Abstract
Objective
Incomplete spinal cord injury (iSCI) disrupts motor control and limits the ability to coordinate muscles for overground walking. Inappropriate muscle activity has been proposed as a source of clinically observed walking deficits after iSCI. We hypothesized that persons with iSCI exhibit lower locomotor complexity compared to able-body (AB) controls as reflected by fewer motor modules, as well as, altered module composition and activation.
Methods
Eight persons with iSCI and eight age-matched AB controls walked overground at prescribed cadences. Electromyograms of fourteen single leg muscles were recorded. Nonnegative matrix factorization was used to identify the composition and activation of motor modules, which represent groups of consistently co-activated muscles that accounted for 90% of variability in muscle activity.
Results
Motor module number, composition, and activation were significantly altered in persons with iSCI as compared to AB controls during overground walking at self-selected cadences. However, there was no significant difference in module number between persons with iSCI and AB controls when cadence and assistive device were matched.
Conclusions
Muscle coordination during overground walking is impaired after chronic iSCI.
Significance
Our results are indicative of neuromuscular constraints on muscle coordination after iSCI. Altered muscle coordination contributes to person-specific gait deficits during overground walking.
Keywords: Spinal cord injury, walking, muscle coordination, modules, motor control
1. Introduction
Incomplete spinal cord injury (iSCI) disrupts motor commands to spinal locomotor circuitry and often severely limits the ability to coordinate muscles for overground walking. While a healthy motor system is capable of coordinating many muscles spanning multiple joints for safe and efficient walking, this ability is impaired following iSCI. More than 75% of persons with motor incomplete injuries regain some walking capacity (van Hedel et al., 2009), but many do not fully return to community walking (Field-Fote et al., 2011, van Hedel et al., 2010). Unfortunately, we do not fully understand the underlying neuromuscular mechanisms that might contribute to this shortcoming nor how specific changes in muscle co-activity impair overground walking after chronic iSCI.
Inappropriate muscle activity is a source of many of the clinically observed walking deficits that emerge in persons with chronic iSCI (Gorassini et al., 2009, Maegele et al., 2002). Locomotor training studies often target impaired muscle activity timing, agonist-antagonist joint level muscle coactivity, and electromyography (EMG) burst durations in an effort to improve walking ability (Gorassini et al., 2009, Grasso et al., 2004, Ivanenko et al., 2003, Ivanenko et al., 2004, Maegele et al., 2002, Visintin et al., 1994). However, these studies primarily focus on treadmill and body-weight support training and not overground walking. Although treadmill walking permits greater experimental control of walking conditions like speed and body-weight support, the ability to coordinate muscles during these more constrained tasks does not necessarily translate to overground or community ambulation which often require assistive devices such as a cane, walker, or crutches (Lee et al., 2008). Even though the mean kinematic trajectories are similar between treadmill and overground walking, overground walking inherently requires greater step-to-step variability (Dingwell et al., 2001). Overground walking is a highly complex motor task that requires flexible motor control strategies that adapt muscle coordination to step-to-step variations in environmental and mechanical demands (Chvatal et al., 2012, Dingwell et al., 2001, Nielsen, 2003), especially compared to more controlled locomotor demands such as single-speed treadmill walking (Dingwell et al., 2001).
The complexity of neuromuscular control required for overground walking is deficient after iSCI, resulting in numerous walking deficits. For example, persons with iSCI present an inability to modulate walking speed outside a small range of slow speeds (Pepin et al., 2003), a dependence on assistive devices (van Hedel et al., 2009), and a failure to adjust to environmental perturbations that subsequently lead to increased falls (Brotherton et al., 2007). The extent of these walking deficits vary widely with injury level, severity, and the pathways damaged, making it difficult to assess the underlying neuromuscular mechanisms (van Hedel et al., 2010).
To date, it is unclear to what extent inappropriate muscle coordination contributes to overground walking deficits after chronic iSCI. Quantifying the contribution of altered muscle coordination is particularly challenging due in part to the large number of muscles that contribute to overground walking. Non-negative matrix factorization (NNMF) quantifies this complexity via extraction of motor modules, or groups of consistently co-activated muscles, that represent the “building blocks” of muscle coordination. Motor modules can be characterized in terms of number, composition (i.e., number of muscles per motor module), and activation (i.e., duration and amplitude). Motor modules can be flexibly activated in combination to produce a wide range of muscle coordination patterns during various motor tasks, with each module achieving a specific biomechanical outcome that subserves the overall biomechanical goal (Cappellini et al., 2006, Chvatal et al., 2012, Chvatal et al., 2011, d’Avella et al., 2011, Drew et al., 2008, Fox et al., 2013, Ivanenko et al., 2004, Neptune et al., 2009, Overduin et al., 2008, Torres-Oviedo et al., 2006). Motor modules also are useful in identifying constraints on muscle coordination related to gait deficits in neurologic pathologies such as stroke, spinal cord injury, and Parkinson’s disease (Allen et al., 2013, Bowden et al., 2010, Cheung et al., 2009b, Clark et al., 2010, Fox et al., 2013, Rodriguez et al., 2013). Following hemiparetic stroke, as well as, Parkinson’s disease, persons exhibit fewer motor modules during walking (Clark et al., 2010, Rodriguez et al., 2013). This reduction is closely related to limited walking speed and walking complexity. Similar findings have been made in pediatric spinal cord injury (Fox et al., 2013), but not explored in adult spinal cord injury. Additionally, most studies have focused on module number without extensive exploration of module composition or activation across the gait cycle.
Thus, the purpose of this study was to quantify neuromuscular deficits in muscle coordination during overground walking in persons with chronic iSCI. We hypothesized that overground muscle coordination is constrained by greater muscle co-activity in persons with iSCI as compared to age-matched (AB) controls. We predict that persons with iSCI have fewer motor modules, as well as, altered composition (i.e., increased number of muscles per motor module) and activation (i.e., increased duration) of motor modules as compared to AB controls. We examined motor modules from 14 muscles during a cadence-matched overground-walking task and revealed that changes in motor module number, composition, and activation contribute to deficits in overground walking after iSCI. Understanding the subject-specific neuromuscular constraints on muscle coordination is critical for effectively developing therapies that are more tailored to a heterogeneous population of persons with chronic iSCI and to the complexities of community ambulation.
2. Methods
2.1. Study Population
Eight persons with iSCI (34.4 ± 3.8 years; mean ± 1 standard error) and eight age-matched AB controls (34.1 ± 4.1 years) participated in this study (Table 1). AB subjects also were selected to match gender and approximate body type. Ethical approval for the study was received from the Emory University Institutional Review Board (IRB protocol STU00044670); informed consent and HIPAA authorization were obtained from all subjects prior to their participation in accordance with the Declaration of Helsinki.
Table 1.
Subject Characteristics
| Subject | Gender | Age (yrs) | AIS Level | Years Since Injury | Assistive Device | SCI-FAI (Total, R) | LEMS (L/R, Total) | Self- Selected Cadence (spm) | 10MWT (sec) | Number of Modules |
|---|---|---|---|---|---|---|---|---|---|---|
| SCI1 | M | 28 | C5 | 8 | 2C | 26, 14 | 11/12, 23 | 55 | 14.4 | 7 |
| SCI2 | M | 30 | C6 | 11 | 1C | 38, 21 | 24/18, 42 | 80 | 5.8 | 3 |
| SCI3 | F | 28 | C6 | 5 | W | 31, 18 | 13/22, 35 | 26 | 46.6 | 3 |
| SCI4 | M | 25 | C5 | 8 | 1C | 29, 20 | 22/19, 41 | 51 | 25.3 | 4 |
| SCI5 | M | 59 | T3 | 24 | 1C | 36, 20 | 24/23, 47 | 49 | 15.3 | 6 |
| SCI6 | M | 35 | T7 | 5 | W | 21, 14 | 9/14, 23 | 26 | 43.4 | 3 |
| SCI7 | M | 31 | C5 | 3 | None | 29, 17 | 19/24, 44 | 29 | 16.2 | 3 |
| SCI8 | M | 39 | C7 | 14 | 2C | 28, 17 | 13/25, 38 | 38* | 28.9 | 4 |
| AB1 | M | 20 | 98 | 7 | ||||||
| AB2 | M | 37 | 1C | 117 | 5 | |||||
| AB3 | F | 23 | W | 110 | 5 | |||||
| AB4 | M | 34 | 101 | 7 | ||||||
| AB5 | M | 58 | 1C | 109 | 5 | |||||
| AB6 | M | 31 | W | 110 | 5 | |||||
| AB7 | M | 33 | None | 106 | 7 | |||||
| AB8 | M | 37 | 2C | 112 | 6 |
Abbreviations: AIS = American Spinal Cord Injury Association Impairment Scale; SCI-FAI = Spinal Cord Injury Functional Ambulation Inventory; LEMS = lower extremity motor scores; spm = steps per minute. Assistive devices are abbreviated as 1C = single cane/crutch, 2C = 2 crutches, W = walker.
