Abstract
Nitric oxide (NO) can be generated by two-step reduction pathway in which nitrate is converted first into nitrite and then into NO via several mechanisms, as well as from arginine by endogenous nitric oxide synthase (NOS). We have recently shown that nitrite ions in the presence of erythrocytes inhibit platelet aggregation and activation, as measured by aggregometry and flow cytometric analysis of P-selectin, through its reduction to NO under partially deoxygenated conditions. In the current study, we investigated how nitrite may affect overall clotting processes via modulating platelet function using thrombelastography (TEG). We measured three major TEG parameters, reaction time (R, time to initial fibrin formation), α angle (velocity of clot growth) and maximum amplitude (MA, maximum clot strength) using blood from healthy volunteers. An NO donor (DEANONOate) showed inhibitory effects on all TEG parameters in platelet rich plasma (PRP) and whole blood, resulting in delayed R, decreased angle, and reduced MA in a dose dependent manner. Nitrite ions also exhibited inhibitory effects in whole blood at 20% hematocrit, and this was greatly enhanced under hypoxic conditions, being demonstrable at 0.1 μM concentration. Neither compound changed any TEG parameters in plasma. Our results suggest that nitrite affects overall blood clotting and that TEG may be used to follow this process. Further the physiological effects of factors which determine NO bioavailability, such as endogenous levels of blood and tissue nitrite, may be useful as biomarkers for predicting hemostatic potential.
Keywords: nitric oxide, nitrites, hypoxia, platelet, erythrocytes, thrombelastography
Introduction
The physiological regulation of platelet reactivity in blood vessels is tightly controlled by balancing prothrombotic and antithrombotic signals through various mechanisms to maintain hemostasis. Nitric oxide (NO), which is a gaseous signaling molecule that can exert various physiological functions, is one of the potent inhibitors of platelet function. Numerous studies since the 1980's showed that NO inhibits platelet aggregation and adhesion to the endothelium via activation of soluble guanylyl cyclase (sGC) and subsequent cyclic guanosine monophosphate (cGMP) production resulting in mobilization of Ca2+ flux [1-3]. NO is generated from L-arginine and molecular oxygen by the action of nitric oxide synthase (NOS) [4]. In addition to this endogenous NO generation mechanism, another alternative pathway for NO production, which involves serial reductive pathways of nitrate, is now understood [5]. A diet rich in vegetables is a good source of inorganic nitrate. Once nitrate is absorbed from the diet, it can be reduced to nitrite mainly by bacterial nitrate reductases in the oral cavity [6] and nitrite can be further reduced to NO in blood and tissues by various mechanisms utilizing deoxyhemoglobin [7,8], deoxymyoglobin [9,10], xanthine oxidase [11,12], and non-enzymatic reduction in the presence of protons [13,14] or vitamin C [15]. These reductive pathways are prominently enhanced under hypoxic conditions making them a good backup system for the shutdown of oxygen-dependent NOS activity under hypoxic situations. Several animal studies showed the therapeutic effects of inorganic nitrite and nitrate in ischemic injury models, which provides compelling evidence of nitrite and nitrate contribution to NO bioavailability [5].
We recently demonstrated that nitrite ions could inhibit aggregation and activation of human platelets in the presence of erythrocytes through its reduction to NO and this effect was promoted at lower oxygenation where deoxyhemoglobin efficiently reduces nitrite to NO [16]. Moreover, we showed in mice that blood nitrite levels are positively correlated with tail bleeding time and inversely correlated with platelet aggregation and granule release upon membrane receptor activation by adenosine diphosphate (ADP) and collagen [17]. Taken together, these results suggest that nitrite might play a critical role in regulating platelet reactivity and overall blood clotting upon its reduction to NO in blood vessels especially under hypoxic conditions.
Regulation of platelet reactivity by NO in hemostatic processes is of importance for vascular homeostasis, however the potential effect of nitrite on clotting pathways has not been explored in detail. It is crucial to understand how nitrite may affect the dynamics of hemostasis in vessels and what is its physiological role in regulating platelet reactivity since it acts as a NO precursor in blood. In the current study, to explore the contribution of nitrite to regulating overall processes of coagulation, we employed thrombelastography (TEG) which is a method widely used in several clinical settings during surgery to monitor hemostasis. TEG measures viscoelastic changes of clotting blood with time assessing coagulation dynamics from the initiation of fibrin formation to clot firmness [18]. We compared DEANONOate (an NO donor) and nitrite effects on three major TEG parameters, reaction time (R, the time until initial fibrin formation), α angle (an indicator of clot growth velocity) and maximum amplitude (MA, clot strength) in platelet rich plasma or 20% hematocrit blood, to simulate blood found in the microcirculation, obtained from healthy volunteers. We observed an inhibition in coagulation by DEANONOate in both platelet rich plasma and whole blood, but nitrite effect was noticeable only in whole blood but this was accentuated in hypoxic conditions. These results suggest that nitrite might play a critical role in modulating hemostatic processes once it is converted to NO by deoxyhemoglobin in blood in the microvasculature.
Materials and methods
Ethics statement
This study was conducted with a clinical protocol reviewed by the NIDDK Institutional Review Board at NIH. Before inclusion in the study, informed written consent was obtained from each subject.
