Abstract
In the last few years, it has become clear that a wide variety of environmental contaminants have specific effects on neuroendocrine systems in fish, amphibians, birds and mammals. While it is beyond the scope of this review to provide a comprehensive examination of all of these neuroendocrine disruptors, we will focus on select representative examples. Organochlorine pesticides bioaccumulate in neuroendocrine areas of the brain that directly regulate GnRH neurons, thereby altering the expression of genes downstream of GnRH signaling. Organochlorine pesticides can also agonize or antagonize hormone receptors, adversely affecting crosstalk between neurotransmitter systems. The impacts of polychlorinated biphenyls are varied and in many cases subtle. This is particularly true for neuroedocrine and behavioral effects of exposure. These effects impact sexual differentiation of the hypothalamic-pituitary-gonadal axis, and other neuroendocrine systems regulating the thyroid, metabolic, and stress axes and their physiological responses. Weakly estrogenic and anti-androgenic pollutants such as bisphenol A, phthalates, phytochemicals, and the fungicide vinclozolin can lead to severe and widespread neuroendocrine disruptions in discrete brain regions, including the hippocampus, amygdala, and hypothalamus, resulting in behavioral changes in a wide range of species. Behavioral features that have been shown to be affected by one or more these chemicals include cognitive deficits, heightened anxiety or anxiety-like, sociosexual, locomotor, and appetitive behaviors. Neuroactive pharmaceuticals are now widely detected in aquatic environments and water supplies through the release of wastewater treatment plant effluents. The antidepressant fluoxetine is one such pharmaceutical neuroendocrine disruptor. Fluoxetine is a selective serotonin reuptake inhibitor that can affect multiple neuroendocrine pathways and behavioral circuits, including disruptive effects on reproduction and feeding in fish. There is growing evidence for the association between environmental contaminant exposures and diseases with strong neuroendocrine components, for example decreased fecundity, neurodegeneration, and cardiac disease. It is critical to consider the timing of exposures of neuroendocrine disruptors because embryonic stages of central nervous system development are exquisitely sensitive to adverse effects. There is also evidence for epigenetic and transgenerational neuroendocrine disrupting effects of some pollutants. We must now consider the impacts of neuroendocrine disruptors on reproduction, development, growth and behaviors, and the population consequences for evolutionary change in an increasingly contaminated world. This review examines the evidence to date that various so-called neuroendocrine disruptors can induce such effects often at environmentally-relevant concentrations.
Introduction
The concept of endocrine disruption became recognized worldwide following the 1991 Wingspread conference organized by Dr. Theo Colborn and colleagues. While much of the early evidence related to sexual and developmental effects (Colborn et al., 1993), it is now well established that pollutants negatively impact many physiological processes. In the last few years, it has also become clear that a subset of pollutants and mass-produced chemicals have significant effects on neuroendocrine systems. Following earlier key initiatives on synthesizing the principles of neuroendocrine disruption (Gore, 2008; Gore and Patisaul, 2010; Zoeller, 2008), a formal definition emerged following the first Symposium on Neuroendocrine Effects of Endocrine Disruptors in July 2010 (Trudeau et al., 2011). At that time, experimental evidence obtained from both invertebrate and vertebrate model systems was reviewed (Waye and Trudeau, 2011). Neuroendocrine responses can be rapidly initiated events that have profound biological consequences or they may represent slower changes in the neuronal morphology of systems controlling numerous physiological processes and behaviors. The following definition was put forth for consideration by the broader research community:
“Neuroendocrine disruptors are defined as pollutants in the environment that are capable of acting as agonists/antagonists or altering the synthesis and/or metabolism of neuropeptides, neurotransmitters, or neurohormones, which subsequently alter diverse physiological, behavioral, or hormonal processes to affect an animal's capacity to reproduce, develop and grow, or deal with stress and other challenges” (Waye and Trudeau, 2011).
The number of publications and reports searchable on the Web of Science for the term “neuroendocrine disruption” has increased dramatically in the last few years, yet it is also clear that the discipline is still in its infancy. One of the earliest publications we identified was from 1992, and examined the diuretic effects of synthetic peptides in the cotton budworm. The study was in the context of targeted disruption of the neuroendocrine control of water balance as a new approach for insect pest control (Keeley et al., 1992). Total citations specifically referring to neuroendocrine disruption reached over 90 in 2012, and there remained a steady citation rate up to 2013 (Figure 1).
Figure 1.
Neuroendocrine disruption has gained more attention from the scientific community in the past decade (Web of Science, November 2013). Published manuscripts and reports that use the specific phrase “neuroendocrine disruption.” In addition, citations of these articles have steadily increased over the past 20 years.
The most recent Neuroendocrine Disruption symposium was held on May 24, 2013 during the Second meeting of the North American Society of Comparative Endocrinology, Quéretaro, Mexico. There, it was proposed to write a comprehensive and current review to delineate clear, existing examples of neuroendocrine effects of endocrine disruptors. Moreover, we wanted to streamline the definition of a ‘neuroendocrine disruptor’. The overarching goal here is to provide a framework for open debate regarding the concept of neuroendocrine disruption. This should also contribute to sound, scientifically-based policies and decisions (e.g., as advocated by (Gore et al., 2013)) regarding the emerging environmental and human health problems resulting from specific disruption of neuroendocrine control systems. This is imperative as evidence grows for associations between environmental contaminant exposures and diseases having strong neuroendocrine components, for example, reduced fertility, obesity, neurodegeneration and cardiac disease, among other conditions (Bellavance and Rivest, 2012; Carro et al., 2013; Go et al., 2013; Straub et al., 2013). Here we outline specific examples of neuroendocrine disruption by various chemicals and highlight some key concepts. Moreover, unless otherwise stated, the doses of the tested chemical are environmentally-relevant.
2. Legacy pesticides as neuroendocrine disruptors
Pesticides that include insecticides, herbicides, and fungicides have historically been assessed primarily for toxicity. As such, adverse effects due to toxicity, as well as the endocrine disrupting actions of pesticides, are well documented (Bretveld et al., 2006; Mrema et al., 2013). However, the effects of pesticides on neuroendocrine axes have been less of a focus. There is good evidence that pesticides are neuroactive in vertebrates, and these chemicals have been shown to affect the expression of neuropeptides (Gore, 2002), alter the reuptake of neurotransmitters such as dopamine (DA) (Miller et al., 1999), and bind to receptors that regulate neuroendocrine signaling (Gore, 2008). We present some of the evidence for neuroendocrine disrupting effects of organopesticides that includes both organochlorines (OCPs) and organophosphates (OPs).
Organochlorine pesticides are considered legacy pesticides, having been restricted or banned in use in the past three to four decades (e.g., methoxychlor, dieldrin); however there remain significant environmental exposure risks because OCPs are highly persistent in the environment and bioaccumulate in tissues (Gallagher et al., 2001; Martyniuk et al., 2013). One criterion to be considered for a chemical being classified as a direct-acting neuroendocrine disruptor is that the parent compound must cross the blood-brain barrier in order to exert central neuroendocrine effects. Both OCPs and OPs have been shown to significantly bioaccumulate in the brain, for example in the rat cortex/midbrain and teleost whole brain (Hatcher et al., 2007; Koch et al., 2006; Tilak et al., 2004). Moreover, the bioaccumulation in the CNS can be significant, in some cases, greater than that in other tissues (Tilak et al., 2004). Thus, studies quantifying OCPs and OPs in peripheral animal tissues (e.g., muscle) may be underestimating their concentrations in the CNS. In Florida, adult brown bullheads (Ameriurus nebulosus; 2–5 years of age) collected from the North marsh of Lake Apopka showed elevated levels of OCPs (mean p,p'-DDE, dieldrin, and toxaphene was 23 μg/kg, 3.6 μg/kg, and 44.3 μg/kg respectively) compared to largemouth bass (Micropterus salmoides) collected from a Lake Woodruff reference site (Gallagher et al., 2001). Hinck et al. (2008) reported that largemouth bass sampled in the Alabama Basin and the Mobile River Basin had mean levels of organochlorine residues ranging from 15.4-73.0 μg/kg wet weight for p,p'-DDE, 1.46-9.13, μg/kg for dieldrin, and 15.0-45.0 μg/kg for toxaphene.
2.1. Adverse effects of OCP and OP in the vertebrate central nervous system
What may classify OCPs and OPs as neuroendocrine disruptors in addition to being endocrine disruptors or neurotoxic compounds are data that demonstrate that a pesticide directly exerts specific effect on well-defined neuroendocrine cells. Perhaps the most notable example is the gonadotropin hormone-releasing hormone (GnRH) neuron. Several OCPs have been shown to affect GnRH1 biosynthesis in the GT1-7 immortalized hypothalamic cell line (Gore, 2002). After 24h exposure, both methoxychlor and chlorpyrifos (CPF; 1-100 μM) significantly affected GnRH primary transcript levels. Methoxychlor suppressed GnRH mRNA levels at 10 and 100 μM while CPF reduced GnRH mRNA at 100 μM. Amounts of the GnRH peptide were also decreased by both pesticides, but only after co-treatment with ICI 182,780, an anti-estrogen. This suggests that there can be interactions between estrogen receptor signaling and organopesticides in neuroendocrine cells. In the same study, qualitative analysis of cell morphology revealed that methoxychlor at >10 μM caused retraction of neuronal processes while 10 and 100 μM CPF appeared to cause an increase in cell density and increased prevalence of neuronal projections. High doses of pesticides resulted in increased cell death, which raises an important point for the study of neuroendocrine disruption – the challenge of discerning direct neurotoxic effects from sub-lethal neuroendocrine effects. Other effects of OCPs on GnRH neurons have been reported using in vivo experimentation. Cichlid fish (Cichlasoma dimerus) larvae exposed to a sub-lethal concentration of 0.1 µg/L endosulfan for 30d showed decreased nucleus/cytoplasm area ratio for GnRH1 neurons whereas pituitary follicle stimulating hormone cells showed a significant increase in nuclear size and mean nuclear diameter (Piazza et al., 2011). The authors hypothesized that these changes may be sufficient to affect sexual differentiation and adult reproduction. Interestingly, there were no notable changes in morphology of GnRH2 or GnRH3 neurons, suggesting that pesticides may differentially affect neuroendocrine cell types. The mechanisms underlying differential sensitivity of neuroendocrine cells to pesticides are largely unexplored and may be one important area for further exploration.
There are also excellent examples for OPs and their neuroendocrine disrupting effects. Chlorpyrifos (CPF) is arguably one of the best studied in this context and there is growing evidence for effects on the neuroendocrine axes regulating reproductive and thyroidal activities (Frye et al., 2012). In mammalian models, it appears that early developmental exposures to CPF results in sexually dimorphic and potentially long lasting effects on the hypothalamic expression of neuropeptides that include oxytocin (OXT) and vasopressin (VP) (Tait et al., 2009). These neurohormones regulate social behavior and reproduction; thus there can be clear links between neuroendocrine disruption and behavioral alterations. Moreover, data demonstrate that CPF can adversely affect neurotransmitter systems that modulate these neurohormones, specifically the dopaminergic and serotonergic (5HT) systems (Slotkin and Seidler, 2007). Classically defined neurotransmitter systems are integral for the neuroendocrine regulation of reproduction, metabolism, stress, and growth. Given the importance of neurotransmitters in this regulation across vertebrate taxa, the sub-lethal effects of pesticides on neurotransmitter systems should also be explored.
2.2 Mechanisms of OCP and OP action in the central nervous system
The mechanisms underlying neuroendocrine disruption by OCPs are likely diverse in the CNS. The OCPs can agonize/antagonize receptors, disrupting crosstalk between neurotransmitter (e.g., via type A gamma-aminobutyric acid (GABA) receptors and DA signaling) and neuroendocrine systems. In an in vitro screen using retinoic acid receptors (RAR), RARγ transfected yeast cells, 16 of 30 tested OCPs that included endosulfan, toxaphene, chlordane, and dieldrin bound to retinoic acid receptors (Kamata et al., 2008). The RARs are found in many cells of all vertebrates, and regulate cell growth, differentiation, among other functions. Some OCPs are also known to act as weak estrogen and androgen receptor agonists in vertebrates (Gore, 2008), and OCPs can elicit biological responses common to estrogenic and anti-androgenic compounds. Therefore, one mechanism for direct effects of OCPs on the neuroendocrine system may be via nuclear or membrane bound estrogen receptor (ER) activation, because some populations of GnRH (Radovick, 2012) and neuropeptide Y (NPY) neurons express ERs (Acosta-Martinez et al., 2007). If acting to bind nuclear and membrane bound ERs the hypothalamus, OCPs have the potential to affect signaling pathways related to reproduction and feeding behavior.
Another example of a mechanism of how OCPs can act on neuroendocrine systems has been demonstrated in largemouth bass, a top apex predator. Male and female bass were fed 3 mg dieldrin/kg food over 2 months (Martyniuk et al., 2013). This concentration was selected to mirror environmental exposures that occur in contaminated sites, specifically the Superfund site of Lake Apopka in Central Florida. Preliminary uptake studies demonstrated that OCP are accumulated rapidly in these bass over a 4-month period after being placed into artificial ponds in the Apopka region (Martyniuk and Denslow, unpublished). For example, p,p'-DDE showed the highest level of bioaccumulation in largemouth bass and was 785-times higher than background levels after the exposure period. Other OCPs that showed high levels of accumulation included dieldrin (15-fold) and endosulfan II (374-fold). Thus feeding regimes were relevant to environmental scenarios. Transcriptomic analysis revealed that neuroendocrine and neurotransmitter signaling were differentially affected in a sex-specific manner in the hypothalamus. Gene network analysis revealed that transcripts associated with activin signaling pathways were significantly reduced 20–30% by dieldrin in males (Martyniuk et al., 2013). Moreover, based upon the bioinformatics approach used, activin signaling was also associated with GnRH, growth hormone 1, and insulin-like growth factor 1 receptor signaling (Martyniuk et al., 2013), suggesting that dieldrin affects multiple neurohormonal systems at the transcript level. A significant challenge is determining the features of the transcriptomic response that are directly regulated by neuroendocrine disruptors versus those responses that are secondary to neurotoxicity (e.g., oxidative stress). Currently, omics-based studies in heterogeneous brain tissues (e.g., whole hypothalamus) are not able to separate these unique and distinct responses. What may be useful in studies of neuroendocrine disruption are direct gene expression profiling in microdissected individual neuroendocrine cells to better elucidate how molecular changes relate to altered neurohormone synthesis, packaging, and release.
