Abstract
In this study, a novel technique was developed in which magnetic microparticles (MMPs) and quantum dots (QDs) were successfully incorporated into fibrin clots. The MMPs were added at concentrations of 0.1 wt% and 1 wt% of the fibrin content in an effort to determine if a magnetic field could be utilized to mechanically stretch the fibrin network, simulating how cells may invade a network. The QDs were added at a dilute concentration of 0.1 wt% to determine if the mechanical properties of the fibrin network would be significantly altered and to ascertain if the overall stretch on the network could be observed. Based on strain sweep and frequency sweep rheological analysis, it was determined that both QDs and MMPs incorporated into fibrin networks at 0.1 wt% caused irreversible plastic deformation in the fibrin clot sample, as evidenced by a precipitous decline in the storage modulus value.
Background
Injury to a blood vessel's endothelial lining is repaired through the hemostatic response, or the formation of a blood clot. A key structural component of this healing process is the formation of the fibrin fiber matrix, which is necessary to obstruct the flow of blood. During injury, when the endothelium is damaged, blood is exposed to the extracellular matrix, specifically to collagen. Activated platelets in the blood are very reactive, as they release contents of stored granules into the blood plasma, which perpetuate more platelets to activate as well as initiate a secession of events that activate the coagulation cascade. Two pathways are involved in the coagulation cascade: the contact activation pathway (intrinsic pathway) and the tissue factor pathway (extrinsic pathway). Both pathways eventually lead to converting factor X (FX) to factor Xa which, along with cofactor FVa, forms the prothombinase complex activating prothrombin into thrombin. Thrombin is the enzyme that catalyzes fibrinogen, or factor I, a soluble plasma glycoprotein, into fibrin. Thrombin cleaves off the N-terminus of the α- and β-chains (within the E domain) of the fibrinogen molecule, leaving the fibrin knobs exposed to bind to the D regions at the end of other fibrin molecules. The resulting protofibril is a half staggered assembly, which has been described in the literature (1, 2). Fibrin serves as the main fiber monomer constituent of a blood clot, which then polymerizes into a fibrin network. This complex series of events results in the formation of a blood clot, which is a dense fibrous material structure, responsible for providing the structural material integrity necessary for maintaining hemostasis.
Objectives
Many researchers have investigated the formation and structure of fibrin networks using microscopy (3–12). Some researchers have also investigated how fibrin networks form in the presence of magnetic fields (13–17); however, it is evident from recent studies that the mechanisms behind the mechanical and viscoelastic properties are still elusive (18–23). In addition, there have been limited studies that have actually assessed the mechanical properties of fibrin matrices with embedded particles. The purpose of this study was to develop an in vitro model that represents the dynamics of the fibrin clot from the perspective of a cell. Using conjugated MMPs in conjunction with a magnetic field, and QDs as particle trackers, the goal was to assess the feasibility of incorporating these foreign particles into a fibrin matrix for mechanical stretching. In addition, the goal of the study was to ascertain whether a dilute particle concentration would alter the mechanical properties of the fibrin matrix. Both goals were achieved for the project. The results showed that the fibrin network with incorporated QDs and MMPs could be stretched in the presence of a magnetic field. The results also showed that particles added to the fibrin matrix even at low concentrations significantly alter the properties of the matrix, as evidenced by mechanical testing results.
Methods
Microfabrication Stamp
A microfabricated stamp was used to secure and mark the fibrin gel to ensure that when the MMPs were added, the network remained situated on a fixated substrate, allowing for easier and accurate data collection (see Supplementary Material, Figure 1). The PDMS gels were fabricated from the Lam Lab at Georgia Institute of Technology, Dept. of Biomedical Engineering. The gels were incubated with labeled fibrinogen diluted to a concentration of 0.03 mg/mL using buffer (25 mM HEPES, 150 mM NaCl, 5 mM CaCl2) for approximately 1 hour (this concentration was enough to thinly cover a surface). The gels were subsequently dried off with nitrogen gas, and immediately placed onto glass coverslips.