Reagents
Sodium nitrite, DEANONOate and 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) were purchased from Sigma (St Louis, MO). PlateletMapping® Assay kit and Biological QC Level I/II kit were purchased from Haemonetics (Braintree, MA).
Blood collection
Venipuncture was done in healthy volunteers (aged 23-49 years) using a 21-gauge butterfly needle into vacutainer tubes containing sodium citrate or sodium heparin (Becton Dickinson, Franklin Lakes, NJ). The first 3 ml of blood was discarded. Blood was kept in a polypropylene tube with a dual-position snap cap (Becton Dickinson, Franklin Lakes, NJ) for room air equilibration unless deoxygenation was performed. The pO2 of blood was maintained at 100.5 ± 11.1 mmHg under room air condition and the oxygenation was measured using a blood gas analyzer (ABL80 FLEX CO-OX, Radiometer, Westlake, OH). All experiments were completed within 4 hours after blood withdrawal.
Blood sample preparation
To obtain platelet-free plasma, whole blood was first centrifuged at 1,600 g for 15 min to get platelet-poor plasma. Then the platelet-poor plasma was filtered using a syringe filter unit (0.22 μm, Millipore, Billerica, MA) to remove remaining platelets. Platelet numbers were under the detection ranges with an analyzer (CELL-DYN 3700, Abbott, Abbott Park, IL) after filtration. For platelet-rich plasma, whole blood was centrifuged at 120 g for 15 min and the upper phase was taken. To prepare blood with different hematocrit, whole blood was diluted with plasma (vol/vol) resulting in 5, 10, 20 and 30% hematocrit respectively. Whole blood of all volunteers participated in this study had an average hematocrit of 37.4 ± 4.6% and this was considered 40% hematocrit.
Preparation of deoxygenated blood
Whole blood in a glass Erlenmeyer flask with a stopper was deoxygenated by blowing helium gas into the flask with gentle stirring for 30min at room temperature. The pO2 was checked using a blood gas analyzer (ABL80 FLEX CO-OX, Radiometer, Westlake, OH) after deoxygenation and the average value was 37.4 ± 12.4 mmHg. The deoxygenated blood was kept in the flask and the stopper was opened only in a glove box for further manipulation.
Evaluation of coagulation by thrombelastography (TEG)
TEG experiments were performed at 37°C using a Haemostasis Analyzer (TEG®5000, Haemonetics, Braintree, MA) according to the manufacturer's guidlines. The TEG analyzer was calibrated and evaluated daily by running quality control samples before experimental sample analysis. For platelet-free plasma samples, 1 mL of plasma was mixed with kaolin (Cat.No.6300, Haemonetics, Braintree, MA). Then a 340 μL volume of kaolin-activated plasma was immediately added to the pre-warmed TEG cups which contained 20 μL of 0.2 M CaCl2 to initiate coagulation. For heparinized blood or platelet-rich plasma, 360 μL of sample was added into the cups and mixed with activator and adenosine diphosphate (ADP) or arachidonic acid (AA) (2 μM and 1 mM respectively, PlateletMapping® assay kit, Cat.No.07-014, Haemonetics, Braintree, MA) to induce platelet activation. DENONOate or nitrite was pre-incubated with plasma or blood at 37°C for 5 min before induction of coagulation.
Statistics
Statistics were analyzed using ANOVA (p-value<0.05, statistical significant) with Origin 8 (Origin Lab Corporation, Northampton, MA). Data shown are presented as the mean ± standard deviation (SD).
Results
NO does not alter intrinsic coagulation pathway
To examine whether NO could directly influence the intrinsic coagulation pathway, we analyzed coagulation processes initiated by kaolin and calcium in platelet-free citrated-plasma using TEG (Fig. 1A). The NO donor, DEANONOate, did not change any of the three major TEG parameters, R, α angle and MA, from 0.01 to 1 μM suggesting that NO itself at physiological concentrations does not interfere with clotting factor pathways initiated through factor XII.
Figure 1.
