Abstract
Introduction
Externalized ventricular drains (EVDs) are commonly used in pediatric intensive care units (PICU) but few data are available regarding infection rates, infection risks, or factors associated with conversion to permanent cerebrospinal fluid (CSF) diversion.
Methods
Retrospective observational study of patients managed with EVDs admitted to a tertiary care PICU from January 2005 to December 2009
Results
Three hundred eighty patients were identified. Neurologic diagnostic groups were externalization of existing shunt (EXSHUNT) in 196 patients (52%), brain tumor in 122 patients (32%), intracranial hemorrhage (ICH) in 23 patients (6%), traumatic brain injury (TBI) in 17 patients (5%), meningitis in 9 patients (2%) or other in 13 patients (3%). Six percent of all patients (24/380) had new infections associated with EVD management for an infection rate of 8.6 per 1000 catheter days. The median time to positive cultures was 7 days (interquartile range 4.75, 9) after EVD placement. Patients with EVD infections had significantly longer EVD duration 6 vs. 11.5 days (p=0.0001), and higher maximum EVD outputs 1.9 vs. 1.5 mL/kg/hr (p=0.0017). Need for permanent CSF diversion was associated with higher maximum EVD drainage (1.3 vs. 1.6 mL/kg/hr p < 0.0001), longer EVD duration (5 vs. 4 days, p < 0.005), and younger age (4.5 vs. 8 years, p < 0.02) but not intracranial hypertension (72% vs. 82% of patients, p = 0.4).
Conclusion
In our large pediatric cohort, EVD infections were associated with longer EVD duration and higher maximum EVD output. Permanent CSF diversion was more likely in patients with higher maximum EVD drainage, longer EVD duration, and younger age.
Keywords: externalized ventricular drain, ventricular catheter infection, pediatric intensive care unit, neurocritical care, ventriculoperitoneal shunt, pediatric
Introduction
Neurosurgical patients represent a large percentage of patients treated in pediatric intensive care units (PICU)1. Externalized ventricular drains (EVD) are commonly used in children with increased intracranial pressure or who require cerebrospinal fluid (CSF) diversion. However, EVDs must be monitored closely due to infection risk 2,3. Infection rates in adult neurosurgical intensive care units have ranged between 0-45% 2,4-6. Few studies of EVD utilization and infections in children have been conducted, and their sample sizes have been relatively small3,7.
With increasing interest in the development of pediatric neurocritical care programs, recent studies have better characterized the pediatric neurocritical care population1,8. Children with EVDs represent a substantial portion of this population and delineation of patient characteristics, risk factors associated with EVD infections and the need for permanent CSF diversion can be utilized to develop future intervention studies on improving EVD management in the pediatric population. Using a large retrospective cohort from a single quaternary care PICU, we aimed to describe the utilization and infection rates of EVDs, determine risk factors associated with EVD infections, and determine risk factors for conversion to permanent CSF diversion.
Patients and Methods
Design: We conducted a retrospective observational study in patients with EVDs admitted to the PICU at The Children’s Hospital of Philadelphia between January 1, 2005 and December 31, 2009. The study was approved by the hospital Institutional Review Board and the requirement for informed consent was waived.
Patients were identified by searching the physician order entry database for EVD orders. Inclusion criteria included admission to the PICU and the presence of an EVD or externalized ventricular shunt. During the dates evaluated, no patients were managed with an antimicrobial impregnated catheter. The medical records of eligible patients were examined. Variables collected included baseline demographics (age, gender, race, and weight), ventriculostomy indication, history of seizures, admission electrolytes, presence of intracranial pressure monitoring, number of days ventriculostomy was present, ventriculostomy disposition, and survival to hospital to discharge. Positive CSF cultures obtained at time of EVD placement or externalization of ventriculo-peritoneal shunt were not included in overall infection rate. Subsequent positive cultures from patients with previous VPS were included in the overall infection rate only if the CSF cultures were negative or the organism identified on subsequent cultures was different than the initial culture. Daily values were recorded for up to the first 14 days of ventriculostomy drain management included ventriculostomy output and use of systemic antibiotics. During the study period, there was no standardized protocol for EVD management. For patients with documented infections, sampling was performed by the pediatric neurosurgical team every 48 hours.