Particles and Magnetization
Quantum dots (QDs) were added to fibrinogen at a concentration that was 0.1% of the final concentration by weight. The QDs are Qdot® 655 ITK™ carboxyl quantum dots manufactured by Life Technologies™. The QDs possess a carboxylate functionalized surface chemistry and the size is on the order of 15–20 nm in diameter. QDs are visible under UV light and emit wavelengths according to their size, which enabled viewing on the confocal microscope. Magnetic microparticles (MMPs) were added at varying concentrations (0.1% and 1% of the final fibrinogen concentration). The MMPs are Dynabeads® M-270 Streptavidin manufactured by Life Technologies™. They are streptavidin coated beads that are 2.8 μm in diameter, with a monolayer of recombinant streptavidin covalently coupled to the surface of the bead. The MMPs are hydrophilic, negatively charged, and display rapid liquid-phase reaction kinetics. An ELISA was performed that showed successful conjugation of the MMPs into the fibrin network. All particles were added prior to polymerization by thrombin. A magnet (Product #D41, K&J Magnetics, Inc., Pipersville, PA) with surface field magnetization strength of 2,952 Gauss was used to induce magnetization in the fibrin sample.
Clot Formation
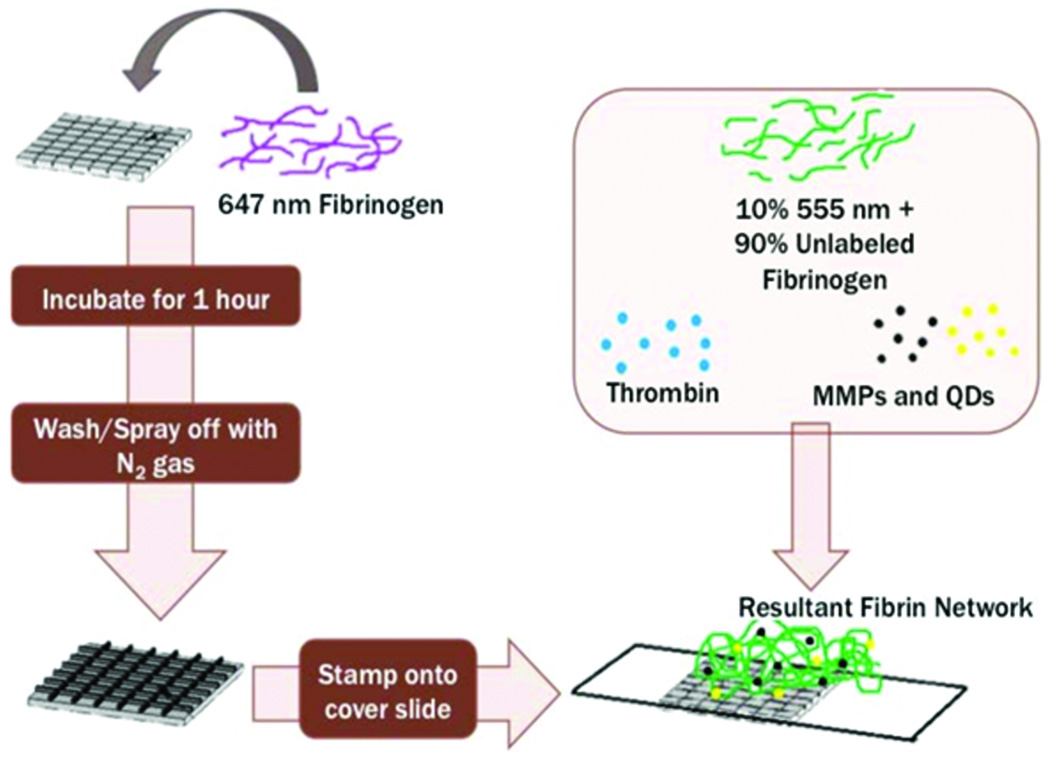
Combining volumes of labeled (647 nm Alexa Fluor dye or 555 nm Alexa Fluor dye) fibrinogen at 10% of the stock fibrinogen concentration with the stock fibrinogen (14.24 mg/mL), a total concentration of 5 mg/mL of fibrinogen was obtained. Next, thrombin was diluted down to 2 U/mL and subsequently added to the fibrinogen solution in a 1:1 ratio, resulting in a final concentration of 2.5 mg/mL of fibrinogen and 1 U/mL of thrombin. Figure 1 shows a schematic of how the microfabrication stamp was utilized, and how this was combined with fibrinogen and the embedded particles (MMPs and QDs) to create a resultant fibrin network.
Figure 1.
Schematic of experimental procedure used to fabricate fibrin networks with incorporated magnetic microparticles (MMPs) and quantum dots (QDs) in preparation for confocal microscopy
Confocal Microscopy
Confocal microscopy was conducted at the Georgia Institute of Technology, Parker H. Petit Institute for Bioengineering & Bioscience, Microscopy and Biophotonics Core Facility. Images were taken on the Zeiss LSM700-405 Confocal Microscope for multiple channel analysis and Zeiss LSM 510 VIS Confocal Microscope for single channel analysis. When MMPs were present, three 2 cm diameter magnets were stacked, and this configuration produced the magnetic field used to manipulate the particles. This magnetic cylinder was placed 3 cm from the center of the sample. The magnetic field was close to homogeneous over the area scanned by the confocal. The fibrin samples were exposed to a magnetic field for a duration of 4–8 minutes.
