Abstract
Background
Impairment in instrumental activities of daily living (IADL) begins as individuals with amnestic mild cognitive impairment (MCI) transition to Alzheimer's disease (AD) dementia. IADL impairment in AD dementia has been associated with inferior parietal, inferior temporal, and superior occipital hypometabolism using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET).
Objective
To investigate the relationship between regional FDG metabolism and IADL in clinically normal (CN) elderly, MCI, and mild AD dementia subjects cross-sectionally and longitudinally.
Methods
One hundred and four CN, 203 MCI, and 95 AD dementia subjects from the Alzheimer's Disease Neuroimaging Initiative underwent clinical assessments every 6 to 12 months for up to three years and baseline FDG PET. The subjective, informant-based Functional Activities Questionnaire was used to assess IADL. General linear models and mixed effects models were used, covarying for demographics, cogniton, and behavior.
Results
The cross-sectional analysis revealed middle frontal and orbitofrontal hypometabolism were significantly associated with greater IADL impairment. Additionally, the interaction of diagnosis with posterior cingulate and with parahippocampal hypometabolism showed a greater decline in IADL performance as metabolism decreased for the AD dementia relative to the MCI group, and the MCI group relative to the CN group. The longitudinal analysis showed that baseline middle frontal and posterior cingulate hypometabolism were significantly associated with greater rate of increase in IADL impairment over time.
Conclusion
These results suggest that regional synaptic dysfunction, including the Alzheimer-typical medial parietal and less typical frontal regions, relates to daily functioning decline at baseline and over time across the early AD spectrum.
Keywords: 18F-fluorodeoxyglucose positron emission tomography, Alzheimer's disease, instrumental activities of daily living, mild cognitive impairment
Introduction
The rapid growth of the aging population in the United States has fueled the rising prevalence of Alzheimer disease (AD) dementia now endemic to this demographic. Currently, AD dementia is estimated to affect nearly 1 out of 10 individuals over the age of 65. The multi-staged disease is believed to transition from clinically normal (CN) to a range of mild cognitive impairment (MCI), followed by an ultimate decline towards AD dementia.[1,2]
As AD progresses, patients experience worsening symptoms of episodic memory, cognition, and daily functioning.[3-5] These symptoms greatly compromise an individual's quality of life, but perhaps none more than impairment in everyday functioning. Daily functioning is measured by performance of activities of daily living (ADL), impairment in which is integral for the diagnosis of AD dementia. AD patients often experience an early loss of independence, which increases the burden of responsibilities on caregivers. ADL are commonly categorized as either basic or instrumental with the former including eating, grooming, bathing, dressing and toileting, while the latter is comprised of more complex tasks such as managing one's own schedule, performing household chores like laundry, preparing meals, handling finances, driving or using public transportation and shopping.[6]
Impaired ADL also play a significant role in understanding disease progression. While impairment in basic ADL is found in the moderate-to-severe stage of AD dementia, decline in instrumental ADL (IADL) has been found to accompany the earlier transition from the MCI stage to AD dementia.[4] The disappointing results from recent AD clinical trials point to the need for earlier intervention in order to slow disease progression and improve treatment outcomes.[1] A better understanding of IADL impairment can help better define early AD trial outcomes.
Clinicians use functional assessment scales to detect the changes in IADL impairment that occur throughout the course of AD. Subjective scales are administered with either caregivers (informant-based) or patients (self-reported), while performance-based tests are administered directly to patients. The Functional Assessment Questionnaire (FAQ)[7] is a ten-item subjective, informant-based scale primarily used to detect IADL impairment in MCI and mild dementia.[6] Recently, two large multicenter studies established that the FAQ clearly distinguishes between the three stages of AD progression: CN individuals potentially in the preclinical stage of AD, MCI and AD dementia.[4,8]
IADL impairment has also been associated with changes in brain metabolism as measured by positron emission tomography (PET). Using 18F-2-fluoro-2-deoxy-D-glucose (FDG) PET, Landau et al. demonstrated an association between FDG hypometabolism in a composite of temporal, lateral parietal and posterior cingulate cortices, a pattern of brain regions typically implicated in AD, and greater IADL impairment in MCI and mild AD dementia subjects participating in the Alzheimer's Disease Neuroimaging Initiative (ADNI) longitudinal study[9]. Cross-sectional analyses have been conducted to localize IADL impairment to specific brain regions. One such study revealed an association between IADL impairment and hypometabolism in the inferior parietal, superior occipital, and inferior temporal cortices in AD dementia patients.[10]
Loss of independence in AD patients due to disease progression is a significant challenge faced by both patients and their caregivers and is attributable to impaired IADL performance in AD patients. Measurement and detection of IADL impairment with the FAQ scale and FDG-PET, respectively, have demonstrated the utility of IADL in tracking disease progression, a critical step for improving treatment outcomes in AD clinical trials.
The objective of this study was to investigate the relationship between glucose metabolism in FDG-PET regions of interest (FDG-ROIs)and IADL as measured by FAQ both cross-sectionally and longitudinally across the AD continuum (CN, MCI, and mild AD dementia), while adjusting for subject demographics, and behavioral and cognitive functions. We intend to expand further on the studies described above by assessing the FDG regional correlates of IADL impairment in the early AD spectrum, including CN elderly at risk for AD. Furthermore, we will systematically assess which brain regions are associated with IADL impairment using a data-driven approach.