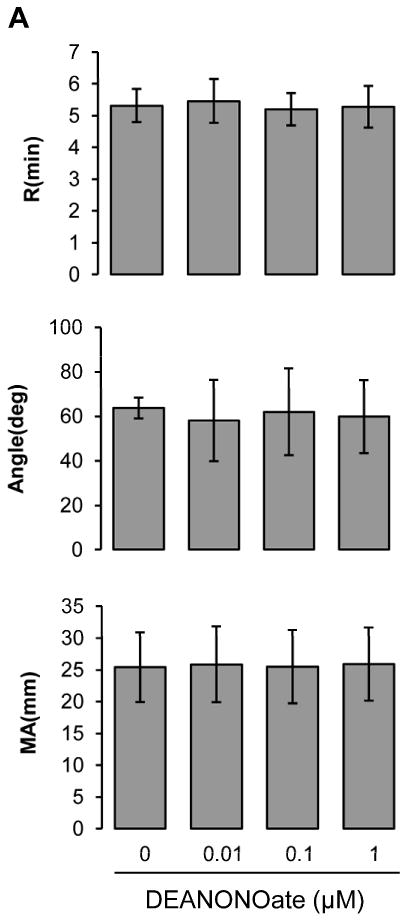
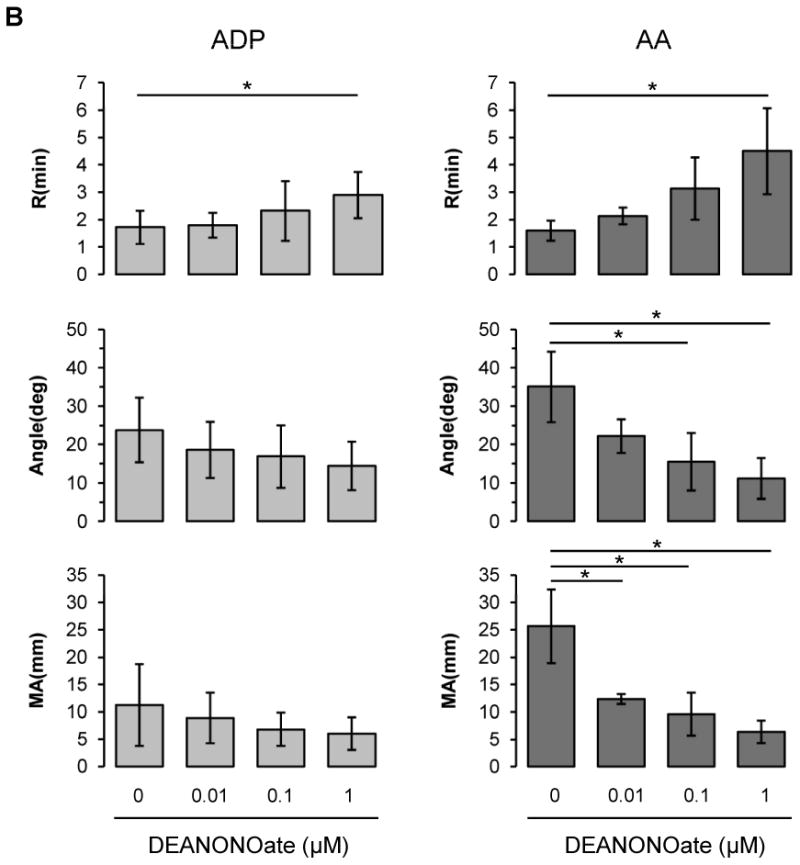
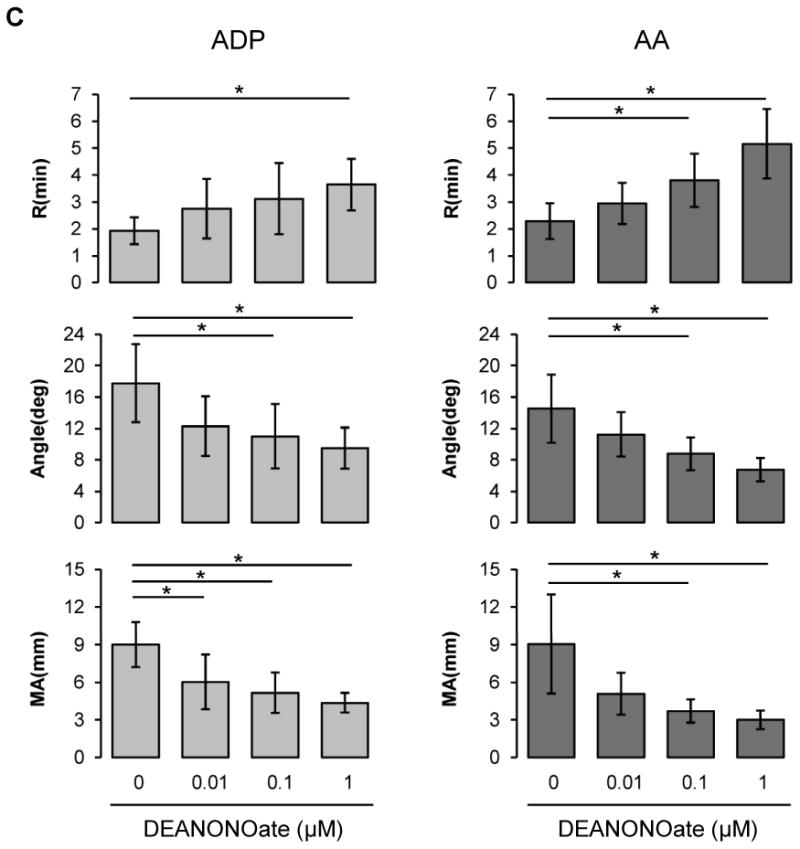
Influences of DEANONOate on coagulation processes in platelet-free plasma (A), platelet-rich plasma (B) and blood at 20% hematocrit (C). Platelet-free plasma was preincubated with DEANONOate (0.01 ∼ 1 μM) for 5 min, then 1 mL of sample was mixed with kaolin. Immediately 0.34 mL was taken and added to a cup containing 20 μL of 0.5 M CaCl2 (A, n=5). For platelet-rich plasma and 20% hematocrit blood, ADP (2 μM) or AA (1 mM) together with activator F was used to induce the coagulation pathway [B; n=5 (ADP), n=3 (AA), C; n=5]. Data are means ± standard deviation. *P < 0.05 compared with control.