Rheometry
Rheometry experiments were conducted in the Fernandez Lab (Soft Condensed Matter Lab) at Georgia Institute of Technology, Atlanta, GA. Rheometry was used to study the bulk mechanical properties of the clot, and to determine the optimum concentration of foreign objects (quantum dots and magnetic particles) in the matrix. An Anton Paar Rheometer, Model 501, which is capable of operation in steady and oscillatory modes was employed. Because of the size of the cone-plate rheometer, the concentration of the fibrinogen had to be increased. 8 mg/mL fibrinogen and 2 U/mL thrombin were used, and the sample was allowed to polymerize on the plate for 2 hours. Next, the fibrin clot was sheared at frequencies corresponding to 0.1, 1, and 10 Hz, and measurements were taken from 0.1% to 1% strain. Using this data, the storage and loss moduli were computed.
Results
Experimental Analysis
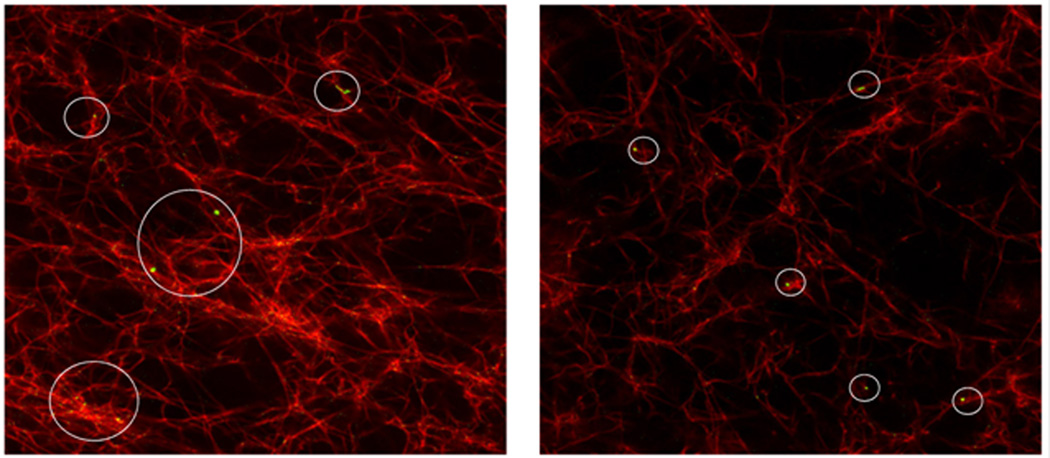
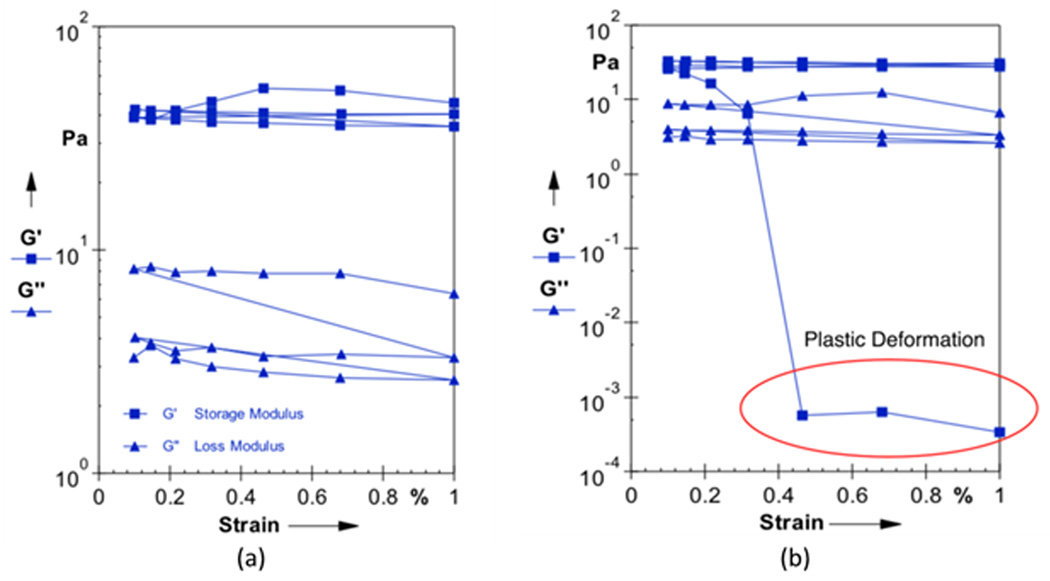
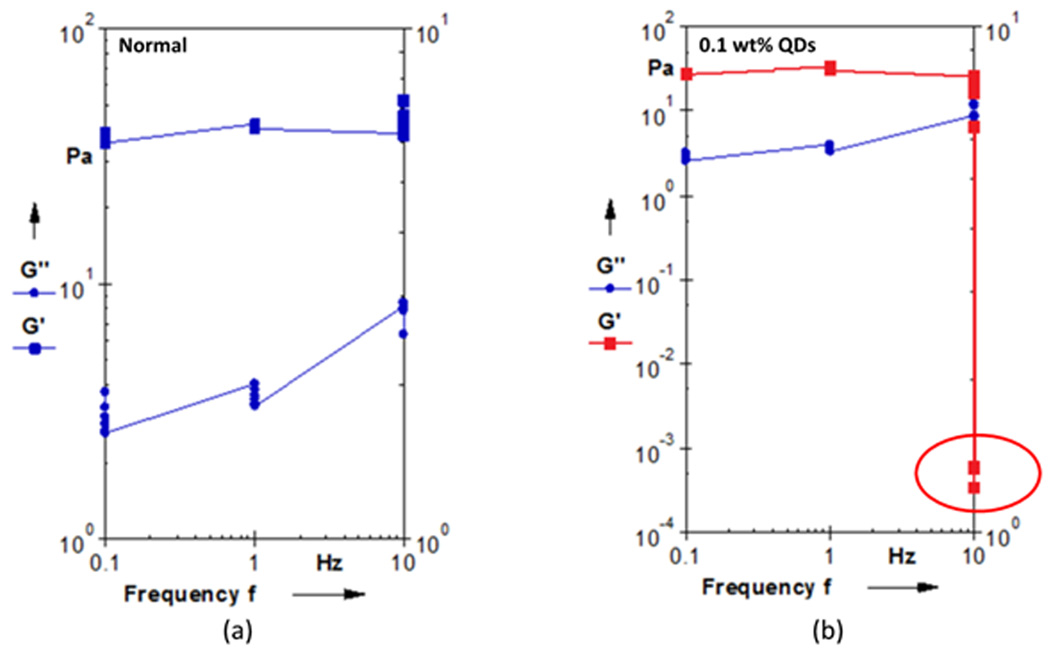
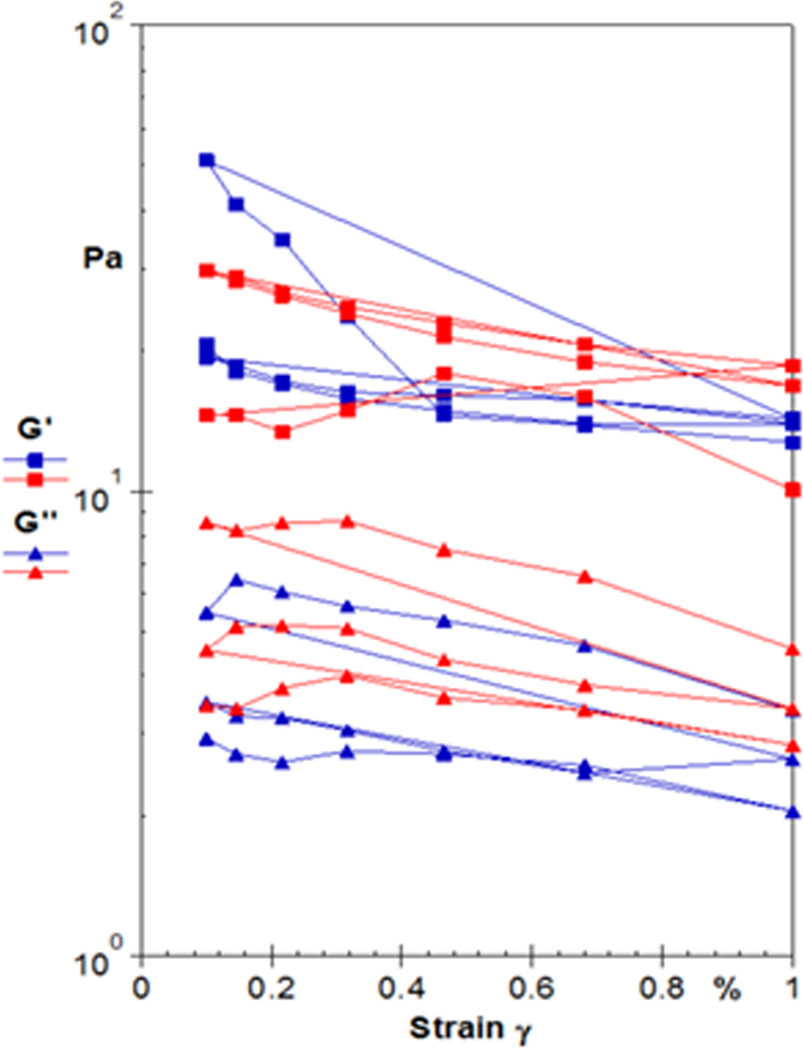
Confocal microscopy images of the fibrin networks with embedded MMPs and QDs showed good incorporation of the QDs to the fibrin network (Figure 2). The rheological data in Figures 3 and 4 showed that adding QDs to the fibrin gels at 0.1% induced a plasticity effect in the fibrin clot, as evidenced by the steep decrease in the storage modulus. Figure 3(a) shows a representative strain sweep for a