Materials and Methods
Participants
This study analyzed data acquired from the ADNI database (www.loni.ucla.edu\ADNI) (See Supplementary Data).[11] Four hundred and two subjects underwent clinical assessments every 6 to 12 months up to 3 years including baseline FDG PET in the ADNI study (diagnoses at baseline: 104 CN, 203 amnestic MCI, 95 mild AD dementia) and were selected as previously described.[4,9] Mean follow-up time was 2.3±0.9 years; CN and MCI subjects had up to 3 years follow-up and AD dementia subjects had up to 2 years follow-up. At screening, subjects were ages 55-91 (inclusive), were medically stable and in generally good health, did not have significant neurological conditions (other than MCI or AD dementia for subjects falling into those groups), and had a study partner able to provide collateral information about the subject's daily functioning, cognition, and behavior. Subjects did not have significant cerebrovascular disease and had a Modified Hachinski Ischemic Score[12] ≤ 4. Subjects did not have active psychiatric disorders and had a Geriatric Depression Scale, short form[13] ≤ 5. Subjects were assigned to diagnostic groups (CN, amnestic MCI, mild AD dementia) as determined by site investigators at screening and baseline visits (See Supplementary Data). The FAQ, which was the dependent variable in our analyses used to assess IADL, was used in the determination of follow-up but not screening diagnoses.
The study was approved by the Institutional Review Board (IRB) of each participating site. Written informed consent was obtained from all subjects and study partners prior to initiation of any study procedures in accordance with local IRB guidelines.
Clinical Assessments
Clinical assessments were performed as previously described.[4] IADL were assessed with the FAQ, where higher scores indicate greater impairment (range 0-30). For other assessments used in this study see the Supplementary Data.
Apolipoprotein E ε4 (APOE4) carrier status (homozygous carrier, heterozygous carrier, or non-carrier) was reported for subjects. Duration of AD dementia symptoms (in years) was reported only for those subjects with a diagnosis of mild AD dementia at screening. Duration of AD dementia symptoms was noted as zero for CN and MCI subjects (a slight normal random error was added, standard deviation=0.1 years, for purposes of analysis to avoid biased error variance).
FDG PET Acquisition
FDG PET images were acquired for subjects using multiple scanners in locations throughout the United States as previously described (see Supplementary Data).[9]
FDG ROI Generation & Selection
Cortical FDG metabolism was expressed as the standardized uptake value (SUV) and normalized to an aggregate of cerebellar grey, pons, and primary (sensorimotor) cortex for each region of interest (ROI). Of note, Landau et al. used a combination of cerebellar vermis and pons as a reference region[9]; we added primary cortex, as has been done by others, in order to maximize the volume of relatively unaffected brain.[14,15] These regions were sampled using the Harvard-Oxford probabilistic atlas (http://neuro.debian.net/pkgs/fsl-harvard-oxford-atlases.html).[16-19] Thirty-five bilateral cortical ROIs were used in the preliminary analyses (see Supplementary Table 1), which were reduced to 6 ROIs significantly associated with total FAQ score. These 6 ROIs were used in the main cross-sectional and longitudinal analyses.
Statistical Analyses
All analyses performed in this study were run using SAS Version 9.3 and JMP Version Pro 10 statistical and graphical software. Associations between diagnostic groups and subject demographics and characteristics were evaluated using analysis of variance (ANOVA) with the Tukey post hoc test for continuous variables and the chi-square test for categorical variables.
Preliminary Multiple Test Protection and Data Reduction Screening Tests
Analyses of covariance (ANCOVA) were run with baseline FAQ score as the dependent variable, a main effect of diagnosis (CN subjects were excluded because of floor effects), and each of the 35 FDG ROIs as covariate in separate respective analyses. The 35 p values for the FDG terms were then adjusted for multiple test chance effects via a conservative stepdown Sidak method [20,21]. Further, a backward elimination (cutoff p<0.05) general linear model (GLM) with baseline FAQ score as the dependent variable, a diagnosis main effect, and all 35 FDG ROIs simultaneously included in the initial predictor pool was run. The FDG ROIs significantly associated with baseline FAQ according to these screening tests were then used subsequently in the cross-sectional and longitudinal analyses of primary interest in this study (see Supplementary Data for more detail).
Cross Sectional Analyses
The FDG ROIs surviving the data reduction above were then entered as simultaneous predictors in another backward elimination GLM regressing baseline FAQ on these predictors as well as diagnosis, and the interaction of each of these FDG ROIs with diagnosis. Covariates included sex, the interaction of sex and diagnosis, baseline age (linear and quadratic terms), duration of AD dementia symptoms, Rey Auditory Verbal Learning Test (RAVLT) total learning, Digit Symbol, the Neuropsychiatric Inventory brief questionnaire form (NPI-Q) apathy and depression items, the number of APOE4 alleles, and American National Adult Reading Test (AMNART) intelligence quotient (IQ). Significance test results (p values) were complemented with effect size estimates such as partial regression coefficient estimates (β) with confidence intervals (CI), covariate adjusted means and estimates of percent variance accounted for in the dependent variable uniquely by individual predictors, as well as by the model as a whole (R2). Residuals were checked for conformance to assumptions of normality and homoscedasticity.
Longitudinal Analyses
A mixed effect longitudinal analysis analogous to the cross sectional analysis above was run with FAQ as the dependent variable including the same covariates as described for the cross-sectional model, a random intercept and linear effect of years in the study, as well as a baseline FAQ covariate and its interaction with time, the interaction of diagnosis with time, and the interaction of time with each of the FDG ROIs surviving the data reduction. Random intercepts and slope terms for time were initially allowed to be correlated, and then all fixed and random covariance terms were subjected to backward elimination at a cutoff of 0.05. Residuals were checked for conformance to assumptions of normality and homoscedasticity.
