Abstract
The introduction of an agricultural pest species into a new environment is a potential threat to agroecosystems of the invaded area. The phytosanitary concern is even greater if the introduced pest’s phenotype expresses traits that will impair the management of that species. The invasive tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), is one such species and the characterization of the insecticide resistance prevailing in the area of origin is important to guide management efforts in new areas of introduction. The spinosad is one the main insecticides currently used in Brazil for control of the tomato borer; Brazil is the likely source of the introduction of the tomato borer into Europe. For this reason, spinosad resistance in Brazilian populations of this species was characterized. Spinosad resistance has been reported in Brazilian field populations of this pest species, and one resistant population that was used in this study was subjected to an additional seven generations of selection for spinosad resistance reaching levels over 180,000-fold. Inheritance studies indicated that spinosad resistance is monogenic, incompletely recessive and autosomal with high heritability (h 2 = 0.71). Spinosad resistance was unstable without selection pressure with a negative rate of change in the resistance level ( = −0.51) indicating an associated adaptive cost. Esterases and cytochrome P450-dependent monooxygenases titration decreased with spinosad selection, indicating that these detoxification enzymes are not the underlying resistance mechanism. Furthermore, the cross-resistance spectrum was restricted to the insecticide spinetoram, another spinosyn, suggesting that altered target site may be the mechanism involved. Therefore, the suspension of spinosyn use against the tomato borer would be a useful component in spinosad resistance management for this species. Spinosad use against this species in introduced areas should be carefully monitored to prevent rapid selection of high levels of resistance and the potential for its spread to new areas.
Introduction
Invasive agricultural pest species are widely recognized as a major threat to agroecosystems and agricultural production [1]–[3]. An additional phytosanitary concern is that the introduced pest’s phenotype could include inheritable traits that could impose management difficulties, such as resistance to insecticides [4]–[7]. The invasive species, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), the tomato borer or tomato leafminer (also tomato pinworm), is one such species. It is of South American origin but was introduced into Europe as early as 2006. This pest has subsequently spread to North Africa and the Middle East and is now threatening the whole of Asia, particularly China and India, the two leading world tomato producers [8]–[11].
From its Peruvian origin, the tomato borer has spread in South America. Its eventual introduction into Brazil, the leading neotropical tomato producer [11], led to drastic changes in tomato production in the country with a dramatic increase in insecticide use in the early 1980’s [10]. Problems with insecticide resistance in the tomato borer were soon detected in the late 1990’s and early 2000’s in Chile, Brazil and Argentina for the insecticides initially used against this species, including organophosphates, pyrethroids, abamectin and cartap [12]–[17]. This resistance led to subsequent registration and large-scale use of new insecticides, particularly in Brazil, including insect growth regulators, indoxacarb, chlorfenapyr, spinosyns, and diamides [10], [18], [19]. Organically produced tomatoes imposed additional restrictions and challenges for tomato borer control, culminating in the use of bioinsecticides, such as the spinosyn spinosad and Bacillus thuringiensis Berliner, aided by alternative supporting control methods [10], [18].
Insecticide registration and use against the tomato borer in South America led to corresponding waves of change in the prevailing patterns of insecticide resistance congruent with the patterns of insecticide use and control failures [13], [19], [20], [21]. The trends, closely followed in Brazil, were intensive use of chitin synthesis inhibitors succeeded the use of abamectin, cartap and pyrethroids against the borer in tomato fields, reaching high levels of resistance (>100-fold), followed by evidences of control failure with this group of insect growth regulators [20], [21]. The bioinsecticide spinosad, a compound of natural origin used in neotropical tomato fields (both organic and conventional fields), has become one of the main compounds used against the tomato borer, but reports of resistance have started to appear both in Brazil and Chile [21], [22], [23].
The appeal of spinosad, a fermentation product of the soil actinomycete Saccharopolyspora spinosa (Mertz and Yao), includes its safety profile and acceptable use in organically produced tomatoes [24]–[26]. However, spinosad resistance counterpoints this appeal and the potential for further use. More seriously, the swift development of insecticide resistance in neotropical field populations of the tomato borer is suggestive of a rapid evolution of spinosad resistance, which remains to be tested [19], [21]. The introduction of the tomato borer from South America into Europe, suggests additional problems for managing this destructive species in newly infested areas, further threatening the current world tomato production [19].
The high genetic homogeneity reported among populations of the tomato borer from South America and Europe give credence to the apparent high level of dispersion of the species and a shared origin [27], [28]. These findings also support the hypothesis of a single invasive event for the tomato borer in Europe [19], [28]. The emerging studies of insecticide resistance in Europe and, particularly, the survey of pyrethroid resistance due to altered target site sensitivity also provides support for the single-introduction event of the tomato borer [29]–[31]. Additionally, the introduced borer phenotype was likely resistant to at least pyrethroid insecticides [19], [31], but may also be capable of rapid development of resistance to other insecticides, including bioinsecticides widely used in traditional and organic tomato production.