Actual mean cadences for SCI8 were slow = 32, self-selected = 38, fast = 39 while expected metronome cadences were slow = 30, self-selected = 35, fast = 40.
We included iSCI subjects with incomplete injuries to the spinal cord between levels C4 and T10 who were at least one year post injury (i.e. chronic), were able to walk overground at least 10 meters with reciprocal pattern and without the assistance of another person, and were able to follow simple verbal, visual, and auditory commands. We excluded subjects if they had a brain injury as defined from chart review, progressive SCI, other concurrent medical condition, and/or history of contraindications to surface electromyography (EMG) such as adhesive allergy. We excluded AB participants if they had a concurrent medical condition and/or neurological impairments.
2.2. Clinical assessments
We recorded injury severity as well as lower extremity strength and mobility using a set of standard clinical tests. The American Spinal Injury Association Impairment Scale (AIS) was used to categorize subject neurological injury level and completeness. Strength was assessed using the Lower Extremity Motor Score (LEMS) from the AIS (Marino et al., 1999). The Spinal Cord Injury Functional Ambulation Inventory (SCI-FAI) was used to identify clinically observable gait deficits (Field-Fote et al., 2001), and the 10 Meter Walk Test identified the maximum walking speed (van Hedel et al., 2007). Walking tests were performed using the minimum assistive device possible for safe walking.
2.3. Equipment
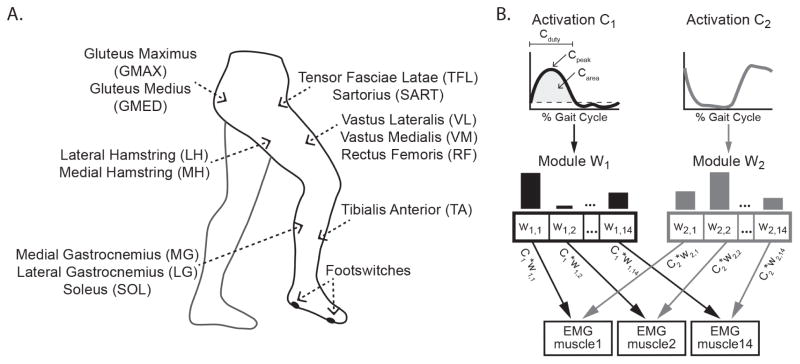
We recorded surface EMGs from 14 muscles on the right leg (Figure 1A), which included the tibialis anterior (TA), medial gastrocnemius (MG), lateral gastrocnemius (LG), soleus (SO), vastus lateralis (VL), vastus medialis (VM), rectus femoris (RF), medial hamstring (MH), lateral hamstring (LH), gluteus medius (GMED), gluteus maximus (GMAX), tensor fascia lata (TFL), sartorius (SART), and adductor magnus (ADDM). These muscles accounted for single and multi-joint actions spanning the ankle, knee, and hip and included at least one antagonistic pair at each joint, similar to previous studies (Chvatal et al., 2012, Ivanenko et al., 2003). Standard skin preparation techniques were applied. Ag/AgCl dual surface electrodes (model #272; Noraxon Inc, Scottsdale, AZ) were placed over the muscle belly parallel to fiber alignment. Four force-sensing resistor footswitches were placed on the plantar surface of the right foot at the heel and 1st, 3rd, and 5th metatarsal heads to identify gait cycle events. The resulting signals were amplified using Zerowire® wireless EMG technology (ZW180/R, Cometa Systems, Italy), which has an input impedance of 20MΩ and a bandwidth between 10–500Hz. All data were sampled at 2500 Hz with an 18-bit data acquisition system (NI PCI-6259; National Instruments, Austin TX).
Figure 1. Schematic of experimental setup and motor module analyses.
A) Schematic of surface EMG recordings from 14 right limb muscles and footswitch placement. B) Schematic of non-negative matrix factorization reconstruction of observed EMG. Each time-invariant motor module (wi), displayed as bar plots in which each bar represents the relative muscle contribution, is flexibly recruited at varying activation levels across time (Ci). The linear sum of the modules multiplied by their activations at each time point accounts for > 90% of the variability in the observed EMG across all time points and muscles. Metrics describing module activations also are illustrated. The dashed horizontal line on C1 indicates the 0.15 threshold. Cpeak is the maximum activation, Carea is the area under the curve in the region where C is above threshold, and Cduty is the percent of the gait cycle in the region where C is above threshold.
We measured self-selected cadences for AB and iSCI from three passes across an instrumented GAITRite® mat (CIR Systems Inc, Clifton, NJ) in which subjects walked at a safe, comfortable speed. In subsequent data collection, subjects matched this cadence. During data collection, footswitch voltages were analyzed using a custom algorithm in Matlab® (Mathworks Inc, USA) to compute cadence. If the difference between the desired and actual cadence was greater than 5%, the trial was repeated. One subject (SCI8) was unable to match the cadences within 5% even after multiple trial repetitions; actual cadences are noted in Table 1 and these actual cadences were matched by the age-matched control. Due to gait deviations, the resolution of footswitch signals was not sufficient for toe off detection. However, average stance-to-swing transitions during self-selected walking were estimated from the instrumented mat collection as percentages of the gait cycle (AB 63.0±0.6%, iSCI 69.1±3.8%). This enabled us to approximate stance and swing phases of the gait cycle.
2.4. Protocols
To quantify the complexity of muscle coordination across a range of overground walking speeds, we identified the number, composition, and activation of motor modules necessary to reproduce each participant’s muscle activation patterns during overground walking. AB and iSCI subjects participated in nine randomized 20-second overground walking trials at three cadences about their self-selected cadence: self-selected, slow cadence of 85% self-selected, and fast cadence of 115% self-selected (see Table 1). We chose to evaluate walking at self-selected cadences for both groups to understand walking coordination under conditions that were most functionally relevant and similar to everyday community ambulation. A variety of speeds were used to acquire a rich set of muscle coordination patterns for exploring muscle coordination and to represent a range of possible community walking speeds (Hof et al., 2002, Pepin et al., 2003, van Hedel et al., 2006). During each of the nine trials, we instructed AB and iSCI subjects to step to the beat of the metronome that was set according to the cadence of that trial. Actual cadences in AB and iSCI subjects matched the metronome within 2% error. Subjects with iSCI walked used their minimum assistive device for all trials. To control for the effects of cadence and assistive device, a subset of the AB control subjects (n=5; Table 1) also performed an additional nine trials at the slow, fast, and self-selected cadences of their iSCI match using the matching assistive device (ABmatch). A licensed physical therapist instructed those subjects on appropriate use of the assistive device to preserve reciprocal gait. Subjects practiced for up to five minutes with the assistive device. The cadences performed by ABmatch control subjects were successfully comparable to their matched subjects with iSCI during slow (paired t-test of actual cadences walked, p=0.51), self-selected (p=0.10), and fast (p=0.22) cadences.