Study data were collected and managed using REDCAP electronic data capture tools hosted at The Children’s Hospital of Philadelphia 9. Continuous variables are presented as medians [IQR25, 75] and categorical variables as numbers and proportions. Univariate analysis was performed using Wilcoxon rank sum for continuous data or chi-squared or Fishers exact test for proportions. All statistics were performed using Stata 10 (College Station, TX).
Results
Three hundred eighty patients were evaluated. Neurologic diagnostic groups for EVD placement were externalization of existing shunt (EXSHUNT) in 196 patients (52%), brain tumor in 122 patients (32%), intracranial hemorrhage (ICH) in 23 patients (6%), traumatic brain injury (TBI) in 17 patients (5%), meningitis in 9 patients (2%) or other in 13 patients (3%). There was no difference in age or sex among neurologic diagnostic groups (Table 1). ICH, TBI, and meningitis had the highest rates of intracranial pressure (ICP) monitoring and intracranial hypertension (ICP > 20 mmHg) (Table 1). Patients with EXSHUNT more commonly had a pre-existing seizure disorder. EVD duration differed significantly between groups: EXSHUNT, ICH, and TBI having the longest durations and tumor having the shortest. EXSHUNT, ICH, and TBI had the highest maximum EVD drain output (Table 1).
Table 1.
Association of variables with neurologic diagnostic group
| Total n=380 |
Exshunt n=196 (52) |
Tumor n=122 (32) |
ICH n=23 (6) |
TBI n=17 (5) |
Meningitis n=9 (2) |
Other n=13 (3) |
p-value | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 5.2 [1.7, 14] |
6.7 [2.2, 12.5] |
9.7 [6.4, 14.7] | 10.3 [2.6, 14.6] |
2.8 [0.7, 8] | 11.4 [3.6, 15] |
0.29 | |
| Sex | ||||||||
| Male | 169 (44) | 89 (45) | 51 (42) | 12 (52) | 9 (53) | 2 (22) | 6 (46) | 0.66 |
| Female | 211 (56) | 107 (55) | 71 (58) | 11 (48) | 8 (47) | 7 (78) | 7 (54) | |
| Weight (kg) | 20.5 [12,50] |
17 [10.5, 48.5] |
23.5 [13, 50] |
44 [18, 55] | 31 [15, 70] | 14.2 [11, 25] | 37 [19.4, 41.8] |
0.099 |
| Seizure disorder | 73 (19) | 56 (29) | 12 (10) | 2 (9) | 0 (0) | 0 (0) | 3 (23) | <0.001 |
| Maximum daily EVD output (mL) |
279 [168, 374] |
309 [205, 389] |
224 [80, 335] |
294 [234, 383] | 299 [241, 389] | 215 [170, 373] | 218 [85, 345] |
<0.001 |
| ICP monitored | 164 (43) | 20 (10) | 89 (73) | 22 (96) | 17 (100) | 8 (89) | 8 (62) | <0.001 |
| ICP > 20 mm Hg | 55 (14) | 2 (10) | 24 (27) | 11 (50) | 14 (82) | 3 (38) | 1 (13) | <0.001 |
| EVD duration (days) | 6 [4, 10] | 8 [5, 12] | 4 [3, 6] | 8 [4,11] | 8 [7,11] | 5 [4, 11] | 4 [3,5] | <0.001 |
| Mortality | 19 (5) | 8 (4) | 5 (4) | 3 (13) | 0 (0) | 2 (22) | 1 (8) | 0.07 |
Externalized ventriculostomy drain (EVD), pediatric intensive care unit (PICU), traumatic brain injury (TBI), spontaneous intracranial hemorrhage (ICH), externalization of ventriculoperitoneal shunt (Exshunt), intracranial pressure (ICP), cerebrospinal fluid (CSF).
