Abstract
Our team is designing and fully characterizing black raspberry (BRB) food products suitable for long-term cancer prevention studies. The processing, scale-up, and storage effects on the consistency, quality, bioactive stability, and sensory acceptability of two BRB delivery systems of various matrices are presented. BRB dosage, pH, water activity, and texture were consistent in the scale-up production. Confections retained >90% of anthocyanins and ellagitannin after processing. Nectars had >69% of anthocyanins and >66% of ellagitannin retention, which varied with BRB dosage due to the processing difference. Texture remained unchanged during storage. BRB products consumed in a prostate cancer clinical trial were well accepted in sensory tests. Thus, this study demonstrates that two different BRB foods can be formulated to meet quality standards with a consistent bioactive pattern and successfully scaled up for a large human clinical trial focusing on cancer risk and other health outcomes.
Keywords: black raspberry, scale-up production, consistency in characteristics, retention of phenolics, storage stability, sensory acceptance
INTRODUCTION
Black raspberries (BRB) (Rubus occidentalis) have gained much attention due to their distinct antioxidant, anti-inflammatory, antiangiogenesis, and other anticancer bioactivities demonstrated in both in vitro1–4 and in vivo5–10 studies. BRB chemopreventive properties are partially attributed to their wide range of phytochemicals including anthocyanins, ellagitannins, ferulic acid, β-sitosterol, bioflavonoids, fiber, vitamins, and minerals.11 Among them, anthocyanins and ellagitannins are considered as the most potent anticancer components6,7,12,13 and are found in higher concentrations in BRB compared to other berries.11,14 These bioactive compounds may be involved in inhibiting chronic inflammatory processes that are now increasingly associated with the initiation and promotion of cancer in various organs.6,10,15
The logical and theoretical advantages of a food-based approach for disease prevention and health are several. However, studies of specific foods have been limited by the variation in bioactive content and incomplete chemical characterization. Our goal is to define specific food products derived from fruits and vegetables that are fully chemically characterized, highly desirable, and easily incorporated into a diet and will be stable over time and storage conditions for long-term human trials of health and disease outcomes. These would represent complex mixtures of bioactive phytochemicals that, when consumed, may affect multiple targets representing defective signaling pathways in mammalian carcinogenesis and thus may have additive and/or synergistic activity to enhance anticancer efficacy. Additionally, active agents, each provided at lower dose, may reduce risk of toxicity.
The food matrix is a key factor that influences the release, absorption, and thus bioavailability of bioactive components.16 However, most studies focused on the absorption of bioactive extracts in water or bioactives as part of a meal with other food components.16 Walton et al. reported that black currant anthocyanins in a more viscous oatmeal matrix had delayed and decreased absorption and excretion of these bioactives but did not change the metabolism in a rat model compared with anthocyanins in water.17 Ahn-Jarvis et al. showed that significantly higher soy isoflavone microbial metabolites were excreted in female subjects consuming soy bread compared to soy beverage.18 However, the effects of food matrix on cancer prevention are largely unknown.
We have developed two different matrices, a pectin-based confection and a nectar (viscous juice), to be used in delivering BRB bioactives in human clinical trials and tested them in a cohort of men with prostate cancer. Food products containing freeze-dried BRB powder have been developed to increase BRB acceptability in clinical trials compared to the BRB powder with water added and a nonfood bioadhesive gel used previously.5,19 Pectin confections have been found to be an appropriate solid matrix to deliver BRB bioactive compounds and characterized for their in vitro dissolution rate, retention of bioactive compounds during processing, and high sensory acceptability.20 Compared to a solid matrix, liquids deliver bioactives more rapidly to the gastrointestinal tract (GIT), hastening digestion and absorption.18 Significantly, ellagic acid was detected in the BRB powder in water.19,21 For nectar formulation, the addition of stabilizer (such as pectin, xanthan, and agar) to the juice would increase viscosity and thus aid in the suspension of particles such as fruit pulp and seeds.22
Aside from the formulation, the processing and storage need to be considered carefully to ensure minimal effect on the levels of berry bioactives in products. For example, the manufacturing and storage of berry jam, juice, and puree as well as canning yielded a decrease in anthocyanins and flavonoids, whereas ellagitannins content remained heat stable and more influenced by the processing related to seeds exclusion.23–25 Formulation and processing of a food delivery system for a human clinical trial and subsequent scale up require consistency of ingredients and final product as well as quality and bioactive retention during storage. As such, the objective of the current study was to assess the consistency, quality, and shelf stability of BRB pectin confections and nectar developed for clinical trials and demonstrate their acceptability among men enrolled in a prostate cancer clinical trial.
MATERIALS AND METHODS
Standardization of All Ingredients and Adjustment of Formulations from Laboratory-Scale to Large-Scale Production
To ensure the homogeneity of the food products for the clinical trial, all ingredients were purchased in a single lot: corn syrup (Gordon Food Service, Springfield, OH, USA), sugar (U.S. Food Service, Cincinnati, OH, USA), and pectin (RS 400, Danisco, USA Inc., New Century, KS, USA). Pectin (CF 130 B) from Danisco was previously used in the laboratory scale for both confections and nectars; however, due to the lack of availability for the scale-up production, RS 400 pectin was applied. Freeze-dried BRB powder was obtained from both Stoke Raspberry Farm (Wilmington, OH, USA) (cultivar ‘Jewel’) and BerriProducts LLC (Corvallis, OR, USA) (cultivar ‘Munger’). Mixed BRB powder (Ohio:Oregon = 2:3) was blended using a mixer (Day mixing, 1608, Federal Equipment Co., Cleveland, OH, USA) in the Food Pilot plant in the Parker Food Science Building at The Ohio State University (OSU). After mixing, the powder was separated into 4 kg/bag, sealed by using a vacuum sealer (MC-30 computer control, Sipromac, Houston, TX, USA), and kept at −40 °C, avoiding light, until use. According to Stoner et al.,11 the levels of 26 nutrients in BRB remained within 10–20% of the original measurements for at least 2 years in the freeze-dried form stored at −20 °C. Scale-up production of confections and nectars required reformulation due to ingredients’ change and processing parameter adjustments with the goal of retaining bioactives and quality. Final selected formulations are shown in Table 1.
Table 1.
