Abstract
Purpose
We determined the effect of an intensive lifestyle intervention on the prevalence, incidence and resolution of bothersome nocturia, increased daytime urinary voiding and urinary incontinence in overweight/obese men with type 2 diabetes after 1 year in the Look AHEAD trial.
Materials and Methods
A subset of male Look AHEAD participants was selected for this secondary data analysis. Overall 1,910 men with an average (mean ± SD) age of 59.9 ± 6.7 years and body mass index of 35.2 ± 5.5 kg/m2 were randomized to an intensive lifestyle intervention or diabetes support and education group. All participants self-reported information regarding incontinence, nocturia and daytime urinary voiding at entry and 1 year.
Results
After 1 year the intensive lifestyle intervention group lost significantly more weight than the diabetes support and education group (9.4% ± 7.0% vs 0.7% ± 4.5%, respectively; p <0.001). The odds of prevalent urinary incontinence at 1 year were reduced by 38% in the intensive lifestyle intervention group compared to the diabetes support and education group. The prevalence of urinary incontinence decreased from 11.3% to 9.0% in the intensive lifestyle intervention group and increased from 9.7% to 11.6% in the diabetes support and education group. The intensive lifestyle intervention group also had increased odds of urinary incontinence resolving (OR 1.93, 95% CI 1.04–3.59, p = 0.04 and 56.0% vs 40.7%, p = 0.03) and trend toward reduced odds of new onset, incident urinary incontinence (OR 0.66, 95% CI 0.42–1.02, p = 0.06) compared with the diabetes support and education arm. In contrast, no differences between intensive lifestyle intervention and diabetes support and education were seen at 1 year for frequency of nocturia or frequency of daytime voiding.
Conclusions
Intensive lifestyle intervention should be considered for the treatment of urinary incontinence in overweight/obese men with type 2 diabetes.
Keywords: diabetes mellitus, weight loss, urinary incontinence, nocturia
Obesity and type 2 diabetes have long been linked to sexual dysfunction in men,1 and are increasingly recognized as independent risk factors for lower urinary tract dysfunction2 such as nocturia3,4 and urinary incontinence.1 Given that obesity is a modifiable risk factor, weight loss interventions targeting physical activity and diet hold promise for LUTD prevention and treatment. While studies have shown the benefits of lifestyle intervention in reducing UI in overweight/obese females with type 2 diabetes,5,6 few studies have examined the impact of weight loss in the treatment of male LUTD. In 2 population based cohorts of males, weight changes (5% of baseline or greater) did not influence the onset or progression of LUTD.7 With respect to type 1 diabetes, in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study randomization to conventional vs intensive treatment did not reduce the risk of moderate to severe LUTD in men.8 However, only preliminary research has been conducted in obese men with type 2 diabetes,9 and whether lifestyle modification would have positive effects on LUTD in overweight/obese men with type 2 diabetes remains unclear.
In the current study we determined the effect of intensive lifestyle intervention focused on weight loss on the prevalence, incidence and resolution of UI, nocturia and daytime voiding in overweight/ obese men with type 2 diabetes. We hypothesized that lifestyle intervention vs control intervention would decrease the prevalence and incidence and increase the resolution rates of UI, bothersome nocturia and increased daytime voiding after 1 year of treatment.
Materials and Methods
Participants
Look AHEAD is a multicenter, randomized controlled study in overweight and obese individuals with type 2 diabetes which was designed to assess the long-term effects of an intensive lifestyle intervention weight loss program vs a diabetes support and educational control program on the primary outcome of cardiovascular morbidity and mortality.10 Participants were eligible if they were age 45 to 76 years and had a BMI of 25 kg/m2 or greater (greater than 27 kg/m2 if currently taking insulin). Exclusion criteria were high blood pressure (160/100 mm Hg or greater), elevated triglycerides (600 mg/dl or greater), uncontrolled hyperglycemia (hemoglobin A1c 11% or greater), a cardiovascular event within the last 3 months, significant renal or pulmonary disease, or conditions likely to limit adherence to the study intervention. Informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki and the guidelines of each center's institutional review board. Participants were randomly assigned within clinic to ILI or DSE with equal probability.