One hundred forty-seven patients had CSF cultures obtained on EVD insertion. Ninety percent (133) were for EXSHUNT (Table 2). Sixty-seven (46%) of cultures sent on EVD insertion were positive and all positive cultures were from the EXSHUNT group. The most common organisms isolated were Staphylococcus aureus (39%) and coagulase negative staphylococcus (34%) (Table 3a). Forty six percent (67/133) of EXSHUNT group had a positive culture on externalization of the shunt. Six percent (4/67) of patients in the EXSHUNT group who had positive cultures on externalization developed subsequent positive cultures with a different organism and were included in statistical analysis of infection rates and associated risk factors. Ninety four percent of all patients in the study received at least 24 hours of parenteral antibiotics while an EVD was in place.
Table 2.
| All | EXSHUNT | Tumor | ICH | TBI | Meningitis | Other | p value | |
|---|---|---|---|---|---|---|---|---|
| Culture sent on EVD insertion | 147 | 133 | 3 | 1 | 0 | 7 | 3 | <0.001 |
| EVD insertion culture positive | 67 | 67 | 0 | 0 | 0 | 0 | 0 | |
| Subsequent positive culture | 24 | 15 | 4 | 1 | 3 | 1 | 0 | 0.145 |
| Infection rate (per 1000 catheter days) | 8.6 | 8.6 | 6.9 | 4.9 | 21 | 14 | 0 | 0.387 |
Externalized ventricular drain (EVD), externalization of previous ventriculoperitoneal shunt (EXSHUNT), spontaneous intracranial hemorrhage (ICH), traumatic brain injury (TBI). EXSHUNT were classified as having a subsequent positive culture only if initial CSF culture on EVD insertion or shunt externalization was negative or the organism identified on subsequent cultures was different than the organism identified on initial culture.
Table 3a.
Initial positive cultures
| All | 67 (100) |
|---|---|
| Staphylococcus aureus | 26 (39) |
| Staphylococcus coagulase | 23 (34) |
| negative | |
| propionibacterium | 5 (7) |
| pseudomonas | 2 (3) |
| streptococcus mitis | 2 (3) |
| E. coli | 2 (3) |
| streptococcus pneumoniae | 1 (1) |
| neisseria meningititidis | 1 (1) |
| Klebsiella | 1 (1) |
| Morganella Morganii | 1 (1) |
| Enterococcus | 1 (1) |
| Listeria | 1 (1) |
| Corneybacterium | 1 (1) |
Six percent of all patients (24/380) had subsequent new infections with an infection rate of 8.6 per 1000 catheter days. The median time for subsequent new positive cultures was 7 days [4.5, 9], and 88% of infections occurred within the first 10 days of management with an EVD (Figure 1). The most common organism isolated was coagulase negative staphylococcus (50%) (Table 3b). There was no difference in infection rates between neurologic diagnostic groups (Table 2). Patients with positive CSF cultures after EVD placement had significantly longer EVD days and higher maximum EVD outputs compared to patients without positive cultures (Table 4).
Figure 1.
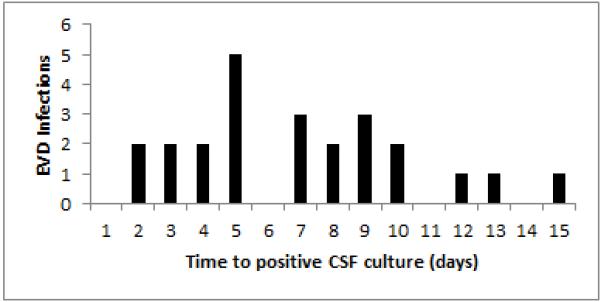
Externalized ventriculostomy drain (EVD), cerebrospinal fluid (CSF).
Table 3b.