NO inhibits clotting in platelet rich plasma and whole blood
Although NO did not affect intrinsic coagulation pathway (Fig. 1A), NO is well known to inhibit platelet aggregation and adhesion. To determine if NO affects coagulation in platelet rich plasma or in whole blood by modulating platelet function, we assessed coagulation in the presence of DEANONOate in platelet rich plasma or in blood at 20% hematocrit (Figs 1B and 1C), a value which we had previously shown to give maximum effects in aggregometry studies. Adenosine diphosphate (ADP) and arachidonic acid (AA) were used to activate platelet membrane receptors, P2 purinergic receptor and thromboxane receptor respectively. In platelet rich plasma, for both ADP and AA, NO inhibited clotting processes in a dose-dependent manner resulting in a prolonged R, decreased α angle and reduced MA, although the AA effect was more pronounced (Fig. 1B). In whole blood diluted with plasma to achieve 20% hematocrit, which best reflects the microcirculation environment, NO also caused inhibition of all three TEG parameters in a dose-dependent manner in response to ADP and AA (Fig. 1C).
Nitrite does not affect clotting processes in platelet rich plasma
Since nitrite is a precursor of NO in biological system, we sought to determine whether nitrite has an impact on coagulation in platelet rich plasma (Fig. 2A). Nitrite at physiological concentrations (0.1 ∼ 10 μM) did not show any inhibitory effects on TEG parameters in platelet rich plasma after ADP or AA stimulation implying that nitrite itself does not affect platelet reactivity or the coagulation pathway.
Figure 2.
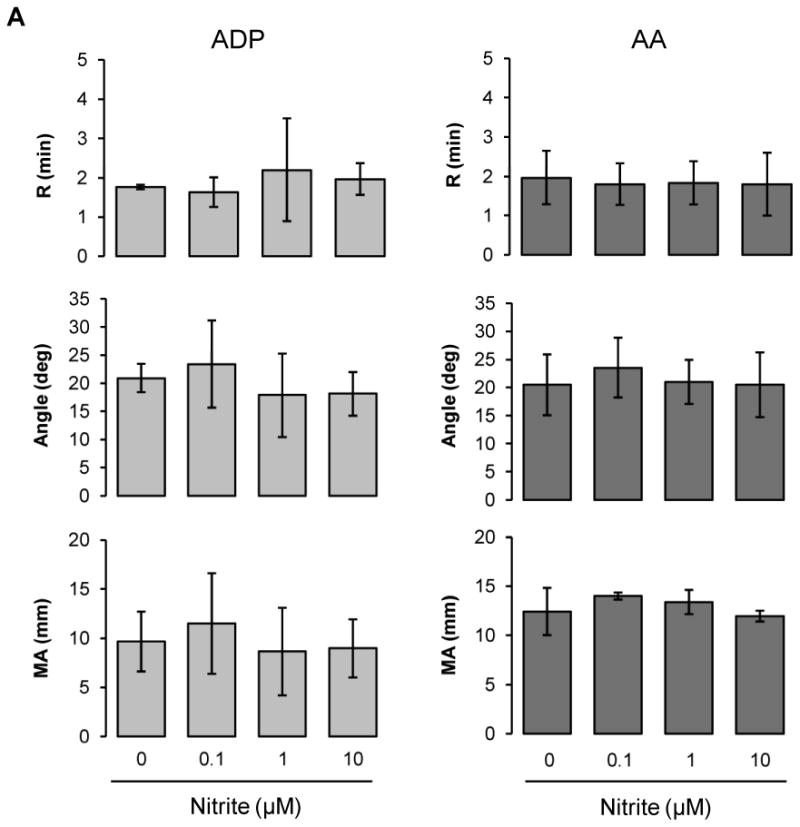
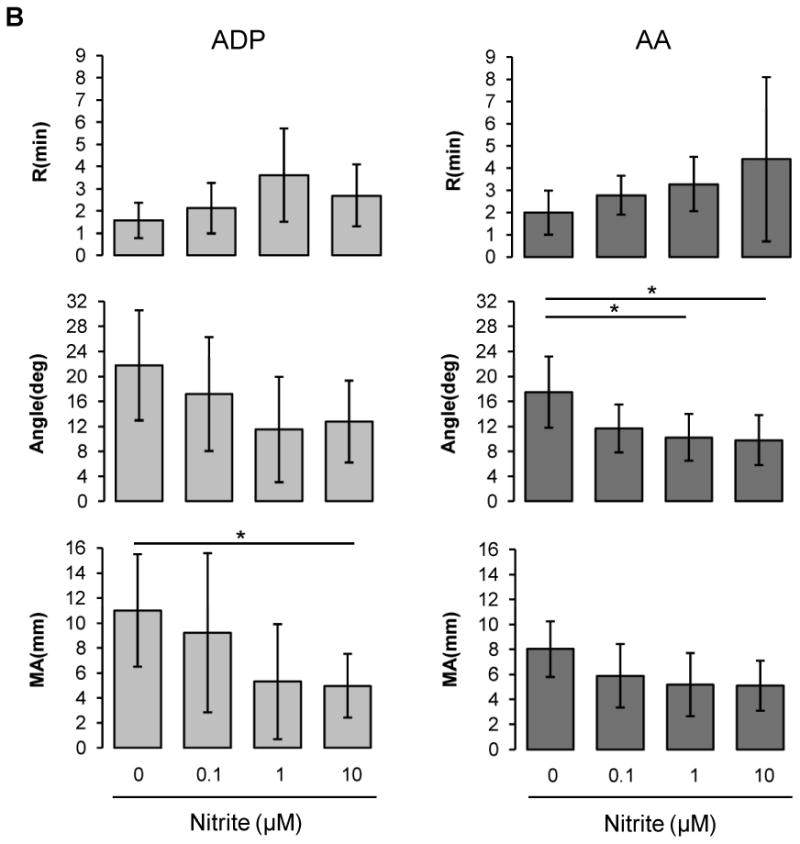
Nitrite effects on ADP- or AA-induced coagulation pathway. Platelet-rich plasma (A) or blood at 20% hematocrit (B) was preincubated with nitrite (0.1 ∼ 10 μM) for 5 min, then coagulation was induced by the addition of activator F and ADP (2 μM) or AA (1 mM) [A; n=3, B; n=6 (ADP), n=5 (AA)]. Data are means ± standard deviation. *P < 0.05 compared with control.