2.5. Data processing for module analysis
We extracted motor modules from EMG recordings during each subject’s overground walking trials as described previously (Chvatal et al., 2012, Clark et al., 2010). First, each trial of EMG recordings was high-pass filtered using a 30 Hz zero-lag fourth-order Butterworth filter and demeaned and then rectified, low-pass filtered (4 Hz, zero lag, fourth-order Butterworth filter), and down-sampled from 2500 Hz to 20 Hz by taking the mean EMG levels in 50 msec bins (Chvatal et al., 2012). Second, to allow for comparison between subjects, we normalized data for each subject to maximum muscle EMG activity for a given muscle across all bins and all trials, such that data ranged from 0 to 1. Third, for each dataset (i.e., AB, iSCI, and ABmatch) for each subject, nine trials (3 trials each at self-selected, slow, and fast cadences) were concatenated into an m x n matrix where m is the number of muscles (14) and n is the number of data points (3600 samples; 20 samples/sec × 20 sec/trial × 9 trials). Forth, before extraction, each muscle was normalized to unit variance such that each muscle’s variability was equally weighted in the extraction. This normalization was removed after extraction (Chvatal et al., 2012, Torres-Oviedo et al., 2007). Finally, we used NNMF to extract motor modules and corresponding activations from step to step (Chvatal et al., 2012, Clark et al., 2010, Gizzi et al., 2011, Lee et al., 1999, Rodriguez et al., 2013). This technique was selected based on previous work demonstrating that it provides robust estimates of EMG muscle coordination with no constraints on the correlation of activations across a broad range of motor tasks (Clark et al., 2010, Rodriguez et al., 2013, Tresch et al., 2006). NNMF algorithm extracts modules such that the activity of each muscle is represented as a linear summation of motor modules:
where EMGr,m is the reconstructed representation of the observed activity (EMGo,m) for muscle m, wi is the motor module vector describing the muscle contributions and weightings for module i, and ci is the module activation coefficient across time points for module i. Each motor module, wi, is fixed across time, but the activation coefficients can vary across time such that modules can be combined to explain a variety of muscle activation patterns. For visualization, module vectors were normalized to the maximum muscle contribution such that each muscle contribution ranged from 0 to 1.
To determine the number of motor modules required to account for the observed muscle coordination patterns, 1 to 14 modules were iteratively extracted from each subject’s data. We quantified goodness of fit of the data reconstruction as the variability accounted for (VAF), or the uncentered correlation coefficient, which describes the amount of variability in EMGo accounted for by EMGr (Zar, 1974). To ensure robustness of module number selection, we reconstructed 10 bootstrapped datasets and computed confidence intervals for the VAF of each module number (Cheung et al., 2009a, Sabatini, 2002). We selected module number (nmod) as the smallest number of modules that account for >90% VAF at the lower limit of the confidence interval. Module number increased if individual muscles were not reconstructed with greater than 75% VAF and the addition of another module increased this muscle’s fit by more than 5%. These criteria are considered conservative to ensure goodness of reconstruction (Chvatal et al., 2012, Clark et al., 2010). Finally, to establish confidence intervals on the muscle contributions in each module, nmod modules were extracted from 10 bootstrapped versions of the dataset and the mean contribution of each muscle to each module computed.
2.6. Quantifying motor modules and activations for between and within group comparisons
To test the hypothesis that persons with iSCI exhibit modules with a greater co-activity, as evidenced by a greater number of muscles in each module compared to AB controls, we compared module composition between subjects within and between groups (i.e. AB versus SCI). For this comparison, similarity between modules was quantified by the Pearson correlation coefficient, r. Modules were considered similar and, thus, aligned if r > 0.532 corresponding the critical r value for 14 muscles (Chvatal et al., 2011, Zar, 1974). In the primary comparison between AB and SCI, the r-value, reported as mean ± 1 standard error, for each AB and SCI module represents the similarity to that module for AB1 or the first AB to exhibit that module type. Module composition was quantified using two co-activity metrics, Wsum and Wmus. Wsum measured the activity of muscles in a module and was defined as the sum of significant muscle contributions (i.e. bar heights) in a module. A muscle was considered significantly active within a module if the confidence interval for that muscle did not include zero (i.e. significantly greater than zero). Wmus measured the number of muscles in a module and was defined as the count of significantly active muscles in a module. We predicted that both Wsum and Wmus would be significantly greater for iSCI compared to AB.
Based on our hypothesis, we predicted altered motor module activation in persons with iSCI as compared to AB controls. Module activations were divided into gait cycles using stance onsets computed from the footswitches. Mean activations for gait cycles at self-selected, slow, and fast cadences were computed for visual representation and statistical comparisons. As illustrated in Figure 1B, we quantified module activation using three metrics: area (Carea), amplitude (Cpeak), and duration (Cduty). Carea defined the overall activation level as the area under the mean activation curve. To further characterize module activation shape, we quantified Cpeak as the average peak of the module activations and Cduty as the average duty cycle of the module activations. For duty cycle, modules were considered active in bins where C > 0.15, i.e. 15% of max activity. Similarly, module composition and activation comparisons were made between walking conditions for AB subjects (AB versus ABmatch, n=5) and between ABmatch and iSCI for matched subjects (n=5).
2.7. Inter-subject and intra-subject data reconstructions
To assess differences in modular organization and the flexibility of motor modules to create a variety of muscle coordination patterns, modules extracted from one dataset were used to reconstruct data from another dataset (Gizzi et al., 2011, Torres-Oviedo et al., 2010). The VAF indicated the degree of similarity, with low VAF indicating different organizations underlying muscle coordination. We computed within-group similarity by iteratively using each individual AB’s modules to reconstruct the data from all other AB subjects concatenated together and meaning the VAF. This was repeated for the iSCI group. Differences in modular organization and flexibility between iSCI and AB groups were similarly quantified by iteratively using each individual AB’s modules to reconstruct the full iSCI dataset. This was repeated for iSCI modules reconstructing the AB dataset.
To quantify the effect of cadence and assistive device, an individual’s AB modules were used to reconstruct data from their own ABmatch conditions (n=5) and vice versa. Such reconstructions also allowed for a direct comparison of module activations because the modules were identical. Uncentered correlation coefficients (r) were used to compare these activations and reported as mean ± 1 standard error. As above, we also compared Carea, Cpeak, and Cduty between groups. For matched subjects, individual AB modules reconstructed data from iSCI match (n=5) and vice versa. Similarly, ABmatch modules reconstructed data from iSCI match and vice versa. These comparisons are shown in Figure 3B.
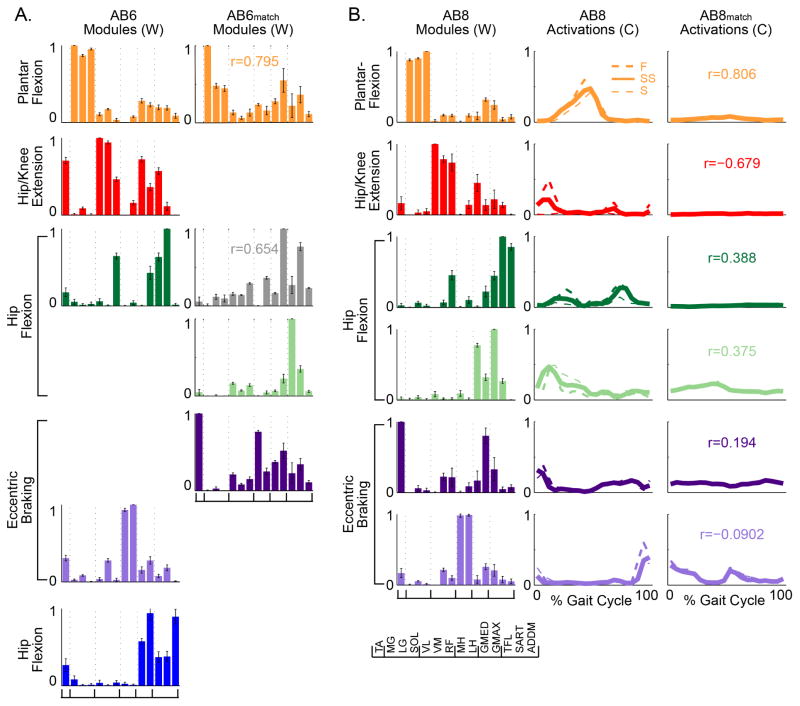
Figure 3. Inter-subject reconstructions show that AB modules can be flexibly combined to reconstruct a wider range of muscle coordination patterns.