Subsequent positive cultures
| All | 24 (100) |
|---|---|
| Staphylococcus coagulase | 12 (50) |
| negative | |
| Staphylococcus | 4 (17) |
| aureus | |
| propionibacterium | 2 (8) |
| E. coli | 1 (4) |
| streptococcus mitis | 1 (4) |
| Bacillus | 1 (4) |
| Enterococcus | 1 (4) |
| Enterobacter | 1 (4) |
| H. influenza | 1 (4) |
Table 4.
Characteristics associated with infection
| No Positive Culture N=356 |
Positive Culture N=24 |
p value | |
|---|---|---|---|
| Age | 6.6 [2, 13.9] | 5.1 [2.3, 14] | 0.99 |
| ICP monitoring | 156 (43) | 8 (33) | 0.396 |
| ICP > 20 mm Hg | 50 (32) | 5 (21) | 0.12 |
| Maximum EVD output (mL/kg/hr) |
1.5 [0.93, 1.9] | 1.9 [1.5, 2.6] | 0.0017 |
| Shunt placement | 225 (63) | 17 (71) | 0.517 |
| Mortality | 19 (5.3) | 0 (0) | 0.621 |
| EVD duration (days) |
6 [4,9] | 11.5 [8,15] | 0.0001 |
EVD, externalized ventricular drain; ICP, intracranial pressure. Patients with positive cultures on initial insertion or externalization were classified in the positive culture group only if the organism identified on subsequent cultures was different than the organism identified on initial culture.
Overall mortality rate prior to hospital discharge was 5% (19/380). Patients with meningitis had the highest mortality at 22% (2/9) (Table 1). Eleven patients died with an EVD in place. Excluding the EXSHUNT group, twenty-nine percent (54/184) of patients had an internalized shunt placed prior to discharge. Thirty-five percent (43/122) of tumor patients required shunt placement prior to discharge. Endoscopic third ventriculostomy without shunt placement was rarely performed 1% (4/380). Shunt placement was associated with higher maximum EVD drainage, longer EVD duration, and younger age (Table 5). Monitoring of ICP and episodes of ICP > 20 mm Hg were not associated with the need for permanent CSF diversion.
Table 5.
Characteristics associated with permanent CSF diversion
| No n = 130 |
Yes n = 54 |
p value | |
|---|---|---|---|
| Age (years) | 8 [2.7, 14.1] | 4.5 [1.2, 12.1] | < 0.02 |
| Maximum 24 hour EVD output (mL/kg/hr) |
1.3 [0.6, 1.8] | 1.6 [1.1, 2.1] | < 0.001 |
| Diagnostic group | |||
| Tumor | 79 (61) | 43 (80) | 0.18 |
| ICH | 19 (15) | 4 (7) | |
| TBI | 15 (11) | 2 (4) | |
| Meningitis | 7 (5) | 2 (4) | |
| Other | 10 (8) | 3 (5) | |
| EVD days | 4 [3, 6] | 5 [4, 8] | < 0.005 |
| ICP monitored | 105 (81) | 30 (72) | 0.2 |
| ICP > 20 | 41 (39) | 12 (31) | 0.4 |
| Mortality | 11 (8) | 0 (0) | < 0.01 |
Cerebrospinal fluid (CSF), externalized ventricular drain (EVD), intracranial pressure (ICP), traumatic brain injury (TBI), intracranial hemorrhage (ICH).
Discussion
Of 380 children managed with EVDs in our PICU, EVD infections were uncommon with 6% of patients developing a positive culture during EVD management, resulting in an infection rate of 8.6 per 1000 catheter days. Higher CSF output and longer duration of EVD were risk factors associated with EVD infections. Excluding patients with existing shunts, 30% of patients had an internalized shunt placed prior to discharge with higher CSF output, longer duration of EVD management, younger age and neoplasm associated with an increased risk for permanent CSF diversion. Interestingly, intracranial hypertension was not a risk factor for EVD infections or permanent CSF diversion in our pediatric patient population.