Nitrite inhibits clotting in whole blood under low oxygen conditions
We examined the effects of nitrite on clotting processes in whole blood diluted with plasma to yield 20% hematocrit (Fig. 2B). For both ADP and AA stimulation, nitrite addition showed a dose-dependent trend of inhibition on all three TEG parameters, although statistical significance was not reached.
Nitrite reduction to NO is enhanced under hypoxic conditions. In blood, deoxyhemoglobin is able to reduce nitrite to NO when oxygen tension falls. To investigate whether the inhibitory effect of nitrite on coagulation is promoted in hypoxic condition, we measured coagulation with deoxygenated blood at 20% hematocrit in a glove box at 5% oxygen (Fig. 3A). Compared to the result in Fig. 2B, nitrite showed a much clearer inhibition in ADP-induced coagulation pathway for all three TEG parameters in a dose-dependent manner at 20% hematocrit blood (pO2 37.4 ± 12.4 mmHg) under these hypoxic conditions (Fig. 3A) suggesting that nitrite reduction has occurred in the hypoxic environment by deoxyhemoglobin leading to NO generation. We further showed that these inhibitory effects of nitrite on ADP-induced clotting were abolished when the blood was preincubated with an NO scavenger, cPTIO, demonstrating that nitrite inhibition of clotting was mediated by its reduction to NO (Fig. 3B). To explore the influences of hematocrit on the nitrite effects, we diluted whole blood with plasma and performed coagulation experiments at 5, 10, 20, 30 and 40% hematocrit in the absence or presence of 1 μM nitrite after ADP stimulation (Fig. 3C). The values of each TEG parameter in nitrite group were normalized to those of the control group to show the relative changes by nitrite at each hematocrit. All three TEG parameters showed a maximal inhibition of clotting processes by nitrite at 20% hematocrit, as we had found before [16], implying that nitrite inhibition of coagulation might impact the microcirculatory vessel system where effective hematocrit is reduced, rather than in the major blood vessels.
Figure 3.
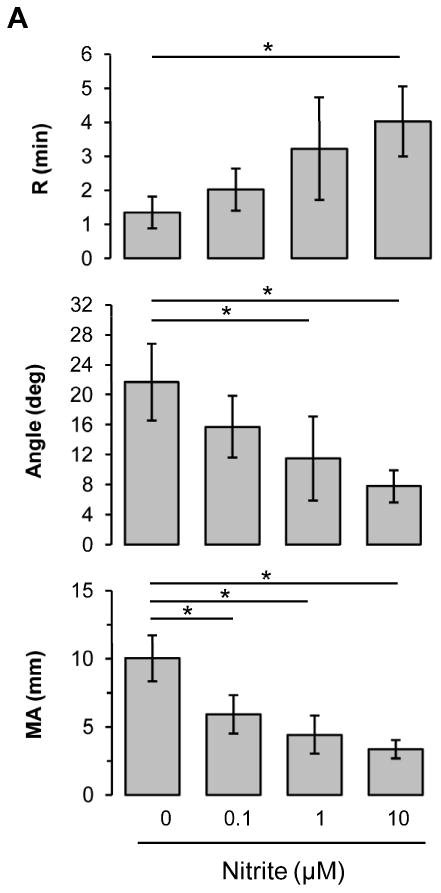
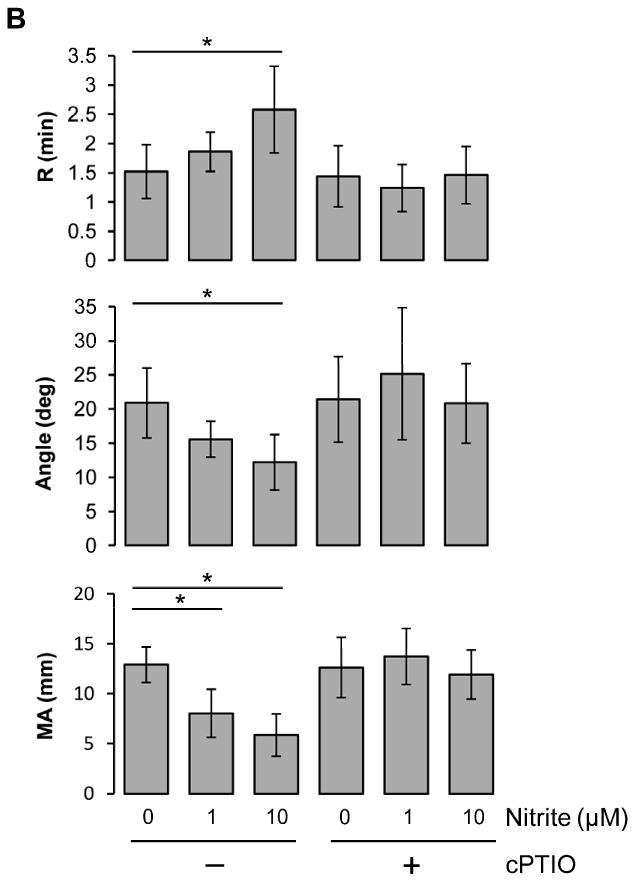
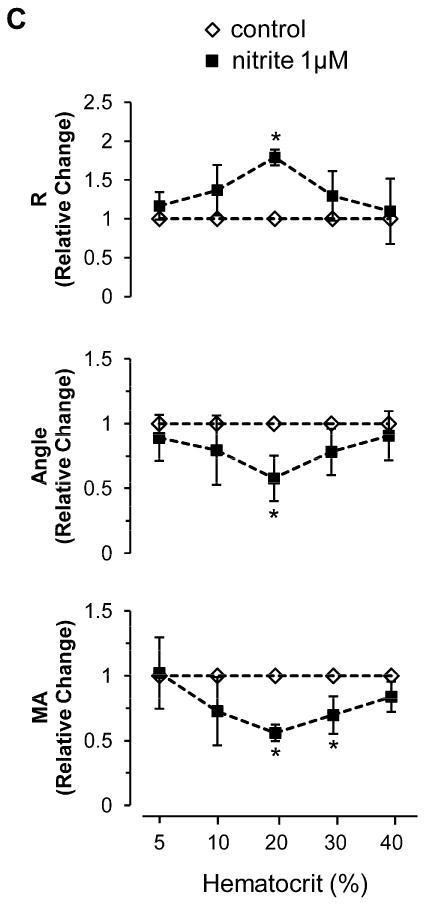
Nitrite and hematocrit effects on coagulation processes under hypoxic condition. Blood in a glass Erlenmeyer flask with a stopper was deoxygenated by blowing helium gas into the flask with gentle stirring for 30min at room temperature. The average value of pO2 after deoxygenation was 37.4 ± 12.4 mmHg. The deoxygenated blood was kept in the flask and the stopper was opened only in a 5% oxygen glove box and the TEG machine was also placed in the glove box for experiments. Nitrite was incubated with the whole blood at 20% hematocrit for 5 min, then activatorF and ADP (2 μM) were used to initiate coagulation (A, n=4). To scavenge NO, 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 200 μM) was incubated with 20% hematocrit blood together with nitrite (1 or 10 μM). Coagulation was initiated by activatorF and ADP (2 μM) (B, n=5). Blood at each hematocrit (5, 10, 20, 30, 40%) was preincubated with nitrite (1 μM) for 5 min, then activator F and ADP (2 μM) were used to initiate coagulation (C, n=3∼4). Data are means ± standard deviation. *P < 0.05 compared with control.
Discussion