normal fibrin clot, indicating fairly constant values of G’ (storage modulus) and G” (loss modulus) as a function of strain at a frequency of 10 Hz. Figure 3(b) shows a representative strain sweep at 10 Hz for a fibrin clot with 0.1 wt% QDs incorporated into the network, where a precipitous decrease in G’ was observed close to 0.3% strain. This decrease in G’ is readily indicative of plastic deformation in the sample, and indicates that the embedded QDs altered the mechanical behavior of the fibrin fiber system. This result is in contradiction with a study in (24), where the researchers were interested in investigating micro-viscosity of lamellar gels with incorporated quantum dots, and associating this with macroscale viscosity. The researchers in (24) concluded that the macroscale viscosity arises from structures much larger than 25 nm, which was approximately twice the hydrodynamic diameter of the QD. Although viscosity was not directly measured in the current study, the results are interesting because it was shown that QDs actually govern the elastic modulus of the fibrin sample on the macroscopic scale. In addition, Figure 4 shows a frequency sweep of both the normal clot (Figure 4(a)) and the clot infused with 0.1 wt% QDs (Figure 4(b)). The results of the rheological frequency sweep are somewhat intuitive for the normal fibrin clot (Figure 4(a)). The storage modulus (G’) of the normal clot was somewhat constant over the range