3. Polychlorinated Biphenyls
Polychlorinated biphenyls (PCBs) encompass 209 compounds that are environmentally persistent, widespread, chemically and thermally stable, insoluble in water, non-flammable, electrically resistant, and toxic to vertebrate and invertebrate organisms (Rattner, 2009; Salice et al., 2013; Wiseman et al., 2011; Zhang et al., 2013). Although PCBs have been banned, the long half-life of this family of persistent organic pollutants continue to exceed tolerable limits recommended by the World Health Organization and present a significant risk to wildlife and humans. Recently, high levels of PCB 11 (3,3 '-dichlorobiphenyl) have been detected in the environment. This PCB is inadvertently produced during the manufacturing of paint pigments (Hu and Hornbuckle, 2010). Lipophilic properties also promote PCB accumulation in vertebrates and biomagnification through the food chain, resulting in high levels in tissues of fish, birds, mammals, top predators, and humans (Bytingsvik et al., 2012; Muir and de Wit, 2010). The PCBs also transfer by maternal deposition into milk during lactation in mammals and via deposition into eggs in oviparous vertebrates (Cromwell et al., 2007; Vigh et al., 2013). Further, exposure continues especially for aquatic organisms from contamination in streams and waterways and for terrestrial vertebrates living in the environment and feeding upon insects and other organisms that have ingested PCBs. Many PCBs have adverse impacts beyond overt toxicity, including neurotoxicity and endocrine impacts primarily on reproductive and thyroid systems. Vulnerable life stages vary across species and classes of organisms, but generally the developing organism is most vulnerable and exposure to PCBs can result in offspring learning disabilities, impaired metabolic and reproductive function, and neurochemical changes (Colciago et al., 2009; Donahue et al., 2004; Rashid et al., 2013; Schantz et al., 1997). The effects of PCBs depends on several factors: dose, sex, susceptibility of each organism, type of congener used (209 congeners, ortho- or non ortho-substituted and hydroxylation), the exposure time and life stage in which the organism is more vulnerable to exposure (Langer, 2008; McKinney and Waller, 1994). Moreover, ancillary effects of PCBs such as promoting oxidative damage also impact physiological processes and neural systems (Westerink, 2013).
3.1. Adverse Effects on Neuroendocrine Systems from PCBs
3.1.1 Effects on the Thyroid System
The PCBs interact with several endocrine systems, especially the thyroid axis, due to structural similarities between thyroid hormones (TH) and some PCB congeners (Brouwer et al., 1998). In general, PCBs decrease circulating TH levels. Some of these effects include increased volume of follicular thyroid cells, hyperplasia of thyrocytes, and reduced levels of circulating THs in most vertebrates studied (Brouwer et al., 1998). Reducing levels of circulating TH may result in an increase in thyroid-stimulating hormone, perhaps related to a reduced negative feedback (Fisher et al., 2006). Thyroid hormones are critical to brain development; deleterious effects of PCBs have been documented for cognitive function, behavioral responses, and neuroendocrine modulation of endocrine responses. Rat pups prenatally treated with PCB 118 (2,3',4,4',5-entachlorobiphenyl), 4 or 16 mg/kg/day. At weaning, serum thyroxine (T4) showed depressed behavioral responses. In a histological evaluation of thyroids, pups revealed changes suggestive of sustained TSH stimulation, including increased follicular cell vacuolization and height, increased nuclear vesiculation, and decreased colloid area. At high doses, decreased body and brain weights were observed and increased liver weights (Ness et al., 1993). Further, PCB exposure has been linked to developmental abnormalities with loss of locomotor activity and hearing (Crofton, 2004). Roelens and his group (2005), investigated the effects of the dioxin-like PCB 77 (1 μg; injected into eggs at day 4 of incubation) as well as those from non-coplanar ortho-substituted PCB 153 (20 μg) on both circulating and intracellular TH levels during the last week of chicken embryonic development and at the moment of hatching. While PCB 77 is one of the most toxic dioxin-like congeners, PCB 153 is one of the most prevalent PCBs in the environment and represents the ortho -substituted PCBs. They concluded that PCB 77 but not PCB 153 severely reduces TH availability in developing chicken embryos around the period of hatching, both in the periphery (plasma and liver) and in the central nervous system (brain). As there was no growth retardation, the delay in the process of hatching and the increased mortality during hatching are most likely the result of this perinatal hypothyroidism. Some key mechanisms resulting from PCB exposure appear to be active across a number of species, including enhanced metabolism and excretion of T4 (Hamers et al., 2006; Meerts et al., 2000). Another mechanism of PCB action is via the activation of the aryl hydrocarbon receptor (AhR), a ligand-dependent cytosolic transcription factor. PCBs act like ligands and, given their lipophilic properties, enter cells by passive diffusion. Two co-chaperone proteins are bound to AhR to form an oligomer that dissociates when binding to a PCB. After ligand binding, a heterodimer is formed which translocates into the nucleus and links to specific DNA regions; this in turn regulates the transcription of specific genes and produces genetic alterations that modify processes and functions in the cell (Gu et al., 2012a; Head and Kennedy, 2007).
3.1.2 Effects on the Vasopressinergic System
Exposure to the PCB mixture Aroclor 1254 has been associated with detrimental effects on VP and OXT systems in the hypothalamus (Kodavanti and Curras-Collazo, 2010; León-Olea et al., 2012). These neuropeptides are neurohypophysial hormones that regulate important functions such as water and electrolytic balance, cardiovascular functions, glycogen metabolism, oviductal and uterine contractions, and milk secretion. Another pathway consists of neurons and fibers that form an extensive central VP system with cell bodies located in different nuclei e.g., the bed nucleus of the stria terminalis, amygdala, and the parvocellular subnuclei of the hypothalamic paraventricular nucleus (PVN). In this pathway VP acts as a neurotransmitter or neuromodulator and participates in central cardiovascular regulation, learning and memory, locomotion, social and sexual behavior, stress responses associated with hypothalamic-pituitary-adrenal axis, circadian rhythmicity and thermoregulation (Kodavanti and Curras-Collazo, 2010). There are numerous studies that support the effects of PCBs on the neuroendocrine functions discussed above, but it remains to be shown if these effects are mediated through changes in VP (Kodavanti and Curras-Collazo, 2010; Schantz et al., 2001). Adult rats exposed to Aroclor 1254 (30 mg/kg/day for 15 days) exhibit marked inhibition of somatodendritic VP release during hyperosmotic activation, which then would interfere with autoregulation of plasma VP release, thereby resulting in an increase in plasma VP. When control rats are subjected to dehydration they displayed an average rise in plasma osmolality of 7–13% and in circulating VP levels of 1.5–4 times. Aroclor 1254 treated, hyperosmotic rats, displayed an increase in plasma VP more than 8-fold increase than the control hyperosmotic rats, suggesting a deregulation in VP release. Altered osmoregulation produces hypertensive responses to hyperosmotic stimuli (Coburn et al., 2005; Kodavanti and Curras-Collazo, 2010; Shah et al., 2011). Furthermore, perinatal exposure (gestational days 10-20) to Aroclor 1254 (30 mg/kg/day) dramatically decreases VP, nitric oxide (NO) and pituitary adenylate cyclase-activating polypeptide (PACAP) immunoreactivity in supraoptic (SON) and PVN of hypothalamus during hyperosmotic stimulation in adult rats. While this dose (30 mg/kg/day) is much higher than the expected daily PCB intake in nature, this dose raises brain PCBs to levels similar to those reported in high risk populations that exhibit neurological symptoms (Dewailly et al., 1999). Aged rats continue to show compromised responses to hyperosmotic stress, suggesting that developmental exposure leads to permanent neuroendocrine disruptions. Nitric oxide and PACAP are also affected and they are involved of VP release regulation and are colocalized in magnocelular neuroendocrine neurons (Gillard et al., 2007; León-Olea et al., 2012). Taken together, these data provide evidence that PCBs impact osmoregulatory neuroendocrine systems.
3.2. PCB Effects on Endocrine and Behavioral Components of Reproduction
Some PCB congeners inhibit hypothalamic tryptophan hydroxylase, the rate-limiting enzyme in 5HT synthesis, and this is associated with decreased gonadal size in the Atlantic croaker (Khan and Thomas, 2006). Some PCBs also have been shown to reduce testosterone production, sperm motility and fertilizing capability, as well as alter male accessory structures and inhibit testicular descent in mammals (Andric et al., 2000; Bush et al., 1986; Inglefield and Shafer, 2000). In humans, effects including early menarche, abnormal menstrual cycles, increased incidence of endometriosis, spontaneous abortion, fetal death, premature delivery, and low-weight in offspring have been associated with exposure to PCBs (Cooper et al., 2005; Simmons et al., 2005). Many of these PCB effects have been related to their antagonist or agonist actions at androgen, progesterone, and estrogen receptors (Hamers et al., 2006; Meerts et al., 2000). Furthermore, sexually dimorphic behaviors were altered in rats exposed to PCBs (Lilienthal et al., 2013). Adverse effects of PCB exposure on reproductive and thyroid systems as well as singing behavior have been observed in birds (Deleon et al., 2013; Ottinger et al., 2013). Other effects, including impaired cardiac development, which may reflect early impacts on growth factors, have the potential to greatly diminish the fitness of the individual (Carro et al., 2013). Field studies of populations in PCB-contaminated regions will provide the data needed to ascertain the long-term impacts to avian populations (Bridge and Kelly, 2013).
3.3. Effects on Neurotransmitter and Behaviorally-Relevant Circuits
There is evidence that PCBs disrupt vesicular transport and release of neurotransmitters that regulate neuroendocrine and cognitive functions. Some PCBs can affect DA release and neurodegenerative processes that may also lead to cellular death (Fielding et al., 2013; Mariussen and Fonnum, 2001; Selvakumar et al., 2013). A PCB-induced reduction in brain 5HT levels has also been observed (Boix and Cauli, 2012; Honma et al., 2009). Exposure to PCBs elicited different and opposing changes in serotonin metabolism depending on the brain region. Honma et al. (2009) investigated the effect of gestational exposure to PCB153 in eight different brain regions of rat dams 3 weeks after delivery. Exposure to PCB 153 at a dose of 64 mg/kg/day increased 5HT concentration in the hypothalamus. The PCB mixtures Aroclor 1242 and 1254 inhibit the uptake of DA, glutamate, GABA and 5HT rat brain synaptosomes (Mariussen and Fonnum, 2001). The mechanism revealed was via selective inhibition of the vesicular monoamine transporter VMAT-2, the common vesicle transporter for all amine neurotransmitters (Bemis and Seegal, 2004). The function of VMAT-2 is to maintain a low level of DA, NE and 5HT in the nerve terminal (Mariussen et al., 1999). Functional aspects such as motor coordination, learning, and memory following chemical-induced developmental effects are associated with changes in intracellular signaling pathways (Fielding et al., 2013; Mariussen and Fonnum, 2001; Selvakumar et al., 2013). Effects on calcium homeostasis and protein kinase C in neurons were suggested to be involved. Disturbances in calcium homeostasis can lead to alterations in neurotransmitter and hormone release, synaptic plasticity and gene expression (Kodavanti and Tilson, 1997).
4. Bisphenol A and Phthalates
4.1 Overview of Bisphenol A and Mechanisms of Action
Bisphenol A (BPA) is a synthetic compound present in a wide variety of products used on a daily basis, including plastic bottles, canned food and thermal paper used for sales receipts. It has been detected in body fluids/tissues and has been associated with a wide range of effects in humans, rodents, and wildlife (Crain et al., 2007; Hunt et al., 2009; vom Saal et al., 2007). Bisphenol A has complex actions in the brain but primarily acts as a weak estrogen receptor agonist and has also been shown to act as an anti-androgen and cause epigenetic changes in gene expression in various brain regions (Wolstenholme et al., 2011). As a result of these actions, environmentally-relevant low doses (<50 μg/kg/day), as well as at higher doses of BPA compromise sexual differentiation in the brain in rodents (Nakamura et al., 2006; Patisaul et al., 2006), decreases synaptogenesis in the hippocampus and prefrontal cortex of monkeys and rats (Leranth et al., 2008a; Leranth et al., 2008b; MacLusky et al., 2005) and disrupts normal cortical development in mice (Nakamura et al., 2007; Nakamura et al., 2006). Low dose perinatal (25 or 250 ng/kg) and postnatal (250 μg/kg) BPA alters sexually dimorphic brain regions within the hypothalamus to make them more similar between the sexes (Patisaul et al., 2007; Rubin et al., 2006) and reduces corticotropin-releasing hormone (CRH) and DA cell number in the midbrain (Funabashi et al., 2004; Tando et al., 2007; Tanida et al., 2009). These data argue against BPA acting solely as a weak-estrogen and instead suggest that it can also act as an estrogen receptor antagonist. Bisphenol A may also impact neural programming indirectly by inhibiting steroidogenic enzymes and production of testosterone and estrogen by the gonads of exposed mice, rats and humans (Akingbemi et al., 2004; Lee et al., 2013; N'Tumba-Byn et al., 2012; Peretz et al., 2011; Zhou et al., 2013).
Bisphenol A at environmentally-relevant doses also has epigenetic actions that can lead to heritable changes in gene expression. Multiple genes in tissues derived from the three embryonic germ layers are differentially methylated in BPA-exposed animals (Dolinoy et al., 2007; Kundakovic et al., 2013; Tang et al., 2012; Weinhouse et al., 2011; Yaoi et al., 2008). These data suggest that BPA might alter DNA methylation quite early in embryonic development and may impact the chromatin state of germ cells (Manikkam et al., 2013). Moreover, studies in rats and mice show that BPA effects on social interactions and spatial memory deficits may be mediated through changes in expression and DNA methylation of nuclear estrogen receptors Esr1, Esr2 and/or signaling via the N-methyl-D- aspartic acid type of glutamate receptor (Kundakovic et al., 2013; Xu et al., 2010). Little is known about how BPA may directly impact other epigenetic mechanisms within the brain. Sparse evidence suggests it may also alter histone modifications, histone subunits and microRNAs (miRNAs) (Avissar-Whiting et al., 2010; Doherty et al., 2010; Zhu et al., 2009), highlighting the need for further work in this area. It is not known at the current time if any of these miRNAs target specific transcripts in neuroendocrine systems.