The Look AHEAD recruitment began in 2001 with planned followup until 2014 from 16 clinical centers. Recently the trial was stopped after the data and safety monitoring board found no significant differences between ILI and DSE in cardiovascular events, which was the primary end point of the trial.11
The ILI was designed to produce an average of 7% or greater weight loss at 1 year.12 During the first 6 months of the program the participants attended weekly meetings and were then seen 3 times per month for the following 6 months. The ILI included individual and group sessions.12 Calorie restriction was the primary action used to achieve weight loss. Participants were given calorie goals of 1,200 to 1,500 if they weighed less than 250 lb and 1,500 to 1,800 if they weighed more than 250 lb, with a maximum of 30% of calories from fat. ILI participants were instructed to consume a portion controlled low fat and low calorie diet that was supplemented with liquid and frozen meal replacements. Participants were encouraged to gradually increase exercise to 175 minutes per week using moderate intensity activities such as brisk walking. The DSE group was invited to 3 group sessions during year 1 to provide basic education about diet and physical activity as well as social support opportunities. Participants in both groups continued to be seen by their own personal physicians during the study period for ongoing health care.
Measures
Measures were completed at baseline and 1 year by trained assessors blinded to participant treatment group. Height and weight were measured in duplicate using a digital scale and standard stadiometer. Demographic and medical history information was obtained by standardized interviewer administered questionnaires. Urinary incontinence, nocturia and daytime voiding were assessed by self-report questions. To classify urinary incontinence the participants were asked if they had leaked even a small amount of urine or wet themselves in the last 7 days (yes/no). To further designate leakage type (stress or urge), participants who responded in the affirmative were asked how often they leaked with “an activity like coughing, sneezing, lifting or exercise” or had “an urge to urinate and couldn't get to the bathroom fast enough.” UI was classified based on the predominant type reported. Nocturia and daytime voiding were determined using a single item question on a continuous scale. Participants were asked, “in the past 7 days, on average, how many times each day have you had to go to the bathroom to urinate a) during the day? b) during the night?” We defined bothersome nocturia and increased daytime voiding to be present (yes/no) when participants urinated 2 or more times at night (nocturia) and 8 or more times during the day (daytime frequency), respectively.13,14
Statistical Analysis
As a sensitivity analysis, men who completed baseline and 1-year assessments were compared with those who did not complete 1-year assessments of relevant baseline variables and demographic characteristics using chi-square tests for categorical variables and ANOVA for continuous variables. A similar analysis was made comparing ILI and DSE participant baseline variables. Subsequent analyses included only participants who completed baseline and 1-year assessments.
For UI, nocturia and daytime urinary voiding we compared the proportion of men in the ILI and DSE groups with prevalent cases at baseline and 1 year, incident cases at 1 year and resolution at 1 year. Chi-square tests were used for unadjusted group comparisons. Multivariable logistic regression models were used to examine the effects of the intervention on 1-year prevalence, incidence and resolution of UI, nocturia and increased daytime voiding. Three models were reported. Model 1 was adjusted for center site only, except not for urgency predominant type incident cases of UI and resolved cases of increased daytime voiding due to scant cases at some sites. Model 2 was adjusted for center site plus baseline incontinence/nocturia/increased daytime voiding (for prevalence only), age, race/ethnicity, BMI, diabetes duration, Beck Depression Inventory and smoking status. The third model pertaining to nocturia included model 2 variables plus sleep apnea and alcohol use (ounces per week). All analyses were performed using SAS® version 9.3 for Windows®.
Results
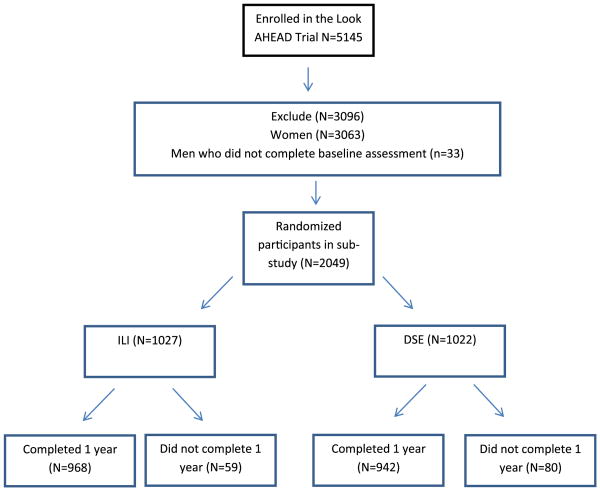
UI, nocturia and increased daytime voiding were assessed in 2,049 men at baseline (ILI 1,027 and DSE 1,022). At baseline no significant differences were observed between the ILI and DSE groups with respect to demographic characteristics. Of these men 1,910 (93%) completed the same measure at 1 year (see figure). The ILI and DSE groups had similar completion rates (94% vs 92%, p = 0.06). Compared to those who did not complete the questionnaires at 1 year, those who did had significantly better blood pressure and Beck Depression Inventory score, and were less likely to smoke at baseline (data not shown).
