Abstract
The insula has been implicated in cue-induced craving and relapse in nicotine-dependent tobacco cigarette smokers. The aims of the present study were to identify brain regions that exhibit greater functional connectivity with the right anterior insula in response to smoking cues than to neutral cues and the role of functional connectivity between these regions in mediating cue-induced craving in healthy (free of Axis I psychiatric disorders) nicotine-dependent tobacco cigarette smokers. Functional magnetic resonance imaging (fMRI) data were collected from 63 healthy nicotine-dependent smokers viewing blocks of smoking and neutral cues. Craving ratings were obtained after each block. A psychophysiologic interaction (PPI) approach was used to identify regions that exhibited significantly greater functional connectivity with the right anterior insula (seed) during the smoking cues than during the neutral (corrected cluster thresholding, Z>2.3, p=0.05). Parameter estimates of the interaction effects from each region were regressed against the mean cue-induced craving scores. Significant task by seed interactions were observed in two clusters centered in the bilateral precuneus and left angular gyrus. The strength of connectivity between the right anterior insula and the precuneus, which is involved interoceptive processing and self-awareness, was positively correlated with the magnitude of the craving response to the smoking cues (r2=0.15; p<0.01). These data suggest that among smokers, cue-induced craving may be a function of connectivity between two regions involved in interoception and self-awareness. Moreover, treatment strategies that incorporate mindful attention may be effective in attenuating cue-induced craving and relapse in nicotine-dependent smokers.
Keywords: tobacco cigarettes, craving, cues, functional connectivity, insula, nicotine-dependence
Introduction
Smoking and exposure to tobacco smoke accounts for approximately 20% of deaths in the United States each year and it is estimated that 30% of all cancers are directly linked to tobacco smoke (Centers for Disease Control and Prevention, 2008). Despite these data, in 2012 approximately 57.5 million individuals aged 12 and older (22.1% of the U.S. population) were current tobacco cigarette smokers (Substance Abuse and Mental Health Services Administration, 2013). The six month abstinence rates for nicotine-dependent cigarette smokers following pharmacological treatment for smoking cessation are low and range from 14 to 36% (Gonzales et al., 2006; Jorenby et al., 1999; Killen et al., 1999). Craving is a common symptom of nicotine withdrawal and often precedes relapse (Allen et al., 2008; Hughes and Hatsukami, 1986; Zhou et al., 2009). Thus, understanding the neurobiologic mechanisms that underscore craving may lead to more effective treatment options for nicotine-dependent smokers. An emerging literature suggests that craving during nicotine withdrawal is mediated in part by interoceptive (visceral) cues (Gray and Critchley, 2007; Naqvi and Bechara, 2010; Naqvi et al., 2007). Data from resting state functional magnetic resonance imaging (rsfMRI) studies suggest that the right anterior insula is a critical component of a network that directs attention away from cognitive tasks towards interoceptive cues, thus this network may be an important neurobiologic mechanism involved in craving in smokers (Fox et al., 2005; Raichle et al., 2001; Seeley et al., 2007; Sridharan et al., 2008; Sutherland et al., 2012).
Data from functional anatomical studies of humans and non-human primates suggest that the anterior insula is responsible for translating interoceptive states to emotional responses (Craig, 2002). Activity in the right anterior insula has been associated with emotional and autonomic responses to mental, pharmacological and physical stressors (Buchel et al., 1998; Cameron and Minoshima, 2002; Critchley et al., 2000). During a heartbeat detection task, interoceptive accuracy was associated with indices of negative affect, demonstrating a link between interoceptive awareness and emotional responses. Activity in right anterior insula was a significant predictor of interoceptive accuracy, providing further support that this region plays an important role in translating interoceptive states to emotional experiences (Critchley et al., 2004).