Adjusted Formulations of BRB Confections and Nectars from Laboratory-Scale to Large-Scale Production
| confections | nectars (two BRB doses) | |||
|---|---|---|---|---|
| composition (%) | lab scale | large scale | lab scale | large scale |
| BRB powder | 20.0 | 20.0 | 4.0/8.0 | 4.0/8.0 |
| sugar | 35.0 | 29.5 | 3.0 | 3.0 |
| corn syrup | 12.0 | 11.0 | 1.0 | 1.0 |
| water | 29.5 | 37.8 | 90.2/86.2 | 91.2/87.2 |
| pectin | 1.5 | 1.2 | 1.8 | 0.8 |
| 50% (w/w) citric acid | 2.0 | 0.5 | ||
| total | 100 | 100 | 100 | 100 |
Scale-up Production of BRB Products
Confection Production
Confections were prepared in 1 kg batches, and formulations adjusted from laboratory scale as shown in Table 1. In the preparation, water, sugar, corn syrup, and pectin were mixed and then stirred and heated on a hot plate (PC-620 D, Corning, Tewksbury, MA, USA) until a final temperature of 95 °C and °Brix from 65 to 68 were reached, which was determined by a hand-held refractometer (Fisher Scientific Japan Ltd., Tokyo, Japan). These processes were the same as laboratory scale and achieved within 45 ± 3 min. When the mixture had cooled to 78 °C at room temperature, freeze-dried BRB powder was added and mixed into the gel. The mixture was deposited at 65 °C with a pastry bag into molds (2115-1521 Truffles, Wilton, Columbus, OH, USA). Confections equilibrated at room temperature (21 ± 2 °C), avoiding lights for 24 h before packaging. All of the products was prepared in the OSU Pilot Plant in the Parker Food Science Building with relative humidity (RH) of 0.1–3.5% and temperature of 22 ± 1 °C. Random samples (n = 3) from each batch (83 ± 4 pieces) were obtained to evaluate their water activity, water content, and pH. Four random batches were chosen to track the stability of bioactives with HPLC (n = 6 per batch), rheological (n = 6 per batch), and textural properties (n = 10 per batch).
Low-Dose BRB Nectar Production
Nectar with 10 g of BRB dose was produced in MicroThermics, Inc. (Raleigh, NC, USA) in a 120 kg batch according to the large-scale formulation shown in Table 1. In the preparation, pectin, sugar, corn syrup, and water were first mixed and heated to 95 °C with a UHT/HTST Lab Direct and Indirect Processing System (MicroThermics Inc.), and then the premix was kept in an extended hold cabinet to maintain a temperature above 60 °C. BRB powder was added into the premix, and an adjustable speed drive (Leeson Electric Corp., Grafton, WI, USA) was used for mixing. The mixture was then pumped into a sterilized UHT/HTST Lab Direct and Indirect Processing System with 3 L/min flow rate and pasteurized at 75 °C for 15 min according to a method adapted from Silva and Gibbs to inactivate enzymes and the majority of molds.26 Nectar was then cooled to 25 °C and filled into 250 mL Nalgene PETG sterilized bottles (Thermo Fisher Scientific Inc.) in a Clean Fill Hood with sterile product outlet (MicroThermics Inc.). Nectars were stored at 4 °C until use. Random bottles (n = 9) were selected to evaluate the pH, Brix, water activity, viscosity, and bioactive compounds with HPLC.
High-Dose BRB Nectar Production
Nectar with 20 g of BRB dose was produced in the OSU Pilot Plant in the Parker Food Science Building because Microthermics equipment was unable to process such high-viscosity nectar. A 100 kg batch of nectar was prepared with the large-scale formulation shown in Table 1. In the preparation, pectin, sugar, corn syrup, and water were first mixed well and heated in a steam-jacketed kettle (A5132-1 ATMOS, Hamilton Kettles, Cincinnati, OH, USA). Once the temperature reached 95 °C, BRB powder was added and mixed well. Temperature was kept at 95 °C for 15 min to pasteurize, a method adopted from the production of BRB puree and strawberry nectar.24,27 After heating, nectar was cooled to 75 °C and filled into 250 mL Nalgene PETG sterilized bottles (Thermo Fisher Scientific Inc.) with a volumetric piston filler (Simplex, AS-1, Napa, CA, USA). A fixed volume of nectar (8 oz) was controlled to fill into bottles. After cooling, nectars were stored at 4 °C until use. Random bottles (n = 9) were selected to evaluate the pH, Brix, water activity, viscosity, and bioactive compounds with HPLC.
Phenolic Analysis of BRB Products
Extraction
One gram of BRB powder or confection/nectar containing 1 g of BRB was fully dispersed into water containing 5% (v/v) formic acid and adjusted to a final volume of 50 mL. Aliquots of 2 mL were immediately removed from the well-mixed solution and then mixed with 8 mL of acetone containing 5% (v/v) of formic acid, followed by bath sonication for 1 min in an FS3OH Sonicator (Fisher Scientific, Fair Lawn, NJ, USA) and then centrifuged at 2000g for 10 min with a IEC HN-SII centrifuge (Damon Corp., Needham Heights, MA, USA). Supernatant was removed to a clean 22 mL glass vial (Fisher Scientific, Pittsburgh, PA, USA) with a disposable glass pipet (Fisher Scientific, Pittsburgh, PA, USA). Solvent of acetone/water (80:20, v/v) containing 5% formic acid was used to extract the pellet twice more until the pellet was colorless. A Speedvac concentrator (SPD 131DDA-115, Thermo Fisher Scientific, Waltham, MA, USA) was used to dry sample extracts.
Identification
A Waters 2695 HPLC with a Waters 996 photodiode array detector (Waters, Milford, MA, USA) combined with a Waters Q-Tof Premier (Micromass MS Technologies, Manchester, UK) was used for the identification of phenolic compounds. Dried extracts were dissolved in acetone/water (20:80, v/v) containing 5% formic acid and filtered through PTFE filters (Fisher Scientific, Pittsburgh, PA, USA; 0.2 µm, 13 mm diameter) before injection. Separation was carried out on a Symmetry C18 (75 mm × 4.6 mm i.d, 3.5 µm particle size) reversed phase column (Waters). The mobile phase for separation consisted of 1% (v/v) formic acid in water (A) and 1% (v/v) formic acid in acetonitrile (B). Initial mobile phase composition, 100% A and 0% B, was followed by a linear gradient to 80% A at 10 min, 70% A at 12 min, 50% A at 14 min, 0% A at 16 min, and returned to 100% A at 16.1 min and re-equilibrated through 20 min. Column temperature was kept at 35 °C, and flow rate was 1.3 mL/min. HPLC flow was split 1:10 prior to MS. The MS analysis was performed in positive and negative ion modes and calibrated with sodium formate in the range of m/z 50–3000. Leucine enkephalin was used as lockspray mass with m/z at 556.2771+/554.2615−. Capillary voltage was at 2.8 kV in negative mode and 3.2 kV in positive mode. Dry gas flow was at 700 L/h, cone voltage at 35 V, and desolvation gas temperature at 480 °C. For MS/MS analysis, the same instrumental parameters were used except that collision energy was set to 25 eV. Standards of cyanidin 3-gucoside, cyanidin 3-sambubioside, cyanidin 3-rutinoside, ellagic acid, and rutin were run to support peak identification.