While NO is well appreciated as a critical factor for modulating platelet function and blood flow regulation under pathophysiological situations, the contribution of nitrite in the circulation to these phenomena has not been studied in detail in terms of its physiological relevance to the regulation of platelet reactivity and hemostasis. Since its discovery, the effects of endothelium-derived NO via the action of NOS on platelet reactivity have been studied extensively [19-21]. However, until recently the roles of nitrite as a precursor of NO in modulating platelet function did not get much attention. Since it has now been established that NO can be generated by the stepwise reduction of nitrate through nitrite [22] as well as by the endogenous NOS system, the prospects for use of nitrite and nitrate for preventing cardiovascular diseases are increasing [23] and many research groups now focus on examining the biological roles of nitrite and nitrate as exogenous NO sources in cardiovascular system [24-26]. Therefore it is of importance to study how these anions influence platelet-dependent hemostasis for maintaining vascular integrity and homeostasis. A recent report by Webb et al. showed that ingestion of nitrate-rich food such as beetroot juice decreased platelet aggregation in healthy people [25] suggesting that NO from exogenous sources might have a substantial impact on platelet function. Our previous report also showed that nitrite anions inhibited human platelet aggregation and granule secretion as well as cGMP production in the presence of deoxygenated erythrocytes and this was completely abolished by NO scavengers, which demonstrates that nitrite inhibits platelet functions through its reduction to NO mediated by partially deoxygenated erythrocytes [16]. To extend this study and prove the potential roles of nitrite in hemostasis, we hypothesized that nitrite might have inhibitory effects on overall blood coagulation once it is converted to NO by deoxyhemoglobin and we utilized TEG as a useful tool to monitor overall processes of coagulation. Although conventional (optical) platelet aggregometry has been the gold standard for the evaluation of platelet function, it does not reflect overall coagulation because it only measures platelet aggregation capability. Since platelets participate in blood coagulation in large part by interacting with plasma proteins, it would be more beneficial for us to investigate the whole coagulation processes from the initiation of fibrin formation to the interaction between fibrin and platelets to closely monitor the roles of nitrite in these events. TEG provides an integrated analysis of hemostasis by assessing viscoelastic changes of blood with quantitative kinetic parameters such as reaction time (R), reaction angle (α) and maximum amplitude (MA) allowing us to monitor global changes in coagulation processes [18]. We analyzed the effects of nitric oxide or nitrite with TEG on two platelet pathways activated by ADP and AA respectively. Many of early studies reported that NO inhibits aggregation in platelet rich plasma using conventional aggregometry [20,21], in agreement with those studies, we showed in the current study that preincubation with an NO donor (DEANONOate) inhibited all three TEG parameters, monitored in a dose-dependent fashion in platelet-rich plasma and 20% hematocrit blood in response to both ADP and AA resulting in delayed fibrin formation and decreased clot-forming speed and maximum amplitude (Figs 1B and C). These results are in agreement with previous reports which showed that DETANONOate decreased hemostatic function in vitro in rabbit blood using TEG [27,28]. However, we demonstrated that NO itself did not affect any of these TEG parameters in platelet-free plasma (Fig. 1A) suggesting that the inhibitory effect of NO on coagulation is mediated by platelet interaction with other coagulation factors in plasma. In dynamic coagulation phases, platelet-platelet, platelet-fibrin and platelet-coagulation factor interactions are required for efficient hemostasis. Our results show that NO influences clotting processes by inhibiting platelet reactivity upon its activation, but NO does not directly modulate intrinsic coagulation