Clinical studies of healthy (free of Axis I psychiatric disorders) nicotine-dependent smokers demonstrate that the anterior insula is involved in behaviors associated with nicotine dependence. For example, a positron emission tomography (PET) neuroimaging study found a significant positive correlation between craving to cigarette paired cues and activity in the insula of smokers (Brody et al., 2002). Smokers with damage to the insula quit faster and report less urges to smoke than do smokers without damage to the insula (Naqvi et al., 2007). Thus, interoceptive processing in the insula appears to have an important link to craving in smokers. Resting state functional connectivity data obtained from smokers suggest that the insula is a critical component of a network involved in mediating cue reactivity (Janes et al., 2012). Compared with food cues, smoking cues increased functional connectivity between the left insula and a larger network encompassing striatal, limbic and sensorimotor brain regions and network connectivity was associated with the severity of nicotine dependence (Claus et al., 2013). Augmented network connectivity involving the anterior insula has been hypothesized to drive craving in smokers undergoing acute nicotine withdrawal (Sutherland et al., 2012). The aims of the present study were to identify brain regions that exhibit greater functional connectivity with the right anterior insula in response to smoking cues than to neutral cues and the role of functional connectivity between these regions in mediating cue-induced craving in healthy (free of Axis I psychiatric disorders) nicotine-dependent tobacco cigarette smokers.
Materials and methods
Participants
Study participants (N=63) were recruited as part of a larger study examining the effects of real-time fMRI neurofeedback on cigarette cue-induced craving in smokers (Hanlon et al., 2013; Li et al., 2012). All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki with approval from the Medical University of South Carolina's (MUSC's) Institutional Review Board. Participants were recruited using advertisements in local print and broadcast media and were screened for eligibility in a brief phone interview that assessed smoking behaviors, mental and physical health, current and past drug and alcohol use, and ability to tolerate an fMRI scan. Prior to enrollment, a screening visit was completed at which informed consent was obtained and participants were assessed for study eligibility using the MINI neuropsychiatric interview (Sheehan et al., 1998), a brief history and physical, urine drug screen and exhaled carbon monoxide levels (using a MicroSmokelyzer; Bedfont Scientific Ltd, Kent, UK). Participants also completed several self-report assessments including the Fagestrom Test for Nicotine Dependence (FTND) (Fagerstrom, 1978), Questionnaire of Smoking Urges-Brief (Cox et al., 2001), Minnesota Withdrawal Scale-Revised (Hatsukami et al., 1984) and a tobacco use history questionnaire.
Enrolled participants smoked greater than 10 cigarettes per day. Nicotine dependence was confirmed using the FTND and exhaled carbon monoxide greater than 10 parts per million (ppm). All participants expressed interest in quitting smoking, but were not currently using any pharmacologic treatment (bupropion, varenicline, NRT). Exclusion criteria included dependence on any substance other than nicotine within the past five years, axis I psychiatric disorders or health conditions that would affect blood flow or brain function (e.g. heart problems or traumatic brain injuries). Participants were also excluded if they could not be scanned because of pregnancy, claustrophobia or metal implants.
Procedures
Participants were instructed not to smoke during the two hours prior to the scanning visit. Neuroimaging data were acquired using a 3 Tesla MRI Trio scanner (Siemens Medical, Erlangen, Germany) at MUSC's Center for Biomedical Imaging. Although the data were obtained from a larger study investigating real-time feedback, the present analysis is limited to the neuroimaging data that were obtained during the first run of the first scanning visit (i.e. prior to feedback). Participants wore headphones and foam padding was used to limit their head motion. A non-ferrous optical hand pad was placed on the participant's right hand. The hand pad was connected via an optical cable to a computer outside the scanner room. Using a block design, images of neutral cues (i.e. pencils and dishes) were matched in frequency and duration with images of smoking cues (i.e. cigarettes and ashtrays) (Geier et al., 2000; Hartwell et al., 2011). The images were projected on a wide screen located at the end of the scanner bore and viewed via a back-projected mirror that was mounted on a 12-channel head coil. The images were presented throughout a ten minute sequence consisting of five, 92.4-second epochs that were preceded by a 132.2-second rest condition (cross-hair). Each epoch contained three, 22-second blocks of the smoking cues, neutral cues and the rest condition which were followed by 4.4 seconds of self-rated craving and 4.4 seconds of an image of a blank thermometer. The smoking and neutral blocks contained five individual pictures and each picture was presented for 4.4 seconds.
Neuroimaging Data Acquisition
Functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) imaging data were acquired using a standard multi-slice gradient echo planar imaging sequence (TR=2200 ms, TE=35 ms, 64 × 64 matrix, 3 mm isotropic voxels, 271 volumes). Data were preprocessed using scripting tools from FMRI Expert Analysis Tool (FEAT) Version 5.63, part of FMRIB's Software Library (FSL;www.fmrib.ox.ac.uk/fsl). First non-brain signal was removed using FSL BET brain extraction. Scans were then corrected for motion using FSL linear registration and scans were spatially smoothed using a Gaussian kernel of 8 mm FWHM. Since head motion can impact functional connectivity analyses, participants with head motion exceeding 0.2 mm were excluded from the analyses (Power et al., 2012). The “fsl_motion_correction” scripting tool was used to identify and scrub motion artifacts from each participant's data (Power et al., 2013). Scans were spatially co-registered with a standardized anatomical template (Montreal Neurological Institute) using a 12 parameter affine transformation (Rorden and Brett, 2000).