of frequencies tested and the loss modulus (G”) showed a slight increasing trend. However, for the fibrin clot infused with 0.1 wt% QDs, the data was in sync with the rheological strain sweep data. The data showed that the storage modulus (G’) was constant at frequencies of 0.1 and 1Hz; however, there was a steep decrease in the storage modulus value at 10 Hz, again indicating that at this higher rate of stress application, the QDs were responsible for engendering material failure in the sample.
Figure 2.
Confocal image of fibrin clots with embedded MMPs (not shown) and QDs (green) indicating the incorporation of QDs into the fibrin network
Figure 3.
Sample rheological strain sweep data displaying the storage modulus (G’) and the loss modulus (G”) at a frequency of 10 Hz for (a) control fibrin gels and (b) fibrin gels with 0.1 wt% QDs incorporated into network
Figure 4.
Sample rheological frequency sweep data (0.1 to 10 Hz) displaying the storage modulus (G’) and the loss modulus (G”) for (a) control fibrin gels and (b) fibrin gels with 0.1 wt% QDs incorporated into network
Incorporation of the magnetic microparticles (MMPs) into the fibrin matrix also affected the mechanical properties of the network. Figure 5 displays sample rheological data that indicates a plasticity effect as the result of incorporating MMPs into the fibrin network at 0.1 wt%, as observed by the decrease in storage modulus G’ as a function of strain.
Figure 5.