Figure. Flowchart of participants in male urinary symptom Look AHEAD substudy.
The demographic characteristics of the participants who completed the 1-year followup instrument are shown in the supplementary table (http://jurology.com/). The overall cohort had an average age of 59.9 ± 6.7 years and BMI of 35.2 ± 5.5 kg/m2. After 1 year of intervention the ILI group lost significantly more weight than the DSE group (9.4% ± 7.0% vs 0.7% ± 4.5%, p <0.001). At baseline the ILI and DSE groups had similar proportions of UI (11.3% vs 9.7%, p = 0.25), bothersome nocturia (37.7% vs 38.2%, p = 0.82) and increased daytime urinary voiding (17.3% vs 19.6%, p = 0.18).
Prevalent UI at 1 Year
The prevalence of UI at 12 months compared to baseline decreased from 11.3% to 9.0% in the ILI group and increased from 9.7% to 11.6% in the DSE group (table 1). Thus, relative to the DSE group the ILI group had 38% reduced odds of prevalent UI at 1 year (OR 0.62, 95% CI 0.43–0.88, p <0.01) after adjusting for relevant demographic and medical comorbidities (table 2).
Table 1. Number and proportion of men with UI, nocturia and increased daytime voiding.
| No./Total No. ILI (%) | No./Total No. DSE (%) | p Value (chi-square test) | |
|---|---|---|---|
| Weekly or greater UI: | |||
| Prevalence at 1 yr | 87/968 (9.0) | 108/942 (11.6) | 0.07 |
| Incidence at 1 yr* | 39/859 (4.5) | 54/851 (6.5) | 0.10 |
| Resolution at 1 yr† | 61/109 (56.0) | 37/91 (40.7) | 0.03 |
| Nocturia (2 or more times/night): | |||
| Prevalence at 1 yr | 404/968 (41.7) | 371/942 (39.4) | 0.30 |
| Incidence at 1 yr* | 118/603 (19.6) | 91/582 (15.6) | 0.08 |
| Resolution at 1 yr† | 79/365 (21.6) | 80/360 (22.2) | 0.85 |
| Increased daytime voiding (8 or more times/day): | |||
| Prevalence at 1 yr | 174/968 (18.0) | 176/942 (18.7) | 0.69 |
| Incidence at 1 yr* | 80/801 (10.0) | 82/757 (10.8) | 0.59 |
| Resolution at 1 yr† | 73/167 (43.7) | 91/185 (50.8) | 0.30 |
Among men without the condition at baseline.
Among men with the condition at baseline.
Table 2. Odds ratios of prevalent and incident weekly or more frequent urinary incontinence, urgency predominant incontinence, nocturia and increased daytime voiding at 1 year for ILI vs DSE.