A psychophysiologic interaction (PPI) approach was used to investigate functional connectivity between the right anterior insula and all other brain regions during exposure to smoking and neutral cues (Friston et al., 1997). A customized square wave form representing the task (1 for smoking cues and 0 for neutral cues) and the duration of the cue presentation was convolved with a double gamma hemodynamic response function. A nine millimeter radius sphere was drawn around a voxel (MNI coordinates; x=38, y=26, z=-10) located in the right anterior insula (seed)(Seeley et al., 2007). The transformation parameters described above were also applied to the mask (Tzourio-Mazoyer et al., 2002). For each participant, the mean corrected and high pass filtered time series of the BOLD signal in right anterior insula was extracted and regressed against the task in a single subject whole brain PPI analysis (Seeley et al., 2007). The first level analysis generated contrast images of the parameter estimates for each regressor for each participant. The contrast images of the parameter estimates of the task and task × seed interaction were combined for group-level t-tests using the contrast smoke > neutral to 1) identify regions that exhibited greater activity during the smoking cues than the neutral cues and 2) identify regions that exhibited greater connectivity with the right anterior insula during the smoking cues as compared with the neutral cues. The left crus of the cerebellum was used as a control seed region for a separate PPI analysis (Engelmann et al., 2012). An anatomical mask of the left crus was derived using the automated anatomical labeling atlas and the preprocessing and PPI model described above were applied. Analyses were conducted using FMRIBs Local Analysis of Mixed Effects (FLAME 1). Group level results were thresholded at Z>2.3 using a corrected cluster threshold of p=0.05.
Linear regression tests were used to assess the relationship between cue-induced craving and functional connectivity with the right anterior insula. The mean craving response to the smoking cues was regressed against the parameter estimate obtained from the center voxel from each cluster that exhibited a significant task × seed interaction with the right anterior insula.
Results
Demographics and Craving
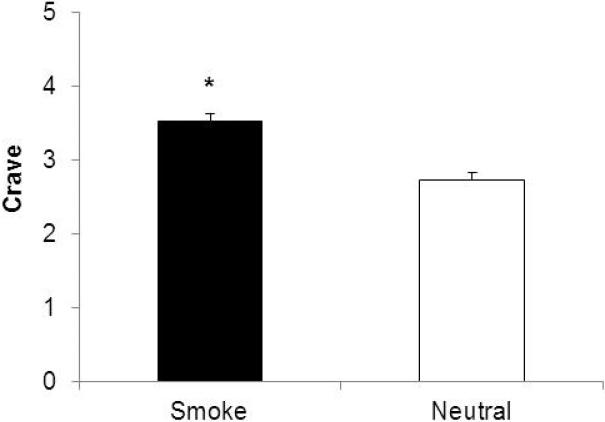
Data from five participants were excluded from the analysis for motion greater than 0.2 mm. Demographic characteristics of the 58 participants included in the analysis are displayed in Table 1. There were no differences in head motion during the smoking cues and neutral cues (Table 1) (t=0.16, p=0.88). The smoking cues produced a significantly greater craving response (mean=3.5; se=0.09) than the neutral cues (mean=2.7; se=1.0) (t=10.0, p<0.001) (Figure 1).
Table 1.
Participant Demographics
| Variable | N=58 |
|---|---|
| Age (years) (mean ±se) | 34.5 ±1.6 |
| Sex (% male) | 60.3 |
| Race (% caucasian) | 80.0 |
| Education (% some college) | 76.0 |
| FTND (mean ±se) | 4.7 ±0.27 |
| Cigarettes/day (mean ±se) | 16.8 ±0.93 |
| CO Level (ppm) (mean ±se) | 14.7 ±1.3 |
| Relative Motion Neutral Cue (mm)(mean ±se) | 0.08 ±.005 |
| Relative Motion Smoking Cue (mm)(mean ±se) | 0.08 ±.005 |
Figure 1.