Sample rheology results displaying storage modulus (G’) and loss modulus (G”) as a function of strain for fibrin clots with MMPs incorporated into network at 0.1 wt%
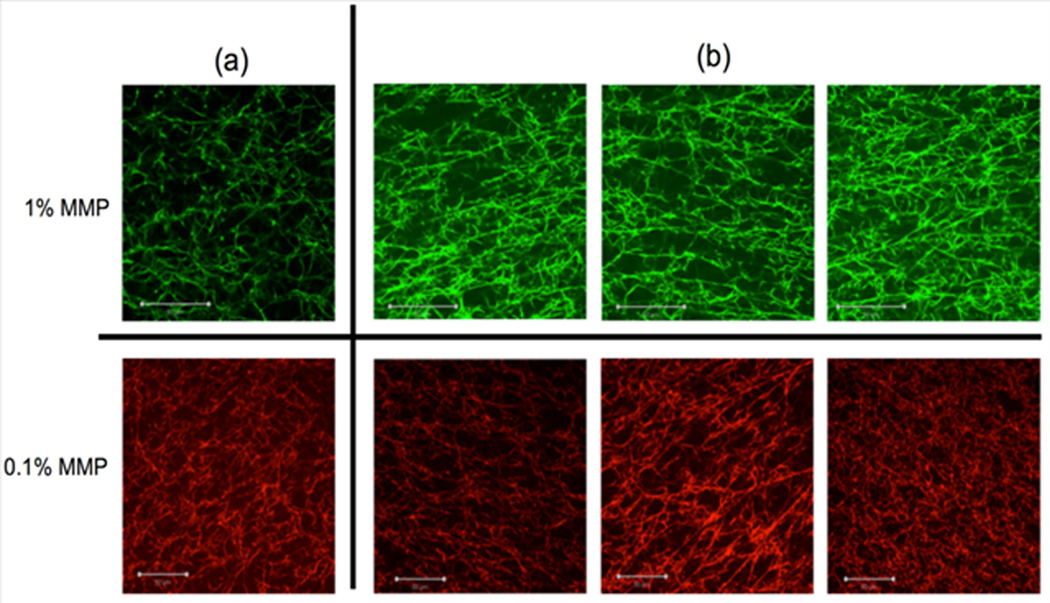
In addition to examining the effects of the concentration of QDs and MMPs on the mechanical properties of fibrin clots, an extensive confocal microscopy study was conducted to determine if MMPs could be used to artificially distort the fibrin network. This was done in an effort to mimic how cells may invade the network and exert forces. In Figure 6(a), the MMPs (not shown) were incorporated into the fibrin network in the absence of a magnetic field at concentrations of 1 wt% (green) and 0.1 wt% (red). In contrast, Figure 6(b) shows images of fibrin networks in which the MMPs were added into the fibrin gels and placed under magnetic field. The results proved that magnetic microparticles, in combination with an ensuing magnetic field, engendered significant distortion to the fibrin network, as evidenced by the anisotropy seen in the confocal images in Figure 6(b). Although there is no quantitative data that can be used to compare the physical relevance of the effect of the magnetic microparticles to the effect of activated cells, such as platelets, there is significant qualitative data from these confocal images to support the use of this method to analyze strain-induced mechanics of fibrin networks. Visible deformations were observed from the confocal images (Figure 6(b)), which indicate that fibrin fibers were reoriented and stretched in the presence of MMPs and a magnetic field. The authors in (25) have developed a similar technique to investigate the mechanical properties of fibrin gels polymerized at 11.7 and 4.7 T conditions and found a higher anisotropy for the gels polymerized under 11.7 T conditions. As a comparison, in the current study, a magnet with a much lower magnetic field strength (surface field strength of 2,952 Gauss (~0.3 T)) was used to induce magnetization and engender anisotropy in the fibrin clot sample.
Figure 6.
Confocal images of fibrin clots with embedded MMPs (not shown) (a) Images of fibrin clots with 1 wt% and 0.1 wt% MMPs with no applied magnetic field and (b) Images of fibrin clots with 1 wt% and 0.1 wt% MMPs with applied magnetic field
The findings of a plasticity induced effect due to incorporation of particles in the fibrin system suggest that the overall system with both QDs and MMPs may plastically deform when stretched in the presence of the magnetic field. There are established micromechanical models that have investigated plasticity induced in fibrous systems with incorporated particles (26, 27). In addition, Yang et al. (28) has proposed a plasticity based mechanical model for polymeric systems with incorporated nanoparticles. One of the main conclusions from that study was that for a system with 0.1 % volume fraction, a negative effect on the modulus was observed in the system for particle sizes greater than 10 nm. In essence, when the nanoparticle size was increased, the overall behavior of the effective modulus was reduced. Based on this observation, it can be concluded that in the current fibrin system with QDs on the order of 15–20 nm and MMPs on the order of 2.8 µm, under applied magnetic field the network may plastically deform when stretched.