4.2. Effects of BPA on Behavioral Patterns in Various Species
4.2.1. Fish
Effects of exposure of fishes to environmental estrogens, particularly 17α-ethinylestradiol (EE2), have been reviewed by others and include both endocrine and neuroendocrine disruption (Frye et al., 2012; Le Page et al., 2011; Söffker and Tyler, 2012). Recently, it was shown that exposure to BPA (15 μg/L) and other endocrine disrupting chemicals (EDCs) induced alternations in the neuroendocrine regulation of fish reproduction. In the rare minnow, Gobiocypris rarus, adult females exposed to BPA had increased expression of GnRH3 and GnRH receptor 1A genes in whole brain homogenates (Qin et al., 2013). Zebrafish embryos exposed to BPA (~ 1,141 – 2,283 μg/L; 5–10 μM) responded with a strong induction of brain aromatase (Chung et al., 2011). In another study, neurodevelopmental exposure of zebrafish to BPA (~23 μg/L; 0.1 μM) induced hyperactivity in larvae and learning deficits in adult zebrafish (Saili et al., 2012). A recent paper suggests that zebrafish exposed during embryonic development to BPA (~228 to 3,424 μg/L;1-15 μM) show decreased touch responses and swimming speed in response to light stimulation; however, part of these behavioral disruptions may be due to BPA-induced axial muscle damage (Wang et al., 2013). Neuroendocrine impacts of BPA exposure can potentially disrupt mate choice between members of the same genus but of different species leading to the breakdown of reproductive isolation mechanisms. Blacktail shiner (Cyprinella venusta) a species native to the USA and the introduced red shiner (Cyprinella lutrensis) were exposed to BPA. Through alterations in behavior and pigmentation, prezygotic barriers to hybridization were weakened, indicating that exposure to BPA (1,280 μg/L) may affect population integrity and enable non-native species to flourish (Ward and Blum, 2012). Given the small number of published studies, it is premature to develop strong conclusions about BPA effects on fish neuroendocrine systems. Nevertheless, it appears that the action of BPA as an estrogen receptor agonist could be contributing to the effects on gnrh3 and gnrhr1a in the rare minnow, aromatase induction in zebrafish, and alteration of secondary sex characteristics in the shiners. Effects of BPA on touch response, swimming speed, hyperactivity, and learning deficits are more difficult to explain at this point.
4.2.2. Birds
Scant information exists on the neuroendocrine effects of BPA in birds (Halldin et al., 2005; Panzica et al., 2005). The Japanese quail (Coturnix japonica) has been the sole avian species used for these few studies. Treatment of quail eggs with 50, 100, or 200 mg BPA arrested embryonic development, precluding additional assessments (Panzica et al., 2005). However, in the same study in ovo treatment with estradiol benzoate (10 or 25 mg) or diethylstilbestrol (DES) (700 ng), and the highest tested dose of genistein (1000 mg) abolished copulatory behavior in treated males and reduced expression of vasotocin (VT) in the nucleus of the stria terminalis and medial preoptic nucleus, and lateral septum. Another study exposed eggs to BPA (200 mg/g), tetrabromophenol A (15 mg/g egg), EE2 (2 and 6 ng/g egg), DES (6, 19, and 57 ng/g) or 1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2,2-trichloroethane (o’p’-DDT, 150 mg/g) (Halldin et al., 2005). While all groups hatched successfully, adult male sexual behavior, as evidenced by mount attempts, was compromised in the high dose EE2 and two highest doses of DES, but not in the BPA or tetrabromophenol A. No assessments of neuroendocrine function were reported in this latter study, although hypothalamic disturbances are a likely cause for the compromised copulatory behaviors. Environmental levels of natural and synthetic estrogens found in sewage effluents (e.g., E2, BPA, phthalates) can impact sexual traits in birds: exposed European starlings (Sturnus vulgaris) developed longer and more complex songs compared to control males (Markman et al., 2008). This resulted from estrogenic effects on the key brain area controlling male song complexity (the song nucleus higher vocal center, HVC) that was significantly enlarged in the contaminated birds.
4.2.3. Rodents, Primates, and Humans
Early life exposure to BPA consistently affects three general categories: learning and memory or cognitive, anxiety, and social-sexual behaviors across mammalian species. While it is beyond the scope of this short review to summarize all of the work to date, this section will focus on a few key examples.
4.2.3.1. Learning and Memory
Estrogens are essential in regulating learning and memory at the level of the hippocampus and cerebral cortex (Shughrue and Merchenthaler, 2000). At doses higher than those frequently found in the environment, but below the EPA exposure guidelines or lowest observe adverse effect level (LOAEL) of 50 μg/kg/day, BPA can selectively inhibit estrogen and testosterone-mediated synptogenesis in the hippocampus of rats and monkeys (Leranth et al., 2008a; Leranth et al., 2008b; MacLusky et al., 2005), and thus, BPA may impair the formation of new memories by interfering with neural plasticity in the brain. For instance, in adult male rats, visual and spatial memory was impaired by 40 μg/kg BPA and was accompanied by reduced spine density in the hippocampus and prefrontal cortex (Eilam-Stock et al., 2012). Likewise, in non-human primates, synaptic spine density was also reduced in the same brain regions with either adult (Leranth et al., 2008a) or prenatal BPA exposure at a human-relevant dose (Elsworth et al., 2013). Other means by which BPA may disrupt learning and memory include induction of long-term depression in the hippocampus by activation of ERRG, MAPK, phosphorylation of NMDA receptors, or the number of DA neurons in the mid-brain region (Elsworth et al., 2013; Hasegawa et al., 2013). The Morris water maze and dry-land Barnes maze have been most commonly used to test learning and memory ability in rodents (Nunez, 2008). Polygamous male deer mice (Peromyscus maniculatus bairdii) rely on enhanced spatial navigational learning and memory ability to locate prospective female partners in the wild (Galea et al., 1996). However, developmental exposure to environmentally-relevant levels of BPA through the maternal diet (5 or 50 mg/ kg feed weight) results in compromised spatial navigation, as assessed when males are tested at adulthood in the Barnes maze (Jasarevic et al., 2011; Jasarevic et al., 2013). In contrast, enhanced spatial navigational ability has not evolved in the closely related species, the monogamous California mouse (Peromyscus californicus) (Jasarevic et al., 2012). Accordingly, developmental exposure to BPA did not affect this trait in male or female California mice (Williams et al., 2013). The human epidemiological studies attempting to correlate BPA exposure with learning and memory ability in children are conflicting. One study reported significant BPA-associated impairments in children 8 to 11 years of age (Hong et al., 2013); whereas another study with children 10-15 years of age did not find any linkage (Maserejian et al., 2012).
4.2.3.2. Anxiety Behaviors
In rats, mice, Peromyscus species, and in human correlative epidemiological studies, the overall findings suggest that developmental exposure to BPA can be anxiogenic (Braun et al., 2011; Braun et al., 2009; Cox et al., 2010; Gioiosa et al., 2013; Goncalves et al., 2010; Harley et al., 2013; Hong et al., 2013; Jasarevic et al., 2013; Jasarevic et al., 2011; Maserejian et al., 2012; Matsuda et al., 2012; Patisaul et al., 2012; Perera et al., 2012; Xu et al., 2013; Xu et al., 2011; Yu et al., 2011). In rodents, anxiogenic behaviors are routinely measured with the Elevated Plus Maze (EPM) where reduced time spent in the open arms or increased time spent in the closed arms suggests that the animals are more anxious and less exploratory (Kulkarni and Sharma, 1991). In rats, human-relevant levels of BPA (1 mg/L in the drinking water) induced anxiety in both the light-dark box and the EPM, and correlated with decreased Esr2 and melanocortin receptor 4 mRNA levels in the amygdala (Patisaul et al., 2012). These genes are associated with anxiety behaviors and regulate expression of OXT, a neuropeptide important in sociosexual behaviors and anxiety. In male mice perinatally exposed to BPA, elevated anxiety behaviors in the open field were associated with increased DA levels and reduced DA turnover (Matsuda et al., 2012). Along with alterations in synaptic plasticity described above, BPA likely affects anxiety and cognitive tasks by also disrupting mesolimbic DA signaling, pathways known to influence mood, anxiety and reward. Developmental timing and dose may alter the potential anxiogenic properties of BPA (Goncalves et al., 2010). These BPA-induced effects on anxiety behaviors can be attributed to decreased acetylcholinesterase activity or increased expression of glucocorticoid receptor expression in the hippocampus, or estrogen-dependent gene decreases in the amygdala (Luo et al., 2013; Patisaul et al., 2012; Poimenova et al., 2010).
4.2.3.3. Sociosexual Behaviors
Developmental exposure to low dose BPA may also lead to epigenetic and gene expression changes, including Esr1 and Esr2 in the hypothalamus, where there is abolishment of the normal sex differences, with males exhibiting elevated expression comparable to females (Kundakovic et al., 2013). These effects may then lead to disruption of various sociosexual behaviors (Dessi-Fulgheri et al., 2002; Jasarevic et al., 2011; Williams et al., 2013; Wolstenholme et al., 2012). Male deer mice developmentally exposed through maternal diet to 50 mg/kg BPA in food are selectively rejected in a mate choice experiment (Jasarevic et al., 2011). In California mice, males developmentally exposed to the same dose of BPA, the normal surge in territorial marking necessary to maintain a home range is suppressed when a control male is visible in the testing arena (Williams et al., 2013). Female Sprague-Dawley rats developmentally exposed from conception to weaning to 40 mg/kg/day BPA showed masculinized play behaviors (Dessi-Fulgheri et al., 2002). In C57BL/6 mice, prenatal exposure to 5 mg/kg feed weight BPA reduced juvenile social interactions and social preference (Wolstenholme et al., 2012). Oxytocin and VP gene expression was reduced in the brains of developing rats and mice by BPA (Patisaul et al., 2012; Wolstenholme et al., 2012). These neuropeptides are well-established mediators of social interactions and affiliation in several mammalian species. Bisphenol A may also transgenerationally affect varying aspects of social behaviors in mice (Wolstenholme et al., 2012), as detailed further below.
4.2.4 Potential Transgenerational Effects of BPA
There is increased recognition that estrogenic and anti-androgenic chemicals may lead to transgenerational neuroendocrine disrupting effects (Crews et al., 2012; Crews et al., 2007; Skinner et al., 2008; Wolstenholme et al., 2012). The transgenerational effects of a classic anti-androgen chemical, viclozolin (VIN) are described in section 6. In this section the focus is on BPA. Treatment of progenitor female mice with a phytoestrogen-free diet supplemented with 5 mg/ kg BPA produced a blood level in mice similar to that found in humans and led to diminished social behaviors in F1 BPA-exposed offspring (Wolstenholme et al., 2012). In contrast, generation F2 and F4 male and female BPA-exposed descendants were for uncertain reasons more socially curious. Along with the phenotypic changes, each generation demonstrated unique brain transcriptome changes. For example, transcript levels for Esr1, vasopressin (Avp), and vasopressin receptor (Avpr1a) expression were reduced, while estrogen related receptor gamma (Esrrg) and G protein-coupled estrogen receptor 1 (Gper) were increased in F1 BPA exposed mice. Even though they were more social, mRNA for Avp and oxytocin (Oxt) were suppressed in F4 BPA-derived males and females. Both OXT and AVP are neuropetides that play important roles in social behavior, anxiety, depression, pair bonding, and parental behaviors (Benarroch, 2013; Feldman, 2012; McCall and Singer, 2012; Neumann and Landgraf, 2012). A more recent follow-up transgenerational study with similar doses of BPA revealed that generation F3 BPA male and female descendants have problems with social memory. Descendants from BPA-exposed mice failed to habituate to repeated presentations of a female and continued to investigate this unrelated female long after controls lost interest. Males also did not increase their investigation of a new, novel female, suggesting that transgenerational exposure to BPA may induce defects in social recognition, as well as social memory (Wolstenholme et al., 2013).
4.3. Phthlate Effects in Vertebrates
Phthalates are synthetic chemicals used in the manufacturing of plastics, medical devices, food packaging, cosmetics, toys, and electronics. Phthalates can easily leach from these products over time, thereby increasing risk for oral, dermal and inhalation exposures in humans and wildlife (Heudorf et al., 2007; Wooten and Smith, 2013). Their major mode of action is to alter steroid hormone synthesis and metabolism, resulting in reproductive problems and altered sexual dimorphism in the brain. At doses frequently found in humans, phthalates possess anti-androgenic activity (Swan, 2008). However, evidence from fish, frog and rodent models indicates that low dose phthalate exposure disrupts the reproductive system and sexual dimorphism in the brain, primarily by acting as an androgen antagonist with weak estrogen agonistic properties (Borch et al., 2004; Borch et al., 2006; Oehlmann et al., 2009; Swan et al., 2008). As with other neuroendocrine disruptors, such non-linear actions, as detailed further below, in target cells, including those in the brain, can lead to low levels of this chemical producing pronounced behavioral disturbances (Swan et al., 2008; Vandenberg et al., 2012). Understanding the neuroendocrine and behavioral consequences of phthalate exposure is complicated by the diverse number of bioactive phthtalates, metabolites produced in the body, and the interactions between such admixtures.
Given the consistent and the large number of studies reporting hormonal disruptions of phthalates on the reproductive tract, there is surprisingly sparse research examining potential neuroendocrine effects. Most studies in wildlife have focused on toxicity (Magdouli et al., 2013; Oehlmann et al., 2009). In fish, butyl benzyl phthalate (BBP, 0.1 mg/L) in water altered shoaling (a social behavior) and feeding behavior in three-spined sticklebacks (Wibe et al., 2002; Wibe et al., 2004). In rats, DEHP during perinatal development caused cognitive deficits in spatial memory (Li et al., 2013), which may be linked to a reduced hippocampal cell density in males (Smith et al., 2011). Single intracisternal injecions of dicyclohexyl phthalate ranging from 0.029 to 2.9 mg/ml (0.087-8.7 mM) five days after birth dose-dependently increased locomotor activity in adult male rats and decreased midbrain DA synthesis, transport and receptor signaling (Ishido et al., 2004). These effects on DA signaling in the brain may be causally linked to reported problems in attention and hyperactivity in human association studies (Engel et al., 2010; Larsson et al., 2009; Tanida et al., 2009). Perinatal low dose DEHP decreased aromatase activity in the hypothalamus of male rats but not female rats (Andrade et al., 2006). In humans, a few convincing association studies link phthalate exposure to cognitive deficits (Cho et al., 2010), slower neurodevelopment, higher incidence of aggression and depression, poor attention, conduct and emotional control (Engel et al., 2010), reduced masculine play behaviors (Swan et al., 2010) and increased behaviors typical of autism spectrum disorders and attention deficit hyperactivity disorder (Kim et al., 2009; Larsson et al., 2009). Many of these behavioral disruptions have only been detected in males, perhaps due to anti-androgenic and thus overall demasculinizing effects of the phthalate (Andrade et al., 2006).
The neuroendocrine effects of phthalates need to be investigated further, especially the underpinning mechanisms of actions leading to the neurobehavioral changes. Animal model studies should focus on examining the effects underlying neuroendocrine mechanisms mediating effects of phthlates on social, anxiety, locomotor and cognitive behaviors, as these are traits apparently vulnerable in children exposed to this chemical.