In our study, a field population of the tomato borer, already exhibiting spinosad resistance, was subjected to further selection for spinosad resistance to assess the rate of development and level of resistance likely to be achieved with intensive use of this insecticide. The spinosad-selected strain of tomato borer was also subsequently used for the genetic characterization of spinosad resistance and assessment of its stability. This strain was also utilized to evaluate the potential involvement of detoxification by esterases and cytochrome P450-dependent monooxygenases as the underlying resistance mechanism and to assess its cross-resistance spectrum. Based on previous findings in Brazil, a fast response to spinosad selection, reaching high levels of resistance (>100-fold) in few generations (<10) and monogenic resistance was expected. The involvement of cytochrome P450-dependent monooxygenases was previously suggested in Chilean populations of the tomato borer [22]. Evidence of cross-resistance has not yet been detected in the tomato borer, except within pyrethroids and chitin synthesis inhibitors [13], [20], [31]. Therefore, cross-resistance is more likely among spinosyns than between spinosad and insecticides from other groups, especially if altered target site sensitivity is involved.
Materials and Methods
Ethics Statement
This study did not involve any endangered or protected species. Although the insect species studied is a pest species, permits were secured for the collection of the original field populations. The laboratory colonies were initially established from over 200 field-collected individuals.
Insects
Populations of the tomato borer were collected from experimental and commercial tomato fields during 2010/2011 in four regions in Brazil. These insect populations were subjected to an initial screening for spinosad resistance and the populations from Iraquara (state of Bahia, Brazil) and Pelotas (state of Rio Grande do Sul, Brazil) were used for our experiments. The insects were laboratory-maintained in wooden cages with anti-aphid mesh. The cages were separate in larvae cage (45×45×45 cm) and adult cage (30×30×30 cm). The adult cage was used for oviposition only, where leaves of tomato were provided daily as substrate. Adults of T. absoluta were fed with 10% glucose solution (Yoki, 10 Brazil), while the larvae were fed with tomato leaves from Santa Clara tomato cultivar (IC11 5500), cultivated under greenhouse conditions without any insecticide application [20], [21]. The insects were maintained under the controlled conditions of 25±1°C temperature, 65±5% relative humidity and 12∶12 (L:D) photoperiod.
Insecticides
The bioinsecticide spinosad was used in its commercial formulation registered for use in tomato fields against the tomato borer (480 g a.i./L, suspension concentrate, Dow AgroSciences, Franco da Rocha, SP, Brazil) [18]. The insecticides used in the cross-resistance bioassays were (the commercial formulations used are indicated between parentheses): abamectin (18 g a.i./L, emulsifiable concentrate, Syngenta Proteção de Cultivos, São Paulo, SP, Brazil), cartap (500 g a.i./Kg, soluble powder, Iharabras, Paulínia, SP, Brazil), chlorantraniliprole (200 g a.i./L, suspension concentrate, DuPont Brazil, Paulínia, SP, Brazil), chlorfenapyr (240 g a.i./L, suspension concentrate, BASF S.A., São Paulo, SP, Brazil), chlorpyrifos (480 g a.i., emulsifiable concentrate, Dow AgroSciences, Santo Amaro, SP, Brazil), indoxacarb (300 g a.i./Kg, water dispersible granule, DuPont Brazil, Paulínia, SP, Brazil), permethrin (384 g a.i./L, emulsifiable concentrate, FMC Química do Brazil, Campinas, SP, Brazil), spinetoram (250 g a.i./Kg, water dispersible granule, Dow AgroSciences, Franco da Rocha, SP, Brazil), and thiamethoxam (250 g a.i./Kg, water dispersible granule, Syngenta Proteção de Cultivos, São Paulo, SP, Brazil). The synergists piperonyl butoxide (PBO-90%) and S,S,S – Tributylphosphorotrithioate (DEF-98%) were purchased from Sigma-Aldrich, Milwaukee, WI, EUA).
Concentration-mortality bioassays
The concentration-mortality bioassays were performed as described previously and validated for the tomato borer, T. absoluta [21]–[23], [32]. The insecticide solutions were diluted in water containing 0.01% Triton X-100 and a control treatment without insecticide was used to record natural mortality. Insecticide-treated tomato leaves were placed in Petri dishes (9 cm diameter) with ten 2nd instar larvae of the tomato borer and were maintained under controlled environmental conditions (25±1°C temperature, 65±5% relative humidity and 12∶12 (L:D) photoperiod). Larval mortality was assessed after 48 hours of exposure by prodding the insects with a fine hairbrush. Larvae were considered dead if they were unable to move the length of their body.
Selection for spinosad resistance
The tomato borer population from Iraquara, previously identified as resistant to spinosad [23], was subjected to spinosad selection after four generations under laboratory conditions. The original Iraquara population was split into two lines, one maintained without insecticide exposure and the other maintained under spinosad selection for 22 generations. Between 1,500 and 2,000 2nd instar larvae of the tomato borer surviving exposure to increasing discriminatory concentrations of spinosad (selected based on the concentration-mortality bioassays) were used for selection in each generation. After the reduction in egg laying by the spinosad-selected population following the 8th generation of selection, the discriminating concentration of 500 µg a.i./mL was maintained. The average mortality of the spinosad-selected population of Iraquara between the F2 and F7 generations was used to estimate the heritability of spinosad resistance. The Pelotas population of the tomato borer, previously identified as susceptible to spinosad, was maintained in the laboratory without insecticide selection as a susceptible standard population.
Stability of spinosad resistance
The spinosad-resistant population selected for 13 generations was split into two lines, one was maintained under spinosad selection as previously described, and the other was maintained without spinosad selection. Both lines were subjected to spinosad concentration-mortality bioassays during each subsequent generation until the 22nd generation to verify the stability of spinosad resistance without the selection pressure of the bioinsecticide.