Quantification
BRB confections and nectars were analyzed by HPLC to quantify anthocyanins, ellagitannins, and ellagic acid content. Freeze-dried BRB powder was quantified to determine the changes of BRB bioactives during production and subsequent storage in products. Quantification in all BRB samples was carried out using an Agilent 1100 series (Agilent, Waldbronn, Germany): G 1322A degasser, G 1328A manual injector, G 1311A quaternary pump, and G 1365A MWD controlled by the ChemStation software (Agilent). Chromatographic separation was performed with the same column and method as those in the identification step. Samples were injected with a volume of 20 µL. Absorbance at 260, 355, and 520 nm wavelength was recorded. Anthocyanins were quantified as one peak at 520 nm with cyanidin 3-glucoside as external calibrant24 with concentrations ranging from 3.125 to 100.0 µg/mL. Ellagic acid was used as the external standard to quantify free ellagic acid and ellagic acid derivatives under 260 nm with a range of 0.25–4.0 µg/mL. Ellagic acid and ellagic acid derivatives were represented as total ellagic acid. Ellagitannin quantification was following the method of Gasperotti et al.,28 in which (sanguiin H-6) ε260 nm = 63615 M−1 cm−1 and (ellagic acid) ε260 nm = 28266 M−1 cm−1 in 88% acetonitrile and 12% 1% formic acid in water (v/v) were reported. The standard curve of sanguiin H-6 was derived from the ellagic acid standard curve using a calculated factor (28266/63615 = 0.44).
Retention of Anthocyanins, Ellagitannins, and Total Ellagic Acid in BRB Products
The retention rate of these compounds from BRB confections and nectars was calculated using the formula
where Cphenolics = concentration of anthocyanins or ellagitannins in products, CBRB = percentage of BRB in products after processing, Cphenolics′ = concentration of anthocyanins or ellagitannins in freeze-dried BRB powder. Cphenolics and Cphenolics′ were obtained from HPLC quantification, and CBRB was obtained from products’ preparation.
Characteristic Consistency of the BRB Products
BRB delivery dosage, water activity (Aqua Lab Water Activity Meter series 3, Pullman, WA, USA), pH, and soluble solid (°Brix) were determined to check the consistency of BRB products needed for the clinical trials. A SevenMulti pH conductivity meter (Mettler Toledo Inc., Columbus, OH, USA) was used to measure the pH of the confection (mashed into a paste) and nectar. °Brix was measured by hand-held refractometers with ranges of 0–32° and 58–90° (Fisher Scientific Japan Ltd., Tokyo, Japan). Water content of confections was obtained from thermogravimetric analysis (TGA) (Q5000, TA Instrument, New Castle, DE, USA) by heating 15–20 mg samples at 5 °C/min from 25 to 160 °C. Moisture content was calculated as described in Siegwein et al.29
Rheological Consistency of BRB Products
Confections were analyzed with an AR 2000ex Controlled Stress Rheometer (TA Instruments) with a 20 mm diameter probe. Dynamic strain sweep (0.01–10%) at 25 °C and different frequencies (0.1, 1, 10, 100 Hz) were first tested to determine the linear viscoelastic region (LVR), and all samples exhibited a linear response for strain values up to 0.15%. Dynamic frequency sweep (0.1–100 Hz) tests were then carried out at 25 °C with 0.1% strain to obtain viscoelastic behavior of confections. Storage modulus (G′), loss modulus (G″), and complex viscosity (η*) were recorded and compared. Changes of apparent viscosity (η) in nectars with a 40 mm diameter probe were recorded with increasing flow shear rate (0.001–1000 s−1) at 1 Hz and 25 °C. Random samples from scale-up production were also analyzed. Texture profile analyses (TPA) of confections were obtained with an Instron 5542 Universal Testing Machine (Instron Corp., Canton, MA, USA) with Bluehill 2 Materials Testing Software (Instron Corp., Norwood, MA, USA) with 40% compression at 1 mm/s rate by 35 mm diameter probe. Hardness, cohesiveness, springiness, and chewiness were obtained according to the methdod of Peleg.30
2-Month Storage of BRB Products
A 2-month shelf life required for BRB products to allow for recruitment and clinical intervention was studied. Both confections and nectars were packaged; plastic 2 oz cups with lids (Gordon Food Service, Springfield, OH, USA) were for confections, and amber bags were used for each nectar bottle; the samples were kept in the dark at 4 °C for 2 months. Fresh samples and samples at 4, 6, and 8 weeks were collected to evaluate the storage stability of bioactive compounds and textural properties in the products. The number of samples used for analysis was the same as those in fresh samples described earlier.
Scale-up Production Design for a Prostate Cancer Clinical Trial
This clinical trial was approved by the OSU Clinical Scientific Review Committee (CSRC) first and subsequently by the Institutional Review Board (IRB) with approval numbers OSU-12125 and 2012C0096, respectively. In total, 56 patients newly diagnosed with resectable prostate cancer (average age = 61.6 ± 1.02 years) were recruited into this study, 32 of which were in BRB functional food intervention groups and 24 in dietary assessment groups (control). Only BRB intervention groups were focused on and discussed in this study. Design of intervention groups in this clinical trial, BRB dosage in products, and total amount of processed products are shown in Figure 1. All patients signed written informed consent forms before participation. The BRB intervention period for each patient lasted 20–30 days depending on the scheduled surgery day. Extra products were given to participants in case of a need to delay surgery. Only the sensory acceptability of the products tested in this study will be discussed herein.
Figure 1.

BRB delivery dosage and total BRB products in a prostate cancer clinical trial.
Sensory Evaluation
To obtain the overall acceptability of BRB products, a sensory evaluation survey was developed and approved by the IRB (2012C0096). At the end of the clinical trial, sensory tests were conducted using a nine-point hedonic scale (1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much; 9, like extremely) to evaluate the overall acceptability and acceptability of aroma, flavor, sweetness, texture, and grittiness.31
Statistical Analysis
A one-way analysis of variance (ANOVA) and Tukey’s post hoc test were used with SPSS 20.0 (Chicago, IL, USA) to determine the significance in the characteristics between confection batches. The means of rheological properties and contents of bioactive compounds in BRB confections and nectars during storage were compared as well. An independent t test compared differences between laboratory-scale and large-scale products with Minitab 15 statistical software (Minitab Inc., State College, PA, USA). A p ≤ 0.05 was considered to be significantly different.