The dashed gray line indicates 90% VAF, the level of reconstruction achieved when modules were extracted from the data. A) Bar plots showing the variance accounted for (VAF, mean ± 1 standard error) when using one subject’s modules to reconstruct muscle activity from other subjects. Each plot represents a mean across 8 subjects. From left to right: Mean VAF for each individual AB modules reconstructing remaining muscle activity of AB subjects, individual iSCI modules reconstructing remaining SCIs’ muscle activity, individual AB modules reconstructing SCIs’ muscle activity, and individual SCI modules reconstructing muscle activity of AB subjects. B–D) Bar plots showing the variance accounted for (VAF, mean ± 1 standard error) by reconstructions for the 5 subjects included in the cadence and assistive device matched subset. B) Mean VAF of each individual ABmatch modules reconstructing their matched iSCI subject’s muscle activity versus individual SCI modules reconstructing their matched ABmatch subject’s muscle activity in the cadence and assistive device condition. C) Individual ABmatch modules reconstructing the same AB subject’s muscle activity from the self-selected condition versus individual iSCI modules reconstructing their matched AB subject’s muscle activity. D) Individual ABmatch modules reconstructing remaining ABmatch muscle activity in the cadence and assistive device condition versus individual iSCI modules reconstructing the remaining iSCIs’ muscle activity. Asterisks indicate significant differences between groups at p < 0.05.
Previous reports have shown that motor modules are robust across from different walking speeds in able-bodied persons (Chvatal et al., 2012, Clark et al., 2010). To determine if this was evident with the cadences used in our study, we used each subjects’ modules to reconstruct data from their individual cadences. For all subjects and conditions, the VAF exceeded 85%, with mean VAFs across all subjects of 91.9±0.4% for self-selected, 91.3±0.6% for slow, and 91.4±0.4% for fast. There was also no statistical difference between the variability that the modules accounted for in the combined cadence dataset compared with variability accounted for in individual cadence datasets (F3,60 = 0.616, p = 0.607). Additionally, modules extracted from the combined dataset accounted for more variance than those extracted from self-selected walking alone, confirming the usefulness of varied cadence. From the models we found the modules extracted from single cadence conditions alone represented either a statistically similar subset of the full dataset modules or included one to two distinct module.
2.8. Statistical analyses
All statistical analyses were performed in SPSS® 21 (IBM SPSS Inc, USA). Results were considered significant at p<0.05 and reported as mean ± 1 standard error. Due to the non-normality and categorical nature of the module number dataset, we used the Mann-Whitney U test for between-group comparisons (iSCI versus AB, iSCI versus ABmatch) and the Wilcoxon Signed Rank test for within group comparisons (AB versus ABmatch). We compared W and C metrics using two-sample t-tests as their distributions were not different from normal. VAF for inter- and intra-subject reconstructions were compared using one-way ANOVA with post-hoc pairwise comparisons with Bonferroni corrections for multiple comparisons. We adjusted our parametric tests depending on the Levene’s test for homogeneity of variances. Finally, linear regressions were used to test for significant relationships between module number and clinical assessment scores.
3. Results
3.1. Reduced number of motor modules during overground walking after chronic iSCI
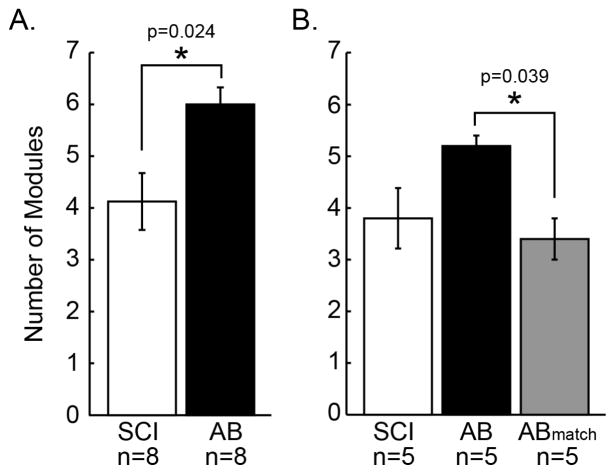
Persons with iSCI required fewer motor modules to account for their muscle coordination patterns during overground walking as compared to AB controls (Figure 2A). AB controls exhibited 5.9 ± 0.4 motor modules as compared to 4.1 ± 0.6 motor modules for persons with iSCI (U14 = 11.000, p = 0.024). Six of eight iSCI subjects required less than 5 modules to account for the variability of muscle coordination patterns, while all AB subjects required more than 5 modules, suggesting AB subjects have greater complexity of and flexibility in constructing muscle coordination during overground walking.
Figure 2. Persons with iSCI exhibit fewer motor modules than AB subjects during overground walking.
A) Bar plots showing the number of modules (mean ± 1 standard error) required to explain muscle activity for SCI subjects (n=8) and AB subjects (n=8). Asterisk indicates significant difference based on a Mann-Whitney U test for independent samples. B) Bars showing number of modules (mean ± 1 standard error) for the subset of matched SCI (n=5) and AB (n=5) subjects. ABmatch indicates the number of modules exhibited when the AB (n=5) subjects were constrained to walk at the cadence and with the assistive device of their iSCI match. Asterisk indicates significant difference based on Wilcoxon Signed Rank test for related samples between AB and ABmatch.
The reduced locomotor complexity in the iSCI subjects was reflected in inter-subject reconstructions. Modules extracted from an AB subject’s EMG muscle activity accounted for a larger percentage (79.5 ± 1.0%) of all iSCIs’ muscle activity (F3,28 = 10.502, p < 0.001), suggesting that AB modules can be combined to create a broader range of muscle coordination patterns as compared to persons with iSCI (Figure 3A). In contrast, modules extracted from an individual iSCI’s muscle activity accounted for a lower amount (68.1 ± 2.6%) of the variability seen across muscle activity in AB controls, suggesting insufficient complexity and flexibility to produce AB-like muscle coordination patterns.
3.2 Altered composition and activation of motor modules emerge after iSCI
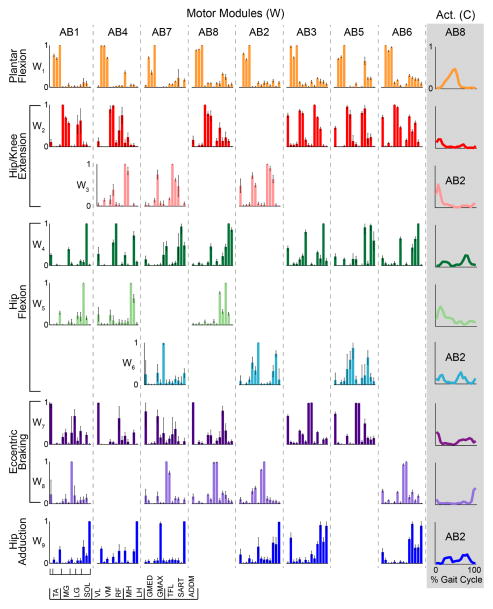
While exact module composition was subject-specific, AB modules exhibited a high degree of similarity in composition and activation of modules (Figure 4). All AB subjects exhibited modules characterized by the same four kinematic descriptors of overground walking. Based on the muscle composition and activation profiles of AB subjects (Figure 4), we characterized the modules as plantar flexion (W1), hip/knee extension (W2 and/or W3), hip flexion (W4, W5, and/or W6), and eccentric braking (W7 and/or W8). 6 of 8 AB subjects also exhibited a module for hip adduction (W9). The plantar flexion module W1 was similar across all 8 AB subjects (r = 0.95 ± 0.015). The hip flexion module (W4) was similar across 7 AB subjects (r = 0.81 ± 0.028). The hip/knee extension module (W2, r = 0.77 ± 0.056), eccentric braking module (W7, r=0.69±0.036), and hip adduction module (W9, r = 0.69 ± 0.063) were similar across 6 AB subjects. The eccentric braking module (W8) was similar across 5 AB subjects (r = 0.75 ± 0.040). Other modules were similar across less than half of AB subjects. Due to this similarity, modules extracted from one AB subject’s data were able to reconstruct the remaining AB subjects’ data with 81.4 ± 0.83% VAF (Figure 3A).