Results
Table 1 provides baseline demographic and clinical data for all subjects and for each of the three diagnostic groups. There were significant differences between diagnostic groups for all variables in expected directions except for age and sex, which were not significantly different across all groups. Supplementary Figure 1 shows longitudinal FAQ scores for each diagnostic group.
Table 1.
Baseline demographic and clinical data for subjects.
| Group | All subjects | CN | MCI | AD dementia |
|---|---|---|---|---|
| N | 402 | 104 | 203 | 95 |
| Age (years) | 75.4±6.7 | 75.9±4.8 | 75.0.±7.2 | 75.6±7.4 |
| Sex (% male) | 63.9 | 62.0 | 67.5 | 59.0 |
| Education (years) | 15.5±3.1‡ | 15.9±3.1 | 15.8±2.9 | 14.6±3.3 |
| AMNART IQ | 117.5±11.5†† | 120.7±11.29 | 117.2 ±11.0 | 114.4±11.9 |
| Duration of AD dementia symptoms (years) | - | 0.0±0.1 | 0.0±0.1 | 3.7±2.4 |
| APOE4 (% non-carrier/heterozygous carrier/homozygous carrier) | 51.0/38.3/10.7† | 75.0/23.1/1.9 | 46.8/40.4/12.8 | 33.7/50.5/15.8 |
| MMSE | 26.8±2.6* | 29.0±1.1 | 27.2±1.7 | 23.5±2.1 |
| RAVLT Total Learning | 32.2±11.1* | 42.2±9.8 | 31.4±9.1 | 22.9±6.9 |
| Digit Symbol | 36.5±13.0* | 44.5±10.4 | 37.4±10.9 | 26.0±12.4 |
| NPI-Q Apathy (% present) | 0.3±0.6 (18.2)** | 0.02±0.1 (1.9) | 0.2±0.6 (15.3) | 0.6±0.8 (42.1) |
| NPI-Q Depression (% present) | 0.2±0.5 (18.7)‡ | 0.1±0.3 (6.7) | 0.2±0.5 (17.2) | 0.4±0.7 (34.7) |
| FAQ | 5.0±6.6* | 0.2±0.8 | 3.4±3.9 | 13.6±6.7 |
AD (Alzheimer's disease), AMNART IQ (American National Adult Reading Test intelligence quotient), APOE4 (Apolipoprotein E ε4), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NPI-Q (Neuropsychiatric Inventory brief questionnaire form), RAVLT (Rey Auditory Verbal Learning Test).
All values (except n, sex, APOE4) represent mean ± standard deviation.
p<0.0001 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.05 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.0001 for CN vs. MCI and CN vs. AD.
p<0.05 for CN vs. MCI and CN vs. AD.
p<0.05 for CN vs. AD and MCI vs. AD.
Preliminary Multiple Test Protection and Data Reduction Screening Tests
The 35 univariate ANCOVAs illustrated the expected negative relations of baseline FAQ with each of the 35 FDG ROIs (greater regional hypometabolism associated with greater IADL impairment), of which 27 were statistically significant at p<0.05 with 25 of these remaining significant after applying the Šidák correction. The backward elimination GLM of the baseline FAQ regressed on all 35 FDG ROIs as simultaneous initial predictors, along with baseline diagnosis, reduced down to 6 FDG ROIs that were significantly associated with total FAQ score and demonstrated an additive significant diagnosis effect (AD FAQ mean>MCI mean) (Supplementary Table 2). The FDG ROIs surviving backward elimination were the posterior cingulate gyrus, orbitofrontal cortex, frontal pole, lingual gyrus, middle frontal gyrus, and parahippocampus (see Supplementary Data).
Cross Sectional Analyses
The second-tier backward elimination GLM, including the 6 FDG ROIs surviving data reduction and other terms described above, yielded significant interactions for diagnosis with posterior cingulate (p<0.0001) and parahippocampal (p=0.0008)FDG hypometabolism. These interactions showed a greater decline in IADL performance as FDG metabolism decreased for the AD dementia group relative to the MCI group, and the MCI group relative to the CN group (Figure 1A). Main effects were found for greater orbitofrontal (p=0.009) and middle frontal (p=0.003, Figure 1B) FDG hypometabolism associated with greater IADL impairment. Greater frontal pole FDG hypometabolism (p=0.008) was associated with less IADL impairment. This latter effect is likely due to the high positive correlations (multicollinearity) of frontal pole FDG metabolism with some of the other FDG ROIs in the model since the univariate unadjusted relationship for frontal pole FDG hypometabolism parallelled the association with greater IADL impairment found with the other FDG ROI main effects, see Figure 1C and 1D. Additionally, significant relations were found for the covariates digit symbol and NPI-Q apathy item in expected directions. The model as a whole accounted for 71 percent of the variance of FAQ (Table 2). The residuals conformed reasonably to assumptions.
Figure 1.