Inheritance of spinosad resistance
The inheritance of spinosad resistance was determined through reciprocal crosses between spinosad-selected insects (after 13 generations of selection) and susceptible insects (from Pelotas). Thirty-five crosses were performed for each reciprocal cross with the adults maintained in separate rearing cages for progeny production and concentration-mortality bioassays. The LC50 values (and the LC90 values) were estimated for both parental strains and reciprocal crosses were used to calculate the degree of dominance (D) of spinosad resistance [38]–[40]. The estimated dominance (h) of spinosad resistance was tested through concentration-mortality bioassays with spinosad for the parental (susceptible and (selected) spinosad-resistant) strains and the F1 progeny from the reciprocal crosses [38]. Five spinosad concentrations (0.005, 0.05, 0.5, 5 and 10 µg a.i./mL), in addition to untreated controls (with the application of only water and adjuvant), were used against individuals of the pooled F1 of reciprocal crosses (n = 180), spinosad-resistant (n = 135), and spinosad-susceptible (n = 177) populations.
The monogenic basis of spinosad resistance was tested using backcrosses with the F1 individuals obtained in the reciprocal crosses between spinosad-selected parental insects. The 2nd instar larvae obtained from such backcrosses were subjected to concentration-mortality bioassays with spinosad and to a direct test of inheritance to recognize their mono or polygenic basis.
Pattern of cross-resistance
The 2nd instar larvae of the 15th and 16th generations of spinosad selection were used in concentration-mortality bioassays with the insecticides abamectin, cartap, chlorantraniliprole, chlorfenapyr, chlorpyrifos, indoxacarb, permethrin, spinetoram and thiamethoxam to detect the potential spectrum of spinosad cross resistance. The bioassay methods used were those previously described for the concentration-mortality bioassays.
Synergism of spinosad
The 2nd instar larvae of spinosad susceptible and resistant colonies were used in concentration-mortality bioassays with the insecticides spinosad + PBO and spinosad + DEF to detect whether metabolism is involved in the resistance. The bioassay methods used were those previously described for the concentration-mortality bioassays, but all larvae were topically treated (0.2 µL/larvae) with a concentration of either PBO (1 µg/µL) or DEF (1 µg/µL) before exposure to spinosad.
Protein extraction and enzyme bioassays
Three batches of ten 3rd instar larvae were collected during each generation of the spinosad selection for triplicate determinations of enzyme activity. The crude insect homogenate was prepared by grinding ten larvae in 0.2 mL of sodium phosphate buffer (0.02 M, pH 7.2). The crude homogenate was filtered through glass-wool and centrifuged at 10,000 gmax for 15 min. The pellet was discarded and the supernatant was used for determining protein content and total esterase activity. The supernatant from the 10,000 gmax centrifugation was further centrifuged at 100,000 gmax to obtain the microsomal fraction, which was resuspended in 500 µL sodium phosphate [0.1 M, pH 7.5+ glycerol (20%)] and used for the determination of cytochrome P450 O-demethylase activity.
Protein concentration was determined following the bicinchoninic acid method using bovine serum albumin as standard [33]. Total esterase activity was determined following the methods of van Asperen [34] using α-naphthyl acetate as substrate and a standard curve of α-naphthol to estimate the esterase activity expressed in nmol α-naphthol/min/mg protein. Cytochrome P450 (O-demethylase) activity was determined using p-nitroanisole as substrate generating p-nitrophenol [35] and enzyme activity was expressed in nmol p-nitrophenol/min/mg protein.
Statistical analyses
The concentration-mortality data were subjected to probit analysis using the software Polo-Plus (LeOra Software Co., Petaluma, CA, USA) with correction for the natural mortality (without insecticide exposure) in the bioassays [36]. The level of resistance was estimated using resistance ratio (RR) estimates, which were considered significant when the 95% confidence interval of the RR did not include the value 1.0 [37].
The stability of spinosad resistance was estimated based on the average response of the spinosad-selected population between the 13th and 18th generations, corresponding to the average rate of change in the absence of the insecticide (RC). Spinosad resistance is unstable if the rate of change in the absence of spinosad is negative (RC<0). The number of generations required for a 10-fold reduction in spinosad resistance (G) can also be used to estimate the average rate of change in resistance without insecticide exposure using the formula G = RC−1 [38], [39].
The degree of dominance (D) of spinosad resistance was calculated according to the method of Hartl [38] and Stone [40], using the formula D = 2 (2.L2−L1−L3)/(L1–L3), where L1, L2, and L3 are the log values of the LC50s (or of LC90s) of the spinosad-selected, F1 (between resistant and selected strains), and spinosad-susceptible strains, respectively. The values of D may range from −1 to +1, with the former corresponding to complete recessive inheritance and the later to complete dominance [40].
The estimated dominance (h) was calculated for each concentration: h = (w12–w22)/(w11–w22); where w11, w12, and w22 represent fitness values determined for resistant homozygotes, heterozygotes, and susceptible homozygotes, respectively [38]. The fitness value of resistant homozygotes was considered 1.0, while the fitness values of heterozygotes and susceptible homozygotes were calculated as the ratio between the observed survival rate of the pooled F1 progeny of the reciprocal crosses and the survival rate of the (selected) spinosad-resistant strain. The h-values varies from 0 (completely recessive) to 1 (completely dominant), where 0.5 corresponds to co-dominance, 0<h<0.5 corresponds to incompletely recessive and 0.5<h<1.0 corresponds to incompletely dominant.