RESULTS AND DISCUSSION
Formulation Adjustment of BRB Products from Laboratory-Scale to Large-Scale Production
Formulations of pectin-based confection and nectar (Table 1) were adjusted from the laboratory scale to achieve similar textural properties as well as a lower caloric content for clinical subjects. Pectin RS 400 used in large-scale production had a higher degree of esterification (70%) compared to CF 130 B used in laboratory scale (31% esterification and 19% of amidation); therefore, pectin was decreased from 1.5 to 1.2% in BRB confection to reach similar rheological properties as indicated by similar G′, G″, and η* values (Figure 2). Sugar in BRB confection and citric acid were both decreased in scale-up versions to achieve the desired lower calorie content (from 29.3 to 23.1 kcal/piece confection). Calorie control was essential so as not to induce weight gain during intervention. For the nectar, calorie adjustment was not required, but pectin addition was decreased from 1.8% in laboratory scale to 0.8% for clinical trial so as to reach similar rheological end points during scale up (Figure 3).
Figure 2.

Frequency sweep of laboratory-scale confection (circles) and large-scale confection (triangles): (solid symbols) storage modulus G′; (open symbols) loss modulus G″; (gray symbols) complex viscosity |η*|.
Figure 3.
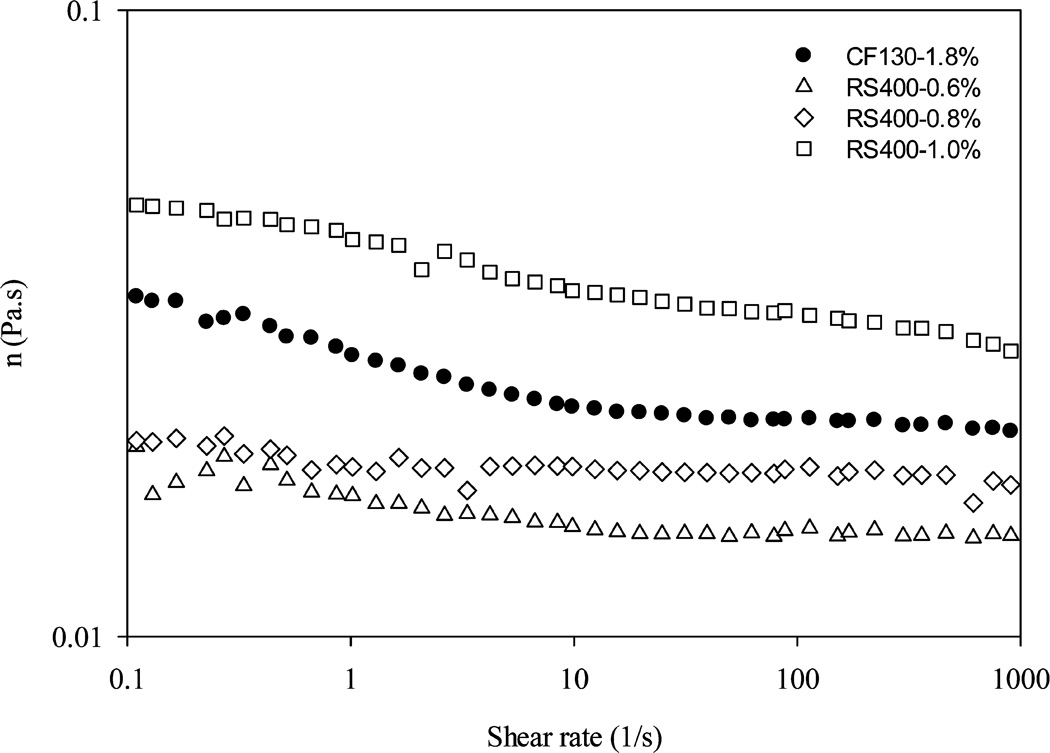
Viscosity of nectar premix with different concentrations of large-scale RS 400 pectin compared to CF 130 B pectin used in laboratory scale.
Identification and Quantification of Phenolics
Phenolics in BRB powder were identified with a combination of HPLC-MS/MS, accessible standards, UV–vis, and reported mass. HPLC chromatograph and peak identification information for phenolics are shown in Figure 4 and the accompanying table. Peaks 1a,b and 1c,d coeluted in the HPLC but were identified by MS as cyanidin 3-glucoside, cyanidin 3-sambubioside, cyanidin 3-xylosylrutinoside, and cyanidin 3-rutinoside with a characteristic fragment of cyanidin aglycon [M – H]− at m/z 285 and other reported fragment ions.32 In this study, pelargonidin 3-rutinoside was detected in a very low content with MS; thus, only the four major cyanidin glycosides were quantified as anthocyanins in BRB powder and the processed products. This HPLC method was not developed to separate each anthocyanin well due to other phenolics including ellagitannins and quercetin glycoside. Peaks shown with a retention time of 7.4–8.6 min were unknown but considered as possible anthocyanin-related compounds due to absorbance at 480 nm and MS [M – H]− m/z 651 and MS/MS m/z at 285 and 593.
Figure 4.

HPLC profile of freeze-dried BRB powder and tentative identification of each peak.