The 8.6 per 1000 catheter day infection rate is lower than that reported for adults with standard non-antimicrobial-impregnated catheters. A recent meta-analysis of antimicrobial-impregnated catheters in adults observed an overall infection rate of 13.7% in standard catheters. In our cohort, intracranial pressure > 20 mm Hg was not a risk factor for EVD infection, but has been reported as a risk factor in adult patients 6. Patients with TBI and meningitis had the highest incidence of EVD infection but this difference did not reach statistical significance, potentially related to the small number of patients in each of these neurologic diagnosis subgroups. Longer EVD durations and higher maximum EVD output were the only risk factors associated with EVD infections (Table 5). Previous studies have reported conflicting results regarding EVD duration as a risk factor for EVD infection3,6,7,10,11. Similar to several other studies, the majority of EVD infections (88%) in our cohort were observed in the first 10 days after EVD placement (Figure 1). The majority of patients with EXSHUNT (63/67, 94%) with initial positive cultures did not have subsequent positive culture with a different organism and were classified as no infection for analysis. Typically these patients would have relatively longer EVD durations, but we still observed a strong association between EVD duration and subsequent positive cultures in our EVD patient population. Clinicians may want to consider evaluating for infection when encountering patients with larger than expected EVD outputs.
Use of systemic prophylactic antibiotics to prevent EVD infections is controversial with conflicting results reported in previous studies12-15. The majority of the patients (94%) in this study received at least 24 hours of prophylactic antibiotics and all patients who developed subsequent EVD infections had received at least 24 hours of systemic antibiotic prophylaxis prior to positive cultures. Due to the variability of systemic antibiotic duration and choice of antibiotics in this cohort, it is difficult to draw any conclusions on the efficacy of antibiotic prophylaxis. Antimicrobial-impregnated catheters have recently demonstrated efficacy in preventing EVD associated infections16,17. Studies of antimicrobial-impregnated ventricular catheters in the pediatric population are lacking and were not in use at our institution during the study period.
Intracranial pressure was measured in all diagnostic groups excluding EXSHUNT, 78% (144/184). Episodes of intracranial hypertension were observed in 14% of all patients and most commonly found in patients with TBI or ICH, but these patients were least likely to require permanent CSF diversion. There is a paucity of data on conversion rates and the risk factors associated with need for permanent CSF diversion in pediatric patients with EVDs. A recent retrospective study of 180 pediatrics patients over a 20 year period reported a conversion rate of 25% to permanent CSF diversion, which varied by diagnosis with TBI having the lowest rate 18. Excluding the EXSHUNT group, we observed a similar conversion rate of 30% (56/184) with the TBI group having the lowest conversion rate (12%) in our similar sized but more contemporary cohort.
We identified several factors that were associated with the need for permanent CSF diversion. Patients requiring permanent CSF diversion were younger, had a higher maximum EVD output, and a longer EVD duration. In a recent study, pediatric patients with neoplasm were also more likely to require a shunt with a 40% conversion to VPS which is similar to our observed rate of 35% for tumor patients18. Interestingly, intracranial hypertension was not associated with the need for permanent CSF diversion in our pediatric population, which is in contrast to adult patients19,20. Patients in our pediatric cohort with ICH or TBI had lower conversion rates as well as shorter durations of EVD placement compared to reported conversion rates of 21-28% in adults 19,20. Our data highlights some of the unique responses of the pediatric brain compared to the adult brain. To our knowledge, this is the first adult or pediatric study, to report an association between maximum EVD drain output and need for permanent CSF diversion.
