig-Delamasure S, Hoffmann JA, Lelièvre E, Gascan H, Ray KP, Morse MA, Imler J-L, Gay NJ. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat. Immunol. 2003;4:794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- 35.McIlroy G, Foldi I, Aurikko J, Wentzell JS, Lim MA, Fenton JC, Gay NJ, Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballard SL, Miller DL, Ganetzky B. Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol. 2014;204:1157–1172. doi: 10.1083/jcb.201308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, Cherry S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity. 2012;36:658–667. doi: 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandergaast R, Schultz KLW, Cerio RJ, Friesen PD. Active depletion of host cell inhibitor-of-apoptosis proteins triggers apoptosis upon baculovirus DNA replication. J. Virol. 2011;85:8348–8358. doi: 10.1128/JVI.00667-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang N, Wu W, Yang K, Passarelli AL, Rohrmann GF, Clem RJ. Baculovirus infection induces a DNA damage response that is required for efficient viral replication. J. Virol. 2011;85:12547–12556. doi: 10.1128/JVI.05766-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell JK, Friesen PD. Baculoviruses modulate a proapoptotic DNA damage response to promote virus multiplication. J. Virol. 2012;86:13542–13553. doi: 10.1128/JVI.02246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Goic B, Vodovar N, Mondotte JA, Monot C, Frangeul L, Blanc H, Gausson V, Vera-Otarola J, Cristofari G, Saleh M-C. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat. Immunol. 2013;14:396–403. doi: 10.1038/ni.2542. This article reveals the existence of viral DNA form in RNA virus infected flies, and its role in the control of viral infection. [DOI] [PubMed] [Google Scholar]
- 42.Patel RK, Hardy RW. Role for the phosphatidylinositol 3-kinase-Akt-TOR pathway during sindbis virus replication in arthropods. J. Virol. 2012;86:3595–3604. doi: 10.1128/JVI.06625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Xu J, Hopkins K, Sabin L, Yasunaga A, Subramanian H, Lamborn I, Gordesky-Gold B, Cherry S. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15025–15030. doi: 10.1073/pnas.1303193110. The first identification of an epithelial barrier to viral infection in flies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell. 2003;5:441–450. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 45.Eleftherianos I, Won S, Chtarbanova S, Squiban B, Ocorr K, Bodmer R, Beutler B, Hoffmann JA, Imler J-L. ATP-sensitive potassium channel (K(ATP))-dependent regulation of cardiotropic viral infections. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12024–12029. doi: 10.1073/pnas.1108926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magwire MM, Bayer F, Webster CL, Cao C, Jiggins FM. Successive increases in the resistance of Drosophila to viral infection through a transposon insertion followed by a Duplication. PLoS Genet. 2011;7:e1002337. doi: 10.1371/journal.pgen.1002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins NE, Faria VG, Nolte V, Schlötterer C, Teixeira L, Sucena E, Magalhães S. Host adaptation to viruses relies on few genes with different cross-resistance properties. Proc. Natl. Acad. Sci. U.S.A. 2014;111:5938–5943. doi: 10.1073/pnas.1400378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasunaga A, Hanna SL, Li J, Cho H, Rose PP, Spiridigliozzi A, Gold B, Diamond MS, Cherry S. Genome-wide RNAi screen identifies broadly-acting host factors that inhibit arbovirus infection. PLoS Pathog. 2014;10:e1003914. doi: 10.1371/journal.ppat.1003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habjan M, Hubel P, Lacerda L, Benda C, Holze C, Eberl CH, Mann A, Kindler E, Gil-Cruz C, Ziebuhr J, et al. Sequestration by IFIT1 impairs translation of 2’O-unmethylated capped RNA. PLoS Pathog. 2013;9:e1003663. doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoggins JW, MacDuff DA, Imanaka N, Gainey MD, Shrestha B, Eitson JL, Mar KB, Richardson RB, Ratushny AV, Litvak V, et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.