pathway. On the other hand, nitrite had no impact on TEG parameters in platelet-rich plasma since the conversion of nitrite to NO did not occur, but it showed a modest effect in 20% hematocrit blood which was equilibrated with room air (Figs 2A and B). However, as expected, nitrite exhibited more strong inhibitory effects on the clotting processes when the deoxygenated blood (20% hematocrit) was used for the TEG analysis (Fig. 3A). Since the average pO2 value of deoxygenated blood was 37.4 ± 12.4 mmHg and the TEG analysis was performed at 5% oxygen in a glove box, this is a suitable condition for deoxyhemoglobin in the deoxygenated blood to reduce nitrite to NO and the produced NO was able to delay fibrin formation, decrease clot kinetics and strength by reducing platelet capability of participating in coagulation. These results are well correlated with a recent report in which coagulation was shown to be reduced at high altitude measured by TEG [29]. The group also reported earlier that blood nitrite and nitrate levels increased on acclimatization to high altitude [30]. These studies together with our results suggest that the blood levels of NO metabolites such as nitrite and nitrate might be important determinants of blood coagulation. Dambisya et al. emphasized the roles of NOS in coagulation by showing that the NOS inhibition by L-NG-nitro arginine methyl ester (L-NAME) had procoagulant effects and the addition of NOS substrate, L-arginine, had anticoagulant effects on the TEG parameters in vitro [31]. However the fact that the endogenous NOS pathway becomes inactive while nitrite reduction pathway is enhanced under hypoxic conditions suggests stepwise reduction of nitrate to nitrite and to NO might contribute to modulating venous clotting system in which deoxyhemoglobin reduces nitrite to NO. In addition, we showed that the maximal inhibitory effects of nitrite were observed at 20% hematocrit (Fig. 3C) which reflects hematocrit levels of microcirculation [32] implying that nitrite concentration in microvessels rather than in major big vessels might significantly affect coagulation pathway under hypoxic conditions. At higher hematocrit values – characteristic of blood in bigger vessels, it is likely that the generated NO from nitrite can be immediately scavenged by the high concentrations of erythrocyte hemoglobin [33,34].
In conclusion, we demonstrated by thrombelastography that nitrite anions as well as NO exhibited inhibitory effects on coagulation processes as measured by increased reaction time of fibrin formation, decreased rate of clot formation and reduced clot strength. The inhibitory effects of nitrite were greatly enhanced by the deoxygenation of blood presumably because deoxyhemoglobin reduced nitrite to NO under hypoxic conditions. The maximal effects of nitrite were seen in blood at 20% hematocrit. These new observations on the nitrite effects on hemostatic potential under hypoxic condition are of physiological importance to understand and help to explain the differences between arterial and venous thrombosis. Although platelet contribution to venous thrombosis is not as much recognized as to arterial thrombosis, our results indicate that the regulation of platelet reactivity by nitrite might play a crucial role in venous thrombosis because deoxygenated blood in hypoxic microvessels can provide a suitable condition for nitrite reduction to NO and this might eventually modulate overall blood flow. It may be a promising and relatively easy intervention to supply nitrite ions through nitrate-rich food or pharmacological preparations to prevent venous thrombosis, however more detailed clinical study will be necessary to determine the effective dosage and kinetics of nitrite.
Highlights.