There are several limitations to our study that must be considered when attempting to generalize our findings. This retrospective study was limited to one large quaternary PICU where clinical practice such as criteria for transfer to the regular inpatient care setting may vary compared to other similar institutions. All patients in this study remained in the ICU during the duration of their EVD management, undergoing daily rounds with the critical care team and daily discussions with the neurosurgical team, which may have contributed to the relatively low infection rate in our report. However, this practice was also a strength in our study, as we were able to fully collect daily data for up to the first 14 days of EVD placement. The distribution of patients by neurologic diagnostic group was heavily weighted to externalization of previous VPS or brain tumors, with smaller numbers of patients with TBI or ICH. Our experience may not be representative of infection and CSF diversion rates at other institutions with different neurocritical patient populations3,18. The TBI patient population in our study was relatively small compared to the EXSHUNT and tumor groups, and is not representative of the total TBI population. This is in part due to the fact that a large portion of TBI patients during the study period were not managed with an EVD. Eleven percent of TBI patients in our cohort underwent conversion to permanent CSF diversion, a higher proportion than has been previously reported 18. However our TBI cohort represents a distinct population as we observed a 0% mortality in the TBI patients managed with EVDs. Furthermore, it has been previously reported that continuous vs. intermittent drainage can alter EVD output, intracranial pressure, as well as CSF biomarkers in patients suffering from traumatic brain injury 21. EVD management was clinician dependent in our small retrospective TBI cohort which precludes any conclusions about the impact of continuous vs. intermittent drainage on EVD output, need for permanent CSF diversion, or infection rates. In the newly funded comparative effectiveness trial in pediatric TBI, CSF diversion is one of the modalities that will be specifically examined to get further insight to optimize care for these patients22. Finally, antibiotic prophylaxis after EVD placement was administered in the majority of our patients, but the variation in antibiotic choice and the duration of antibiotic prophylaxis in our study population, limited any conclusions about its efficacy.
Conclusion
In summary, to the best of our knowledge this is the largest cohort of pediatric patients with EVDs assessing EVD utilization and risk factors for infection and need for permanent CSF diversion. Positive CSF cultures during EVD management were uncommon, with a 6% incidence in our cohort. Higher maximum drain output was found to be associated with EVD infections. Clinicians should consider infectious etiologies for unexplained high volume draining EVDs in pediatric patients. Permanent CSF diversion was more likely in patients with higher maximum EVD drain output, younger age, and longer EVD duration but not in patients with intracranial hypertension during their hospitalization. Future prospective studies using a comparative effectiveness approach may be able to establish whether varying infection prevention and management strategies impact outcome and whether varying practices regarding permanent CSF diversion impact long-term outcome.
Acknowledgments
Supported by NIH K23-NS075363 (AAT), K23-NS076550 (NSA), RO1-HD061963 (JWH), and K08-NS064051 (SHF).
Footnotes
Conflict of Interest
Alexis A Topjian, Amber Stuart, Alyssa A. Pabalan, Ashleigh Clair, Todd J. Kilbaugh, Nicholas S. Abend, Robert A. Berg, Gregory G. Heuer, Phillip B. Storm Jr,, Jimmy W. Huh, and Stuart H. Friess declare that they have no conflict of interest.