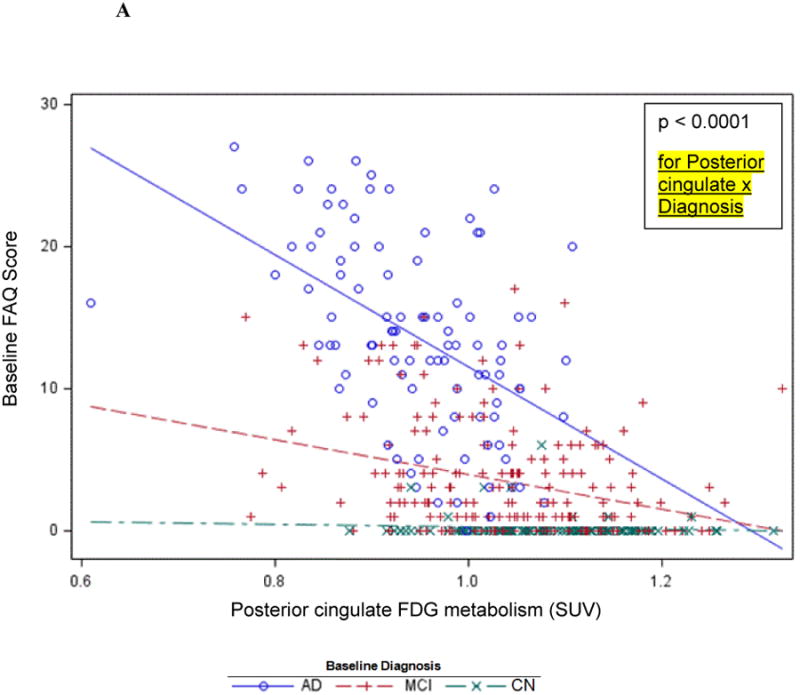

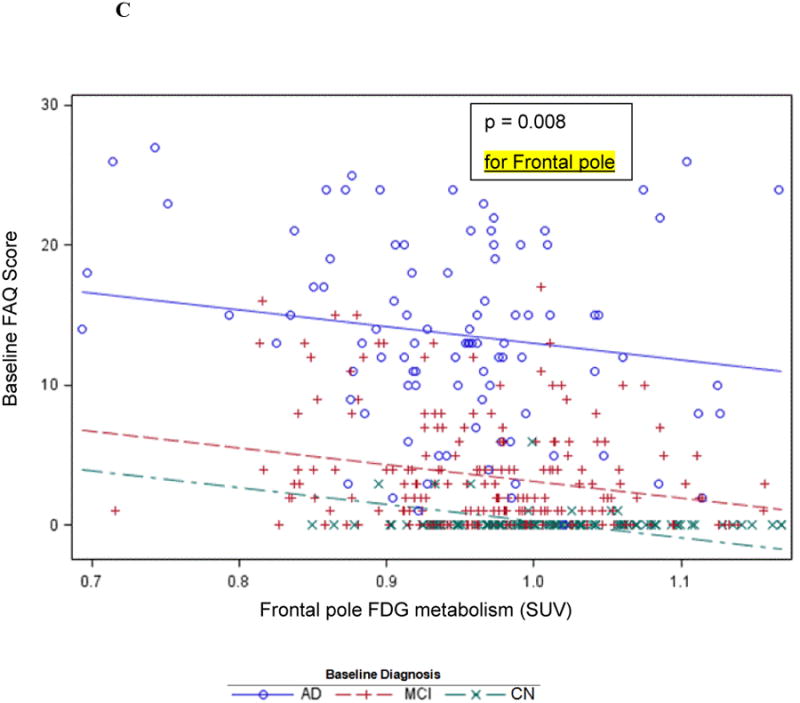
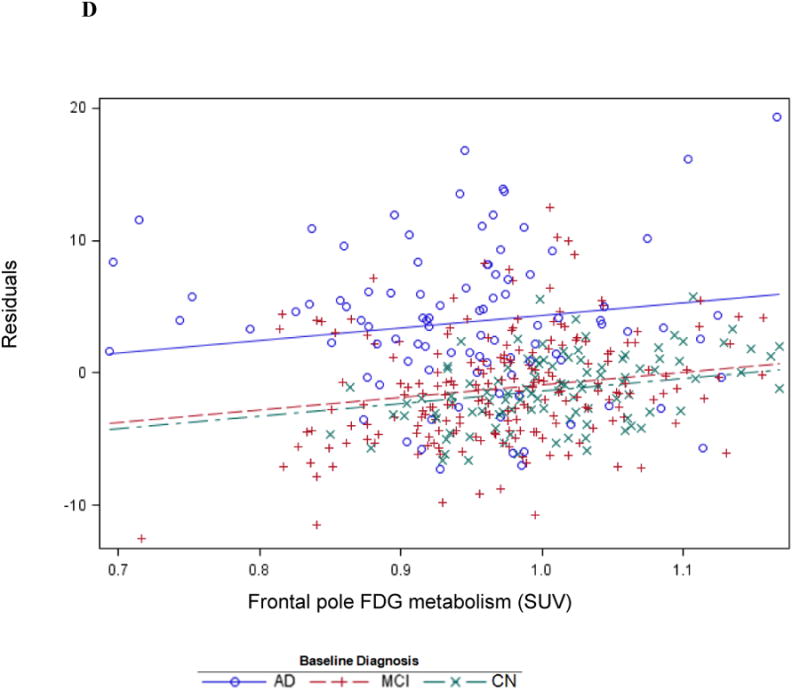
Cross-sectional graphs for predictor FDG ROIs and baseline diagnosis vs. baseline FAQ. Associations of FAQ with posterior cingulate (A), middle frontal (B), and frontal pole FDG metabolism (C and D) are shown. Actual observations (symbols) and values predicted (lines) by the indicated FDG ROI are shown (for simplicity, graphs are not adjusted for other predictors in the model, but those adjusted relations, if displayed, would be essentially the same as the ones shown). The indicated p value is that corresponding to the interaction or main effect for the FDG ROI as the case may be. The unadjusted association with frontal pole FDG (C) is contrasted with adjusted association in the reverse direction with all predictors except diagnosis × time residualized out first (D).