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| No. Events | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Prevalent cases: | |||||||
| Urinary incontinence | 195 | 0.77 (0.57, 1.04) | 0.08 | 0.62 (0.43, 0.88) | <0.01 | ||
| Urgency predominant type | 103 | 0.53 (0.35, 0.80) | <0.01 | 0.36 (0.22, 0.60) | <0.0001 | ||
| Nocturia | 775 | 1.09 (0.91, 1.31) | 0.34 | 1.21 (0.96, 1.53) | 0.12 | ||
| Daytime voiding 8 or more times daily | 350 | 0.96 (0.76, 1.22) | 0.76 | 1.02 (0.79, 1.33) | 0.88 | ||
| Incident cases: | |||||||
| Urinary incontinence | 93 | 0.71 (0.46, 1.09) | 0.12 | 0.66 (0.42, 1.02) | 0.06 | ||
| Urgency predominant type | 45 | 0.40 (0.21, 0.76) | <0.01 | 0.37 (0.19, 0.72) | <0.01 | ||
| Nocturia | 209 | 1.31 (0.96, 1.77) | 0.08 | 1.26 (0.92, 1.73) | 0.15 | ||
| Daytime voiding 8 or more times daily | 162 | 0.93 (0.67, 1.29) | 0.68 | 0.93 (0.67, 1.30) | 0.66 | ||
| Resolved cases: | |||||||
| Urinary incontinence | 98 | 1.90 (1.05, 3.44) | 0.04 | 1.93 (1.04, 3.59) | 0.04 | ||
| Urgency predominant type | 54 | 3.10 (1.29, 7.42) | 0.01 | 2.93 (1.19, 7.24) | 0.02 | ||
| Nocturia | 159 | 1.14 (0.43, 3.05) | 0.95 | 0.96 (0.66, 1.40) | 0.84 | ||
| Daytime voiding 8 or more times daily | 164 | 0.80 (0.53, 1.22) | 0.30 | 0.79 (0.51, 1.21) | 0.27 | ||
The differences in prevalence at 1 year appeared largely due to differences in resolution and, to a lesser extent, new onset incidence rates. Specifically, among those with UI at entry, men in the ILI group had a greater likelihood of UI resolving (OR 1.93, 95% CI 1.04–3.59, p = 0.04, table 2) compared to the DSE arm (56.0% vs 40.7%, p = 0.03, table 1). Also, among those without UI at study entry the ILI group had a marginally significant reduction in odds of new onset UI developing (OR 0.66, 95% CI 0.42–1.02, p = 0.06, table 2) compared with the DSE arm (4.5% vs 6.5%, p = 0.10, table 1).
Examining UI prevalence by type, the odds of prevalent cases of urge incontinence at 1 year were lower in the ILI vs the DSE group (OR 0.36, 95% CI 0.22–0.60, p <0.0001). The prevalence of urge UI decreased from 6.9% to 4.0% in the ILI group and increased from 5.0% to 7.3% in the DSE group. Similarly ILI was associated with reduced incidence (OR 0.37, 95% CI 0.19–0.72, p <0.01) and increased resolution (OR 2.93, 95% CI 1.19–7.24, p = 0.02) of urge UI over 1 year. There were too few cases to model the odds of the prevalence, incidence and resolution of stress incontinence in these male subjects. We examined the relationship of weight loss and UI with multivariable logistic regression. We found that each 1 kg of weight loss reduced the odds of weekly incontinence by 3% (OR 0.97, 95% CI 0.95–0.99, p = 0.04).
Prevalent Nocturia and Increased Daytime Voiding at 1 Year
The frequency of increased nocturia (2 or more times per night) and increased daytime voiding (8 or more times per day) was similar in each of the groups at baseline and at the conclusion of the study period. In the multiple logistic regression model being in the ILI vs DSE group did not significantly reduce the odds of prevalent (OR 1.21, 95% CI 0.96–1.53, p = 0.12, table 2) or incident (19.6% vs 15.6%, p = 0.08, table 1) nocturia at 1 year. Similarly no significant treatment effect was observed for prevalence (OR 1.02, 95% CI 0.79–1.33, p = 0.88), incidence (OR 0.93, 95% CI 0.67–1.30, p = 0.66) or resolution (OR 0.79, 95% CI 0.51–1.21, p = 0.27) of increased daytime urinary voiding (table 2).
Discussion
In this study of overweight/obese men with type 2 diabetes, randomization to ILI was associated with a 38% reduced odds of having UI at 1 year relative to DSE. The fact that 10% of our cohort of obese men with type 2 diabetes reported at least weekly incontinence is noteworthy, as is the reduction in rates of incontinence at 1 year in the intervention group. The reduced prevalence of incontinence in those in our cohort randomized to ILI seemed to be associated primarily with increased rates of remission. However, there was also a trend toward lower rates of incident UI among ILI participants.
It is informative to compare these results with the UI female cohort in Look AHEAD.5 Among men and women the 1-year prevalent cases of UI were fewer in the ILI groups. Interestingly while ILI primarily improved resolution rates in men, it improved incident rates in women. Thus, weight loss interventions may be more effective for the treatment of UI in men and the prevention of UI in women with type 2 diabetes.