The monogenic or polygenic basis of spinosad resistance in the tomato borer was initially estimated by comparing the slopes of the concentration-mortality curves of the F1 reciprocal crosses (between resistant and susceptible lines) and their backcrosses. The results from the backcrosses were compared with the monogenic expectation using the χ2 test [41], [42]. The minimal number of effective genes (nE) was estimated with the formula nE = (L 2 –L1)2/8σs 2 [43], where L2 and L1 are the log of LC50 for the spinosad-selected strain and for the susceptible strain, respectively. The phenotypic variance (σs 2) was estimated with the formula σs 2 = σB1 2+σB2 2–(σF1 2+½.σP1 2+½.σP2 2), where σF1 2, σB1 2, σP1 2 and σP2 2 are the phenotypic variances of the F1 progeny, of the F1-spinosad resistance backcross progeny, of the spinosad resistant strains, and of the spinosad susceptible strain, respectively. The F1-spinosad susceptible backcross was not carried out and, therefore, we considered σB2 2 = 0.
The heritability (h2) of spinosad resistance was estimated using the formula h2 = R/S, where R is the response to selection and S is the differential selection; the 10-fold increase in spinosad resistance was estimated using the formula G = R−1 [38], [39]. The response to selection (R) was calculated by the formula R = (L f–Li)/n, where Lf and Li are the log LC50 of the 2nd and 7th generations and n is the number of generations under selection. The differential selection (S) was estimated with the formula S = i.σF, where i is the selection intensity and σF is the phenotypic standard deviation [39]. The selection intensity (i) was estimated for p, which is the percentage of individuals surviving the selection [40]. The phenotypic standard deviation (σF) was estimated using the formula σF = ½.(βi+βf)−1, where βi is the initial slope and βf is the final slope of the concentration-mortality curve.
The synergism ratio was calculated dividing the LC50 unsynergized by the LC50 synergized for each colony and synergist. The results of enzyme activity were subjected to analyses of variance and Tukey’s HSD test (P<0.05) when appropriate, after ascertaining normality and homoscedasticity assumptions (PROC GLM and PROC UNIVARIATE) [44]. Linear regression analyses between enzyme activity and LC50s at each generation of spinosad selection were performed using the procedure PROC REG in SAS [44].
Results
Selection for spinosad resistance
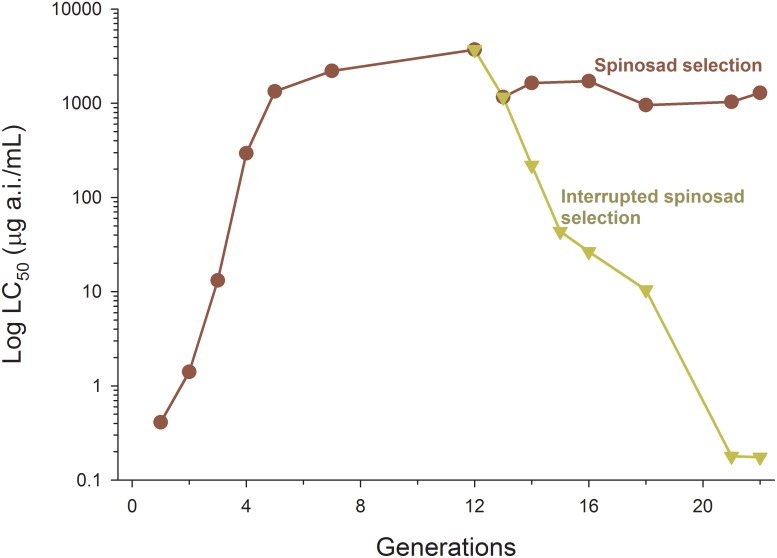
The selection for spinosad resistance at each generation of the initial Iraquara population led to a steady increase of the level of spinosad resistance until the 7th generation of selection, reaching a 5,000-fold increase in the level of resistance (>180,000-fold based on standard susceptible stain) (Table 1, Fig. 1). The selection response (R) was 0.53 and the differential selection (S) was estimated at 0.75, leading to a high heritability of spinosad resistance (h2 = 0.71) and representing a 10-fold increase in the level of resistance at each 1.88 generations (Table 2).
Table 1. Relative toxicity of spinosad to successive generations of spinosad–selected and –unselected strains of the tomato borer Tuta absoluta.