Figure 4. Motor modules exhibited by each AB subject and representative activations.
Bar plots represent the relative muscle contributions to each module, with muscles ordered as indicated by the legend at the bottom. Standard deviation bars show the variation about the mean muscle contribution across 10 bootstrapped extractions. Each column represents modules from a single AB subject. Modules were considered similar and, thus, group in a row if r > 0.532 when compared to AB1 modules, the subject with the most modules. Functional labels to the left indicate the purported mechanical function of that module or group of modules (Neptune et al., 2001). On the far right, representative activations for each module are shown as line plots from one of 2 representative subjects that exhibited that module (AB8 unless indicated as AB2). The lines show the mean module activation across the gait cycle (5% bins) for self-selected walking trials.
Most AB modules were recruited during one or two specific phases of the gait cycle (Figure 4, last column). During stance, one or more of the hip/knee extension modules, composed of quadriceps (VL, VM, RF) and gluteals (GMAX, GMED), were first recruited likely to support the weight of the body followed by the plantar flexion module toward the end of stance likely for propulsion. The hip flexion modules, composed largely of TFL, SART, and RF, were recruited during stance and again during swing, with larger activation early in the gait cycle for modules with greater RF contributions. The eccentric braking modules were composed largely of the ankle dorsiflexion (TA) and hamstrings (MH, LH) and also recruited during both stance and swing. Early stance phase activation likely controlled foot placement through eccentric braking of the foot by the TA and of the shank by the hamstrings, while swing phase activation flexed and shortened the limb for forward progression and foot-ground clearance. Finally, the hip adduction module, composed of the ADDM with or without other proximal muscles, was likewise recruited during both phases possibly to control limb placement during mid-stance and in preparation for heel contact during swing.
In contrast, iSCI subjects exhibited a wider range of module compositions, reflective of the heterogeneity inherent to iSCI. An example of modules and their activations for a representative subject with the mean number of modules is shown in Figure 5. Modules extracted from iSCI subjects were each compared to the set of modules extracted from the AB subjects. All iSCI subjects exhibited a plantar flexion module (W1) that was statistically similar to the AB plantar flexion module (r = 0.77 ± 0.040). The hip/knee extension module (W2, r = 0.76 ± 0.048) and eccentric braking module (W8, r = 0.71 ± 0.057) were similar across 5 of 8 iSCI subjects and similar to their AB counterparts. All other modules were shared by less than half of iSCI subjects or statistically distinct from all AB modules. A total of 3 iSCI modules were statistically distinct from all AB modules, mostly characterized by extensive co-activity. Due to reduced similarity between modules in iSCI subject, modules extracted from one iSCI subject’s data reconstructed the remaining iSCI subjects’ data with 74.3 ± 2.3% VAF, significantly less than AB subjects (Figure 3A; F3,28 = 11.431, p = 0.001). Interestingly, the eccentric braking module (W7) recruited by AB for controlled foot placement in early swing was absent in all iSCI, contributing to foot drop or slap often observed after iSCI. Some subjects appeared to use other TA-containing modules to provide eccentric TA control, but many lacked this control.
Figure 5. Motor modules and activations exhibited during overground walking in a representative subject with iSCI.
Modules and activations from a representative iSCI subject (SCI4) who exhibited 4 modules, the mean number for iSCI subjects. As in Figure 4, bar plots represent the muscle contributions to each module and line plots show the corresponding mean activations across the gait cycle. Modules were assigned the same color and functional label if they were statistically similar to those shown in Figure 4 (r<0.532). Gray modules indicate modules that were dissimilar from all AB modules.
Module composition in subjects with iSCI modules differed from that of AB controls (Figure 6). In subjects with iSCI, motor modules consisted of greater muscle co-activity (Wmus) (t14 = 2.836, p = 0.013), as well as, higher contributions (Wsum) from the composing muscles (t14 = 2.606, p = 0.021) as compared to AB controls. This can be seen in the plantar flexion and eccentric braking modules in the representative comparison shown in Figure 6. AB modules contained primarily muscles from one functional group, such as plantar flexors or hamstrings, whereas the iSCI modules contained a combination of multiple functional groups merged together from multiple AB modules, similar to reports in persons with stroke (Clark et al., 2010). For example, muscles across multiple joints, such as the hamstrings and gluteals or the ankle plantar flexors and knee extensors, were co-active in the iSCI eccentric braking module and plantar flexion module. Some iSCI subjects also showed antagonist muscles in a single module. In contrast, AB controls appeared to separate muscle contributions to motor modules, which may allow for differential control of these muscles and, thus, greater locomotor complexity.
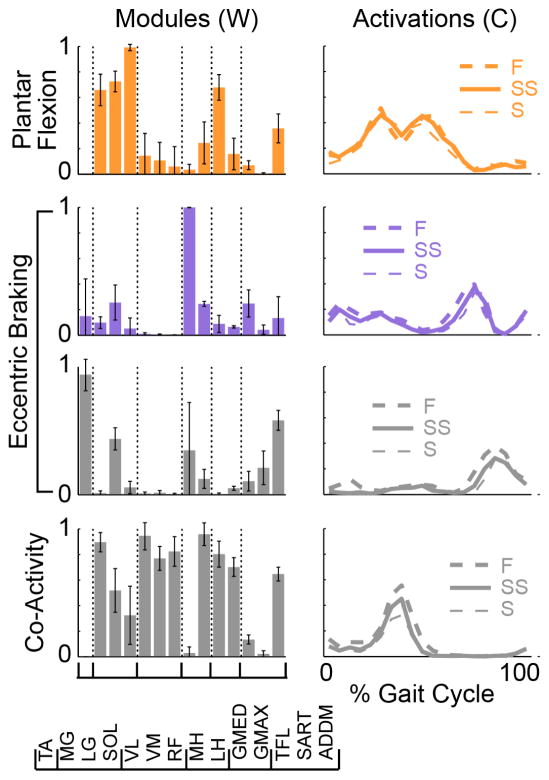
Figure 6. Modules differ between subject groups in number, composition, and activation.
Comparison of modules and activations for a representative AB (AB6) and iSCI (SCI6) matched pair. SCI6 modules were considered similar and aligned to AB6 modules if r > 0.532. The r-values for each module and activation represent the similarity to AB#. Colors and functional labels indicate similarity to as described for Figure 4. The gray modules were dissimilar in the Figure 4 comparison but aligned when only compared to AB6’s hip flexion module.
The iSCI modules exhibited broader activation patterns during overground walking as compared to AB controls. Activation area (Carea, t14 = 3.539, p=0.007) and duty cycle (Cduty, t14 = 4.243, p = 0.003) were greater for iSCI modules compared to AB modules. The plantar flexion and eccentric braking modules in particular demonstrated this difference (Figure 6). Although peak activation level tended to be slightly higher for iSCI modules, it did not differ significantly between the two groups (Cpeak, t14 = 1.071, p=0.302). Broad activations of iSCI modules overlapped between modules, as seen in Figures 5 and 6, where multiple modules were co-active across much of the gait cycle; while overlap in AB module activation tended to be most active during distinct phases of the cycle.