Table 2.
Cross-sectional general linear model of the association of FAQ to regional FDG metabolism and covariates.
| Model: R2=0.71, p<0.0001 | ||||||
|---|---|---|---|---|---|---|
| Predictor | β | 95% CI for β | p | % Variance Accounted for | ||
| Total | Partial | |||||
| Baseline Diagnosis | AD | 60.4 | 44.92, 75.89 | <0.0001 | 5.2 | 14.7 |
| MCI | 19.1 | 5.09, 33.03 | ||||
| CN | 0 | |||||
| Posterior cingulate | 2.99 | -5.93, 11.91 | 0.0004 | 0.9 | 2.9 | |
| Parahippocampus | 3.37 | -11.58, 18.32 | 0.009 | 0.5 | 0.5 | |
| Posterior cingulate × Diagnosis | AD | -28.62 | -41.18, -16.07 | <0.0001 | 1.5 | 4.6 |
| MCI | -8.64 | -19.05, 1.77 | ||||
| CN | 0 | |||||
| Parahippocampus × Diagnosis | AD | -31.91 | -49.77, -14.04 | 0.0008 | 0.9 | 3.0 |
| MCI | -10.32 | -27.19, 6.54 | ||||
| CN | 0 | |||||
| Middle frontal | -12.81 | -21.32, -4.30 | 0.003 | 0.6 | 1.9 | |
| Orbitofrontal | -13.86 | -24.28, -3.43 | 0.009 | 0.4 | 1.4 | |
| Frontal pole | 16.34 | 4.38, 28.29 | 0.008 | 0.5 | 1.5 | |
| Digit Symbol | -0.04 | -0.08, -0.01 | 0.02 | 0.3 | 1.1 | |
| NPI-Q apathy | 1.68 | 1.02, 2.33 | <0.0001 | 1.8 | 5.7 | |
AD (Alzheimer's disease), β (partial unstandardized regression coefficient estimate), CI (confidence interval), CN (clinically normal elderly), MCI (mild cognitive impairment), NPI-Q (Neuropsychiatric Inventory brief questionnaire form), ‘×’ indicates an interaction. CN was used as a reference group in predictors including diagnosis.
“%Variance Total” represents percent of total variance of FAQ uniquely associated with the indicated predictor (unbiased population estimate), “%Variance Partial” represents percent of variance of FAQ with portion associated with other predictors pre-removed, which is uniquely associated with the indicated predictor (unbiased population estimate).
Longitudinal Analyses
The mixed effects backward elimination model resulted in a significant interaction for both posterior cingulate (p=0.004) and middle frontal (p=0.0005) FDG hypometabolism with time whereby individuals with greater baseline hypometabolism demonstrated a greater rate of worsening IADL impairment (increasing FAQ scores) over time. The faster trajectory of deterioration in FAQ scores for these FDG-ROIs is depicted for each population across the AD spectrum in Figure 2. There was also a significant partialed interaction of orbitofrontal FDG metabolism (p=0.0006) with time whereby greater hypometabolism was associated with lesser rate of worsening of IADL impairment over time. This latter finding was possibly suggestive of multicollinearity due to the moderate positive correlations of orbitofrontal cortex with posterior cingulate gyrus and middle frontal gyrus. Additionally, significant relations were found in the expected direction for APOE4, RAVLT total learning, NPI-Q Apathy, baseline FAQ, sex (female higher), and interactions of diagnosis with time such that the AD dementia and MCI groups deteriorated faster than the CN group. The percent variance accounted for by the overall model fixed effects was 75%, and the percent variance accounted for by the entire mixed random and fixed coefficient model was 95% (Table 3). The residuals reasonably conformed to assumptions. Supplementary Figure 2 shows predicted longitudinal FAQ scores by diagnostic group.
Figure 2.
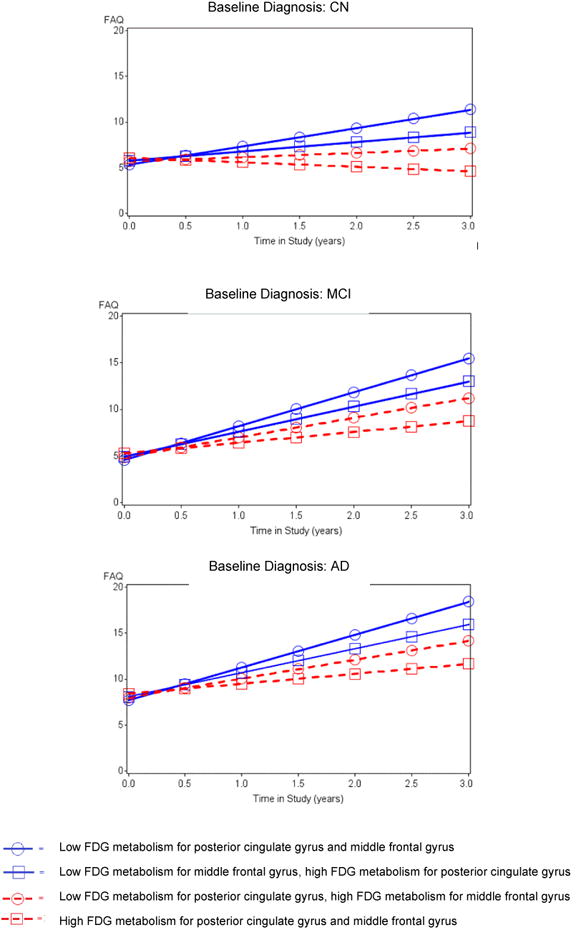
Predicted FAQ scores from fixed effects longitudinal model across time for posterior cingulate and middle frontal regions by diagnostic groups, showing a longitudinal association between hypometabolism and increased rate of progression of IADL impairment. ‘Low”=1 standard deviation below the mean; ‘High’= 1 above.