5. Phytoestrogens and Other Phytochemicals
Plants produce a significant array of secondary metabolites, some of which are classified at phytosteroids. Many plants make compounds that directly, or following microbial degradation, bind and activate estrogen, androgen or progesterone receptors (Adlercreutz et al., 1993; Durhan et al., 2002; Jenkins et al., 2003; MacLatchy and Van Der Kraak, 1995; Patisaul and Jefferson, 2010). Given the documentation of effects in mammals, and the known binding affinities of teleost ERs for phytoestrogens, it is reasonable to predict that there will be effects of phytochemicals on neurochemistry and behaviors (Latonnelle et al., 2002). In one study, male Siamese fighting fish exposed to genistein (1-1,000 μg/L) and other phytoestrogens exhibited decreased intensity of aggressive behavior (Clotfelter et al., 2006).
The evidence that phytoestrogens and other phytochemicals may act as neuroendocrine disruptors stems mostly from studies on the effects of the paper mill/bleached kraft mill effluent on fish. One of the earliest studies examined neuroendocrine regulation of reproductive function in wild white sucker (Catostomus commersoni) exposed in situ to bleached kraft mill effluent and found that pituitary luteinizing hormone (LH) content and GnRH-stimulated LH was significantly depressed relative to control fish (Van der Kraak et al., 1992). Wild female mosquitofish (Gambusia holbrooki) collected from Florida rivers, where they were exposed to paper mill effluent, display masculinized secondary sex characteristics including reproductive behavior, where effluent exposed females court and appear to attempt spawning with control female fish (Bortone and Davis, 1994). In another study, social but not reproductive behavior was decreased in the effluent-exposed mosquitofish (Toft et al., 2004). Interestingly, phytosterols incubated with certain bacteria have been hypothesized to be converted to progesterone in the field (Jenkins et al., 2003) and can be converted to the androgen androstenedione in a lab study (Denton et al., 1985). Female mosquitofish exposed to microbially degraded phytosterols, stigmastanol and β-sitosterol, courted and pursued control females in a laboratory study (Krotzer, 1990).
Some pulp and paper mill effluents (dose range 3-50%) reduce gonad size, rapidly inhibit egg production in fish, and life-cycle exposures alter sex ratios (McMaster et al., 2006; Parrott et al., 2006). Pioneering research shows anti-reproductive effects of the phytoestrogen β-sitosterol (20 or 100 μg/g i.p.) and that effluents contain ligands for sex steroid receptors (Hewitt et al., 2006; MacLatchy and Van Der Kraak, 1995). Although steroid-dependent pathways clearly are affected (Orrego et al., 2010), disruption of other pathways could impair fish reproduction (Basu et al., 2009; Popesku et al., 2010; Van der Kraak et al., 1992), because many effluents are strongly anti-reproductive but are neither estrogenic nor androgenic in standard assays. In vitro studies have revealed that extracts from effluents and trees contain chemicals that can interfere with the GABA synthesis enzyme glutamic acid decarboxylase and the DA metabolism enzyme monoamine oxidase (Basu et al., 2009; Milestone et al., 2012; Waye et al., 2013). Given the importance of GABA to stimulate and DA to inhibit LH release, such data led to the neuroendocrine disruption hypothesis for the reversible effects of pulp mill effluents to inhibit spawning in teleost fish (Waye and Trudeau, 2011). Comparative transcriptomic studies between female goldfish (Carassius auratus) and fathead minnow (Pimephales promelas) further indicate that effluents contain chemicals that induce hypothalamic gene expression profiles similar to those caused by injection of specific DA agonists (Popesku et al., 2012). This lends strong support for a dopaminergic mechanism of anti-reproductive effects; however, field-based studies are required to fully validate this hypothesis. Pupl and paper mill effluents are complex mixtures released to the aquatic environment and act at a number or points in the hypothalamic-pituitary-gonadal axis and affects reproductive behavior (Basu et al., 2009; Bortone and Davis, 1994; Van der Kraak et al., 1992). As such, exposure to pulp and paper mill effluent is a key example of neuroendocrine disruption that impacts wild fish populations and aquatic ecosystem health.
6. Anti-androgens as Neuroendocrine Disruptors
6.1 Vinclozolin Effects in Vertebrates
Various taxa, including fish, amphibians, birds, and rodents have been used to test the neuroendocrine disrupting effects of the fungicide VIN (Andre and Markowski, 2006; Bayley et al., 2002; Bayley et al., 2003; Colbert et al., 2005; Crews et al., 2012; Crews et al., 2007; Flynn et al., 2001; Hoffmann and Kloas, 2010; Hotchkiss et al., 2003; Hotchkiss et al., 2002; McGary et al., 2001; Satre et al., 2009; Skinner et al., 2008). The doses tested include those that are environmentally-relevant and those that may exceed human and animal levels of exposure, as indicated below. Nonetheless, these latter studies demonstrate important proof of principles that merit follow-up experiments testing whether lower concentrations cause similar disruptions. The traits most affected by VIN include sociosexual, anxiety, cognition, appetitive, and locomotor behaviors. We will consider example studies in each of these categories.
6.1.1. Sociosexual Behaviors
Adult guppies (Poecilia reticulata) fed VIN in concentrations ranging from 1.8 to 180 mg/kg had disrupted courtship display behaviors (Bayley et al., 2003). Mate calling behavior of male South African clawed frogs (Xenopus laevis) is also disrupted when they are exposed as adults to ~286 μg/L (10−6M) VIN (Hoffmann and Kloas, 2010). Social disruptions following VIN exposure have also been demonstrated in birds; GnRH-1 is reduced in male Japanese quail exposed in ovo to VIN (25, 50, or 100 mg/kg) (McGary et al., 2001). Female dark-eyed juncos selectively prefer wild caught adult dark-eyed juncos (Junco hyemalis) chronically orally gavaged for 10 weeks with ~572 mg/kg VIN compared to control males (Satre et al., 2009). This female preference for anti-androgenized males may lead to compromised offspring fitness with potential population ramifications. In contrast, female rodents selectively reject males, whose ancestors were exposed to VIN, albeit at concentrations that exceed normal environmental exposures, three generations previously (Crews et al., 2007). Copulatory behavior is diminished in Long-Evans male rats gestationally dosed orally with VIN (1.5, 3, 6, or 12 mg/kg) (Colbert et al., 2005). However, in this same study the 12 mg/kg dose of VIN increased male play behavior. Other studies with Sprague-Dawley rats reported the opposite findings of reduced play behavior in neonate males injected on post-natal days 2 and 3 with 200 mg/kg/day VIN (Hotchkiss et al., 2003; Hotchkiss et al., 2002). These deficits may be partially due to VIN-induced neuroendocrine disruption in the hypothalamus, such as altered expression of GnRH1 in males (McGary et al., 2001).
6.1.2. Anxiety Behaviors
The neuroendocrine effects of estrogenic and anti-androgenic chemical exposure may extend beyond the immediate exposed generations (F0, F1, and germ cells representing the F2 generation). Vinclozolin has been the predominant chemical studied and reported to induce transgenerational effects when F0 gestating dams are intraperitoneally injected with of 100 mg/kg/day from embryonic day 8–14 (E8–E14), a concentration considered beyond typical human and animals exposure but still within the range of exposure considered acceptable by the US Environmental Protection Agency. Besides the sociosexual effects on descendants described above, F3 male and female rats of the original exposed founders show altered anxiety-like behaviors relative to controls with F3 males less and females more anxious (Skinner et al., 2008). Correspondingly, these male and female descendants each possessed unique transcriptomic signatures in the hippocampus and amygdala relative to their respective controls. A follow-up study with a similar approach also showed that ancestral exposure to VIN (three generations removed) alters the physiology, behavior, metabolic activity, and transcriptome in discrete brain nuclei in descendant males, leading to differential responses to chronic restraint stress (Crews et al., 2012).
6.1.3. Cognitive, Appetitive, and Locomotor Behaviors
Long-Evans male rats exposed through the dams from mid-gestation through the early post-natal period to 6 or 12 mg/kg VIN fail to learn when food is no longer available at a previous location (Andre and Markowski, 2006). Daughters of pregnant rats administered 60 mg/kg/day VIN moved less on a running wheel relative to controls, suggestive of hypoactivity (Flynn et al., 2001). Sons and daughters of these same rats drank more, particularly water supplemented with saccharin solution. These behavioral alterations may be partially attributable to VIN-induced alterations in gene expression (such as Esr1, Esr2, and Ar) in several hypothalamic nuclei, hippocampus, and striatum (Loutchanwoot et al., 2008).
6.2. Dichlorodiphenyldichloroethylene in Fishes and Birds
Dichlorodiphenyldichloroethylene, p,p’DDE, is a metabolite of DDT, an insecticide for which the production and use has been banned by many industrialized nations (Rogan and Chen, 2005). Dichlorodiphenyldichloroethylene is lipophilic, bioaccumulates in organisms, and has been identified as an androgen receptor antagonist (Kelce et al., 1998). Few studies have examined the potential for DDE to alter neuroendocrine function. Juvenile guppies (Poecilia reticulata) were exposed to antiandrogenic pesticides, including DDE, (0.01, 0.1 μg/mg) singly in their feed from birth to adulthood. All tested anti-androgens caused a reduction in male-specific pigmentation, phallus development, sperm count, and courtship behaviors (Bayley et al., 2002). Generally, embryonic and juvenile life stages are more sensitive. However, another study has revealed that adult guppies exposed to anti-androgenic pesticides, including DDE, (0.1, 1, 10 μg/mg) singly in their feed for only 30 days exhibit decreased sperm count, testis mass, male-specific pigmentation, and disruption of male courtship behaviors (Baatrup and Junge, 2001). In contrast, DDE (0.01, 0.1 μg/mg in the feed) did not affect the same reproductive endpoints or courtship behaviors measured in the two above studies when tested another strain of male guppies treated as juveniles for approximately 30 weeks (Kristensen et al., 2006). These studies are difficult to compare, for in the latter study behavior was directly observed, instead of taped and analyzed by quantitative computer imaging software used in the earlier studies and in the former studies Columbian guppy strain was used versus a Nigerian strain of guppy in the latter study (Baatrup and Junge, 2001; Bayley et al., 2002) and (Kristensen et al., 2006), respectively.
Birds exposed either in ovo through DDE fed to parents or through topical application to the eggshell, display disruption in behavior, neural circuitry, and neurotransmitter-specific neurons (Heinz, 1976; Quinn et al., 2008). Mallard ducklings (Anas platyrhynchos) born to parents fed a diet containing 3 mg/kg DDE displayed altered behavior (Heinz, 1976). Treated ducklings were hyper-responsive to maternal vocalizations and their avoidance behavior was decreased as they traveled shorter distances from a frightening stimulus relative to control ducklings. Japanese quail (Coturnix japonica) exposed to DDE in ovo a single time (20 or 40 μg/egg) was assessed at puberty for a number of reproductive endpoints, including reproductive behaviors. Females treated with DDE exhibited accelerated puberty while in males copulatory behaviors were decreased (Quinn et al., 2008). A subsequent study following the same in ovo exposure protocol, investigated the effects of DDE on the parvocellular VT system, a part of the limbic circuitry that plays a role in male copulatory behavior. The DDE-treated quail had decreased density of parvocellular VT expressing neurons in the medial preoptic nucleus, bed nucleus of the stria terminalis, and lateral septum, whereas no effect was found the magnocellular neurons of the supraoptic nucleus (Mura et al., 2009).
7. Neuroactive Pharmaceuticals: the case of selective serotonin reuptake inhibitors
Globally, it is now evident that many hundreds of pharmaceuticals, including neuroactive drugs, are detected in aquatic environments and water supplies through the release of sewage effluents (Boxall et al., 2012; Elorriaga et al., 2013; Kolpin et al., 2002; Kostich et al., 2013; Subedi et al., 2013). In particular the antidepressant fluoxetine (FLX) serves as an important example of a neuroendocrine disruptor. As a selective serotonin reuptake inhibitor (SSRI) FLX is neuroactive, it has a specific mechanism of action, and because of effects on central serotoninergic systems can affect multiple neuroendocrine pathways and behavioral circuits at environmentally-relevant concentrations (Mennigen et al., 2011).
In addition to intended therapeutic effects as antidepressants, there are beneficial neurogenic effects of SSRIs in mammalian brains (Olivier et al., 2011). Why should fluoxetine or other SSRIs be considered as disruptors of neuroendocrine systems? There are reported effects on numerous hormone systems (Raap and Van de Kar, 1999; Weintrob et al., 2002) that could be considered as side effects rather than main therapeutic targets in mammals. Antidepressants are well-known to significantly decrease libido in both men and women (Montejo et al., 2001). Moreover, there is placental transfer of SSRIs to the mammalian fetus, and SSRIs are detectable in breast milk and breast-fed infants. Therefore, unborn and newborn children of depressed mothers taking SSRIs may be unintentionally exposed during critical phases of neurodevelopment (Olivier et al., 2011). Data from rodent models indicate paradoxical anxiety- and depression- like symptoms during adulthood in animals exposed to SSRIs in utero or in the neonatal period, although the impacts in humans are debatable (Olivier et al., 2011). While these situations of early exposure indicate disruption of neuronal development, the consequences for specific neuroendocrine systems remain to be addressed. The presence of very low levels of SSRIs in drinking water also raises important issues about: (1) the efficacy of current sewage treatment, and (2) the effects of very low, chronic unintended exposure to pharmaceuticals to human populations.
The case for the neuroendocrine disrupting effects of SSRIs in fish is perhaps more compelling (Mennigen et al., 2011). Several SSRIs and metabolites have been detected in wild fish, indicating the potential to bioconcentrate (Brooks et al., 2005; Metcalfe et al., 2010). There are now a range of clear effects of environmentally-relevant FLX levels (range: 12-540 ng/L) on behaviour, physiology, neuroendocrine function, reproduction, feeding and growth in several teleost models (Mennigen et al., 2011). Risk assessments for FLX already conducted indicate environmental concern (Oakes et al., 2010). The total load of all measured SSRIs may reach 3.2 μg/L near sewage outfalls, with levels decreasing downstream (Mennigen et al., 2011).
In a recent study of fathead minnow (Thomas et al., 2012), exposure to high levels of FLX (10 μg/L) resulted in transcriptome-level changes in genes in whole brain homogenates brain coding for products capable of transmitting (or inhibiting the transmission of) a nerve impulse from a neuron to another cell (e.g., the ontology group defined as “neurotransmitter binding”). Chronic waterborne exposure of zebrafish to pharmacological levels (100 μg/L) of FLX (racemic) indicated effects on stress- and anxiety- related neuropeptides that are perhaps indicative of neuroendocrine disruption. RNAseq analysis of whole brain revealed subtle but statistically significant increases in NPY and isotocin (IST), and a decrease in urocortin 3 mRNA levels (Wong et al., 2013). Unfortunately, these studies used whole brain as the tissue for analysis, thus no conclusions can be drawn regarding effects on specific neuroendocrine tissues or systems. However, given the extent of effects, one can speculate that neurotransmitter- and neuropeptide-dependent processes in neuroendocrine regions such as the hypothalamus would be disrupted.