Peak 2 was tentatively identified as quercetin xylosylrutinoside with [M – H]− m/z at 741 and MS/MS m/z 301.036 and 355 nm UV–vis. Peak 3 was identified as sanguiin H-6 with MS [M – H]2− m/z at 934; MS/MS m/z at 935, 633, and 301 with UV–vis featured at 260 nm and comparison with previous reports.33–35 Lambertianin C with MS [M – H]2− m/z at 1401 and a true mass of 2803 was identified according to Hager et al.,35 but it was detected in a minor peak at 8.57 min and coeluted with some unknown anthocyanin degradation compounds in this HPLC method. However, lambertianin C was reported as one of the major ellagitannins (>32%) besides sanguiin H-6 (>42%) in six raspberry (Rubus idaeus L.) cultivars.28 Several ellagitannins eluted before anthocyanins with MS of 783, 933, and 633, which were likely pedunculagin isomer, castalagin/vescalagin isomer, and galloyl-HHDP glucose isomer according to Hager et al.35 In this study, sanguiin H-6 was the most abundant ellagitannin detected and thus quantified as ellagitannin and compared during processing and storage. In peaks 4–6, rutin, ellagic acid, and quercetin hexuronic acid were identified with a combination of available standards (rutin and ellagic acid), accurate MS, MS/MS, UV–vis, and reported data.36 Methyl-ellagic acid-pentose, acetylellagic acid-pentose, malonyl-methyl-ellagic acid-pentose, and acetyl-methyl-ellagic acid-pentose were identified according to the accurate masses of their molecular ions and fragment m/z (MeEA [M – H]− m/z at 315, EA at 301) by MS/MS in peaks 7–10.28,33
Changes of Phenolics during Laboratory-Scale and Large-Scale Processing
HPLC profiles of BRB confections and nectars were the same as that of BRB powder, but in some cases were present at different concentrations due to the effect of processing. Anthocyanins, ellagitannin, and total ellagic acid in BRB products were quantified according to the identification above and then were compared to that of laboratory scale to ensure similar delivery of these bioactives for the clinical trial. Retention rates of phenolics in both laboratory-scale and large-scale products are shown in Table 2. Confections contained 6.8 mg/g of anthocyanins and proved to have the highest anthocyanin retention rates (94%) after processing. In addition, there were no distinct changes of anthocyanins and ellagitannin between laboratory-scale and large-scale confections, demonstrating consistency in the processing steps (p > 0.05). This was mainly due to the low mixing temperature of the pectin gel with freeze-dried BRB powder (78 °C). For the nectars with 10 g of BRB dosage, 86% of anthocyanins were retained after large-scale processing, whereas only 69% were left in the 20 g of BRB dose. This was related to the different processing conditions of the low and high dose of BRB nectars: 75 °C for 15 min and 95 °C for 15 min, respectively. Higher temperature and longer heating time were required to pasteurize the 20 g of BRB dose nectars. Both anthocyanin retention rates in large scale were significantly lower than those in the laboratory scale (p < 0.05), mainly due to the longer mixing time of 10 g BRB powder with premix at 60 °C in the hold cabinet and higher heating temperature for the 20 g dose nectar in the large-scale production. Anthocyanin degradation is known to follow firstorder reaction kinetics, and processing at lower temperature for shorter time improves the retention rates.37 In addition, multiple processing steps influence the retention of anthocyanins. Significantly lower retention of monomeric anthocyanins was found in nonclarified and clarified juices (31 and 27%) than in BRB puree (63%) and berries canned in water (58%).24 The comparatively higher retention rates of anthocyanins in BRB confections and nectars (69–94%) may be due to the use of whole BRB powder and single mixing and/or heating steps. Other factors such as the presence of oxygen, light, enzymes, and pH fluctuations may also influence the loss of anthocyanins.38
Table 2.
Retention of Phenolics in BRB Products after Processinga
| product | processing scale | anthocyanins (%) | ellagitannins (%) | total ellagic acid (%) |
|---|---|---|---|---|
| confection | lab | 94.3 ± 0.3a | 93.4 ± 1.5a | 101.3 ± 2.3a |
| large | 94.4 ± 0.98a | 95.6 ± 1.9a | 106.4 ± 3.3a | |
| nectar, 10 g of BRB | lab (75 °C, 10 min) | 92.5 ± 0.3a | 95.1 ± 1.0a | 105.2 ± 3.2a |
| large (75 °C, 15 min) | 86.1 ± 2.7b | 66.4 ± 3.5b | 113.6 ± 6.8b | |
| nectar, 20 g of BRB | lab (85 °C, 10 min) | 81.7 ± 0.3a | 89.5 ± 0.8a | 110.4 ± 4.2a |
| large (95 °C, 15 min) | 69.0 ± 2.8b | 91.0 ± 3.6a | 127.9 ± 6.2b |
Values represent means ± standard error (n = 24 for confections and n = 9 for nectars). Means with different letters in the same column within each BRB product are significantly different (p ≤ 0.05).
Ellagitannins were retained at 95 and 91% after processing of confection and nectar with the 20 g of BRB dose in large-scale production. Nectar with lower BRB dose had 66% of ellagitannin retention rate, which was significantly lower than those of confection and nectar with 20 g of BRB as well as that of laboratory scale (p < 0.05). This may be due to partial seed sedimentation in the Microthermics flow processing system because higher BRB dose nectar would cause blockage of the system, which resulted in the higher BRB dose nectar being processed in the OSU pilot plant. This was consistent with previous results where BRB ellagitannins were retained in higher quantities in purees (65%) and berries canned in water or syrup (79%) but not juice (only 31–33% retained), where the exclusion of seeds accounted for the significant loss.39 Similar results were also found in blackberry processing, where 30 and 18% of ellagitannins were recovered in nonclarified and clarified juices, respectively, whereas canning and pureeing had little effect.25
All BRB products demonstrated above 100% ellagic acid retention rates. Ellagic acid increased in raspberry juice after heat processing due to better extraction from the cell matrix.40 It was possible that the heat treatment of BRB products in this study may have improved ellagic acid extraction compared to the BRB powder.25 Additionally, increased ellagic acid may result from hydrolysis of ellagitannins during processing.
Consistency of Scale-up Production of BRB Products
Characteristics of BRB confections from different batches and nectars from randomly selected bottles were measured to evaluate the production consistency (Table 3). Confections were prepared within 4 weeks; 50 batches (83 ± 4 pieces per batch) totaled 4162 pieces. The average weight of the confections was 8.42 ± 0.35 g with a BRB concentration of 24.95 ± 0.48%. Dosage of BRB was within 5% of desired concentration (Table 3). Physical properties such as pH and water activity also showed consistency in the products (Table 3).
Table 3.
Characteristics of BRB Confections and Nectars in Scale-up Processinga
| characteristic | BRB confections (per piece) |
nectar, 10 g of BRB (per bottle) |
nectar, 20 g of BRB (per bottle) |
|---|---|---|---|
| BRB dosage (g) | 2.10 ± 0.09 | 10.48 ± 0.31 | 20.22 ± 0.45 |
| pH | 3.44 ± 0.02 | 3.64 ± 0.02 | 3.56 ± 0.02 |
| Aw | 0.72 ± 0.02 | 0.95 ± 0.00 | 0.95 ± 0.00 |
Values represent means ± standard error (n = 3 per batch for confections and n = 9 for nectars).
Rheological properties of confections from different batches (randomly selected) were evaluated (Figure 5A,B). There were no distinct differences in G′, G″, and η* between different batches at 0.1, 1, 10, and 100 Hz (p > 0.05), suggesting the confections were made with consistent textural properties. Viscosity of samples from 10 and 20 g of BRB nectar was Viscosity of samples from 10 and 20 g of BRB nectar was measured with shear flow tests, and results are shown in Figure 5C. With increased shear rate, viscosity showed smaller difference between replicates. Nectars with 10 and 20 g of BRB had 0.15 ± 0.02 and 0.72 ± 0.13 Pa·s viscosity at 1.00 s−1 shear rate, respectively.