References
- 1.Bell MJ, Carpenter J, Au AK, et al. Development of a pediatric neurocritical care service. Neurocritical care. 2009;10:4–10. doi: 10.1007/s12028-008-9061-3. [DOI] [PubMed] [Google Scholar]
- 2.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170–81. doi: 10.1097/00006123-200207000-00024. discussion 81-2. [DOI] [PubMed] [Google Scholar]
- 3.Ngo QN, Ranger A, Singh RN, Kornecki A, Seabrook JA, Fraser DD. External ventricular drains in pediatric patients. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2009;10:346–51. doi: 10.1097/PCC.0b013e3181a320cd. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Desai NS, Ricci J, et al. Factors contributing to ventriculostomy infection. World neurosurgery. 2012;77:135–40. doi: 10.1016/j.wneu.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent JL. Ventriculostomy-related infections in critically ill patients: a 6-year experience. Journal of neurosurgery. 2005;103:468–72. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 6.Mayhall CG, Archer NH, Lamb VA, et al. Ventriculostomy-related infections. A prospective epidemiologic study. The New England journal of medicine. 1984;310:553–9. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 7.Khalil BA, Sarsam Z, Buxton N. External ventricular drains: is there a time limit in children? Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2005;21:355–7. doi: 10.1007/s00381-004-1080-6. [DOI] [PubMed] [Google Scholar]
- 8.LaRovere KL, Graham RJ, Tasker RC. Pediatric Critical Nervous System P. Pediatric neurocritical care: a neurology consultation model and implication for education and training. Pediatric neurology. 2013;48:206–11. doi: 10.1016/j.pediatrneurol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aucoin PJ, Kotilainen HR, Gantz NM, Davidson R, Kellogg P, Stone B. Intracranial pressure monitors. Epidemiologic study of risk factors and infections. The American journal of medicine. 1986;80:369–76. doi: 10.1016/0002-9343(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 11.Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;33:2028–33. doi: 10.1086/324492. [DOI] [PubMed] [Google Scholar]
- 12.Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta neurochirurgica Supplement. 1998;71:146–8. doi: 10.1007/978-3-7091-6475-4_43. [DOI] [PubMed] [Google Scholar]
- 13.Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. Journal of neurosurgery. 1985;62:694–7. doi: 10.3171/jns.1985.62.5.0694. [DOI] [PubMed] [Google Scholar]
- 14.Wyler AR, Kelly WA. Use of antibiotics with external ventriculostomies. Journal of neurosurgery. 1972;37:185–7. doi: 10.3171/jns.1972.37.2.0185. [DOI] [PubMed] [Google Scholar]
- 15.Wong GK, Poon WS, Lyon D, Wai S. Cefepime vs. Ampicillin/Sulbactam and Aztreonam as antibiotic prophylaxis in neurosurgical patients with external ventricular drain: result of a prospective randomized controlled clinical trial. Journal of clinical pharmacy and therapeutics. 2006;31:231–5. doi: 10.1111/j.1365-2710.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 16.Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly ES., Jr. Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: a systematic review. Neurosurgery. 2011;68:996–1005. doi: 10.1227/NEU.0b013e3182096d84. [DOI] [PubMed] [Google Scholar]
- 17.Keong NC, Bulters DO, Richards HK, et al. The SILVER (Silver Impregnated Line Versus EVD Randomized trial): a double-blind, prospective, randomized, controlled trial of an intervention to reduce the rate of external ventricular drain infection. Neurosurgery. 2012;71:394–403. doi: 10.1227/NEU.0b013e318257bebb. discussion-4. [DOI] [PubMed] [Google Scholar]
- 18.Walker CT, Stone JJ, Jacobson M, Phillips V, Silberstein HJ. Indications for Pediatric External Ventricular Drain Placement and Risk Factors for Conversion to a Ventriculoperitoneal Shunt. Pediatric neurosurgery. 2013 doi: 10.1159/000353608. [DOI] [PubMed] [Google Scholar]
- 19.Bauer DF, McGwin G, Jr., Melton SM, George RL, Markert JM. Risk factors for conversion to permanent ventricular shunt in patients receiving therapeutic ventriculostomy for traumatic brain injury. Neurosurgery. 2011;68:85–8. doi: 10.1227/NEU.0b013e3181fd85f4. [DOI] [PubMed] [Google Scholar]
- 20.Miller C, Tsivgoulis G, Nakaji P. Predictors of ventriculoperitoneal shunting after spontaneous intraparenchymal hemorrhage. Neurocritical care. 2008;8:235–40. doi: 10.1007/s12028-007-9018-y. [DOI] [PubMed] [Google Scholar]
- 21.Shore PM, Thomas NJ, Clark RS, et al. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: effect on biochemical markers. J Neurotrauma. 2004;21:1113–22. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- 22.Bell MJ, Adelson PD, Hutchison JS, et al. Differences in medical therapy goals for children with severe traumatic brain injury-an international study. Pediatric critical care medicine: a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:811–8. doi: 10.1097/PCC.0b013e3182975e2f. [DOI] [PMC free article] [PubMed] [Google Scholar]