Indeed, this is the case in an earlier microarray analysis of the effects of therapeutic levels of injected FLX (5 μg/g; 5 injections over ~2 week period). A broad range of effects on signaling and metabolic pathways in the hypothalamus of female goldfish was observed (Mennigen et al., 2008). However, severe reductions in the expression of the reproductive and behavioural neuropeptide IST in both hypothalamus and telencephalon were indicative of neuroendocrine disruption. Isotocin is the teleost homolog of mammalian OXT, and depressed IST was linked to reduced E2 levels in female goldfish. Moreover, FLX injections also depressed Esr1 and Esr2 mRNA levels in both telencephalon and hypothalamus. Waterborne exposures of breeding groups of female and male zebrafish to high levels of FLX (32 µg/L) were also anti-reproductive (Lister et al., 2009). The number of eggs laid was reduced 4.5-fold. Following the FLX treatment, ovarian E2 levels were depressed, as were the expression levels of gonadal aromatase, follicle stimulating hormone receptor and luteinizing hormone receptor in these zebrafish (Lister et al., 2009). The authors concluded that disruptions to the synthesis of ovarian steroids and the actions of gonadotropins may underlie the negative influence of FLX on ovarian E2 and spawning levels (Lister et al., 2009). Experiments with male goldfish further support the hypothesis that FLX is an anti-reproductive neuroendocrine disruptor (Mennigen et al., 2010a). Sexually mature male goldfish were exposed up to 2 weeks to environmentally-relevant levels of FLX in the water (540 ng/L). Following this, males were treated with a pulse of female primer pheromone 17,20α-dihydroxy-4-pregnene-3-one or the releaser pheromone, prostaglandin F2α, and sperm release and neuroendocrine responses assessed. Remarkably, both basal and pheromone-stimulated milt production were suppressed by pre-exposure to FLX. Together, these data in teleost fish indicate that FLX can suppress both female and male reproductive function, in part by modulating IST and ER signaling (Mennigen et al., 2010a). The hypothesis that environmental FLX exposure and neuroendocrine disruption leads to deleterious effects on natural fish populations requires rigorous testing.
There are other emerging concerns with SSRIs in the environment. Not only is there suppressed reproduction, but waterborne FLX (540 ng/l and 54 μg/L) also decreases food intake, induces body weight loss and a fasting-like response in goldfish (Mennigen et al., 2010b). Hypothalamic gene expression studies support potential anorexigenic effects, with increased expression of CRH and decreased expression of NPY (Mennigen et al., 2010b). In striped bass, high levels of waterborne FLX (35-150 μg/L) reduced prey capture ability (Gaworecki and Klaine, 2008). In addition to FLX, numerous other antidepressants (e.g., venlafaxine, bupropion, fluoxetine, sertraline, citalopram, paroxetine and their metabolites) have been detected in wastewater and may bioconcentrate in exposed fish (Metcalfe et al., 2010). Sertraline at levels above those expected in the environment (0.12, 89, and 300 μg/L) dose-dependently reduces foraging in juvenile perch (Hedgepeth et al., 2013). Some SSRIs have additional mechanisms of action, for example, venlafaxine is a pharmaceutical whose prescription rates are increasing, and has effects to inhibit reuptake of both 5HT and norepinephrine. Few studies had address potential neuroendocrine disrupting effects of these other antidepressants. In studies comparing the effects of FLX and venlafaxine on whole brain transcriptome in fathead minnow using microarrays, it was clear that there were more extensive effect of venlafaxine, and distinct gene sets are affected by the two antidepressants (Thomas et al., 2012).
Studies of mixtures containing SSRI and other neuroactive pharmaceuticals are beginning to appear (Galus et al., 2013; Silva de Assis et al., 2013), yet their impacts on neuroendocrine systems have not been fully characterized or assessed. Regardless, these studies support the idea that SSRIs significantly disrupt reproduction and metabolism in fish through neuroendocrine mechanisms.
8. Conclusion and future perspectives
Due to the pervasive use and increased mass production of many of these chemicals, there is sufficient evidence for neuroendocrine disruption by organopesticides, PCBs, pulp and paper mill effluents, and chemicals that have estrogenic or anti-androgenic modes of action. Laboratory and field sampling studies illustrate the transfer of some EDCs from the environment to the circulation and into the brain. Many of these compounds can persist and may bioconcentrate or bioaccumulate, ensuring widespread and continued exposure of animals including humans to these chemicals. Further, neuroactive pharmaceuticals (e.g., fluoxetine, among others), found in the environment are now also emerging as neuroendocrine disruptors because effects have been observed in experiments using environmentally-relevant exposure scenarios. While there are a few existing examples, a key issue that should be addressed in future research is the relationship between exposure route and concentration, and subsequent neuroendocrine alterations and effects on apical endpoints.
To guide future experiments and policymaking decisions, it is essential to develop a consensus statement that encompasses the current discussion and debate amongst the broader scientific and regulatory communities. Our goal is that this will aide in classifying other environmental pollutants as neuroendocrine disruptors and will increase the scope of risk assessments across taxa. Based on this review, and in the spirit of being cogent and succinct for a wider audience, we propose the following modification to the definition of Waye and Trudeau (2011):
Neuroendocrine disruptors are exogenous substances found in the environment that alter normal neuroendocrine function and result in an adverse effect on the organism or population.
Exposure to many neuroendocrine dirsuptors, especially during development, likely interferes with sexual differentiation and maturation, behavior, adult endocrine function. As such, neuroendocrine disruptors are potential risk factors across vertebrate species and may pose an environmental health hazard for humans and wildlife. Future investigations related to mechanisms of action of these neuroendocrine disruptors are needed for a better understanding of how they alter the neurophysiology of the organisms. Critically missing is research specifically addressing direct effects of environmental chemicals on anterior pituitary function. New experimental approaches used to build a case for neuroendocrine disruption by environmentally-relevant levels of contaminants must strive to link direct actions in neuroendocrine cells to altered physiology, as well as to behaviors related to reproduction, cognition and memory, etc. These studies may serve as a framework for investigating other emerging neuroendocrine disruptors. It will be important to link these outcomes with effects at the population-level. Additionally, this information should aid in establishing guidelines and public policy decisions for targeted reductions in production levels for these various chemicals, financial support for remediation strategies for existing contaminated environments, and ultimately minimizing animal and human exposure to suspected neuroendocrine disruptors. Given the persistence, ubiquitous and lipophilic (easily transferable) nature of many of these compounds, exposure to neuroendocrine disruptors may start in embryonic life, be it a fish larva, free-living amphibian tadpole, hatchling bird, or the human conceptus.
Future studies in neuroendocrine disruption need to be designed to carefully test a wide range of concentrations. It is essential to assess low environmentally-relevant levels in addition to higher concentrations currently considered acceptable by the various National regulatory agencies and policy-makers. It is essential to determine if low concentrations of the various chemicals lead to neuroendocrine disruptions. Alternatively, it may be that some of these chemicals demonstrate a U-shape or non-monotonic response curve where only the lowest and highest doses tested result in significant neuroendocrine disruption (Vandenberg et al., 2012). These types of studies may aid in establishing new guidelines for acceptable exposure with minimal risk to wildlife and humans. Additionally, further studies are needed to determine whether there are sexually dimorphic differences in vulnerability to neuroendocrine disruption by the various chemicals. It may be that each sex has contrasting neural pathways and associated behaviors that are differentially susceptible to environmental pollutants (Jasarevic et al., 2011).
Epigenetic or inheritable changes that do not alter the DNA sequence itself but affect the expression of certain genes are not analyzed in most risk-assessment studies. Select pesticides, estrogenic and anti-androgenic chemicals have been linked to epigenetic changes, including DNA methylation, histone protein alterations and non-coding RNA changes. However, it is not clear how DNA methylation, especially through the male germline, might withstand the normal embryonic reprogramming mechanisms and be passed on to subsequent generations to account for the ascribed transgenerational inheritance. Nor is it clear in many cases whether the mode of transgenerational inheritance is through the female or male germline and what groups of genes are affected. Additional studies should examine impacts of neuroendocrine disruptors on microRNAs and other non-coding RNAs such as Piwi-interacting RNA (piRNA), because they are likely to be vulnerable to changing environmental conditions in vertebrates (Zhang et al., 2010), as has been shown in Caenorhabditis elegans (Ashe et al., 2012; Gu et al., 2012b; Vandegehuchte and Janssen, 2013).
Neuroendocrine disruption is more than just disturbances in hormones and feedback mechanisms. Some neuroendocrine disruptors can induce morphological changes in neuroendocrine neurons (Panzica et al., 2011), whereas others may reduce or eliminate sexually dimorphic behaviors (Jasarevic et al 2011; Patisaul et al., 2007; Rubin et al., 2006) at the center of mate choice, and thus successful reproductive events. By altering reproductive and sexually-selected behaviors, for example, those that involve intrasex competition or intersex traits and provide honest indicators of fitness, neuroendocrine disruptors may already be shaping evolutionary change in an increasingly contaminated world (Crews and Gore, 2012).
Environmental contaminants have specific effects on vertebrate neuroendocrine systems
Neuroendocrine disruption is linked to infertility, obesity and cardiac disease
Epigenetic and transgenerational effects of neuroendocrine disruptors are a concern
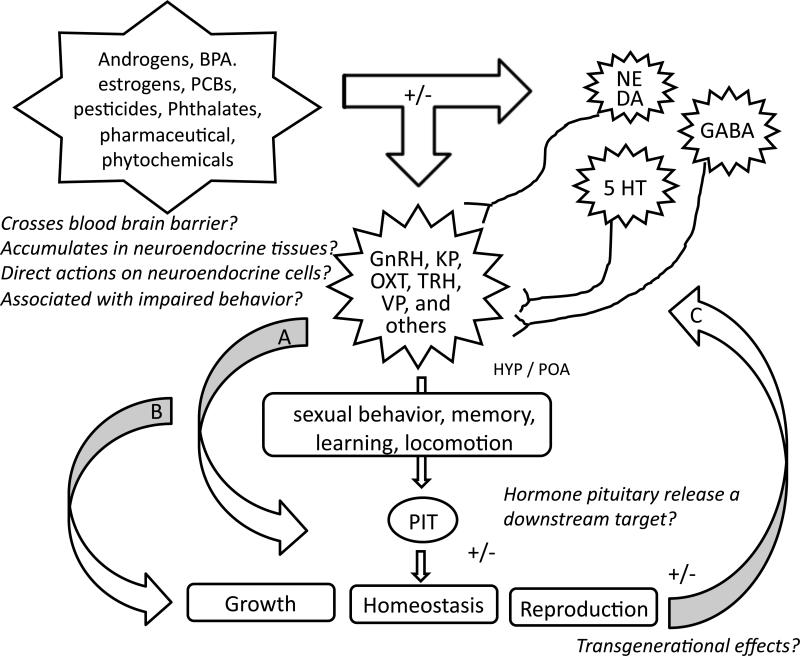
Figure 2.
Building a case for neuroendocrine disruption. Environmental pollutants (e.g. , pesticides, PCBs, phthalates, pharmaceuticals, etc.) can act directly on cells within neuroendocrine tissues of the central nervous system (CNS) as well as on neurotransmitter systems that regulate neurohormone release, for example dopamine (DA), gamma-aminobutyric acid (GABA), norepinephrine (NE) and serotonin (5HT), among others. Altered neuropeptide synthesis and release will have downstream consequences on pituitary hormone release (arrow A), affecting homeostasis, growth and reproduction. Neuroendocrine disruptors are also proposed to regulate behaviors by modulating neuropeptide synthesis and release, the mechanisms of which are not fully characterized but likely involve membrane bound receptor (e.g., estrogen, progesterone) signaling and/or nuclear receptor (e.g., androgen, glucocorticoid, estrogen) pathways and post-translational protein modifications (i.e., phosphorylations) within neuroendocrine cells. Changes in behaviors (e.g., feeding, sociosexual) will adversely impact homeostasis, growth and reproductive output (arrow B). The CNS will be responsive to these physiological changes, and may be altered by longer acting effects of neuroendocrine disruptors, such epigenetic modifications (e.g., DNA methylation state) resulting in transgenerational effects (arrow C). Focused studies that address some of the questions proposed will assist to better define the scope of neuroendocrine disruption. Abbreviations: 5-HT, 5-hydroxytryptamine; GnRH, gonadotropin-releasing hormone; KP, kisspeptin; OXT, oxytocin (known as isotocin in fish), PCBs, polychlorinated biphenyls; PIT, pituitary; POA, preoptic area; TRH, thyrotropin-releasing hormone; VP, vasopressin.