Mean (se) craving responses to the smoking and neutral cues. *Denotes a significant difference from neutral cues.
Neuroimaging Data
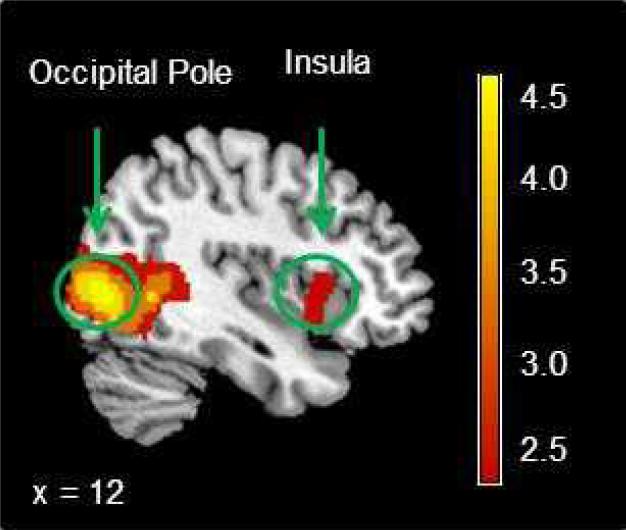
A significant effect of the task was observed in a cluster centered in the right occipital pole (Figure 2) that included the bilateral posterior cingulate gyri, left frontal pole and right lateral occipital cortex (Table 2). Using a corrected cluster threshold (Z>2.3, p=0.05.), there was no significant main effect of task on activity in the right anterior insula. However, there appeared to be activation in the right anterior insula at a level below the statistical threshold (Figure 2). In a post-hoc analysis, the contrast between the smoking and neutral cues expressed as the parameter estimate (COPE) from a voxel centered in the right anterior insula was extracted for each participant. A one-sample t-test was used to test the null hypothesis that there was no difference between the mean COPE in the right anterior insula and zero. The COPE in the right anterior insula (mean=3.7, se=1.3) was significantly greater than zero (t=2.8, p=0.007).
Figure 2.
A significant main effect of task was observed in the right occipital cortex and sub-threshold activation was observed in the right anterior insula.
Table 2.
Regions exhibiting a significant main effect of task (smoke > neutral)
| Peak activation (MNI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Anatomical region | Cluster | Cluster (voxels) | p Value | Side | x | y | z | Z |
| Occipital Pole | 1 | 18471 | 4.11e-15 | R | 12 | −96 | 6 | 5.8 |
| Posterior Cingulate Gyrus | L | −4 | −42 | 8 | 5.8 | |||
| L/R | 0 | −36 | 14 | 4.98 | ||||
| Frontal Pole | L | −32 | 46 | −12 | 5.73 | |||
| Occipital Pole | R | 22 | −94 | 16 | 5.41 | |||
| Lateral Occipital Cortex | R | 36 | −80 | 4 | 5.1 | |||
| R | 32 | −86 | 12 | 4.97 | ||||
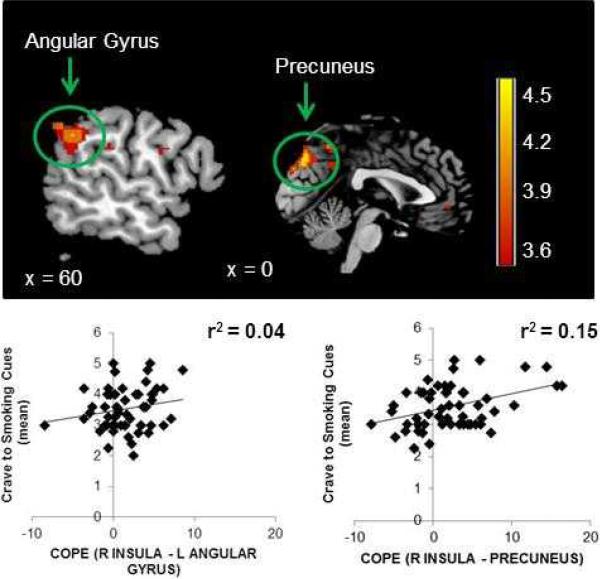
A significant task × seed interaction was observed in a cluster centered in the bilateral precuneus and included the right frontal operculum cortex, angular gyrus and precentral gyrus. A significant task × seed interaction was also found in a second cluster centered in the left angular gyrus that included the left central opercular cortex and lateral occipital cortex (Table 3, Figure 3). No significant task × seed interactions were observed when the left crus of the cerebellum was used as the seed region in the control PPI analyses.