Figure 5.
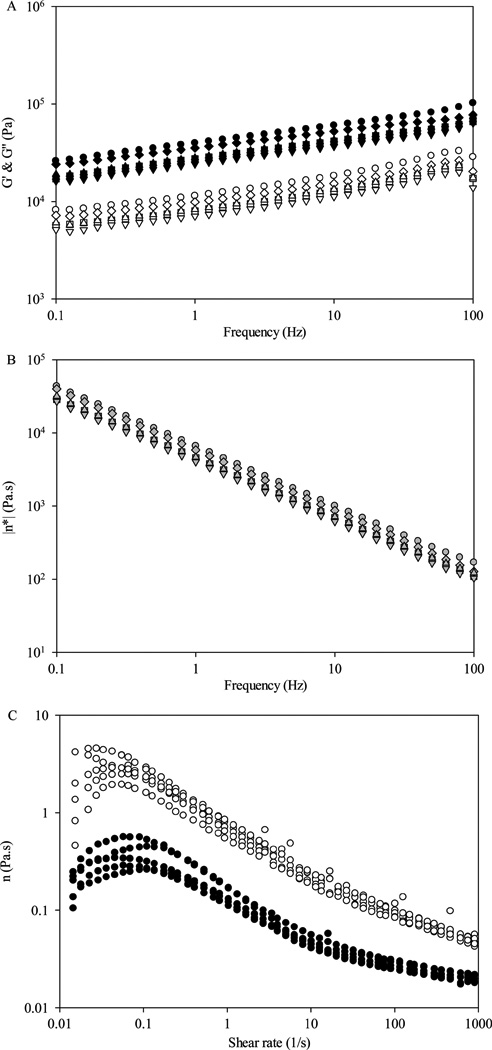
Rheological consistency of BRB products in the scale-up production: (A) confections G′ (solid symbols) and G″ (open symbols); (B) confection |η*| (gray symbols); (C) nectar η, 10 g (solid symbols) and 20 g (open symbols).
Stability of Phenolics during Storage
Concentration and daily delivery amount of anthocyanins, ellagitannins, and total ellagic acid in fresh products are shown in Table 4. Confections delivered higher daily amount of anthocyanins and ellagitannins than nectars for equivalent BRB doses due to higher initial value of these bioactives in confections (Table 2). The retention rate of these phenolics in BRB products during storage is shown in Table 5. During storage, anthocyanins in confections decreased significantly from 94 to 87% in 4 weeks but were fairly constant (no statistically significant change) up to 8 weeks of storage. A similar trend was observed for the anthocyanins in the low nectar dose, whereas the 20 g dose had no significant change until 8 weeks of storage. The depletion of anthocyanins in the confections may be a consequence of residual enzymatic activity, as was observed in processing of blueberry and storage of strawberry nectar.27,41 Gössinger et al. reported that enzymes were not fully inactivated even after a heat treatment of 90 °C for 60 min.27 Therefore, to promote retention of anthocyanins, a balance may need to be reached between initial heat processing to achieve enzyme inactivation (high heat for longer time results in lower anthocyanin concentration) and stability during storage (higher heat initially applied, less degradation during storage). Additionally, storage conditions may also play an important role in the stability of anthocyanins. Refrigerated storage resulted in lowering of the polymeric color percentage of blueberry juice42 and reducing the rate of anthocyanin degradation43 compared to room temperature.
Table 4.
Concentration and Daily Delivery of Phenolics in Fresh BRB Products
| products for BRB deliverya | ||||
|---|---|---|---|---|
| confections (5 pieces/day) |
confections (10 pieces/day) |
nectar, 10 g of BRB (one bottle/day) | nectar, 20 g of BRB (one bottle/day) | |
| concentration | ||||
| anthocyanins (mg/g) | 6.8 ± 0.1 | 6.8 ± 0.1 | 1.0 ± 0.03 | 1.6 ± 0.1 |
| ellagitannins (µg/g) | 593.0 ± 12.1 | 593.0 ± 12.1 | 66.4 ± 3.5 | 182.2 ± 9.3 |
| total ellagic acid (µg/g) | 113.7 ± 3.5 | 113.7 ± 3.5 | 19.6 ± 3.1 | 44.1 ± 2.1 |
| estimated daily amount of deliveryb | ||||
| anthocyanins (mg) | 285.6 | 571.2 | 253.7 | 399.2 |
| ellagitannins (mg) | 24.9 | 49.8 | 16.8 | 45.5 |
| total ellagic acid (mg) | 4.8 | 9.6 | 5.0 | 11.0 |
Average weight of products: confections, 8.4 g/piece; nectar, 10 g of BRB, 253.7 g/bottle; nectars, 20 g of BRB, 249.5 g/bottle.
Estimated daily amount of delivery was obtained from the average weight of confections/nectars; thus, no SD was provided.
Table 5.
Retention of Phenolics in BRB Products during 2 Months of Storage at 4 °Ca
| product | storage time (weeks) |
anthocyanins (%) |
ellagitannins (%) |
total ellagic acid (%) |
|---|---|---|---|---|
| confection | 0 | 94.4 ± 1.0a | 95.6 ± 1.9a | 106.4 ± 3.3a |
| 4 | 87.0 ± 1.0b | 94.4 ± 2.7a | 101.4 ± 7.3a | |
| 6 | 84.5 ± 1.6b | 95.7 ± 4.8a | 100.0 ± 1.4a | |
| 8 | 83.4 ± 2.5b | 93.6 ± 4.5a | 106.9 ± 6.9a | |
| nectar, 10 g of BRB | 0 | 86.1 ± 2.7a | 66.4 ± 3.5a | 113.6 ± 6.8a |
| 4 | 78.9 ± 3.6b | 59.6 ± 1.3b | 105.0 ± 2.3a | |
| 6 | 77.9 ± 3.2b | 57.2 ± 2.6b | 103.2 ± 7.0a | |
| 8 | 76.3 ± 2.7b | 60.2 ± 1.7b | 104.1 ± 4.8a | |
| nectar, 20 g of BRB | 0 | 69.0 ± 2.8a | 91.0 ± 4.6a | 128.0 ± 6.2a |
| 4 | 68.5 ± 3.2ab | 91.4 ± 3.7a | 129.4 ± 3.0a | |
| 6 | 66.3 ± 1.0ab | 89.9 ± 3.1a | 125.3 ± 2.5a | |
| 8 | 65.1 ± 3.3b | 93.3 ± 2.4a | 127.8 ± 3.3a |
Values represent means ± standard error (n = 24 for confections and n = 9 for nectars). Means with different letters in the same column within each BRB product are significantly different (p ≤ 0.05).