Acknowledgments
MLO acknowledges support from UCMEXUS/CONACYT. A Tier II Canada Research Chair to CJM funded this research. EFO acknowledges research support from the Morris Animal Foundation Grant (D12ZO-046) and the Maryland Agricultural Experiment Station Grant (1-10833). MAO is supported by EPA grants R826134010 (Star Grant) and R-82877801; Battelle contract for EPA-EDSTAC validation studies, NRI 92-37203 and NSF 9817024; Fish and Wildlife Service and Hudson River NRDA Trustees. CSR acknowledges support from a US National Institutes of Health Challenge Grant (RC1 ES018195), Mizzou Advantage Grant, University of Missouri CVM Faculty Award, and a US National Institute of Health Sciences fellowship (U01 ES020929). JTW is supported by a the US National Institute of Environmental Health Science (F32 ES019404). VLT acknowledges with appreciation support from the Natural Sciences and Engineering Research Council of Canada (NSERC-DG, NSERC-SGP), Canadian Water Network, Environment Canada, and the University of Ottawa Research Chair in Neuroendocrinology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol. Metab. 2007;18:48–50. doi: 10.1016/j.tem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Bannwaert C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Jr JK, Vickery LE. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993;44(2):147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology. 2006;225:64–74. doi: 10.1016/j.tox.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Andre SM, Markowski VP. Learning deficits expressed as delayed extinction of a conditioned running response following perinatal exposure to vinclozolin. Neurotoxicol. Teratol. 2006;28:482–488. doi: 10.1016/j.ntt.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Andric SA, Kostic TS, Dragisic SM, Andric NL, Stojilkovic SS, Kovacevic RZ. Acute effects of polychlorinated biphenyl-containing and -free transformer fluids on rat testicular steroidogenesis. Environ. Health Perspect. 2000;108:955–959. doi: 10.1289/ehp.00108955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, Pintacuda G, Sakaguchi A, Sarkies P, Ahmed S, Miska EA. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, Gagne LA, Moen EL, Marsit CJ. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010;29:401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baatrup E, Junge M. Antiandrogenic pesticides disrupt sexual characteristics in the adult male guppy (Poecilia reticulata). Environ. Health Perspect. 2001;190:1063–1070. doi: 10.1289/ehp.011091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Ta CA, Waye A, Mao J, Hewitt M, Arnason JT, Trudeau VL. Pulp and paper mill effluents contain neuroactive substances that potentially disrupt neuroendocrine control of fish reproduction. Environ. Sci. Technol. 2009;43:1635–1641. doi: 10.1021/es802552m. [DOI] [PubMed] [Google Scholar]
- Bayley M, Junge M, Baatrup E. Exposure of juvenile guppies to three antiandrogens causes demasculinization and a reduced sperm count in adult males. Aquat. Toxicol. 2002;56:227–239. doi: 10.1016/s0166-445x(01)00210-7. [DOI] [PubMed] [Google Scholar]
- Bayley M, Larsen PF, Baekgaard H, Baatrup E. The effects of vinclozolin, an anti-androgenic fungicide, on male guppy secondary sex characters and reproductive success. Biol. Reprod. 2003;69:1951–1956. doi: 10.1095/biolreprod.103.017780. [DOI] [PubMed] [Google Scholar]
- Bellavance MA, Rivest S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol. Rev. 2012;248:36–55. doi: 10.1111/j.1600-065X.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Oxytocin and vasopressin: social neuropeptides with complex neuromodulatory functions. Neurology. 2013;80:1521–1528. doi: 10.1212/WNL.0b013e31828cfb15. [DOI] [PubMed] [Google Scholar]
- Boix J, Cauli O. Alteration of serotonin system by polychlorinated biphenyls exposure. Neurochem. Int. 2012;60:809–816. doi: 10.1016/j.neuint.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Borch J, Ladefoged O, Hass U, Vinggaard AM. Steroidogenesis in fetal male rats is reduced by DEHP and DINP, but endocrine effects of DEHP are not modulated by DEHA in fetal, prepubertal and adult male rats. Reprod. Toxicol. 2004;18:53–61. doi: 10.1016/j.reprotox.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Bortone SA, Davis WP. Fish intersexuality as an indicator of environmental stress. Bioscience. 1994;44:165. [Google Scholar]
- Boxall AB, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley JP, Verslycke T, Ankley GT, Beazley KF, Belanger SE, Berninger JP, Carriquiriborde P, Coors A, Deleo PC, Dyer SD, Ericson JF, Gagne F, Giesy JP, Gouin T, Hallstrom L, Karlsson MV, Larsson DG, Lazorchak JM, Mastrocco F, McLaughlin A, McMaster ME, Meyerhoff RD, Moore R, Parrott JL, Snape JR, Murray-Smith R, Servos MR, Sibley PK, Straub JO, Szabo ND, Topp E, Tetreault GR, Trudeau VL, Van Der Kraak G. Pharmaceuticals and personal care products in the environment: what are the big questions? Environ. Health Perspect. 2012;120:1221–1229. doi: 10.1289/ehp.1104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretveld RW, Thomas CM, Scheepers PT, Zielhuis GA, Roeleveld N. Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod. Biol. Endocrinol. 2006;4:30. doi: 10.1186/1477-7827-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge ES, Kelly JF. Reproductive success of belted kingfishers on the upper Hudson River. Environ. Toxicol. Chem. 2013;32:1855–1863. doi: 10.1002/etc.2263. [DOI] [PubMed] [Google Scholar]
- Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 2005;24:464–469. doi: 10.1897/04-081r.1. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser TJ. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol. Ind. Health. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Bush B, Bennett AH, Snow JT. Polychlorobiphenyl congeners, p,p'-DDE, and sperm function in humans. Arch. Environ. Contam. Toxicol. 1986;15:333–341. doi: 10.1007/BF01066399. [DOI] [PubMed] [Google Scholar]
- Bytingsvik J, van Leeuwen SP, Hamers T, Swart K, Aars J, Lie E, Nilsen EM, Wiig O, Derocher AE, Jenssen BM. Perfluoroalkyl substances in polar bear mother-cub pairs: a comparative study based on plasma levels from 1998 and 2008. Environ. Int. 2012;49:92–99. doi: 10.1016/j.envint.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Carro T, Dean K, Ottinger MA. Effects of an environmentally relevant polychlorinated biphenyl (PCB) mixture on embryonic survival and cardiac development in the domestic chicken. Environ. Toxicol. Chem. 2013;32:1325–1331. doi: 10.1002/etc.2178. [DOI] [PubMed] [Google Scholar]
- Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, Yoo HJ, Cho IH, Kim HW. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ. Health Perspect. 2010;118:1027–1032. doi: 10.1289/ehp.0901376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Genco MC, Megrelis L, Ruderman JV. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17732–17737. doi: 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotfelter ED, Curren LJ, Murphy CE. Mate choice and spawning success in the fighting fish Betta splendens: The importance of body size, display behavior and nest size. Ethology. 2006;112:1170–1178. [Google Scholar]
- Coburn CG, Gillard ER, Curras-Collazo MC. Dietary exposure to aroclor 1254 alters central and peripheral vasopressin release in response to dehydration in the rat. Toxicol. Sci. 2005;84:149–156. doi: 10.1093/toxsci/kfi046. [DOI] [PubMed] [Google Scholar]
- Colbert NK, Pelletier NC, Cote JM, Concannon JB, Jurdak NA, Minott SB, Markowski VP. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ. Health Perspect. 2005;113:700–707. doi: 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5α-reductases in the offspring. Toxicol. Appl. Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Klebanoff MA, Promislow J, Brock JW, Longnecker MP. Polychlorinated biphenyls and menstrual cycle characteristics. Epidemiology. 2005;16:191–200. doi: 10.1097/01.ede.0000152913.12393.86. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 2010 doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette Jr LJ. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC. Epigenetic synthesis: a need for a new paradigm for evolution in a contaminated world. F1000 biology reports. 2012;4:18. doi: 10.3410/B4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM. Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Anal. 2004;24:1665–1671. doi: 10.1111/j.0272-4332.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Johnson A, McKnight L, Horinek M, Asbrock C, Burt S, Jolous-Jamshidi B, Meserve LA. Effects of polychlorinated biphenyls on maternal odor conditioning in rat pups. Physiol. Behav. 2007;91:658–666. doi: 10.1016/j.physbeh.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleon S, Halitschke R, Hames RS, Kessler A, Devoogd TJ, Dhondt AA. The effect of polychlorinated biphenyls on the song of two passerine species. PloS One. 2013;8:e73471. doi: 10.1371/journal.pone.0073471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton TE, Howell WM, Allison JJ, McCollum J, Marks B. Masculinization of female mosquitofish by exposure to plant sterols and Mycobacterium smegmatis. Bull. Environ. Contam. Toxicol. 1985;35:627–632. doi: 10.1007/BF01636565. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S.s, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ. Health Perspect. 110 Suppl. 2002;3:403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC. Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland. Environ Health Perspect. 1999;107:823–828. doi: 10.1289/ehp.99107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer. 2010;1:146–155. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue DA, Dougherty EJ, Meserve LA. Influence of a combination of two tetrachlorobiphenyl congeners (PCB 47; PCB 77) on thyroid status, choline acetyltransferase (ChAT) activity, and short- and long-term memory in 30-dayold Sprague-Dawley rats. Toxicology. 2004;203:99–107. doi: 10.1016/j.tox.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Durhan EJ, Lambright C, Wilson V, Butterworth BC, Kuehl DW, Orlando EF, Guillette LJ, Jr., Gray LE, Jr., Ankley GT. Evalutation of androstendione as an androgenic component of river water downstream of pulp and paper mill effluent. Environ. Toxicol. Chem. 2002;21:1973–1976. [PubMed] [Google Scholar]
- Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav. Neurosci. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorriaga Y, Marino DJ, Carriquiriborde P, Ronco AE. Human pharmaceuticals in wastewaters from urbanized areas of Argentina. Bull. Environ. Contam. Toxicol. 2013;90:397–400. doi: 10.1007/s00128-012-0919-x. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, Jr DE, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Fielding JR, Rogers TD, Meyer AE, Miller MM, Nelms JL, Mittleman G, Blaha CD, Sable HJ. Stimulation-evoked dopamine release in the nucleus accumbens following cocaine administration in rats perinatally exposed to polychlorinated biphenyls. Toxicol. Sci. 2013;136(1):144–53. doi: 10.1093/toxsci/kft171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW, Campbell J, Muralidhara S, Bruckner JV, Ferguson D, Mumtaz M, Harmon B, Hedge JM, Crofton KM, Kim H, Almekinder TL. Effect of PCB 126 on hepatic metabolism of thyroxine and perturbations in the hypothalamic-pituitary-thyroid axis in the rat. Toxicol. Sci. 2006;90:87–95. doi: 10.1093/toxsci/kfj069. [DOI] [PubMed] [Google Scholar]
- Flynn KM, Delclos KB, Newbold RR, Ferguson SA. Behavioral responses of rats exposed to long-term dietary vinclozolin. J. Agric. Food Chem. 2001;49:1658–1665. doi: 10.1021/jf0008893. [DOI] [PubMed] [Google Scholar]
- Frye C, Bo E, Calamandrei G, Calzà L, Dessì-Fulgheri F, Fernández M, Fusani L, Kah O, Kajta M, Le Page Y, Patisaul HB, Venerosi A, Wojtowicz AK, Panzica GC. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29:475–485. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles, Microtus pennsylvanicus, and deer mice, Peromyscus maniculatus. J. Exp. Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Gross TS, Sheehy KM. Decreased glutathione S-transferase expression and activity and altered sex steroids in Lake Apopka brown bullheads (Ameiurus nebulosus). Aquat. Toxicol. 2001;55:223–237. doi: 10.1016/s0166-445x(01)00158-8. [DOI] [PubMed] [Google Scholar]
- Galus M, Jeyaranjaan J, Smith E, Li H, Metcalfe C, Wilson JY. Chronic effects of exposure to a pharmaceutical mixture and municipal wastewater in zebrafish. Aquat. Toxicol. 2013;132-133:212–222. doi: 10.1016/j.aquatox.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Gaworecki KM, Klaine SJ. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008;88:207–213. doi: 10.1016/j.aquatox.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Gillard ER, Coburn CG, de Leon A, Snissarenko EP, Bauce LG, Pittman QJ, Hou B, Curras-Collazo MC. Vasopressin autoreceptors and nitric oxide-dependent glutamate release are required for somatodendritic vasopressin release from rat magnocellular neuroendocrine cells responding to osmotic stimuli. Endocrinology. 2007;148:479–489. doi: 10.1210/en.2006-0995. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Parmigiani S, Vom Saal FS, Palanza P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm. Behav. 2013;63:598–605. doi: 10.1016/j.yhbeh.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Go O, Freeman RH, Reams GP, Spear R, Liu K, Villarreal D. Obesity hypertension: Pathophysiological role of leptin in neuroendocrine dysregulation. Am. J. Med. Sci. 2013 doi: 10.1097/MAJ.0b013e31827ad5cf. [DOI] [PubMed] [Google Scholar]
- Goncalves CR, Cunha RW, Barros DM, Martinez PE. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environ. Toxicol. Pharmacol. 2010;30:195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Gore AC. Organochlorine pesticides directly regulate gonadotropin-releasing hormone gene expression and biosynthesis in the GT1-7 hypothalamic cell line. Mol. Cell. Endocrinol. 2002;192:157–170. doi: 10.1016/s0303-7207(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Gore AC. Neuroendocrine systems as targets for environmental endocrine-disrupting chemicals. Fertil. Steril. 2008;89(Suppl. 2):e101–102. doi: 10.1016/j.fertnstert.2007.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Balthazart J, Bikle D, Carpenter DO, Crews D, Czernichow P, Diamanti-Kandarakis E, Dores RM, Grattan D, Hof PR, Hollenberg AN, Lange C, Lee AV, Levine JE, Millar RP, Nelson RJ, Porta M, Poth M, Power DM, Prins GS, Ridgway EC, Rissman EF, Romijn JA, Sawchenko PE, Sly PD, Soder O, Taylor HS, Tena-Sempere M, Vaudry H, Wallen K, Wang Z, Wartofsky L, Watson CS. Policy decisions on endocrine disruptors should be based on science across disciplines: a response to Dietrich et al. Eur. J. Endocrinol. 2013;169:E1–4. doi: 10.1530/EJE-13-0763. [DOI] [PubMed] [Google Scholar]
- Gore AC, Patisaul HB. Neuroendocrine disruption: historical roots, current progress, questions for the future. Front. Neuroendocrinol. 2010;31:395–399. doi: 10.1016/j.yfrne.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Goodarzi M, Yang X, Bian Y, Sun C, Jiang X. Predictive insight into the relationship between AhR binding property and toxicity of polybrominated diphenyl ethers by PLS-derived QSAR. Toxicol. Lett. 2012a;208:269–274. doi: 10.1016/j.toxlet.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Gu SG, Pak J, Guang S, Maniar JM, Kennedy S, Fire A. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 2012b;44:157–164. doi: 10.1038/ng.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldin K, Axelsson J, Brunstrom B. Effects of endocrine modulators on sexual differentiation and reproductive function in male Japanese quail. Brain Res. Bull. 2005;65:211–218. doi: 10.1016/j.brainresbull.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Ogiue-Ikeda M, Tanabe N, Kimoto T, Hojo Y, Yamazaki T, Kawato S. Bisphenol A significantly modulates long-term depression in the hippocampus as observed by multi-electrode system. Neuroendocrinol. Lett. 2013;34:129–134. [PubMed] [Google Scholar]
- Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp. Neurol. 2007;204:619–630. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JA, Kennedy SW. Differential expression, induction, and stability of CYP1A4 and CYP1A5 mRNA in chicken and herring gull embryo hepatocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:617–624. doi: 10.1016/j.cbpc.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Hedgespeth ML, Nilsson PA, Berglund O. Ecological implications of altered fish foraging after exposure to an antidepressant pharmaceutical. Aquat Toxicol. 20132013 doi: 10.1016/j.aquatox.2013.12.011. pii: S0166-445X(13)00352-4. doi: 10.1016/j.aquatox.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Heinz GH. Behavior of mallard ducklings from parents fed 3 ppm DDE. Bull. Environ. Contam. Toxicol. 1976;16:640–651. doi: 10.1007/BF01685567. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int. J. Hyg. Environ. Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hewitt LM, Parrott JL, McMaster ME. A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: sources and characteristics of bioactive substances. J. Toxicol. Environ. Health B Crit. Rev. 2006;9:341–356. doi: 10.1080/15287390500195976. [DOI] [PubMed] [Google Scholar]
- Hinck JE, Blazer VS, Denslow ND, Echols KR, Gale RW, Wieser C, May TW, Ellersieck M, Coyle JJ, Tillitt DE. Chemical contaminants, health indicators, and reproductive biomarker responses in fish from rivers in the Southeastern United States. Sci. Total Environ. 2008;390:538–557. doi: 10.1016/j.scitotenv.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Kloas W. An environmentally relevant endocrine-disrupting antiandrogen, vinclozolin, affects calling behavior of male Xenopus laevis. Horm. Behav. 2010;58:653–659. doi: 10.1016/j.yhbeh.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Hong SB, Hong YC, Kim JW, Park EJ, Shin MS, Kim BN, Yoo HJ, Cho IH, Bhang SY, Cho SC. Bisphenol A in relation to behavior and learning of school-age children. J. Child Psychol. Psychiatry. 2013;54:890–899. doi: 10.1111/jcpp.12050. [DOI] [PubMed] [Google Scholar]
- Honma T, Suda M, Miyagawa M, Wang RS, Kobayashi K, Sekiguchi S. Alteration of brain neurotransmitters in female rat offspring induced by prenatal administration of 16 and 64 mg/kg of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153). Ind. Health. 2009;47:11–21. doi: 10.2486/indhealth.47.11. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE., Jr. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol. Behav. 2003;79:151–156. doi: 10.1016/s0031-9384(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenburgh JG, Gray LE., Jr. Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ. Health Perspect. 110 Suppl. 2002;3:435–439. doi: 10.1289/ehp.02110s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 2009;81(5):807–13. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglefield JR, Shafer TJ. Perturbation by the PCB mixture aroclor 1254 of GABA(A) receptor-mediated calcium and chloride responses during maturation in vitro of rat neocortical cells. Toxicol. Appl. Pharmacol. 2000;164:184–195. doi: 10.1006/taap.2000.8898. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y, Sayato-Suzuki J, Oka S, Niki E, Morita M. Dicyclohexylphthalate causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. J. Neurochem. 2004;91:69–76. doi: 10.1111/j.1471-4159.2004.02696.x. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Jr., Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Williams SA, Roberts RM, Geary DC, Rosenfeld CS. Spatial navigation strategies in Peromyscus: a comparative study. Anim. Behav. 2012;84:1141–1149. doi: 10.1016/j.anbehav.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Williams SA, Vandas GM, Ellersieck MR, Liao C, Kannan K, Roberts RM, Geary DC, Rosenfeld CS. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 2013;63:180–189. doi: 10.1016/j.yhbeh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Wilson EM, Angus RA, Howell WM, Kirk M. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol. Sci. 2003;73:53–59. doi: 10.1093/toxsci/kfg042. [DOI] [PubMed] [Google Scholar]
- Kamata R, Shiraishi F, Nishikawa J.-i., Yonemoto J, Shiraishi H. Screening and detection of the in vitro agonistic activity of xenobiotics on the retinoic acid receptor. Toxicol. In Vitro. 2008;22:1050–1061. doi: 10.1016/j.tiv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Keeley LL, Chung JS, Hayes TK. Diuretic and antifeedant actions by manduca-sexta diuretic hormone in Lepidopteran larvae. Experientia. 1992;48:1145–1148. [Google Scholar]
- Kelce WR, Gray J, L E, Wilson EM. Antiandrogens as environmental endocrine disruptors. Reprod. Fertil. Dev. 1998;10:105–111. doi: 10.1071/r98051. [DOI] [PubMed] [Google Scholar]
- Khan IA, Thomas P. PCB congener-specific disruption of reproductive neuroendocrine function in Atlantic croaker. Mar. Environ. Res. 2006;62(Suppl.):S25–28. doi: 10.1016/j.marenvres.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, Kim JW, Yang YH, Kim HW, Bhang SY, Hong YC. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol. Psychiatry. 2009;66:958–963. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Koch BT, Garvey JE, You J, Lydy MJ. Elevated organochlorines in the brain–hypothalamic–pituitary complex of intersexual shovelnose sturgeon. Environ. Toxicol. Chem. 2006;25:1689–1697. doi: 10.1897/05-474r.1. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Curras-Collazo MC. Neuroendocrine actions of organohalogens: thyroid hormones, arginine vasopressin, and neuroplasticity. Front. Neuroendocrinol. 2010;31:479–496. doi: 10.1016/j.yfrne.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Tilson HA. Structure-activity relationships of potentially neurotoxic PCB congeners in the rat. Neurotoxicology. 1997;18:425–441. [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kostich MS, Batt AL, Lazorchak JM. Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ. Pollut. 1987. 2013;184C:354–359. doi: 10.1016/j.envpol.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Kristensen T, Baatrup E, Bayley M. p,p2-DDE fails to reduce the competitive reproductive fitness in Nigerian male guppies. Ecotoxicol. Environ. Saf. 2006;63:148–157. doi: 10.1016/j.ecoenv.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Krotzer MJ. The effects of induced masculinization on reproductive and aggressive behaviors of the female mosquitofish, Gambusia affinis affinis. Environ. Biol. Fishes. 1990;29:127–134. [Google Scholar]
- Kulkarni SK, Sharma AC. Elevated plus-maze: a novel psychobehavioral tool to measure anxiety in rodents. Methods Find. Exp. Clin. Pharmacol. 1991;13:573–577. [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U.S.A. 2013 doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. Persistent organochlorinated pollutants (PCB, DDE, HCB, dioxins, furans) and the thyroid--review 2008. Endocr. Regul. 2008;42(2-3):79–104. [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latonnelle K, Fostier A, Le Menn F, Bennetau-Pelissero C. Binding affinities of hepatic nuclear estrogen receptors for phytoestrogens in rainbow trout (Oncorhynchus mykiss) and Siberian sturgeon (Acipenser baeri). Gen. Comp. Endocrinol. 2002;129:69–79. doi: 10.1016/s0016-6480(02)00512-9. [DOI] [PubMed] [Google Scholar]
- Le Page Y, Vosges M, Servili A, Brion F, Kah O. Neuroendocrine effects of endocrine disruptors in teleost fish. J. Toxicol. Environ. Health B Crit. Rev. 2011;14:370–386. doi: 10.1080/10937404.2011.578558. [DOI] [PubMed] [Google Scholar]
- Lee SG, Kim JY, Chung JY, Kim YJ, Park JE, Oh S, Yoon YD, Yoo KS, Yoo YH, Kim JM. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect. 2013;121(6):663–9. doi: 10.1289/ehp.1205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Olea M, Sánchez-Islas E, Mucio-Ramírez S, Miller-Pérez C, Garduño-Gutiérrez R. Contaminantes ambientales neurotóxicos cercanos a nuestra vida diaria. Salud. Mental. 2012;35:395–403. [Google Scholar]
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 2008a;105:14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008b;149:988–994. doi: 10.1210/en.2007-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Jiang L, Chen L, Chen HS, Li X. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environ. Toxicol. Pharmacol. 2013;36:392–402. doi: 10.1016/j.etap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Heikkinen P, Andersson PL, Viluksela M. Sexually dimorphic behavior after developmental exposure to characterize endocrine-mediated effects of different non-dioxin-like PCBs in rats. Toxicology. 2013;311:52–60. doi: 10.1016/j.tox.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Lister A, Regan C, Van Zwol J, Van Der Kraak G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: a mechanistic evaluation. Aquat. Toxicol. 2009;95:320–329. doi: 10.1016/j.aquatox.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Loutchanwoot P, Wuttke W, Jarry H. Effects of a 5-day treatment with vinclozolin on the hypothalamo-pituitary-gonadal axis in male rats. Toxicology. 2008;243:105–115. doi: 10.1016/j.tox.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Luo G, Wei R, Niu R, Wang C, Wang J. Pubertal exposure to Bisphenol A increases anxiety-like behavior and decreases acetylcholinesterase activity of hippocampus in adult male mice. Food Chem. Toxicol. 2013;60:177–180. doi: 10.1016/j.fct.2013.07.037. [DOI] [PubMed] [Google Scholar]
- MacLatchy DL, Van Der Kraak GJ. The phytoestrogen beta-sitosterol alters the reproductive endocrine status of goldfish. Toxicol. Appl. Pharmacol. 1995;134:305–312. doi: 10.1006/taap.1995.1196. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ. Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdouli S, Daghrir R, Brar SK, Drogui P, Tyagi RD. Di 2-ethylhexylphtalate in the aquatic and terrestrial environment: a critical review. J Environ. Manag. 2013;127:36–49. doi: 10.1016/j.jenvman.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PloS One. 2013;8(1):e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Morch Andersen J, Fonnum F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol. Appl. Pharmacol. 1999;161:274–282. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- Markman S, Leitner S, Catchpole C, Barnsley S, Muller CT, Pascoe D, Buchanan KL. Pollutants increase song complexity and the volume of the brain area HVC in a songbird. PloS One. 2008;3(2):e1674. doi: 10.1371/journal.pone.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Kroll KJ, Barber DS, Denslow ND. Sexually dimorphic transcriptomic responses in the teleostean hypothalamus: A case study with the organochlorine pesticide dieldrin. Neurotoxicology. 2013;34:105–117. doi: 10.1016/j.neuro.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Bellinger DC. Dental composite restorations and neuropsychological development in children: treatment level analysis from a randomized clinical trial. Neurotoxicology. 2012;33:1291–1297. doi: 10.1016/j.neuro.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012a;39:273–279. doi: 10.1016/j.pnpbp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- McGary S, Henry PF, Ottinger MA. Impact of vinclozolin on reproductive behavior and endocrinology in Japanese quail (Coturnix coturnix japonica). Environ. Toxicol. Chem. 2001:2487–2493. doi: 10.1897/1551-5028(2001)020<2487:iovorb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McKinney JD, Waller CL. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Health Perspect. 1994;102:290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster ME, Hewitt LM, Parrott JL. A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: field studies and mechanistic research. J. Toxicol. Environ. Health B Crit. Rev. 2006;9:319–339. doi: 10.1080/15287390500195869. [DOI] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Lado WE, Zamora JM, Duarte-Guterman P, Langlois VS, Metcalfe CD, Chang JP, Moon TW, Trudeau VL. Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus. Aquat Toxicol. 2010a;100:354–64. doi: 10.1016/j.aquatox.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Martyniuk CJ, Crump K, Xiong H, Zhao E, Popesku J, Anisman H, Cossins AR, Xia X, Trudeau VL. Effects of fluoxetine on the reproductive axis of female goldfish (Carassius auratus). Physiol. Genomics. 2008;35:273–282. doi: 10.1152/physiolgenomics.90263.2008. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Sassine J, Trudeau VL, Moon TW. Waterborne fluoxetine disrupts feeding and energy metabolism in the goldfish Carassius auratus. Aquat. Toxicol. 2010b;100:128–137. doi: 10.1016/j.aquatox.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Stroud P, Zamora JM, Moon TW, Trudeau VL. Pharmaceuticals as neuroendocrine disruptors: lessons learned from fish on Prozac. J. Toxicol. Environ. Health B Crit. Rev. 2011;14:387–412. doi: 10.1080/10937404.2011.578559. [DOI] [PubMed] [Google Scholar]
- Metcalfe CD, Chu S, Judt C, Li H, Oakes KD, Servos MR, Andrews DM. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010;29:79–89. doi: 10.1002/etc.27. [DOI] [PubMed] [Google Scholar]
- Milestone CB, Orrego R, Scott PD, Waye A, Kohli J, O'Connor BI, Smith B, Engelhardt H, Servos MR, MacLatchy DL, Smith DS, Trudeau VL, Arnason JT, Kovacs T, Heid Furley T, Slade AH, Holdway DA, Hewitt LM. Evaluating the potential of effluents and wood feedstocks from pulp and paper mills in Brazil, Canada, and New Zealand to affect fish reproduction: chemical profiling and in vitro assessments. Environ. Sci. Technol. 2012;46:1849–1858. doi: 10.1021/es203382c. [DOI] [PubMed] [Google Scholar]
- Miller GW, Kirby ML, Levey AI, Bloomquist JR. Hepatachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999;20:631–637. [PubMed] [Google Scholar]
- Montejo AL, Llorca G, Izquierdo JA, Rico-Villademoros F. Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry. 62 Suppl. 2001;3:10–21. [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology. 2013;307:74–88. doi: 10.1016/j.tox.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Muir DC, de Wit CA. Trends of legacy and new persistent organic pollutants in the circumpolar arctic: overview, conclusions, and recommendations. The Sci. Total Environ. 2010;408:3044–3051. doi: 10.1016/j.scitotenv.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Mura E, Barale C, Quinn MJ, Panzica G, Ottinger MA, Viglietti-Panzica C. Organizational effects of DDE on brain vasotocin system in male Japanese quail. Neurotoxicology. 2009;30:479–484. doi: 10.1016/j.neuro.2009.01.012. [DOI] [PubMed] [Google Scholar]
- N'Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud'homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, Rouiller-Fabre V, Habert R. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS One. 2012;7(12):e51579. doi: 10.1371/journal.pone.0051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci. Lett. 2007;420:100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J. Neurosci. Res. 2006;84:1197–1205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- Ness DK, Schantz SL, Moshtaghian J, Hansen LG. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol. Lett. 1993;68:311–323. doi: 10.1016/0378-4274(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nunez J. Morris Water Maze Experiment. J. Vis. Exp. 2008;(19):897. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes KD, Coors A, Escher BI, Fenner K, Garric J, Gust M, Knacker T, Kuster A, Kussatz C, Metcalfe CD, Monteiro S, Moon TW, Mennigen JA, Parrott J, Pery AR, Ramil M, Roennefahrt I, Tarazona JV, Sanchez-Arguello P, Ternes TA, Trudeau VL, Boucard T, Van Der Kraak GJ, Servos MR. Environmental risk assessment for the serotonin re-uptake inhibitor fluoxetine: Case study using the European risk assessment framework. Integr. Environ. Assess. Manag. 2010;6(Suppl):524–539. doi: 10.1002/ieam.77. [DOI] [PubMed] [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ, Tyler CR. A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2047–2062. doi: 10.1098/rstb.2008.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: A review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1400–1408. doi: 10.1016/j.pnpbp.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Orrego R, Guchardi J, Krause R, Holdway D. Estrogenic and anti-estrogenic effects of wood extractives present in pulp and paper mill effluents on rainbow trout. Aquat. Toxicol. 2010;99:160–167. doi: 10.1016/j.aquatox.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Carro T, Bohannon M, Baltos L, Marcell AM, McKernan M, Dean KM, Lavoie E, Abdelnabi M. Assessing effects of environmental chemicals on neuroendocrine systems: potential mechanisms and functional outcomes. Gen. Comp. Endocrinol. 2013;190:194–202. doi: 10.1016/j.ygcen.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Panzica G, Mura E, Pessatti M, Viglietti-Panzica C. Early embryonic administration of xenoestrogens alters vasotocin system and male sexual behavior of the Japanese quail. Domest. Anim. Endocrinol. 2005;29:436–445. doi: 10.1016/j.domaniend.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Bo E, Martini MA, Miceli D, Mura E, Viglietti-Panzica C, Gotti S. Neuropeptides and enzymes are targets for the action of endocrine disrupting chemicals in the vertebrate brain. J. Toxicol. Environ. Health B Crit. Rev. 2011;14:449–472. doi: 10.1080/10937404.2011.578562. [DOI] [PubMed] [Google Scholar]