Also of note, urgency UI was more prevalent than stress UI in men. Moreover, ILI significantly improved remission rates and incident rates of urgency incontinence in these men. In contrast, randomization to ILI did not affect the prevalence of nocturia at 1 year or the incidence of new onset or resolution of bothersome nocturia and increased daytime voiding.
Mechanisms are unknown for why weight loss may reduce urgency UI in men. Diabetes and obesity are known risk factors for LUTD.2,3,15–18 The pathophysiology of LUTD in overweight/obese men with type 2 diabetes is likely complex and potentially has interrelated, overlapping or disparate mechanisms. Diabetes is known to affect the bladder and prostate. Diabetes results in elevated insulin levels which can stimulate or accelerate benign prostatic hyperplasia development.19 Higher serum concentrations of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 have been associated with an increased risk of benign prostatic hyperplasia diagnosis and surgery.20 Obesity and diabetes generated systemic inflammation may result in LUTD.21 Vascular insufficiency secondary to pelvic atherosclerotic disease caused by the metabolic syndrome may lead to ischemia and bladder dysfunction. Ischemia results in increased levels of transforming growth factor beta, leading to fibrosis, collagen deposition, smooth muscle atrophy, and subsequent reduced compliance and bladder size.22 Similar changes have been suggested in the prostate.23 In addition to inflammatory and hormonal factors, increased central obesity and the abdominal mass effect may contribute to LUTD. Urodynamic studies have verified higher filling and voiding pressures in obese individuals.1 Finally, obesity is a risk factor for sleep apnea, which can lead to increased nighttime voiding, and potentially to urinary urgency and frequency.24
To our knowledge this study was the first to examine the impact of intensive lifestyle intervention on LUTD in overweight/obese men with type 2 diabetes. Our findings suggest that in overweight/ obese men with type 2 diabetes a lifestyle modification program promoting weight loss should be considered a first line intervention to promote the resolution of UI. Given that the weight loss intervention did not improve nocturia and increased daytime urinary voiding, it is less clear how effective weight loss would be in preventing and treating LUTD other than urinary incontinence.
Our study should be considered within its limitations. These results may not be generalizable to the general population of overweight/obese people with type 2 diabetes or to overweight/obese people without diabetes. Our study participants were clinical trial volunteers and the exclusion criteria were relatively strict. We did not investigate how weight loss impacts other LUTDs such as post-void dribbling, weak stream or urinary urgency without UI. Our outcomes were based on self-report and were vulnerable to recall bias.25
In addition, our single item questions assessing daytime frequency of urination and nocturia differed slightly from previous self-report instruments, which may have impacted the validity of the responses.13,14 For example, our nocturia question addressed urinating at night but did not specify that the urination event caused a sleep interruption. Future investigations should include more extensive urinary outcome phenotyping at multiple points and objective measures such as voiding diaries. In addition, while the study completion rate was high (92% to 94%), completers had better blood pressure and depression scores, and were less likely to smoke compared to noncompleters. These differences may have biased our results. Lastly, we performed a subgroup analysis (male participants of Look AHEAD) which also could have introduced bias.26 However, given our large sample size and single subgroup, the subgroup analysis bias was likely minimal.
Conclusions
ILI was associated with reduced 1-year prevalent UI and improved resolution rates. Bothersome nocturia and increased daytime urinary voiding were not improved in the treatment group. Lifestyle interventions focused on weight loss should be considered for the treatment of UI in overweight/obese men with type 2 diabetes.
Supplementary Material
Acknowledgments
Supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135 and DK56992.
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases and its Intramural Research Program; National Heart, Lung and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; National Institutes of Health Office of Research on Women's Health; and the Centers for Disease Control and Prevention.