Blood clotting is assessed by thrombelastography in the presence of NO or nitrite
NO does not affect intrinsic coagulation, but inhibits overall clotting processes
Nitrite inhibits clotting in the presence of partially deoxygenated red blood cells
Nitrite reduction to NO, which inhibits platelet aggregation, is likely mechanism
Nitrite reduction to NO may modulate thrombotic processes in the microcirculation
Abbreviations
- NO
nitric oxide
- TEG
thrombelastography
- ADP
adenosine diphosphate
- AA
arachidonic acid
Footnotes
Conflict of Interest: Dr. Alan Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. The authors declare that they have no conflicts of interest.
Addendum: J. W. Park designed and performed research, analyzed data and wrote the manuscript. B. Piknova and A. N. Schechter designed research, interpreted data and wrote the manuscript. K. Nghiem provided technical support. J. N. Lozier helped design research, provided critical advice and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mellion BT, Ignarro LJ, Ohlstein EH, Pontecorvo EG, Hyman AL, Kadowitz PJ. Evidence for the inhibitory role of guanosine 3′, 5′-monophosphate in ADP-induced human platelet aggregation in the presence of nitric oxide and related vasodilators. Blood. 1981;57:946–55. [PubMed] [Google Scholar]
- 2.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181–7. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naseem KM, Roberts W. Nitric oxide at a glance. Platelets. 2011;22:148–52. doi: 10.3109/09537104.2010.522629. [DOI] [PubMed] [Google Scholar]
- 4.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–30. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- 6.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–7. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 8.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–56. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 9.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 10.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–54. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Naughton DP, Blake DR, Benjamin N, Stevens CR, Winyard PG, Symons MC, Harrison R. Human xanthine oxidase converts nitrite ions into nitric oxide (NO) Biochem Soc Trans. 1997;25:524S. doi: 10.1042/bst025524s. [DOI] [PubMed] [Google Scholar]
- 12.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–8. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–6. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlsson S, Wiklund NP, Engstrand L, Weitzberg E, Lundberg JO. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide. 2001;5:580–6. doi: 10.1006/niox.2001.0371. [DOI] [PubMed] [Google Scholar]
- 16.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JW, Piknova B, Huang PL, Noguchi CT, Schechter AN. Effect of blood nitrite and nitrate levels on murine platelet function. PLoS One. 2013;8:e55699. doi: 10.1371/journal.pone.0055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chitlur M, Lusher J. Standardization of thromboelastography: values and challenges. Semin Thromb Hemost. 2010;36:707–11. doi: 10.1055/s-0030-1265287. [DOI] [PubMed] [Google Scholar]
- 19.Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986;88:411–5. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987;47:561–71. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- 21.Bult H, Fret HR, Van den Bossche RM, Herman AG. Platelet inhibition by endothelium-derived relaxing factor from the rabbit perfused aorta. Br J Pharmacol. 1988;95:1308–14. doi: 10.1111/j.1476-5381.1988.tb11769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 23.Machha A, Schechter AN. Dietary nitrite and nitrate: a review of potential mechanisms of cardiovascular benefits. Eur J Nutr. 2011;50:293–303. doi: 10.1007/s00394-011-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raat NJ, Noguchi AC, Liu VB, Raghavachari N, Liu D, Xu X, Shiva S, Munson PJ, Gladwin MT. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic Biol Med. 2009;47:510–7. doi: 10.1016/j.freeradbiomed.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen VG, Geary BT, Baird MS. Effects of DETANONOate, a nitric oxide donor, on hemostasis in rabbits: an in vitro and in vivo thrombelastographic analysis. J Crit Care. 2000;15:30–5. doi: 10.1053/jcrc.2000.0150030. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen VG. Nitric oxide decreases coagulation protein function in rabbits as assessed by thromboelastography. Anesth Analg. 2001;92:320–3. doi: 10.1097/00000539-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Martin DS, Pate JS, Vercueil A, Doyle PW, Mythen MG, Grocott MP. Reduced coagulation at high altitude identified by thromboelastography. Thromb Haemost. 2012;107:1066–71. doi: 10.1160/TH12-01-0004. [DOI] [PubMed] [Google Scholar]
- 30.Levett DZ, Fernandez BO, Riley HL, Martin DS, Mitchell K, Leckstrom CA, Ince C, Whipp BJ, Mythen MG, Montgomery HE, Grocott MP, Feelisch M. The role of nitrogen oxides in human adaptation to hypoxia. Sci Rep. 2011;1:109. doi: 10.1038/srep00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dambisya YM, Lee TL. A thromboelastography study on the in vitro effects of L-arginine and L-NG-nitro arginine methyl ester on human whole blood coagulation and fibrinolysis. Blood Coagul Fibrinolysis. 1996;7:678–83. doi: 10.1097/00001721-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Sarelius IH, Duling BR. Direct measurement of microvessel hematocrit, red cell flux, velocity, and transit time. Am J Physiol. 1982;243:H1018–26. doi: 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- 33.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 34.Megson IL, Sogo N, Mazzei FA, Butler AR, Walton JC, Webb DJ. Inhibition of human platelet aggregation by a novel S-nitrosothiol is abolished by haemoglobin and red blood cells in vitro: implications for anti-thrombotic therapy. Br J Pharmacol. 2000;131:1391–8. doi: 10.1038/sj.bjp.0703731. [DOI] [PMC free article] [PubMed] [Google Scholar]