Table 3.
Mixed effects model of longitudinal FAQ association with baseline regional FDG metabolism and covariates.
| Model: R2=0.75 for fixed effects, p<0.0001; R2=0.95 including random terms, p<0.0001 | ||||
|---|---|---|---|---|
| Predictor | β | 95% CI for β | p | |
| Posterior cingulate gyrus | 2.00 | -3.00, 7.00 | 0.43 | |
| Middle frontal gyrus | 1.66 | -4.55, 7.86 | 0.60 | |
| Orbitofrontal cortex | -5.72 | -12.6, 1.15 | 0.10 | |
| Time in study (years) | 5.58 | 0.95, 10.2 | 0.004 | |
| Posterior cingulate gyrus × time | -4.80 | -8.10, -1.51 | 0.004 | |
| Middle frontal gyrus × time | -7.67 | -11.95, -3.39 | 0.0005 | |
| Orbitofrontal cortex × time | 8.42 | 3.64, 13.20 | 0.0006 | |
| Baseline FAQ | 0.79 | 0.71, 0.87 | <0.0001 | |
| RAVLT total learning | -0.05 | -0.09, -0.02 | 0.005 | |
| NPI-Q apathy | 0.60 | 0.01, 1.18 | 0.05 | |
| APOE4 | 0.52 | 0.05, 1.00 | 0.03 | |
| Sex | Female | 0.67 | 0.02, 1.33 | 0.04 |
| Male | 0 | |||
| Baseline Diagnosis | AD | 2.32 | 0.69, 3.96 | <0.0001 |
| MCI | -0.81 | -1.84, 0.21 | ||
| CN | 0 | |||
| Baseline Diagnosis × time | AD | 1.57 | 0.68, 2.47 | <0.0001 |
| MCI | 1.64 | 1.04, 2.25 | ||
| CN | 0 | |||
AD (Alzheimer's disease), APOE4 (Apolipoprotein E ε4), β (partial unstandardized regression coefficient estimate), CI (confidence interval), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), NPI-Q (Neuropsychiatric Inventory brief questionnaire form), RAVLT (Rey Auditory Verbal Learning Test). ‘×’ indicates an interaction.
CN and male sex were used as reference groups in predictors including diagnosis or sex.
As a follow-up to the above longitudinal mixed effects model, we re-ran the model with 3-way interactions for FDG-ROI with time and with diagnosis for the FDG ROIs found to have significant relations with time in the above model. We found no such 3-way interaction effects except for a barely significant one, suggesting that the association of posterior cingulate FDG hypometabolism with greater rate of worsening IADL impairment over time was slightly stronger in the MCI group when compared to the other diagnostic groups.
Discussion
This data-driven analysis sought to characterize the relationship between regional FDG metabolism and IADL cross-sectionally and longitudinally across CN, MCI, and mild AD dementia subjects. The cross-sectional results suggest that orbitofrontal and middle frontal FDG hypometabolism are associated with greater IADL impairment, independent of diagnosis at baseline, while there were significant interactions for FDG posterior cingulate and parahippocampal hypometabolism with diagnosis illustrated by the greater decline in IADL performance (increasing FAQ scores) as metabolism decreased for AD dementia relative to MCI, and MCI relative to CN. The longitudinal analysis showed that baseline middle frontal and posterior cingulate FDG hypometabolism were associated with a greater rate of increase in IADL impairment over time across the AD spectrum.
It is notable that although regional hypometabolism accounted for only a modest proportion (percent variance) of IADL impairment when compared to diagnostic group in these analyses, the contribution of regional hypometabolism was independent of diagnostic group for many of the regions. Considering that in early AD, IADL play a major role in the determination of diagnosis, it is challenging to find other important variables that are associated with IADL. Moreover, in these analyses, the FAQ (the IADL measure), was not included in the initial diagnosis determination, which provided the baseline diagnosis variable for all analyses.
Localization of IADL impairment to medial parietal and medial temporal synaptic dysfunction is consistent with regions typically prominently affected in MCI and AD dementia, while prominent involvement of frontal regions is less typical early on in AD. However, multiple studies have shown frontal hypometabolism in AD dementia though always to a lesser extent than temporo-parietal hypometabolism.[22-25] Moreover, progression from MCI to AD dementia, which is often driven by worsening IADL, has been associated with greater frontal hypometabolism[26,27]. Finally, frontal regions have been associated with executive function, which is essential for the performance of IADL.[4,28] Our results reinforce this association in early AD.
Salmon et al. demonstrated an association between IADL impairment and inferior temporal, inferior parietal, and superior occipital hypometabolism in patients with mild to moderate AD dementia in a cross-sectional study using FDG PET.[10] Our results demonstrated involvement of other temporal and parietal regions and did not show occipital involvement. Our sample was much earlier on the AD trajectory, possibly accounting for this difference.