Retention of ellagitannins was not significantly changed in confection and 20 g of BRB nectar during storage (p > 0.05). Ellagitannins in the 10 g of BRB nectar decreased significantly, but the change was <10% after 8 weeks of storage. The high stability of ellagitannins in confections and nectar during storage was consistent with those previously reported in other berries.25,39 Total ellagic acid concentration remained stable during storage of all products (p > 0.05). This was consistent with previous reports in which ellagic acid derivatives were not affected by thermal processing and remained stable during storage in red raspberry jams.23
Textural Stability during Storage
Rheological properties of BRB confections showed no significant change in complex viscosity η* (Figure 6) during storage with similar trends for G′ and G″ (data not shown). Texture profile analysis (TPA) showed significant decrease (p < 0.05) in cohesiveness and springiness but not in hardness and chewiness after 2 months of storage (data not shown). Chewiness is the combined results of hardness, cohesiveness, and springiness. This indicated that minor changes in cohesiveness and springiness of BRB confections did not influence chewiness. Water content was 27.85 ± 0.55% at day 1 and did not significantly change during 8 weeks of storage (p > 0.05). However, water activity (Aw) increased significantly from 0.72 ± 0.02 to 0.76 ± 0.02 after 8 weeks of storage (p < 0.05). This increase in water activity indicated water migration in the gel structure, which resulted in changes of cohesiveness and springiness but no other textural properties. Nectars with both BRB doses showed no significant changes in viscosity during storage at 4 °C (p > 0.05).
Figure 6.

Viscosity of BRB scale-up products during storage at 4 °C: (A) confections, |η*|; (B) nectar, 10 g of BRB dosage, η; (C) nectar, 20 g of BRB dosage, η.
Sensory Acceptability of BRB Products during Dietary Intervention
Sensory evaluation was obtained from 15 of 16 subjects in the BRB nectar group and from 12 of 16 in the BRB confection group. Results are shown in Table 6. Nectar and confections scored 7.27 and 7.08 in overall acceptability (7, like moderately on a 9-point hedonic scale). Both products scored above 6 on flavor, aroma, sweetness, and texture. Grittiness was below 6 (like slightly) in nectars, likely due to the seeds in the whole freeze-dried BRB. Grittiness may influence the overall liking score of both products. When subjects were asked in the surveys what factors contributed to or hindered the consumption of BRB products, 33% in the nectar group and 20% in the confection group mentioned grittiness. Other factors such as dose frequency in confection may have influenced the overall liking (40% mentioned that high dose frequency, 10 pieces per day, was a hindrance).
Table 6.
Sensory Acceptance of BRB Confections and Nectars with 9-Point Hedonic Scalea
| product | overall liking | flavor | aroma | sweetness | hardness or thickness | grittiness |
|---|---|---|---|---|---|---|
| confections | 7.08 ± 1.68 | 7.08 ± 1.78 | 6.25 ± 1.36 | 6.92 ± 1.98 | 6.83 ± 1.75 | 6.08 ± 1.78 |
| nectars | 7.27 ± 1.44 | 7.00 ± 1.60 | 6.93 ± 1.44 | 6.53 ± 1.77 | 6.47 ± 1.60 | 5.33 ± 1.76 |
Values represent means ± standard error (n = 12 for confections and n = 15 for nectars).
In this study, both BRB confections and nectars were formulated successfully to deliver a specific dose of bioactives in a consistent manner. After scale up, rheological properties did not vary within a product category, and ellagitanin and ellagic acid proved to be more heat stable and consistent than anthocyanins. During 8 weeks of storage, high-dose nectar showed the least changes in bioactives. Final products were well accepted (>like moderately) by men enrolled in a prostate cancer clinical trial. Thus, this study demonstrates that two different BRB delivery vehicles consisting of different matrices can be formulated to meet quality standards and bioactive stability and successfully scaled up for a human clinical trial. The use of these food products can prove to be instrumental in studying the efficacy of BRB bioactives in the prevention of or as adjuvant therapy in a variety of cancers (prostate, esophageal, oral, etc.).
ABBREVIATIONS USED
- BRB
black raspberry
- HPLC
high-performance liquid chromatography
- IRB
Institutional Review Board
- ESI
electrospray ionization
- MS
mass spectrometry
- UV–vis
ultraviolet–visible spectroscopy
- TGA
thermogravimetry analysis
- TPA
textural profile analysis
- LVR
linear viscoelastic region
- ANOVA
analysis of variance
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Mallery SR, Stoner GD, Larsen PE, Fields HW, Rodrigo KA, Schwartz SJ, Tian Q, Dai J, Mumper RJ. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: implications for oral cancer chemoprevention. Pharm. Res. 2007;24:728–737. doi: 10.1007/s11095-006-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeram NP, Adams LS, Zhang Y, Lee P, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 3.Keatley KE. Thesis. Columbus, OH: The Ohio State University; 2008. Chemoprevention of Bladder Cancer with Anthocyanin-Rich Extract from Black Raspberries. [Google Scholar]
- 4.Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee R, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 5.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, Lu B, Fields HW, Mumper RJ, Stoner GD. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein human premalignant oral lesions. Cancer Res. 2008;68:4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang LS, Hecht SS, Garmella SG, Yu N, Larue B, Henry C, Mclntyre C, Rocha C, Lechner JF, Stoner GD. Anthocyanins in black raspberries prevent esophageal tumor in rat. Cancer Prev. Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LS, Hecht S, Carmella S, Seguin C, Rocha C, Yu N, Stoner K, Chiu S, Stoner G. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J. Agric. Food Chem. 2010;58:3992–3995. doi: 10.1021/jf9030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravoori S, Vadhanam MV, Aqil F, Gupta RC. Inhibition of estrogen-mediated mammary tumorigenesis by blueberry and black raspberry. J. Agric. Food Chem. 2012;60:5547–5555. doi: 10.1021/jf205325p. [DOI] [PubMed] [Google Scholar]
