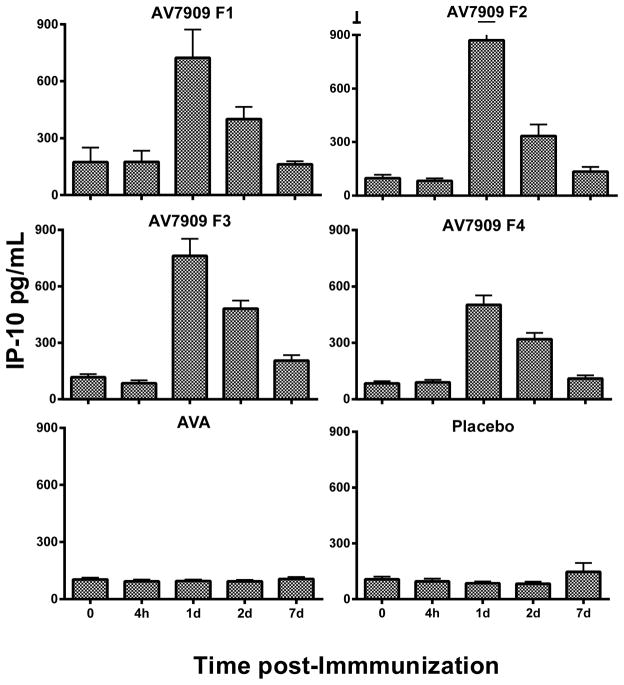
Fig. 1.
Average of the subject’s individual IP-10 Cytokine responses (pg/mL) among all groups of the EBS.AVA.201 Study. Vaccines: AV7909 F1 (AVA 0.5 ml + CpG 0.5 ml); AV7909 F2 (AVA 0.5 ml + CpG 0.25 ml); AV7909 F3 (AVA 0.25 ml + CpG 0.5 ml); AV7909 F4 (AVA 0.25 ml + CpG 0.25 ml); AVA= BioThrax (0.5 mL); placebo (0.5 ml saline). Statistical differences (p < 0.05 following post-hoc analysis using MANOVA) of IP-10 in serum were identified between AV7909 (Formulations 1 – 4) relative to AVA or placebo.