In their seminal work using FDG PET to associate cognitive and functional impairments, Landau et al. showed that a composite of FDG regions typically affected in AD at baseline can be predictive of future increases in FAQ score indicative of IADL decline in MCI and AD dementia.[9] Similarly, our results suggest a faster trajectory of decline in IADL performance over time across the AD spectrum. However, we demonstrated which regions drove this association, and in addition to associations with posterior cingulate and temporal region (parahippocampus), we found associations with frontal regions. These results reinforce the cortical regional localization of progressive functional impairment in the AD spectrum.
Cross-sectional studies using other imaging modalities have also looked at the regional association with IADL in mild AD dementia and have found similar regional involvement to that found in our study. One study utilizing single-photon emission computed tomography showed an association between IADL impairment and lateral superior parietal, dorsolateral prefrontal, medial frontal, and occipital hypoperfusion.[29] Two studies employing structural magnetic resonance imaging (MRI) showed an association between IADL impairment and medial frontal and temporoparietal atrophy.[30,31] Of note, the FDG-PET results presented in our study were not corrected for partial volume effects and were therefore likely influenced by underlying atrophy. As such, our imaging results might be more representative of overall neurodegeneration, as seen with structural MRI, rather than regional synaptic dysfunction, which is more specific to FDG-PET.
Recent work by Royall et al. has shown that the cognitive correlates of IADL have been associated specifically with atrophy in the Default Mode Network (DMN)[32]. Our results also support this link in light of the association we found between IADL impairment and FDG hypometabolism in lateral and medial frontal, medial temporal, and posterior cingulate regions, all parts of the DMN. Future studies using resting-state functional MRI can directly assess the association between IADL and DMN connectivity.
The preliminary data reduction from 35 to 6 FDG ROIs allowed the data to drive the localization of IADL impairment, and subsequently allowed for the most critical ROIs to be identified. However, ROIs may not fully encompass significant regions, which can be captured in whole brain voxel-based analyses. Moreover, we looked at averaged bilateral regions and not separate left and right regions because that would have doubled the number of ROIs, making the data reduction process potentially too challenging. That said, the combined use of data-derived ROIs and a mixed effects longitudinal model allows the results to be more readily adaptable to patient populations.
This study features some limitations. The ADNI sample is not representative of the general population because subjects were carefully selected to have limited general health issues, psychiatric conditions, and cerebrovascular disease.[11,33] Moreover, subjects were highly intelligent premorbidly and had a high proportion of APOE4 carriers. However, we adjusted for these elements in all analyses. Additionally, this population resembles that of most AD spectrum clinical trials, making it easier to compare the results to clinical trial outcomes. The IADL scale used in these analyses, the FAQ, has been shown to be a sensitive measure for differentiating between CN, MCI, and mild AD dementia, but nearly all CN subjects had a score of 0 at baseline, representing a major floor effect.[4,8] Therefore, the cross-sectional results were driven by the MCI and AD dementia groups. However, the longitudinal results were significant across all diagnostic groups indicating that the FAQ is sensitive to the development of functional decline over time even in CN subjects. Drawbacks to the data-driven regression models used in these analyses are the instances of multicollinearity in which counter-intuitive findings may falsely indicate significant interactions. We observed such instances cross-sectionally for the main effect of frontal pole FDG hypometabolism with FAQ and longitudinally with orbitofrontal FDG hypometabolism and FAQ decline. In each case, the univariate unadjusted relationship for these FDG regions was in the expected direction, but the adjusted relationship in the model was in the reverse direction possibly due to positive inter-correlations with other FDG ROIs in the model. However, since significance tests run on the study results encompass numerous image pixels, and the mean SUV of the ROI and the maximum pixel SUV are determined when generating FDG ROIs, positive inter-correlations are expected and results are inherently conservative.[34,35] In their study of time series analysis using functional MRI, Locascio et al. determined possible sources of positive correlation as attributable to physiologically based associations, close spatial proximity of pixels, image smoothing, or image resolution that is finer than areas of (non–task-related) activation.[35]
In conclusion, orbitofrontal and middle frontal FDG hypometabolism are associated with IADL impairment in mild AD dementia cross-sectionally, while baseline posterior cingulate and middle frontal FDG hypometabolism predict worsening IADL impairment over time across the AD spectrum. These results demonstrate the association between patterns of regional FDG hypometabolism and complex daily functioning decline in early AD, and subsequently reinforce the importance of measuring IADL impairment throughout the course of AD progression. Frontal regions are not typically affected prominently in early AD. Our findings suggest that these regions are affected in the context of IADL impairment in early AD. In order to better develop early interventions, it is essential to understand the pathophysiological underpinnings of important clinical outcomes such as IADL. Our results move us closer to this goal.
Supplementary Material
Supplementary Table 1. 35 FDG ROIs used in analyses with corresponding data reduction results.
Supplementary Table 2. FDG ROIs surviving reduction analysis using backward elimination general linear model with FAQ as the dependent variable.
Supplementary Figure 1. Longitudinal raw FAQ scores by diagnostic group. Each line connects scores for the same person.
Supplementary Figure 2. Predicted longitudinal FAQ scores from mixed effects model by diagnostic group. Visit level error variance and random subject intercepts and slopes were removed, leaving only fixed effects.