The Indian Health Service (IHS) provided personnel, medical oversight and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the IHS or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719), the Massachusetts General Hospital Mallinckrodt General Clinical Research Center (M01RR01066), the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520), the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140), the University of Pittsburgh General Clinical Research Center (M01RR000056 44) and National Institutes of Health (Grant DK 046204), the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corp.; Health Management Resources; LifeScan, Inc., a Johnson and Johnson Company; Optifast® of Nestlé HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Abbreviations and Acronyms
- BMI
body mass index
- DSE
diabetes support and education
- ILI
intensive lifestyle intervention
- Look AHEAD
Action for Health in Diabetes
- LUTD
lower urinary tract dysfunction
- UI
urinary incontinence
References
- 1.Natarajan V, Master V, Ogan K. Effects of obesity and weight loss in patients with nononcological urological disease. J Urol. 2009;181:2424. doi: 10.1016/j.juro.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 2.Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012;110:540. doi: 10.1111/j.1464-410X.2011.10819.x. [DOI] [PubMed] [Google Scholar]
- 3.Tikkinen KA, Auvinen A, Huhtala H, et al. Nocturia and obesity: a population-based study in Finland. Am J Epidemiol. 2006;163:1003. doi: 10.1093/aje/kwj139. [DOI] [PubMed] [Google Scholar]
- 4.Asplund R. Obesity in elderly people with nocturia: cause or consequence? Can J Urol. 2007;14:3424. [PubMed] [Google Scholar]
- 5.Phelan S, Kanaya AM, Subak LL, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol. 2012;187:939. doi: 10.1016/j.juro.2011.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360:481. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Sarma AV, Hollingsworth JM, et al. Associations between modest weight changes and onset and progression of lower urinary tract symptoms in two population-based cohorts. Urology. 2011;78:437. doi: 10.1016/j.urology.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Den Eeden SK, Sarma AV, Rutledge BN, et al. Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2009;32:664. doi: 10.2337/dc07-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 10.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 11.Després JP, Poirier P. Diabetes: looking back at Look AHEA–Degiving lifestyle a chance. Nat Rev Cardiol. 2013;10:184. doi: 10.1038/nrcardio.2013.16. [DOI] [PubMed] [Google Scholar]
- 12.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne KS, Payne C, Bhattacharyya SK, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7:455. doi: 10.1111/j.1524-4733.2004.74008.x. [DOI] [PubMed] [Google Scholar]
- 14.Tikkinen KA, Johnson TM, 2nd, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010;57:488. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Parsons JK, Sarma AV, McVary K, et al. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions. J Urol, suppl. 2009;182:S27. doi: 10.1016/j.juro.2009.07.086. [DOI] [PubMed] [Google Scholar]
- 16.Sarma AV, Wei JT, Jacobson DJ, et al. Comparison of lower urinary tract symptom severity and associated bother between community-dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men's Health Study. Urology. 2003;61:1086. doi: 10.1016/s0090-4295(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 17.Dahle SE, Chokkalingam AP, Gao YT, et al. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002;168:599. [PubMed] [Google Scholar]
- 18.Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 19.Hammarsten J, Hogstedt B. Clinical, anthropometric, metabolic and insulin profile of men with fast annual growth rates of benign prostatic hyperplasia. Blood Press. 1999;8:29. doi: 10.1080/080370599438365. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011;21:1. doi: 10.1097/MOU.0b013e32834100c9. [DOI] [PubMed] [Google Scholar]
- 21.Rohrmann S, De Marzo AM, Smit E, et al. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III) Prostate. 2005;62:27. doi: 10.1002/pros.20110. [DOI] [PubMed] [Google Scholar]
- 22.Azadzoi KM, Tarcan T, Siroky MB, et al. Atherosclerosis-induced chronic ischemia causes bladder fibrosis and non-compliance in the rabbit. J Urol. 1999;161:1626. [PubMed] [Google Scholar]
- 23.Kozlowski R, Kershen RT, Siroky MB, et al. Chronic ischemia alters prostate structure and reactivity in rabbits. J Urol. 2001;165:1019. [PubMed] [Google Scholar]
- 24.Kemmer H. The relationship between sleep apnea and overactive bladder. Curr Urol Rep. 2009;10:448. doi: 10.1007/s11934-009-0071-2. [DOI] [PubMed] [Google Scholar]
- 25.Gopal M, Sammel MD, Pien G, et al. Investigating the associations between nocturia and sleep disorders in perimenopausal women. J Urol. 2008;180:2063. doi: 10.1016/j.juro.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis SC, Warlow CP. How to spot bias and other potential problems in randomised controlled trials. J Neurol Neurosurg Psychiatry. 2004;75:181. doi: 10.1136/jnnp.2003.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.