| Population | Generation | Degrees of freedom | Slope ± SE | LC50 (95% CI) (µg a.i./mL) | LC99 (95% CI) (µg a.i./mL) | RR50 (95% CI) | ?2 |
| Unselected | F1 | 5 | 1.85±0.23 | 0.41 (0.30–0.52) | 7.37 (4.36–17.04) | – | 1.51 |
| Spinosad-selected | F2 | 6 | 1.21±0.18 | 1.41 (0.882–2.14) | 117.77 (42.813–703.52) | 3.43 (3.25–3.61) | 1.51 |
| F3 | 6 | 1.06±0.20 | 13.13 (4.08–43.74) | 2048.90 (270.67–0.28×107) | 32.01 (32.44–32.58) | 9.91 | |
| F4 | 5 | 1.14±0.26 | 294.15 (147.85–520.62) | 3166.00 (7419.20–0.13×107) | 717.14 (716.26–718.01) | 2.66 | |
| F5 | 5 | 1.98±0.31 | 1333.67 (718.99–2109.73) | 19760.00 (9020.30–0.11×106) | 3256.03 (3255.62–3258.03) | 5.24 | |
| F7 | 6 | 1.16±0.18 | 2200.41 (1051.24–3944.73) | 22.09×107 (6.0×107–3.9×109) | 5380.88 (5379.52–5382.24) | 6.74 | |
| F12 | 5 | 1.70±0.36 | 3706.34 (2055.99–6152.69) | 86640.00 (32808.00–0.82×106) | 9030.57 (9028.39–9032.74) | 1.98 | |
| F13 | 4 | 1.45±0.27 | 1180.48 (698.73–1756.64) | 47952.00 (18525.00–0.35×106) | 2880.06 (2878.68–2881.44) | 0.79 | |
| F14 | 4 | 1.62±0.20 | 1637.63 (1240.59–2117.19) | 44902.00 (23312.00–0.13×106) | 3993.27 (3993.08–3993.37) | 3.16 | |
| F16 | 4 | 1.47±0.19 | 1717.33 (998.16–2764.20) | 65180.00 (21576.00–0.90×106) | 4191.55 (4192.49–4192.61) | 5.46 | |
| F18 | 5 | 1.08±0.19 | 956.66 (542.62–1537.35) | 0.13×106 (38335.00–0.10×107) | 2338.54 (2338.42–2338.65) | 1.78 | |
| F21 | 4 | 1.86±0.23 | 1034.05 (664.63–1462.17) | 18271.00 (8876.20–76320.00) | 2526.35 (2526.20–2526.49) | 4.35 | |
| F22 | 4 | 2.04±0.23 | 1290.22 (1017.12–1601.71) | 17848.00 (11037.00–37045.00) | 3150.06 (3149.90–3150.21) | 1.56 |
All of the concentration-mortality curves followed the probit model based on the χ2 goodness-of-fit test (P>0.05).
Figure 1. LC50s for spinosad with successive selections for spinosad resistance of the tomato borer Tuta absoluta.
After 12 generations of spinosad selections, the selected line was split into two, one line maintaining selection and one line with interrupted selection.
Table 2. Heritability estimate (h2) of spinosad resistance for a seven-generation spinosad-selected strain of the tomato borer Tuta absoluta.
| Parameters | Generations F1–F7 | |
| Response estimate | F1 CL50 (log) (µg a.i./mL) | 0.41 (−0.39) |
| F7 CL50 (log) (µg a.i./mL) | 2200.00 (3.34) | |
| Response to selection (R) | 0.53 | |
| Estimate of differential selection | Selection-surviving Individuals (p; %) | 31.25 |
| Intensity of selection (i) | 1.12 | |
| Initial slope | 1.85 | |
| Final slope | 1.16 | |
| Phenotypic standard deviation (sF) | 0.66 | |
| Differential selection (S) | 0.75 | |
| Generations for 10-fold increase in resistance (G) | 1.88 | |
| Herdability (h 2) | 0.71 | |
Stability of spinosad resistance
Although the selection for spinosad resistance was rapid, it reached a plateau after the 7th generation of selection with no further increases in the level of resistance with additional selection maintained until the 22nd generation (Fig. 1). After reaching a plateau in the selection for spinosad resistance, the selection was interrupted in a line of selected insects exhibiting high spinosad resistance (>100,000-fold based on standard-susceptible strain) and such high resistance levels quickly eroded with a negative rate of change in subsequent generations without selection (RC = −1.06). Spinosad resistance was, therefore, unstable without spinosad exposure and resulted in a 10-fold reduction in the level of spinosad resistance at each 1.57 generations with a return to susceptibility resembling the original strains after eight generations without selection (Fig. 1).
Inheritance of spinosad resistance
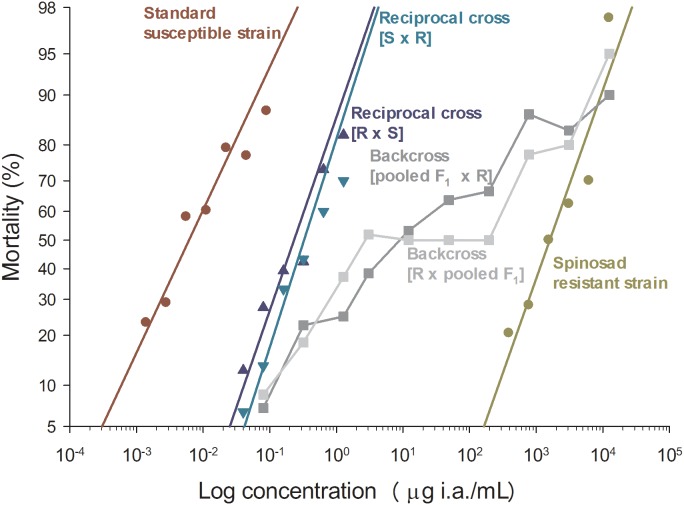
The spinosad-selected strain exhibited very high levels of spinosad resistance (180,000-fold) compared with the standard susceptible strain, both of which exhibited similar variability of responses based on the overlapping standard errors of the slope from the concentration-mortality curves of both strains, indicating their relatively similar homogeneity of responses to spinosad (i.e., they exhibit similar levels of homozygosis). The F1 progeny of the reciprocal crosses between spinosad-susceptible and –resistant strains exhibited intermediate levels of spinosad resistance (ca. 27- and 40-fold) (Table 3; Fig. 2), which were not significantly different based on the Polo-Plus χ2 test of equality of the concentration-mortality curves (χ2 = 3.53; df = 2, P>0.05). Therefore, spinosad resistance is an autosomal trait (i.e., not sex-linked) for the tomato borer.