Table 3.
Regions exhibiting a significant task × seed interaction (smoke > neutral)
| Anatomical region | Cluster | Cluster (voxels) | p Value | Side | MNI Coordinates | Z | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Precuneus | 2 | 18331 | 1.89e-15 | L/R | 0 | −80 | 44 | 4.42 |
| L/R | 0 | −76 | 44 | 4.42 | ||||
| L/R | 0 | −78 | 36 | 4.37 | ||||
| L | −10 | −54 | 38 | 3.99 | ||||
| Frontal Operculum Cortex | R | 44 | 16 | −6 | 4.29 | |||
| Angular Gyrus | R | 60 | −40 | 32 | 3.83 | |||
| Precentral Gyrus | R | 56 | 4 | 32 | 3.83 | |||
| Angular Gyrus | 1 | 5427 | 1.49e-06 | L | −58 | −54 | 34 | 4.36 |
| L | −66 | −42 | 36 | 4.36 | ||||
| L | −60 | −52 | 34 | 4.24 | ||||
| Central Opercular Cortex | L | −40 | −2 | 20 | 4.32 | |||
| L | −38 | −16 | 22 | 4.09 | ||||
| Lateral Occipital Cortex | L | −58 | −62 | 40 | 3.98 | |||
| L | −54 | −64 | 44 | 3.85 | ||||
Figure 3.
Significant task × seed interactions were observed in in the left angular gyrus and bilateral precuneus. Association between cue-induced craving and functional connectivity between the right anterior insula and the left angular gyrus (p>0.05) and the precuneus (p<0.01).
There was no significant correlation between the mean craving responses to the smoking cues and the parameter estimate of the task × seed interaction observed between the right anterior insula and the left angular gyrus (Figure 3). There was a significant positive correlation between the parameter estimate of the task × seed interaction between the right anterior insula and the precuneus and the mean craving response to the smoking cues (Figure 3).
Discussion
The present study demonstrates enhanced functional connectivity during the presentation of smoking cues in recently abstinent nicotine-dependent smokers. Smoking cues produced significantly greater functional connectivity between the right anterior insula and the precuneus and the left angular gyrus as compared to neutral cues. Cue-induced craving was positively associated with the magnitude of functional connectivity between the right anterior insula and precuneus. To our knowledge, this is the first study to demonstrate a link between enhanced functional connectivity and cue-induced craving in healthy (free of Axis I psychiatric disorders) nicotine-dependent smokers.
In agreement with previous neuroimaging studies of nicotine-dependent smokers, smoking cues produced significantly greater activity than neutral cues in brain regions located within both the extended visual system (occipital cortex) and the default mode network (posterior cingulate cortex and prefrontal cortex) (Brody et al., 2002; Engelmann et al., 2012; Greicius et al., 2003). Previous neuroimaging studies of nicotine-dependent smokers have found cue elicited increases in activity in the left and bilateral anterior insula, thus we had anticipated to find a main effect of task on activity in the right anterior insula (Brody et al., 2002; Franklin et al., 2007). We did observe activation in the right anterior insula that was sub-threshold and statistically greater than zero, suggesting that activity in the seed region was greater during the smoking cue blocks than the neutral cue blocks. A recent meta-analysis of neuroimaging studies compared cue induced brain activity between non-deprived smokers (mean CO level = 17.5 ppm) and deprived smokers (mean CO level = 2.7 ppm) and found a greater number of active clusters in the insula of deprived smokers compared with non-deprived smokers. The mean CO level in the present study was 14.7 ppm, suggesting this cohort was relatively non-deprived and thus it is possible that activity in the right anterior insula to the smoking cues was attenuated by the short duration of abstinence.