3.3. AB subjects flexibly alter muscle coordination for iSCI matched walking conditions
AB control subjects walked faster overground at faster cadences than subjects with iSCI. Mean SCI self-selected cadence was 44.3 ± 6.5 steps per minute, significantly slower than that of AB (t12 = 8.779, p < 0.001). AB self-selected cadence was 107.9 ± 2.2 (mean ± 1 standard error) steps per minute. Due to this between-group discrepancies in cadences, as well as, differences in assistive devices used during overground walking, we assessed to what extent slower cadences and use of assistive devices affect motor module number, composition, and activation in AB controls. When a subset of AB subjects (n=5) were constrained to walk at iSCI-matched cadences using the matched assistive device, the number of motor modules was significantly reduced from 5.9 ± 0.35 to 3.4 ± 0.32 (p = 0.039; Figure 2B). The number of modules did not differ significantly between the subset of matched iSCI and AB subjects in this condition (U8 = 12.000, p = 0.911; Figure 2B). However, matched iSCI modules exhibited greater muscle co-activity as reflected by higher muscle contributions within modules (Wsum, t8 = 2.806, p=0.023); other metrics were not significantly different. Additionally, while the number of modules were not statistically different, the inability of iSCI modules to reconstruct ABmatch data and vice versa demonstrates that the modules differed in composition and are not interchangeable for creating muscle coordination patterns (Figure 3B). Although not statistically different, ABmatch modules were better at reconstructing AB muscle coordination patterns compared to iSCI modules, despite having the same number of available modules (Figure 3C). This suggests that number is not the only indicator of flexibility and that AB subjects retain greater flexibility with a reduced number of modules compared to iSCI subjects with the same number. Finally, ABmatch modules are significantly less effective at reconstructing ABmatch from the other subjects (t8 = 2.543, p = 0.035), suggesting their coordination strategies varies more across the cadences and assistive devices compared to persons with iSCI (Figure 3D).
In addition to using a reduced number of modules, AB subjects (n=5) reduced their activation of stance-phase modules, reflecting reliance on the assistive device for body weight support and propulsion. For instance, two AB subjects that walked with a rolling walker no longer exhibited a hip/knee extension module active during stance (Figure 7A). When the AB self-selected modules were used to reconstruct the ABmatch dataset, all activations decreased, particularly of weight-bearing and propulsive modules like the plantar flexion and hip/knee extension modules. Moreover, three AB subjects that walked with cane or crutches (Table 1) at slower cadences (<50% of self-selected) no longer exhibited a plantar flexion module for propulsion while the higher cadence subject (>50% of self-selected) returned this module at a reduced activation level. When AB modules were used to reconstruct the ABmatch data for either assistive device type, all module activations decreased in Carea, Cpeak, and Cduty (all t4 > 3.770 and p < 0.020); figure 7B illustrates this in a representative subject (AB8). This was particularly evident for weight-bearing and propulsive modules like the plantar flexion and hip/knee extension modules.
Figure 7. AB subjects reduce their module number and activation when walking at matching iSCI cadence and assistive device.
A) Modules extracted from muscle coordination patterns during walking at self-selected cadences (112 steps per minute) for a representative subject (AB6) compared to modules extracted from the ABmatch condition of walking for that same subject (38 steps per minute with loftstrand crutches). Module number was reduced. B) Modules (left) exhibited by a representative subject (AB8) walking at self-selected cadences. Module activations required to reconstruct muscle coordination during the self-selected condition (middle, 110 steps per minute) versus the cadence and assistive device match condition (right, 26 steps per minute with rolling walker). All activations were reduced, particularly during stance. Colors and functional labels again indicate similarity to those shown in Figure 4.
3.4. Constraints on muscle coordination reflect walking deficits after iSCI
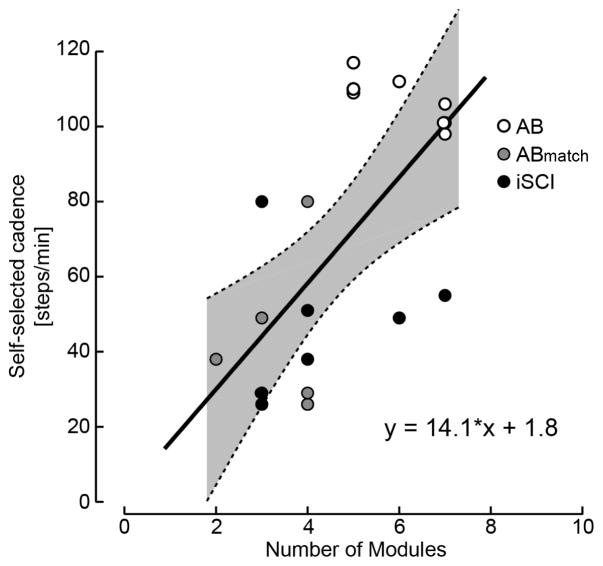
All iSCI subjects showed limited maximum walking speeds and significantly lower self-selected cadences. All but one subject required an assistive device to walk overground safely. The reduced module number, co-activity within modules, and broad inappropriate module activation reveal neuromuscular mechanisms that likely constrain these aspects of overground walking ability. However, the specific neuromuscular mechanisms underlying their deficits reflected in module composition and activation appear to be highly subject-specific and heterogeneous likely due to the inherent heterogeneity of iSCI. As a result, the number of modules alone did not predict self-selected cadence or maximum speed (R2 < 0.16, p > 0.33) and no linear relationship was seen between limb strength (LEMS) or gait deviations in the SCI-FAI score (R2 < 0.09, p > 0.50). This is in contrast to previous reports in stroke and Parkinson’s disease in which the number of motor modules scales with locomotor speed (Clark et al., 2010, Rodriguez et al., 2013). Rather, the neuromuscular mechanisms underlying reduced speed and SCI-FAI deficits appear to be subject-specific, as explored below in the discussion, and cannot be seen in module number alone. Nevertheless, across all participants, module number correlated to walking cadence (Figure 8). There was a linear relation between the number of motor modules extracted for overground walking and cadence (r2 = 0.40; p = 0.003); subjects with slower cadences had fewer motor modules. In contrast, subjects with essentially no impairments (i.e., AB control subjects) and correspondingly higher cadences had more motor modules.
Figure 8. Motor module number versus overground self-selected cadence in subjects with iSCI and age-matched controls.
Linear regression models were used to describe the relationship between self-selected cadence and module number for AB subjects walking at self-selected cadence, subjects with iSCI walking at self-selected cadence, and AB subjects walking at matched iSCI cadences and assistive devices (ABmatch). Data are pooled across all subjects. Data from AB subjects are represented with open circles, data from iSCI subjects are represented as black circles, and data from ABmatch subjects are represented with gray circles. Thin dashed lines above and below the regression line define 95% confidence intervals. The model was significant for describing a relationship between self-selected cadence and motor module number (r2 = 0.4; p < 0.003).
4. Discussion
The purpose of this study was to quantify altered muscle coordination during overground walking in persons with chronic iSCI. In particular, we predicted that during overground walking, persons with iSCI have fewer motor modules, greater number of muscles within each motor module (composition), and altered activation of motor modules as compared to age-matched AB controls. Indeed, our results showed that persons with iSCI required fewer motor modules to explain the observed muscle coordination patterns during overground walking. Reduced module number, along with altered composition and activation within and across modules, reflected constraints on muscle coordination that likely limit safe and effective walking. These constraints are evidenced by the universal reduction in walking speed (10MWT) and self-selected cadence and the requirement of an assistive device in all but one iSCI. Below we discuss how motor module number, composition, and activation reveal common as well as subject-specific mechanisms. The identification of neuromuscular deficits have implications for developing targeted rehabilitation strategies to improve community ambulation in persons with chronic iSCI.
4.1 Constraints on muscle coordination during overground walking after chronic iSCI
Persons with iSCI have fewer “building blocks” for constructing muscle activity, limiting the complexity of muscle coordination for overground walking. Additionally, the co-activation within modules and overlapping broad activation across modules limits the ability to activate individual muscles or muscle groups to perform specific biomechanical subtasks. Therefore, the range of possible muscle coordination patterns and the flexibility to modulate patterns is reduced. This lack of flexibility is evidenced by the intersubject reconstructions. While AB modules can be flexibly combined to reconstruct other AB and iSCI muscle coordination patterns, iSCI modules can only reconstruct a limited range of activity and, thus, yield significantly poorer reconstructions of other iSCI and AB patterns.