Table 3. Relative toxicity of spinosad in spinosad-susceptible and (selected) spinosad-resistant strains, the progeny of reciprocal crosses (F1: ♀R×♂S and ♀S×♂R) and of backcrosses [F1 (pooled)×(selected) spinosad-resistant strain] of the tomato borer Tuta absoluta.
| No. insectstested | LC50 (95% CI) | Degree ofdominance (D) | LC90 (95% CI) | Degree ofdominance (D) | |||||
| Strain | (degrees offreedom) | Slope ± SE | (µg a.i./mL) | RR50 (95% CI) | at LC50 (±SE) | (µg a.i./mL) | RR90 (95% CI) | at LC90 (±SE) | ?2 |
| Spinosad susceptible | 320 | 1.18±0.13 | 0.01 | 1.00 | - | 0.113 | 1.00 | - | 5.32 |
| (standard) | (6) | (0.007–0.01) | (0.81–1.19) | (0.071–0.222) | (0.81–1.19) | ||||
| Spinosad-resistant (F15 of | 238 | 1.47±0.19 | 1717.30 | 183122.81 | - | 12730.00 | 1354308.97 | - | 5.46 |
| selected strain) | (4) | (998.00–2764.00) | (183123.17–182847.10) | (6481.00–56151.00) | (1354309.17–1354309.25) | ||||
| Reciprocal cross ♂ S×♀ R | 216 | 1.51±0.19 | 0.26 | 27.27 | −0.45±0.03 | 1.81 | 15.96 | −0.52±0.06 | 5.32 |
| (5) | (0.17–0.39) | (27.65–27.44) | (1.02–4.98) | (27.65–27.44) | |||||
| Reciprocal cross ♂ R×♀ S | 211 | 1.60±0.19 | 0.38 | 40.31 | −0.39±0.03 | 2.38 | 21.03 | −0.47±0.06 | 5.83 |
| (5) | (0.25–0.59) | (40.12–40.29) | (1.30–7.16) | (20.68–21.37) | |||||
| Pooled F1 of reciprocal | 427 | 1.55±0.13 | 0.31 | 33.15 | −0.42±0.02 | 2.11 | 18.67 | −0.49±0.05 | 9.18 |
| crosses (RC) | (5) | (0.21–0.46) | (33.48–33.25) | (1.22–5.32) | (18.34–18.94) | ||||
| Backcross (Pooled F1 RC× | 347 | 0.57±0.05 | 18.15 | 1947.35 | - | 4314.1 | 38374.52 | - | 7.21 |
| Spinosad-resistant) | (9) | (9.40–35.36) | (1947.03–1947.66) | (1479.10–18945.00) | (38373.96–38375.12) |
All of the concentration-mortality curves followed the probit model based on the χ2 goodness-of-fit test (P>0.05).
Figure 2. Spinosad concentration-mortality curves (with observed data as symbols) for the (standard) spinosad susceptible strain, (selected) spinosad resistant strain, the F1 progeny of the reciprocal crosses and the backcross progeny (pooled F1 RC×spinosad-resistant) of the tomato borer Tuta absoluta.
The degree of dominance of spinosad resistance was estimated for the F1 progeny of both reciprocal crosses between spinosad-susceptible and –resistant strains, and also for the pooled data from both progenies, providing values ranging from –0.39 to –0.45 (at the LC50). The estimates of degree of dominance at the LC90 for the same progenies were similar ranging from –0.47 and –0.52. These findings indicate that spinosad resistance is incompletely recessive, which was further confirmed by estimating the dominance using a range of five concentrations against the spinosad-susceptible and -resistant strains and their F1 progeny (pooled together from both reciprocal crosses strain) (Table 4). At high concentrations, full recessiveness prevailed, while at low concentrations full dominance prevailed and the incompletely recessive pattern prevailed at intermediate spinosad concentrations as would be expected for an incompletely recessive pattern of inheritance.
Table 4. Dominance of spinosad resistance based on a range of spinosad concentrations including LC50s from the susceptible parental strains and pooled F1 progeny of reciprocal crosses estimated for the tomato borer Tuta absoluta.