A PPI analysis was used to identify brain regions that exhibited greater functional connectivity with the right anterior insula in response to the smoking cues as compared to neutral cues. We identified two significant clusters that were centered in the bilateral precuneus and left angular gyrus. Data from resting state function connectivity studies demonstrate that the right anterior insula acts as a switch tethering between the default mode network at rest and the executive control network during tasks requiring attention, cognition and executive control (Fox et al., 2005; Raichle et al., 2001). Acute nicotine administration increases cognitive performance and inhibits activity in regions of the default mode network including the angular gyrus and precuneus of smokers (Hahn et al., 2007). Sutherland et al. proposed that enhance network connectivity between the right anterior insula and the default mode network plays a role in behaviors associated with addiction (Naqvi and Bechara, 2010). In support, we found that cues increased functional connectivity between the right anterior insula and components of the default mode network (angular gyrus and Precuneus). A growing literature suggests a critical role for the precuneus within the default mode network in processing self-consciousness, self-reflection and episodic memory retrieval (Cabanis et al., 2013; Cavanna, 2007; Cavanna and Trimble, 2006). It is plausible that the present findings of enhanced connectivity between the right anterior insula and the precuneus in recently abstinent smokers reflects the coordinated processing of negative somatic cues and self-referential thinking directed towards evaluating the salience of the smoking cues. In support, we found a significant positive association between cue-induced craving and the strength of functional connectivity between the right anterior insula and the precuneus. Of note, there is significant anatomical overlap between the anterior insula and parietal cortex (Mufson and Mesulam, 1982; Selemon and Goldman-Rakic, 1988). Previous neuroimaging studies have linked activity in both the precuneus and the right anterior insula with cue-induced craving in nicotine-dependent smokers (Brody et al., 2002; Franklin et al., 2007). Although purely speculative, ameliorating the strength of connectivity between the right anterior insula and the precuneus may attenuate cue-induced craving and thus could have potential treatment implications, for example using non-invasive brain stimulation methods and/or mindfulness.
An important question pertains to whether the present findings were specific to smoking cues. While it would have been ideal to include a task of executive function, the data were collected from participants enrolled in larger study investigating the impact of real-time fMRI feedback on cue-induced craving in smokers. Comparisons of brain regions that shared functional connectivity with the right anterior insula during exposure to the smoking cues versus those sharing connectivity with the right anterior insula during tasks that require executive function may have provided a better assessment of network dynamics in smokers. Non-smoking controls were not included in the study, thus it is difficult to discern whether the present findings were specific to smokers or a function of switching attention between groups of disparate images. However, no significant task by seed interactions were found when a seed region that appears to be the least relevant to smoking cues was used in a control PPI analysis, supporting the present findings as specific to the smoking cues. Moreover, functional connectivity was significantly associated with craving to the smoking cues, which would not be expected in a group of non-smoking healthy controls. Participants with axis I psychiatric disorders were excluded from study participation, thus the present findings are specific to healthy non-psychiatric smokers. Future studies examining function connectivity during smoking cues in psychiatric populations of smokers are warranted.
These novel findings were collected from a relatively large sample of healthy nicotine-dependent smokers and suggest that enhanced functional connectivity between the right anterior insula and the precuneus may be an important neurologic substrate of cue-induced craving in nicotine-dependent smokers. In addition, interoceptive states and self-consciousness may be important therapeutic targets for ameliorating craving in nicotine dependent smokers.
References
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr., Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of general psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cabanis M, Pyka M, Mehl S, Muller BW, Loos-Jankowiak S, Winterer G, Wolwer W, Musso F, Klingberg S, Rapp AM, Langohr K, Wiedemann G, Herrlich J, Walter H, Wagner M, Schnell K, Vogeley K, Kockler H, Shah NJ, Stocker T, Thienel R, Pauly K, Krug A, Kircher T. The precuneus and the insula in self-attributional processes. Cognitive, affective & behavioral neuroscience. 2013;13:330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosomatic medicine. 2002;64:851–861. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS spectrums. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008:1226–1228. [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association Between Nicotine Dependence Severity, BOLD Response to Smoking Cues, and Functional Connectivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523 Pt. 2000;1:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study G Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Hartwell KJ, Canterberry M, Li X, Owens M, Lematty T, Prisciandaro JJ, Borckardt J, Brady KT, George MS. Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry research. 2013;213:79–81. doi: 10.1016/j.pscychresns.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, Brady KT. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16:654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hughes JR, Pickens RW, Svikis D. Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of general psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and alcohol dependence. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England journal of medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Experimental and clinical psychopharmacology. 1999;7:226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, Lematty T, Brady KT, George MS. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addiction biology. 2012 doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. The Journal of comparative neurology. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain structure & function. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2013;84C:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl. 1998;20:22–33;quiz 34-57. [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addictive behaviors. 2009;34:365–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]