- Parrott JL, McMaster ME, Hewitt LM. A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: development and application of fish bioassays. J. Toxicol. Environ. Health B Crit. Rev. 2006;9:297–317. doi: 10.1080/15287390500195752. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol. Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza Y, Pandolfi M, Lo Nostro F. Effect of the organochlorine pesticide endosulfan on GnRH and gonadotrope cell populations in fish larvae. Arch. Environ. Contam. Toxicol. 2011;61:300–310. doi: 10.1007/s00244-010-9621-3. [DOI] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167:741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Popesku JT, Martyniuk CJ, Trudeau VL. Meta-type analysis of dopaminergic effects on gene expression in the neuroendocrine brain of female goldfish. Front. Endocrinol. 2012;3:130. doi: 10.3389/fendo.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popesku JT, Tan EY, Martel PH, Kovacs TG, Rowan-Carroll A, Williams A, Yauk C, Trudeau VL. Gene expression profiling of the fathead minnow (Pimephales promelas) neuroendocrine brain in response to pulp and paper mill effluents. Aquat. Toxicol. 2010;99:379–388. doi: 10.1016/j.aquatox.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Qin F, Wang L, Wang X, Liu S, Xu P, Wang H, Wu T, Zhang Y, Zheng Y, Li M, Zhang X, Yuan C, Hu G, Wang Z. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2013;157:192–202. doi: 10.1016/j.cbpc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, Summitt CL, Ottinger MA. Consequences of in ovo exposure to p,p2-DDE on reproductive development and function in Japanese quail. Horm. Behav. 2008;53:249–253. doi: 10.1016/j.yhbeh.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65:1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- Radovick S. Estrogenic regulation of the GnRH neuron. Front. Endocrinol. 2012;3:52. doi: 10.3389/fendo.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid CS, Carter LG, Hennig B, Pearson KJ. Perinatal polychlorinated biphenyl 126 exposure alters offspring body composition. J. Pediatr. Biochem. 2013;3:47–53. doi: 10.3233/JPB-120072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner BA. History of wildlife toxicology. Ecotoxicology. 2009;18:773–783. doi: 10.1007/s10646-009-0354-x. [DOI] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013;42C:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Roelens SA, Beck V, Maervoet J, Aerts G, Reyns GE, Schepens P, Darras VM. The dioxin-like PCB 77 but not the ortho-substituted PCB 153 interferes with chicken embryo thyroid hormone homeostasis and delays hatching. Gen. Comp. Endocrinol. 2005;143:1–9. doi: 10.1016/j.ygcen.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Chen A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet. 2005;366:763–773. doi: 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291:83–92. doi: 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salice CJ, Rowe CL, Eisenreich KM. Integrative demographic modeling reveals population level impacts of PCB toxicity to juvenile snapping turtles. Environ. Pollut. 2013;184C:154–160. doi: 10.1016/j.envpol.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Satre D, Reichert M, Corbitt C. Effects of vinclozolin, an anti-androgen, on affiliative behavior in the Dark-eyed Junco, Junco hyemalis. Environ. Res. 2009;109:400–404. doi: 10.1016/j.envres.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gasior DM, Polverejan E, McCaffrey RJ, Sweeney AM, Humphrey HE, Gardiner JC. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ. Health Perspect. 2001;109:605–611. doi: 10.1289/ehp.01109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Wong PW, Pessah IN. Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology. 1997;18:457–467. [PubMed] [Google Scholar]
- Selvakumar K, Bavithra S, Ganesh L, Krishnamoorthy G, Venkataraman P, Arunakaran J. Polychlorinated biphenyls induced oxidative stress mediated neurodegeneration in hippocampus and behavioral changes of adult rats: anxiolytic-like effects of quercetin. Toxicol. Lett. 2013;222:45–54. doi: 10.1016/j.toxlet.2013.06.237. [DOI] [PubMed] [Google Scholar]
- Shah A, Coburn CG, Watson-Siriboe A, Whitley R, Shahidzadeh A, Gillard ER, Nichol R, Leon-Olea M, Gaertner M, Kodavanti PR, Curras-Collazo MC. Altered cardiovascular reactivity and osmoregulation during hyperosmotic stress in adult rats developmentally exposed to polybrominated diphenyl ethers (PBDEs). Toxicol. Appl. Pharmacol. 2011;256:103–113. doi: 10.1016/j.taap.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen is more than just a “sex hormone”: novel sites for estrogen action in the hippocampus and cerebral cortex. Front. Neuroendocrinol. 2000;21:95–101. doi: 10.1006/frne.1999.0190. [DOI] [PubMed] [Google Scholar]
- Silva de Assis HC, Simmons DB, Zamora JM, Lado WE, Al-Ansari AM, Sherry JP, Blais JM, Metcalfe CD, Trudeau VL. Estrogen-like effects in male goldfish co-exposed to fluoxetine and 17α-ethinylestradiol. Environ. Sci. Technol. 2013;47:5372–5382. doi: 10.1021/es3044888. [DOI] [PubMed] [Google Scholar]
- Simmons SL, Cummings JA, Clemens LG, Nunez AA. Exposure to PCB 77 affects the maternal behavior of rats. Physiol. Behav. 2005;84:81–86. doi: 10.1016/j.physbeh.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects. Reprod. Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Smith CA, Macdonald A, Holahan MR. Acute postnatal exposure to di(2-ethylhexyl) phthalate adversely impacts hippocampal development in the male rat. Neuroscience. 2011;193:100–108. doi: 10.1016/j.neuroscience.2011.06.082. [DOI] [PubMed] [Google Scholar]
- Söffker M, Tyler CR. Endocrine disrupting chemicals and sexual behaviors in fish – a critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012;42:653–668. doi: 10.3109/10408444.2012.692114. [DOI] [PubMed] [Google Scholar]
- Straub RH, Bijlsma JW, Masi A, Cutolo M. Role of neuroendocrine and neuroimmune mechanisms in chronic inflammatory rheumatic diseases-The 10-year update. Semin. Arthritis Rheum. 2013;43:392–404. doi: 10.1016/j.semarthrit.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Subedi B, Lee S, Moon HB, Kannan K. Psychoactive pharmaceuticals in sludge and their emission from wastewater treatment facilities in Korea. Environ. Sci. Technol. 2013;47:13321–13329. doi: 10.1021/es404129r. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, Sparks A, Weiss B. Prenatal phthalate exposure and reduced masculine play in boys. Int. J. Androl. 2010;33:259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Ricceri L, Venerosi A, Maranghi F, Mantovani A, Calamandrei G. Long-term effects on hypothalamic neuropeptides after developmental exposure to chlorpyrifos in mice. Environ. Health Perspect. 2009;117:112–116. doi: 10.1289/ehp.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tando S, Itoh K, Yaoi T, Ikeda J, Fujiwara Y, Fushiki S. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 2007;29:352–356. doi: 10.1016/j.braindev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153:42–55. doi: 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi WM, Inamoto T, Yokoyama T, Kitagawa H, Hoshi N. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol. Lett. 2009;189:40–47. doi: 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Joshi PP, Klaper RD. Gene-class analysis of expression patterns induced by psychoactive pharmaceutical exposure in fathead minnow (Pimephales promelas) indicates induction of neuronal systems. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2012;155:109–120. doi: 10.1016/j.cbpc.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilak KS, Veeraiah K, Rao DK. Toxicity and Bioaccumulation of Chlorpyrifos in Indian Carp Catla catla(Hamilton), Labeo rohita (Hamilton), and Cirrhinus mrigala (Hamilton). Bull. Environ. Contam. Toxicol. 2004;73:933–941. doi: 10.1007/s00128-004-0516-8. [DOI] [PubMed] [Google Scholar]
- Toft G, Baatrup E, Guillette LJ. Altered social behavior and sexual characteristics in mosquitofish (Gambusia holbrooki) living downstream of a paper mill. Aquat. Toxicol. 2004;70:213–222. doi: 10.1016/j.aquatox.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Trudeau VL, Kah O, Bourguignon JP. Neuroendocrine disruption: the emerging concept. J. Toxicol. Environ. Health B Crit. Rev. 2011;14:267–269. doi: 10.1080/10937404.2011.578272. [DOI] [PubMed] [Google Scholar]
- Van der Kraak GJ, Munkittrick KR, McMaster ME, Portt CB, Chang JP. Exposure to bleached kraft pulp mill effluent disrupts the pituitary-gonadal axis of white sucker at multiple sites. Toxicol. Appl. Pharmacol. 1992;115:224–233. doi: 10.1016/0041-008x(92)90327-o. [DOI] [PubMed] [Google Scholar]
- Vandegehuchte MB, Janssen CR. Epigenetics in an ecotoxicological context. Mutat. Res. 2013 doi: 10.1016/j.mrgentox.2013.08.008. pii:S1383-5718(13)00232-5.doi: 0.1016/j.mrgentox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh E, Colombo A, Benfenati E, Hakansson H, Berglund M, Bodis J, Garai J. Individual breast milk consumption and exposure to PCBs and PCDD/Fs in Hungarian infants: a time-course analysis of the first three months of lactation. Sci. Total Environ. 2013;449:336–344. doi: 10.1016/j.scitotenv.2013.01.024. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr., Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dong Q, Chen Y, Jiang H, Xiao Q, Wang Y, Li W, Bai C, Huang C, Yang D. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat. Toxicol. 142. 2013;143C:104–113. doi: 10.1016/j.aquatox.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Ward JL, Blum MJ. Exposure to an environmental estrogen breaks down sexual isolation between native and invasive species. Evol. Appls. 2012;5:901–912. doi: 10.1111/j.1752-4571.2012.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye A, Annal M, Tang A, Picard G, Harnois F, Guerrero-Analco JA, Saleem A, Hewitt LM, Milestone CB, MacLatchy DL, Trudeau VL, Arnason JT. Canadian boreal pulp and paper feedstocks contain neuroactive substances that interact in vitro with GABA and dopaminergic systems in the brain. Sci. Total Environ. 468. 2013;469C:315–325. doi: 10.1016/j.scitotenv.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Waye A, Trudeau VL. Neuroendocrine disruption: more than hormones are upset. J. Toxicol. Environ. Health B Crit. Rev. 2011;14:270–291. doi: 10.1080/10937404.2011.578273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse C, Anderson OS, Jones TR, Kim J, Liberman SA, Nahar MS, Rozek LS, Jirtle RL, Dolinoy DC. An expression microarray approach for the identification of metastable epialleles in the mouse genome. Epigenetics. 2011;6 doi: 10.4161/epi.6.9.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintrob N, Cohen D, Klipper-Aurbach Y, Zadik Z, Dickerman Z. Decreased growth during therapy with selective serotonin reuptake inhibitors. Arch. Pediatr. Adolesc. Med. 2002;156:696–701. doi: 10.1001/archpedi.156.7.696. [DOI] [PubMed] [Google Scholar]
- Westerink RH. Modulation of cell viability, oxidative stress, calcium homeostasis, and voltage- and ligand-gated ion channels as common mechanisms of action of (mixtures of) non-dioxin-like polychlorinated biphenyls and polybrominated diphenyl ethers. Environ. Sci. Pollut. Res. Intl. 2013 doi: 10.1007/s11356-013-1759-x. [DOI] [PubMed] [Google Scholar]
- Wibe AE, Billing A, Rosenqvist G, Jenssen BM. Butyl benzyl phthalate affects shoaling behavior and bottom-dwelling behavior in threespine stickleback. Environ. Res. 2002;89:180–187. doi: 10.1006/enrs.2002.4360. [DOI] [PubMed] [Google Scholar]
- Wibe AE, Fjeld E, Rosenqvist G, Jenssen BM. Postexposure effects of DDE and butylbenzylphthalate on feeding behavior in threespine stickleback. Ecotoxicol. Environ. Saf. 2004;57:213–219. doi: 10.1016/S0147-6513(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PLoS One. 2013;8(2):e55698. doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman SB, Wan Y, Chang H, Zhang X, Hecker M, Jones PD, Giesy JP. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011;63:179–188. doi: 10.1016/j.marpolbul.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm. Behav. 2013;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RY, Oxendine SE, Godwin J. Behavioral and neurogenomic transcriptome changes in wild-derived zebrafish with fluoxetine treatment. BMC Genomics. 2013;14:348. doi: 10.1186/1471-2164-14-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten KJ, Smith PN. Canine toys and training devices as sources of exposure to phthalates and bisphenol A: Quantitation of chemicals in leachate and in vitro screening for endocrine activity. Chemosphere. 2013 doi: 10.1016/j.chemosphere.2013.07.075. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu X, Zhang Q, Zhang G, Lu Y, Ruan Q, Dong F, Yang Y. Sex-specific effects of bisphenol-A on memory and synaptic structural modification in hippocampus of adult mice. Horm. Behav. 2013;63:766–775. doi: 10.1016/j.yhbeh.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Xu X, Tian D, Hong X, Chen L, Xie L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology. 2011;61:565–573. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm. Behav. 2010;58:326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Yu C, Tai F, Song Z, Wu R, Zhang X, He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ. Toxicol. Pharmacol. 2011;31:88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang D, Duarte-Guterman P, Langlois VS, Trudeau VL. Temporal expression and steroidal regulation of piRNA pathway genes (mael, piwi, vasa) during Silurana (Xenopus) tropicalis embryogenesis and early larval development. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2010;152:202–206. doi: 10.1016/j.cbpc.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dickhut R, DeMaster D, Pohl K, Lohmann R. Organochlorine pollutants in Western Antarctic peninsula sediments and benthic deposit feeders. Environ. Sci. Technol. 2013;47:5643–5651. doi: 10.1021/es303553h. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Miao M, Ran M, Ding L, Bai L, Wu T, Yuan W, Gao E, Wang J, Li G, Li DK. Serum bisphenol-A concentration and sex hormone levels in men. Fertil. Steril. 2013;100:478–482. doi: 10.1016/j.fertnstert.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Edwards RJ, Boobis AR. Increased expression of histone proteins during estrogen-mediated cell proliferation. Environ. Health Perspect. 2009;117:928–934. doi: 10.1289/ehp.0800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT. Environmental neuroendocrine and thyroid disruption: relevance for reproductive medicine? Fertil Steril. 2008;89:e99–e100. doi: 10.1016/j.fertnstert.2007.12.038. [DOI] [PubMed] [Google Scholar]