| Concentrations (µg a.i./mL) | Strains | No. insects | Mortality (%) | Survival performance | Estimated dominance (h) |
| 0.005 | Spinosad-resistant | 19 | 0.00 | 1.00 | - |
| Spinosad-susceptible | 29 | 3.45 | 0.97 | - | |
| Pooled F1 of reciprocal crosses | 30 | 0.00 | 1.00 | 1.00 | |
| 0.05 | Spinosad-resistant | 25 | 0.00 | 1.00 | - |
| Spinosad-susceptible | 28 | 25.00 | 0.75 | - | |
| Pooled F1 of reciprocal crosses | 29 | 0.00 | 1.00 | 1.00 | |
| 0.50 | Spinosad-resistant | 22 | 0.00 | 1.00 | - |
| Spinosad-susceptible | 30 | 100.00 | 0.00 | - | |
| Pooled F1 of reciprocal crosses | 31 | 58.06 | 0.42 | 0.42 | |
| 5.00 | Spinosad-resistant | 21 | 0.00 | 1.00 | - |
| Spinosad-susceptible | 29 | 100.00 | 0.00 | - | |
| Pooled F1 of reciprocal crosses | 30 | 100.00 | 0.00 | 0.00 | |
| 10.00 | Spinosad-resistant | 29 | 13.79 | 0.86 | - |
| Spinosad-susceptible | 31 | 100.00 | 0.00 | - | |
| Pooled F1 of reciprocal crosses | 30 | 100.00 | 0.00 | 0.00 |
The concentration range used also discriminates for high spinosad resistance, as observed in the spinosad-selected strain. The estimated dominance (h) varies from 0 (completely recessive) to 1 (completely dominant), where 0.5 corresponds to co-dominance, 0<h<0.5 corresponds to incompletely recessive and 0.5<h<1.0 corresponds to incompletely dominant.
The direct test for monogenic inheritance of spinosad resistance provided non-significant variation between expected and observed frequencies at increasing spinosad concentrations (Table 5). The overall χ2 test for the 11 spinosad concentrations tested was not significant (χ2 = 14.88, df = 10, P>0.05) (Table 5). The minimal number of effective genes (nE) involved in spinosad resistance was 0.63 indicating, again, a monogenic trait.
Table 5. Direct test of monogenic inheritance for spinosad resistance in the tomato borer Tuta absoluta by comparing expected and observed mortality of the progeny of the backcrosses between the pooled F1 progeny of the reciprocal crosses and the (selected) spinosad-resistant strain.
| Concentration (mg a.i./L) | Observed mortality (%) | Expected mortality (%) | ?2 * | P |
| 0.04 | 0.00 | 0.00 | 0.00 | 1.00 |
| 0.08 | 6.90 | 8.62 | 0.11 | 0.74 |
| 0.32 | 22.58 | 18.33 | 0.37 | 0.54 |
| 1.28 | 25.00 | 37.27 | 2.06 | 0.15 |
| 3.05 | 38.46 | 52.00 | 2.86 | 0.09 |
| 12.21 | 53.33 | 50.00 | 0.13 | 0.72 |
| 48.83 | 63.89 | 50.00 | 2.78 | 0.10 |
| 195.31 | 66.67 | 50.00 | 3.33 | 0.07 |
| 781.25 | 86.67 | 77.50 | 1.45 | 0.23 |
| 3125.00 | 83.33 | 80.00 | 0.21 | 0.65 |
| 12500.00 | 90.00 | 95.00 | 1.58 | 0.21 |
| Total | Σχ2 = 14.89 | 0.14 |
*Non-significant at P>0.05.
Spinosad cross-resistance spectrum
The concentration-mortality curves for nine different insecticides (of different groups) used against the tomato borer were estimated for the parental spinosad-resistant strain (Iraquara) and its spinosad-selected strain after 15 generations of selection to allow the recognition of potential patterns of cross-resistance (i.e., a single resistance mechanism leading to resistance to two or more insecticides). An eventual increase in resistance with the increase of spinosad resistance by selection indicates cross-resistance. However, among the insecticides tested, only spinetoram exhibited a significant increase in resistance with selection for spinosad resistance (Table 6). Therefore, cross-resistance was observed only between spinosad and spinetoram, another spinosyn insecticide.