4.2 Adapting muscle coordination to novel task demands
The uninjured human neuromotor system can flexibly adjust motor commands to coordinate muscles during various walking demands. For example, when AB subjects were challenged with the novel task of walking at approximately 25% of their self-selected cadence using an assistive device, they adapted muscle coordination strategies to fit the task. AB subjects either reduced activation of their self-selected modules and/or reduced the number of modules required. Adjusting control strategies to account for body-weight support through an assistive device eliminated the need for modules observed during self-selected cadence conditions. In many cases, AB subjects eliminated hip/knee extension or plantar flexion modules or greatly reduced their activation. The reduction in the number of modules required to walk at slower cadences with an assistive device suggests that the assistive device may serve as a substitute module for iSCIs, allowing iSCI subjects to compensate through the use of upper body muscle activation and mechanical properties of the device to walk overground.
The inability of iSCI subjects to walk across a broad range of speeds prevents us from directly testing whether iSCI retain this degree of flexibility. However, their inability to modulate speed by modulating muscle coordination suggests that they lack the flexibility and locomotor complexity of the intact nervous system. While AB subjects have a greater number of modules available and the ability to modulate activation of these modules, iSCI may be limited to just the reduced set exhibited at their self-selected cadences. Further, the increased co-activity (Wsum) in their modules compared to AB and ABmatch conditions may prevent them from performing and individuating the biomechanical subtasks necessary for modulating speed. For example, their plantar flexion modules were often compounded with hip flexors and knee extensors, which may reduce the propulsive forces produced. The plantar flexion module also was co-activated with other modules that could interfere further with propulsion, thereby limiting speed. Alternatively, the reduction in the number of modules used by AB at slower cadences with an assistive device could suggest that the reduced number of modules exhibited by iSCI subjects simply reflects the task of a slower cadence and assistive device use. However, the inability of iSCI subjects to walk at higher speeds or without their assistive device implies that the reduced number of modules and increased co-activity constrains task performance. This firm ceiling on their walking speed suggests that, even if reduced module number is due to speed alone, they do not have access to the additional modules required to produce the coordination required for higher walking speeds. Further, previous work in able-bodied persons found that modules (also referred to as synergies) are robust across speeds within AB subjects (Chvatal et al., 2012, Clark et al., 2010), providing credence to the notion that speed is not the only factor accounting for a reduction in module number when AB subjects walk slower. Most likely, the two are inextricably linked, such that the injury reduces the ability of the nervous system to produce muscle coordination, requiring iSCI subjects to substitute an assistive device for certain biomechanical subtasks and to walk at slower speeds.
4.3 Limitations
Despite careful experimental design, our study has limitations. As noted above, because iSCIs cannot walk at faster speeds and forcing AB to walk at nearly half their cadence represents a novel and somewhat unnatural task, it is difficult to know for sure whether the reduced module number is an artifact of speed. However, this study does provide insight into the differences in coordination between iSCI and AB persons at both self-selected speeds and slow cadences, allowing us to infer the underlying neuromuscular mechanisms that constrain iSCI overground walking. The small sample size and heterogeneity of injury compromise the ability to identify relationships between module number and gait deficits. While subject-specific relationships were seen, future studies in which subjects are stratified by injury characteristics may reveal a stronger correlation between modules and gait deficits. Additionally, while previous work on AB subjects allowed us to infer the mechanical function of these modules (Neptune et al., 2009), how the absence or altered composition and activation of modules in iSCI affects mechanical output remains unconfirmed and deserves future investigation. Given the significant asymmetries exhibited by iSCI subjects (see Table 1), the number of modules may likely be affected by whether the more or less impaired limb was tested. Severe impairment in one limb may not be fully reflected by an outcome measure, like the 10MWT, if significant asymmetry allows for compensation with a much stronger contralateral limb. This was certainly true of our fastest subject iSCI2 and calls to attention the need to extend modular analysis to both limbs. Finally, subjects with a low number of modules and high co-activity within modules may present similarly to subjects who fail to appropriately activate a high number of AB-like modules. Both may display a low step rhythm score on the SCI-FAI and slow walking speed on the 10MWT, but the number of modules may vary significantly. Further exploration, including injury stratification, mechanical measures, and interlimb interactions, will allow us to more fully understand how neuromuscular mechanisms influence observable gait deficits and how therapies may be prescribed to target these deficits.
4.3 Clinical implications
The heterogeneity of spinal injuries, which vary with the neural pathways affected and the severity of the injury, demands subject-specific analysis of the neuromuscular mechanisms that produce walking deficits. Understanding and quantifying these mechanisms is vital for the development of more targeted therapies. Current clinical walking tests effectively describe observable gait deficits and track improvements, but are limited in their ability to identify the underlying mechanisms and to predict adaptability to complex environments or perturbations (Barbeau et al., 2002, van Hedel et al., 2005). In many cases, the same observable deficit can have multiple causes that vary between subjects. Additionally, many clinical tests rely on 7 qualitative ordinal scales while even the most quantitative tests, such as the 10-meter or 6-minute walk tests, fail to detect small deficits in higher functioning walkers (van Hedel et al., 2005). This limited sensitivity is evidenced by the absence of correlation between clinical scores and the modules that serve as “building blocks” for muscle coordination. Our analyses also showed that modular constraints are highly heterogeneous among persons with iSCI, as demonstrated by the low similarity among iSCI modules and poor inter-subject reconstructions. Thus, it is not surprising that a single metric like module number cannot fully predict walking impairment.
Module analysis may provide a more sensitive tool for identifying the diversity of neuromuscular mechanisms that constrain overground walking after iSCI. For example, the SCI-FAI identified deviations in step rhythm or relative time to advance the swing limb. The two subjects who show delayed advancement, iSCI3 and iSCI6, both exhibited prolonged activation of the hip/knee extension module, suggesting that inappropriate prolongation of this module prevents hip flexion delaying advancement. Other SCI-FAI deviations had multiple underlying mechanisms that varied depending on the subject. For example, SCI-FAI deviations in step height in which the toe fails to clear the ground were related to the weak activation of the ankle and hip flexion modules in iSCI1, but not in iSCI3 and iSCI6 who possessed greater TA activation. Reduced step height in iSCI3 and 6 is more likely explained by the activation of a co-activity module with hip extensors and flexors during swing phase. These co-activity modules are dissimilar from all AB modules and likely constrain hip flexion leading to failed toe clearance. Additionally, iSCI6 exhibited particularly prolonged flexion module activation that may further contribute to an inability to dorsiflex the foot for clearance (Capaday et al., 1999). There was also variability in foot contact deviations, with 3 subject showing appropriate heel strike and 5 showing forefoot or flat foot contact. iSCI subjects with heel strike used eccentric activation of modules containing TA, allowing for controlled foot placement at stance onset, while the others showed little or no eccentric activation. This variability likely reflects differing preservation of corticospinal tract projections to the TA, as TA receives greater cortical input compared to other lower extremity muscles particularly during early stance-phase eccentric control (Capaday et al., 1999, Perez et al., 2004, Schubert et al., 1997). Finally, it should be noted the subject who is the most different from AB in terms of number of modules and module similarity (iSCI6) walked at the slowest cadence, slowest speed, and had the lowest SCI-FAI score, suggesting that similarity to AB modules may be the best predictor of overground walking function.