A future study would include incorporating anti-fibrinogen labeled with biotin antibodies that will attach to the streptavidin-coated MMPs. This may help to ensure that the MMPs are properly affixed to the fibrin fibers, which will allow for more realistic stretching under the magnetic field. One drawback to the current method is that the magnets move through the fibrin network upon application of the magnetic field. Another future study will be aimed at understanding the magnetic forces in the fibrin system using an appropriate magnetic field model. This could help yield quantitative data that would allow comparison of the experimental mechanics and quantitative model to systems such as those observed in platelet-fibrin clot dynamics. This information could be used to assess quantitatively how platelets induce strains within a fibrin network and to estimate the ensuing forces associated with such strains.
Conclusions
An experimental technique has been presented to demonstrate that quantum dots (QDs) and magnetic microparticles (MMPs) can be successfully incorporated into fibrin gel networks. Rheology experiments were used to determine the mechanical properties of the gels, where it was shown that a noticeable plastic deformation occurs in the samples at 0.1 wt% of content for both the QDs and MMPs, under 10 Hz oscillatory conditions. This was evidenced by the noticeable drop in elastic modulus at both higher strain and frequency during oscillatory shear. This plastic deformation was directly the result of the foreign objects being dispersed into the matrix. Based on the individual observation of induced plasticity in the fibrin system with incorporated QDs or MMPs, it is likely that the combined fibrin system (with inclusion of both QDs and MMPs) plastically deforms when the magnetic field is applied and the network is stretched. For future experiments involving the incorporation of foreign particles in fibrin, it may be necessary to use new surface chemistry techniques to modify the properties of the particle in an effort to increase compatibility with the matrix. This result is important for researchers aiming to develop in-situ models, where particles are incorporated into the matrix for mechanical testing. Another important conclusion from the study is that MMPs can be successfully conjugated to fibrin networks and used in conjunction with a magnetic field to produce strains and alignment of the fibrin fiber network. This is important for researchers and clinicians that have an interest in quantifying localized mechanical phenomena within fibrin clots, which are often caused by pathological states.
Supplementary Material
Supplementary Material
Figure 1S. Representative confocal images of fibrin stamps formed by using 555nm-dyed fibrinogen (green) and 647nm-dyed fibrinogen (red) used as fibrin gel, which polymerized subsequent to the addition of thrombin.
Acknowledgments
The author would like to thank the Barker Lab and Lam Lab at Georgia Tech, Department of Biomedical Engineering for help with sample preparation and development of the fibrin stamps. In addition, the author would like to thank Nancy Zhong (John Hopkins University) who helped perform experiments for this research. Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number K01HL115486 and NIH grant R01EB011566.
References
- 1.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophysical Chemistry. 2004;112(2-3):267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Weisel JW. Fibrinogen and Fibrin. In: David ADP, John MS, editors. Advances in Protein Chemistry. Academic Press; 2005. pp. 247–299. [DOI] [PubMed] [Google Scholar]
- 3.Brown AC, Barker TH. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomaterialia. (0) doi: 10.1016/j.actbio.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai VK, Frey CR, Kerandi AM, Lake SP, Tranquillo RT, Barocas VH. Microstructural and mechanical differences between digested collagen-fibrin co-gels and pure collagen and fibrin gels. Acta Biomaterialia. 2012;8(11):4031–4042. doi: 10.1016/j.actbio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blombäck B, Carlsson K, Hessel B, Liljeborg A, Procyk R, Åslund N. Native fibrin gel networks observed by 3D microscopy, permeation and turbidity. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1989;997(1-2):96–110. doi: 10.1016/0167-4838(89)90140-4. [DOI] [PubMed] [Google Scholar]