Acknowledgments
This study was supported by R01 AG027435, K23 AG033634, K24 AG035007, the Massachusetts Alzheimer's Disease Research Center (P50 AG005134), the Harvard Aging Brain Study (P01 AGO36694), and the Alzheimer's disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) (See Supplementary Data). Drs. Keith Johnson and Alex Becker at the Department of Radiology, MGH; David Kennedy and Christian Haselgrove at the Centre for Morphometric Analysis, Harvard; Bruce Fischl, Martinos Center for Biomedical Imaging, MGH; Janis Breeze and Jean Frazier, Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance; Larry Seidman and Jill Goldstein, Department of Psychiatry of Harvard Medical School; Barry Kosofsky, Weill Cornell Medical Center.
The authors have received research salary support from Janssen Alzheimer Immunotherapy (DMR and GAM), Wyeth/Pfizer Pharmaceuticals (DMR and GAM), and Bristol-Myers-Squibb (RAS).
References
- 1.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3(111):111cm133. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. Thies W, Bleiler L. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012:8131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Luck T, Luppa M, Angermeyer MC, Villringer A, Konig HH, Riedel-Heller SG. Impact of impairment in instrumental activities of daily living and mild cognitive impairment on time to incident dementia: results of the Leipzig Longitudinal Study of the Aged. Psychol Med. 2011;41(5):1087–1097. doi: 10.1017/S003329171000142X. [DOI] [PubMed] [Google Scholar]
- 4.Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 6.Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: where do they fit in the diagnosis of Alzheimer's disease? Neurodegener Dis Manag. 2012;2(5):483–491. doi: 10.2217/nmt.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch Neurol. 2012;69(6):700–708. doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmon E, Lespagnard S, Marique P, Peeters F, Herholz K, Perani D, Holthoff V, Kalbe E, Anchisi D, Adam S, Collette F, Garraux G. Cerebral metabolic correlates of four dementia scales in Alzheimer's disease. J Neurol. 2005;252(3):283–290. doi: 10.1007/s00415-005-0551-3. [DOI] [PubMed] [Google Scholar]
- 11.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7(5):486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 13.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 14.Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, Arahata Y, Kato T, Gjedde A. Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010;214(4):303–317. doi: 10.1007/s00429-010-0246-0. [DOI] [PubMed] [Google Scholar]
- 15.Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz HG, Peters J, Bartenstein P, Lieb K, Schreckenberger M. SPM-based count normalization provides excellent discrimination of mild Alzheimer's disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009;44(1):43–50. doi: 10.1016/j.neuroimage.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 17.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83(2-3):155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness VS, Jr, Kennedy DN, Faraone SV, Tsuang MT. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61(8):935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Finner H. On a monotonicity problem in step-down multiple test procedures. J Amer Statist Assoc. 1993;88(423):920–923. [Google Scholar]
- 21.Šidák Z. Rectangular confidence regions for the means of multivariate normal distributions. J Amer Statist Assoc. 1967;62(318):626–633. [Google Scholar]
- 22.Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47(4):1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, Mathis CA, Klunk WE, Price J, DeKosky S, Wester HJ, Schwaiger M, Kurz A. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. Neuroimage. 2008;39(2):619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, Camus V, Abbas A, Landeau B, Guilloteau D, de La Sayette V, Eustache F, Desgranges B, Chetelat G. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer's disease dementia. J Neurosci. 2012;32(46):16265–16273. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(8):1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 27.Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, de la Sayette V, Viader F, Baron JC, Eustache F, Chetelat G. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain. 2009;132(Pt 8):2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 29.Nadkarni NK, Levy-Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild Alzheimer's disease. Neurobiol Aging. 2012;33(1):53–60. doi: 10.1016/j.neurobiolaging.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Gois Vasconcelos L, Jackowski AP, Oliveira MO, Flor YM, Bueno OF, Brucki SM. Voxel-based morphometry findings in Alzheimer's disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (Sao Paulo) 2011;66(6):1045–1050. doi: 10.1590/S1807-59322011000600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimers Dis. 2010;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royall DR, Palmer RF, Vidoni ED, Honea RA, Burns JM. The default mode network and related right hemisphere structures may be the key substrates of dementia. J Alzheimers Dis. 2012;32(2):467–478. doi: 10.3233/JAD-2012-120424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitwell JL, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Roberts RO, Boeve BF, Petersen RC, Jack CR., Jr Comparison of imaging biomarkers in the Alzheimer Disease Neuroimaging Initiative and the Mayo Clinic Study of Aging. Arch Neurol. 2012;69(5):614–622. doi: 10.1001/archneurol.2011.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, Larson S, Mankoff DA, Siegel BA, Van den Abbeele A, Yap J, Sullivan D. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47(6):1059–1066. [PubMed] [Google Scholar]
- 35.Locascio JJ, Jennings PJ, Moore CI, Corkin S. Time series analysis in the time domain and resampling methods for studies of functional magnetic resonance brain imaging. Hum Brain Mapp. 1997;5(3):168–193. doi: 10.1002/(SICI)1097-0193(1997)5:3<168::AID-HBM3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. 35 FDG ROIs used in analyses with corresponding data reduction results.
Supplementary Table 2. FDG ROIs surviving reduction analysis using backward elimination general linear model with FAQ as the dependent variable.
Supplementary Figure 1. Longitudinal raw FAQ scores by diagnostic group. Each line connects scores for the same person.
Supplementary Figure 2. Predicted longitudinal FAQ scores from mixed effects model by diagnostic group. Visit level error variance and random subject intercepts and slopes were removed, leaving only fixed effects.