One striking finding from this study was that module number was correlated with self-selected cadence during overground walking. This result implies that slower cadences (i.e., iSCI subjects) required fewer muscle coordination patterns. Our findings are consistent with a prior study that showed persons with chronic stroke had fewer motor modules and walked slower (Clark et al., 2010). Indeed, persons with chronic iSCI walked slower and were constrained by fewer motor modules, as well as, abnormal co-activity between muscles in each motor module and prolonged, overlapping activation of these modules. Thus, we suspect that altered composition and activation of motor modules contributed to our subject-specific gait deficits (e.g., reduced speed and reliance on assistive devices), which have been implicated as possible mechanisms for gait deficits in other neurologic disorders such as stroke (Bowden et al., 2010) or Parkinson’s disease (Rodriguez et al., 2013). Based on these complimentary discoveries, we propose that therapies directed at facilitating increased complexity of muscle coordination also may facilitate increased self-selected walking speed and subsequently lead to improved community ambulation in persons with iSCI.
f4.4 Conclusions
This study quantified the constraints on overground muscle coordination that occur after chronic iSCI. In agreement with our hypotheses, persons with iSCI were constrained by fewer motor modules characterized by increased co-activity and broad overlapping activation, both of which contribute to increased muscle co-activity. iSCI modules cannot explain the diversity of muscle coordination patterns expressed by AB controls, reflecting limited flexibility in neuromuscular control that likely contributes to constraints on gait speed and overground walking independent of walking aids. Further, subject-specific alterations in module composition and activation reveal neuromuscular mechanisms underlying gait deficits, potentially providing guidance for targeted and individualized rehabilitation plans.
Highlights.
Persons with chronic incomplete spinal cord injury (iSCI) exhibit significant reduced muscle coordination during overground walking as compared to age-matched adults.
Neuromuscular constraints following iSCI contribute to person-specific deficits in overground walking.
Neuromuscular mechanisms underlying gait deficits may provide guidance for targeted SCI rehabilitation.
Acknowledgments
This work was supported in part by NIH grant K12 HD055931, DOD grant SC090355, as well as, a grant from the Petit Institute for Bioengineering & Bioscience. The authors are very grateful to the participants. The authors would also like to thank Monica Danneman, Jillian Dean, David Gustafson, and Caitlin Manuel for their assistance with data collection and Victoria Stahl for her assistance with technical issues and manuscript proofing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JL, Kautz SA, Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech. 2013;28:697–704. doi: 10.1016/j.clinbiomech.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Fung J, Leroux A, Ladouceur M. A review of the adaptability and recovery of locomotion after spinal cord injury. Prog Brain Res. 2002;137:9–25. doi: 10.1016/s0079-6123(02)37004-3. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: Relationship of the fugl-meyer assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24:328–37. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton SS, Krause JS, Nietert PJ. Falls in individuals with incomplete spinal cord injury. Spinal Cord. 2007;45:37–40. doi: 10.1038/sj.sc.3101909. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol. 1999;81:129–39. doi: 10.1152/jn.1999.81.1.129. [DOI] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F. Motor patterns in human walking and running. J Neurophysiol. 2006;95:3426–37. doi: 10.1152/jn.00081.2006. [DOI] [PubMed] [Google Scholar]
- Cheung VCK, d’Avella A, Bizzi E. Adjustments of motor pattern for load compensation via modulated activations of muscle synergies during natural behaviors. J Neurophysiol. 2009a;101:1235–57. doi: 10.1152/jn.01387.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VCK, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. P Natl Acad Sci. 2009b;106:19563–8. doi: 10.1073/pnas.0910114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci. 2012;32:12237–50. doi: 10.1523/JNEUROSCI.6344-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol. 2011;106:999–1015. doi: 10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–57. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Portone A, Lacquaniti F. Superposition and modulation of muscle synergies for reaching in response to a change in target location. J Neurophysiol. 2011;106:2796–812. doi: 10.1152/jn.00675.2010. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. Journal of biomechanical engineering. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- Drew T, Kalaska J, Krouchev N. Muscle synergies during locomotion in the cat: A model for motor cortex control. J Physiol. 2008;586:1239–45. doi: 10.1113/jphysiol.2007.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC, Fluet GG, Schafer SD, Schneider EM, Smith R, Downey PA, et al. The spinal cord injury functional ambulation inventory (sci-fai) Journal of rehabilitation medicine: official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2001;33:177–81. doi: 10.1080/165019701750300645. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Phys Ther. 2011;91:48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EJ, Tester NJ, Kautz SA, Howland DR, Clark DJ, Garvan C, et al. Modular control of varied locomotor tasks in children with incomplete spinal cord injuries. J Neurophysiol. 2013;110:1415–25. doi: 10.1152/jn.00676.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizzi L, Nielsen JF, Felici F, Ivanenko YP, Farina D. Impulses of activation but not motor modules are preserved in the locomotion of subacute stroke patients. J Neurophysiol. 2011;106:202–10. doi: 10.1152/jn.00727.2010. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol. 2009;101:969–79. doi: 10.1152/jn.91131.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Castellano V, et al. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1019–34. doi: 10.1093/brain/awh115. [DOI] [PubMed] [Google Scholar]
- Hof AL, Elzinga H, Grimmius W, Halbertsma JP. Speed dependence of averaged emg profiles in walking. Gait Posture. 2002;16:78–86. doi: 10.1016/s0966-6362(01)00206-5. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Zago M, Molinari, Marco, Scivoletto G, Castellano V, et al. Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J Neurophysiol. 2003;90:3555–65. doi: 10.1152/jn.00223.2003. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. 2004;556:267–82. doi: 10.1113/jphysiol.2003.057174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–91. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Hidler J. Biomechanics of overground vs. Treadmill walking in healthy individuals. J Appl Physiol. 2008;104:747–55. doi: 10.1152/japplphysiol.01380.2006. [DOI] [PubMed] [Google Scholar]
- Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotraum. 2002;19:1217–29. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr Neurologic recovery after traumatic spinal cord injury: Data from the model spinal cord injury systems. Archives of physical medicine and rehabilitation. 1999;80:1391–6. doi: 10.1016/s0003-9993(99)90249-6. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: A simulation study. J Biomech. 2009;42:1282–7. doi: 10.1016/j.jbiomech.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. How we walk: Central control of muscle activity during human walking. The Neuroscientist. 2003;9:195–204. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci. 2008;28:880–92. doi: 10.1523/JNEUROSCI.2869-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin A, Ladouceur M, Barbeau H. Treadmill walking in incomplete spinal-cord-injured subjects: 2. Factors limiting the maximal speed. Spinal Cord. 2003;41:271–9. doi: 10.1038/sj.sc.3101453. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez KL, Roemmich RT, Cam B, Fregly BJ, Hass CJ. Persons with parkinson’s disease exhibit decreased neuromuscular complexity during gait. Clin Neurophysiol. 2013;124:1390–7. doi: 10.1016/j.clinph.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini AM. Identification of neuromuscular synergies in natural upper-arm movements. Biol Cybern. 2002;86:253–62. doi: 10.1007/s00422-001-0297-7. [DOI] [PubMed] [Google Scholar]
- Schubert M, Curt A, Jensen L, Dietz V. Corticospinal input in human gait: Modulation of magnetically evoked motor responses. Exp Brain Res. 1997;115:234–46. doi: 10.1007/pl00005693. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol. 2006;96:1530–46. doi: 10.1152/jn.00810.2005. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. 2007;98:2144–56. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Ting LH. Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol. 2010;103:3084–98. doi: 10.1152/jn.00960.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Cheung VCK, d’Avella A. Matrix factorixation algorithms for the identification of muscle synergies: Evaluation on simulated and experimental data sets. J Neurophysiol. 2006;95:2199–212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Dietz V, Curt A. Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil Neural Repair. 2007;21:295–301. doi: 10.1177/1545968306297861. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: Validity and reliability of 3 walking tests. Arch Phys Med Rehab. 2005;86:190–6. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- van Hedel HJA, Dietz V. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neuros. 2010;28:123–34. doi: 10.3233/RNN-2010-0508. [DOI] [PubMed] [Google Scholar]
- van Hedel HJA, Dietz V Group EMSoHSCIS. Walking during daily life can be validly and responsively assessed in subjects with a spinal cord injury. Neurorehabil Neural Repair. 2009;23:117–24. doi: 10.1177/1545968308320640. [DOI] [PubMed] [Google Scholar]
- van Hedel HJA, Tomatis L, Müller R. Modulation of leg muscle activity and gait kinematics by walking speed and bodyweight unloading. Gait Posture. 2006;24:35–45. doi: 10.1016/j.gaitpost.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Paraplegia. 1994;32:540–53. doi: 10.1038/sc.1994.86. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall; 1974. [Google Scholar]