- 6.Soon ASC, Stabenfeldt SE, Brown WE, Barker TH. Engineering fibrin matrices: The engagement of polymerization pockets through fibrin knob technology for the delivery and retention of therapeutic proteins. Biomaterials. 2010;31(7):1944–1954. doi: 10.1016/j.biomaterials.2009.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baradet TC, Haselgrove JC, Weisel JW. Three-dimensional reconstruction of fibrin clot networks from stereoscopic intermediate voltage electron microscope images and analysis of branching. Biophysical Journal. 1995;68(4):1551–1560. doi: 10.1016/S0006-3495(95)80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Stasio E, Nagaswami C, Weisel JW, Di Cera E. Cl- Regulates the Structure of the Fibrin Clot. Biophysical Journal. 1998;75(4):1973–1979. doi: 10.1016/S0006-3495(98)77638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magatti D, Molteni M, Cardinali B, Rocco M, Ferri F. Modeling of Fibrin Gels Based on Confocal Microscopy and Light-Scattering Data. Biophysical Journal. 2013;104(5):1151–1159. doi: 10.1016/j.bpj.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molteni M, Magatti D, Cardinali B, Rocco M, Ferri F. Fast Two-Dimensional Bubble Analysis of Biopolymer Filamentous Networks Pore Size from Confocal Microscopy Thin Data Stacks. Biophysical Journal. 2013;104(5):1160–1169. doi: 10.1016/j.bpj.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu AL, Gersh KC, Safer D, Weisel JW. Visualization of the Dynamics of Fibrin Clot Growth One Molecule at a Time by Total Internal Reflection Fluorescence Microscopy. Biophysical Journal. 2013;104(2) Supplement 1:393a. doi: 10.1182/blood-2012-08-451518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann A, Boukamp P, Friedl P. Confocal reflection imaging of 3D fibrin polymers. Blood Cells, Molecules, and Diseases. 2006;36(2):191–193. doi: 10.1016/j.bcmd.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Dubey N, Letourneau PC, Tranquillo RT. Neuronal contact guidance in magnetically aligned fibrin gels: effect of variation in gel mechano-structural properties. Biomaterials. 2001;22(10):1065–1075. doi: 10.1016/s0142-9612(00)00341-0. [DOI] [PubMed] [Google Scholar]
- 14.Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials. 2011;32(26):6111–6118. doi: 10.1016/j.biomaterials.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Yamagishi A. Biological systems in high magnetic field. Journal of Magnetism and Magnetic Materials. 1990;90-91(0):43–46. [Google Scholar]
- 16.Yamagishi A, Takeuchi T, Date M, Higashi T. Polymerization of biological molecules under high magnetic fields. Physica B: Condensed Matter. 1989;155(1-3):433–436. [Google Scholar]
- 17.Yamagishi A, Takeuchi T, Higashi T, Date M. Magnetic field effect on the polymerization of fibrin fibers. Physica B: Condensed Matter. 1990;164(1-2):222–228. [Google Scholar]
- 18.Helms Christine C, Ariëns Robert AS, Uitte de Willige S, Standeven Kristina F, Guthold M. α-α Cross-Links Increase Fibrin Fiber Elasticity and Stiffness. Biophysical Journal. 2012;102(1):168–175. doi: 10.1016/j.bpj.2011.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litvinov RI, Faizullin DA, Zuev YF, Zhmurov A, Kononova O, Barsegov V, et al. Structural Molecular Origins of Fibrin Mechanics. Biophysical Journal. 2013;104(2) Supplement 1:59a. [Google Scholar]
- 20.Piechocka IK, Bacabac RG, Potters M, MacKintosh FC, Koenderink GH. Structural Hierarchy Governs Fibrin Gel Mechanics. Biophysical Journal. 2010;98(10):2281–2289. doi: 10.1016/j.bpj.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural Origins of Fibrin Clot Rheology. Biophysical Journal. 1999;77(5):2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez M, Weisel JW, Ischiropoulos H. Functional impact of oxidative posttranslational modifications on fibrinogen and fibrin clots. Free Radical Biology and Medicine. 2013;65(0):411–418. doi: 10.1016/j.freeradbiomed.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pivalizza EG, Abramson DC, Harvey A. Perioperative hypercoagulability in uremic patients: A viscoelastic study. Journal of Clinical Anesthesia. 1997;9(6):442–445. doi: 10.1016/s0952-8180(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 24.Szymański J, Wilk A, Hołyst R, Roberts G, Sinclair K, Kowalski A. Micro- and macroshear viscosity in dispersed lamellar phases. Journal of Non-Newtonian Fluid Mechanics. 2008;148(1-3):134–140. [Google Scholar]
- 25.Namani R, Wood MD, Sakiyama-Elbert SE, Bayly PV. Anisotropic mechanical properties of magnetically aligned fibrin gels measured by magnetic resonance elastography. Journal of Biomechanics. 2009;42(13):2047–2053. doi: 10.1016/j.jbiomech.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gengkai H. A method of plasticity for general aligned spheroidal void or fiberreinforced composites. International Journal of Plasticity. 1996;12(4):439–449. [Google Scholar]
- 27.Liu X, Hu G. A continuum micromechanical theory of overall plasticity for particulate composites including particle size effect. International Journal of Plasticity. 2005;21(4):777–799. [Google Scholar]
- 28.Yang BJ, Hwang YY, Lee HK. Elastoplastic modeling of polymeric composites containing randomly located nanoparticles with an interface effect. Composite Structures. 2013;99(0):123–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure 1S. Representative confocal images of fibrin stamps formed by using 555nm-dyed fibrinogen (green) and 647nm-dyed fibrinogen (red) used as fibrin gel, which polymerized subsequent to the addition of thrombin.