- 9.Wang LS, Arnold M, Huang YW, Sardo C, Seguin C, Martin E, Huang TH, Riedl K, Schwartz S, Frankel W, Pearl D, Xu Y, Winston J, Yang GY, Stoner G. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin. Cancer Res. 2011;17:598–610. doi: 10.1158/1078-0432.CCR-10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoner GD, Wang LS, Zikri N, Chen T, Hecht SS, Huang C, Sardo C, Lechner JF. Cancer prevention with freeze-dried berries and berry components. Semin. Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev. Res. 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruits and vegetables – does bright colour signal cancer chemopreventive activity? Eur. J. Cancer. 2005;41:1931–1940. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Ross HA, Gordon JM, Stewart D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry. 2007;68:218–228. doi: 10.1016/j.phytochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res. Int. 2011;44:1150–1160. [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Koo SI, Song WO, Chun OK. Food matrix affecting anthocyanin bioavailability: review. Curr. Med. Chem. 2011;18:291–300. doi: 10.2174/092986711794088380. [DOI] [PubMed] [Google Scholar]
- 17.Walton MC, Hendriks WH, Broomfield AM, McGhie TK. Viscous food matrix influences absorption and excretion but not metabolism of blackcurrant anthocyanins in rats. J. Food Sci. 2009;74:22–29. doi: 10.1111/j.1750-3841.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahn-Jarvis JH, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food Funct. 2012;3:1051–1058. doi: 10.1039/c2fo10284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, Singh A, Sanders J, Aziz R, Casto B, Sun X. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J. Clin. Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Vodovotz Y. Functionality of novel black raspberry confections as a potential targeted delivery system for oral health; Presented at the 11th Internaltional Hydrocolloids Conference; May 14–18; West Lafayette, IN, USA. 2012. [Google Scholar]
- 21.González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, Gómez-Sánchez MB, García-Talavera NV, Gil-lzquierdo A, Sánchez-Álvarez C, Fontana-Compiano LO, Morga-Egea JP, Pastor-Quirante FA, Martínez-Díaz F, Tomás-Barberán FA, Espín JC. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 22.Neidhart S, Reiter M, Mensah-Wilson M, Stemmer G, Braig C, Sevinç S, Carle R. Possibilities for improving quality of fruit juices and drinks from tropical fruits by homogenization and addition of pectin; Presented at the International Symposium Sustaining Food Security and Managing Natural Resources in Southeast Asia; Jan 8–11; Chiang Mai, Thailand. 2002. [Google Scholar]
- 23.Zafrilla P, Ferreres F, Tomás-Barberán FA. Effects of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001;49:3651–3655. doi: 10.1021/jf010192x. [DOI] [PubMed] [Google Scholar]
- 24.Hager A, Howard RL, Brownmiller C. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed black raspberry products. J. Food Sci. 2008;73:134–140. doi: 10.1111/j.1750-3841.2008.00855.x. [DOI] [PubMed] [Google Scholar]
- 25.Hager TJ, Howard LR, Prior RL. Processing and storage effects on the ellagitannin composition of processed blackberry products. J. Agric. Food Chem. 2010;58:11749–11754. doi: 10.1021/jf102964b. [DOI] [PubMed] [Google Scholar]
- 26.Silva FVM, Gibbs P. Target selection in designing pasteurization processes for shelf-stable high-acid fruit products. Crit. Rev. Food Sci. Nutr. 2004;44:353–360. doi: 10.1080/10408690490489251. [DOI] [PubMed] [Google Scholar]
- 27.Gössinger M, Ullram T, Hermes M, Wendelin S, Berghold S, Halbwirth H, Stich K, Berghofer E. Effects of pre-freezing, puree content and pasteurization regime on colour stability of strawberry nectar made from puree. J. Sci. Food Agric. 2009;89:144–149. [Google Scholar]
- 28.Gasperotti M, Masuero D, Vrhovsek U, Guella G, Mattivi F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. Food Chem. 2010;58:4602–4616. doi: 10.1021/jf904543w. [DOI] [PubMed] [Google Scholar]
- 29.Siegwein AM, Vodovotz Y, Fisher EL. Concentration of soy protein isolate affects starch-based confections’ texture, sensory, and storage properties. J. Food Sci. 2011;76:422–428. doi: 10.1111/j.1750-3841.2011.02241.x. [DOI] [PubMed] [Google Scholar]
- 30.Peleg M. Texture profile analysis parameters obtained by an Instron universal testing machine. J. Food Sci. 1976;41:721–722. [Google Scholar]
- 31.Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. New York: Chapman and Hall; 1998. [Google Scholar]
- 32.Tian Q, Giusti MM, Stoner GD, Schwartz SJ. Characterization of a new anthocyanin in black raspberries (Rubus occidentalis) by liquid chromatography electrospray ionization tandem mass spectrometry. Food Chem. 2006;94:465–468. [Google Scholar]
- 33.Mullen W, Yokota T, Lean MEJ, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MS/MS. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 34.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 35.Hager TJ, Howard LR, Liyanage R, Lay JO, Prior R. Ellagitannin composition of blackberry as determined by HPLC-ESIMS and MALDI-TOF-MS. J. Agric. Food Chem. 2008;56:661–669. doi: 10.1021/jf071990b. [DOI] [PubMed] [Google Scholar]
- 36.Paudel L, Wyzgoski FJ, Scheerens JC, Chanon AM, Reese RN, Smiljanic D, Wesdemiotis C, Blakeslee JJ, Riedl KM, Rinaldi PL. Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) fruits: identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS analysis. J. Agric. Food Chem. 2013;61:12032–12043. doi: 10.1021/jf4039953. [DOI] [PubMed] [Google Scholar]
- 37.Kechinski CP, Guimarães PVR, Noreňa CPZ, Tessaro IC, Marczak LDF. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J. Food Sci. 2010;75:173–176. doi: 10.1111/j.1750-3841.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- 38.McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 39.Hager AC. Thesis. Fayetteville, AR: University of Arkansas; 2008. Processing and Storage Effects on Black Raspberry Polyphenolics and Antioxidant Capacity. [Google Scholar]
- 40.Rommel A, Wrolstad RE. Ellagic acid content of red raspberry juice as influenced by cultivar, processing, and environmental factors. J. Agric. Food Chem. 1993;41:1951–1960. [Google Scholar]
- 41.Skrede G, Wrolstad RE, Durst RW. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.) J. Food Sci. 2000;65:357–364. [Google Scholar]
- 42.Howard LR, Prior RL, Liyanage R, Lay JO. Processing and storage effect on berry polyphenols: challenges and implications for bioactive properties. J. Agric. Food Chem. 2012;60:6678–6693. doi: 10.1021/jf2046575. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava A, Akoh CC, Yi W, Fischer J, Krewer G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry packed in glass bottles. J. Agric. Food Chem. 2007;55:2705–2713. doi: 10.1021/jf062914w. [DOI] [PubMed] [Google Scholar]