Table 6. Relative toxicity of insecticides to the parental spinosad-resistant strain and its derived strain after 15-generations of selection for spinosad resistance.
| Insecticides | No. insects | Slope ± SE | LC50 (95% CI) (µg a.i./mL) | RR50 (95% CI) | ?2 (degrees of freedom) |
| Parental spinosad-resistant strain | |||||
| Spinosad | 264 | 1.85±0.23 | 0.410 (0.31–0.51) | - | 1.51 (5) |
| Spinetoram | 276 | 1.72±0.18 | 0.29 (0.23–0.38) | - | 2.91 (5) |
| Abamectin | 282 | 1.56±0.24 | 0.54 (0.31–0.78) | - | 2.07 (5) |
| Chlorantraniliprole | 243 | 2.84±0.38 | 12.18 (9.38–15.10) | - | 2.40 (4) |
| Cartap | 265 | 2.25±0.25 | 173.65 (137.26–214.16) | - | 0.95 (5) |
| Chlorfenapyr | 283 | 1.65±0.21 | 1.08 (0.74–1.44) | - | 0.77 (5) |
| Indoxacarb | 279 | 3.25±0.47 | 0.86 (0.69–1.04) | - | 0.41 (5) |
| Thiamethoxam | 300 | 1.65±0.17 | 1008.86 (717.56–1389.27) | - | 5.49 (5) |
| Permethrin | 281 | 1.87±0.21 | 269.15 (204.91–342.36) | - | 5.49 (6) |
| Chlorpyrifos | 273 | 2.30±0.23 | 509.16 (416.99–623.34) | - | 2.30 (5) |
| Selected spinosad-resistant strain | |||||
| Spinosad | 238 | 1.47±0.19 | 1717.30 (998.16–2764.20) | 4191.55 (4191.38–4191.72) | 5.46 (4) |
| Spinetoram | 211 | 1.62±0.23 | 195.94 (140.94–261.88) | 656.99 (656.82–657.15) | 2.32 (4) |
| Abamectin | 282 | 1.66±0.18 | 2.85 (2.12–3.66) | 5.25 (5.00–5.50) | 2.15 (6) |
| Chlorantraniliprole | 244 | 1.80±0.22 | 0.42 (0.30–0.55) | 0.03 (0.01–0.20) | 1.26 (5) |
| Cartap | 243 | 1.21±0.20 | 105.34 (72.22–164.21) | 0.61 (0.41–0.80) | 0.32 (4) |
| Chlorfenapyr | 432 | 1.19±0.12 | 3.80 (2.94–4.89) | 3.53 (3.35–3.71) | 3.86 (5) |
| Indoxacarb | 313 | 1.68±0.19 | 1.19 (0.73–1.72) | 1.38 (1.22–1.54) | 6.64 (6) |
| Thiamethoxam | 212 | 1.91±0.23 | 3573.35 (2414.90–5147.74) | 3.54 (3.40–3.69) | 4.40 (4) |
| Permethrin | 238 | 1.62±0.19 | 662.07 (497.68–864.87) | 2.46 (2.30–2.62) | 2.12 (5) |
| Chlorpyrifos | 299 | 1.82±0.19 | 951.97 (758.56–1221.65) | 1.87 (1.73–2.00) | 4.75 (5) |
All of the concentration-mortality curves followed the probit model based on the χ2 goodness-of-fit test (P>0.05).
Synergism of spinosad
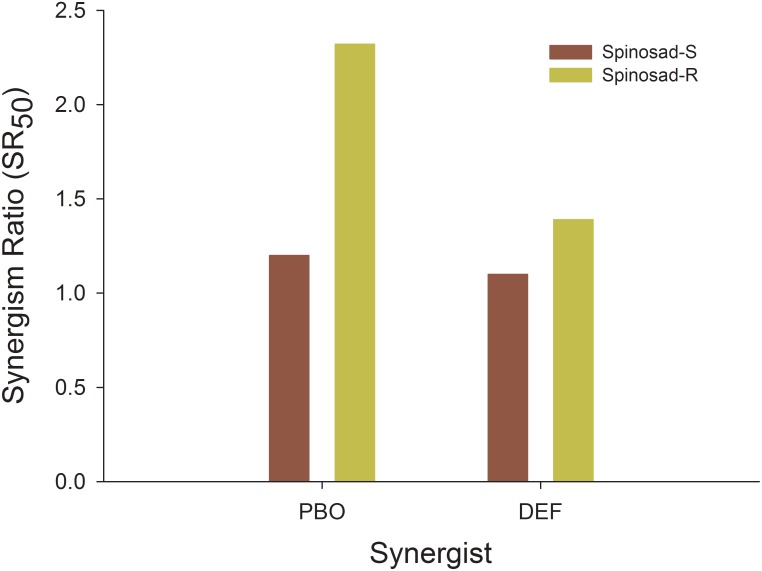
The synergism ratios with PBO and DEF were 1.2- and 1.1-fold, respectively (Fig. 3) for susceptible colony, while it was 2.32- and 1.39-fold in the resistant colony for PBO and DEF, respectively. Therefore, the synergisms caused by PBO and DEF against spinosad for the resistant colony were 1.93- and 1.26-fold greater compared to the susceptible colony, respectively. This suggests that such enzymes play a minimal role in the spinosad resistance.
Figure 3. Synergism of spinosad toxicity in spinosad-susceptible and -resistant strains of the tomato borer Tuta absoluta.
Activity of detoxification enzymes
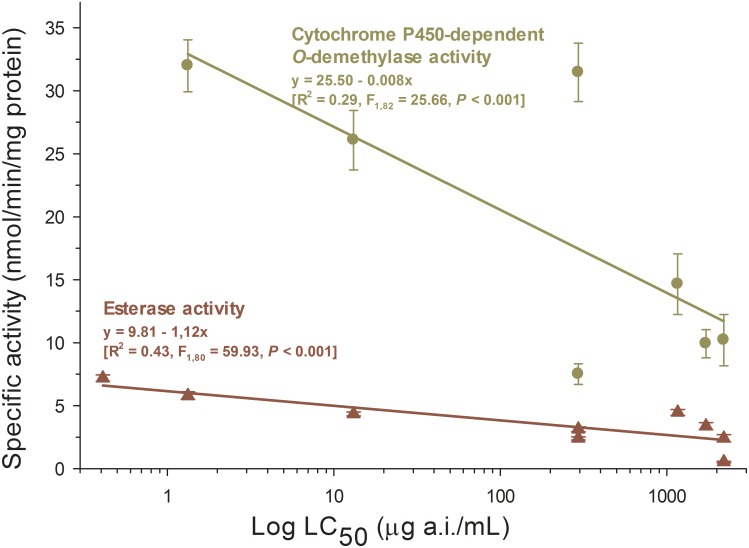
The activity of esterases and cytochrome P450-dependent monooxygenases was determined for the spinosad-selected strain after different generations of selection to identify a potential increase in detoxification activity with the increase in spinosad resistance. However, the activity of both detoxification enzymes significantly decreased with selection for spinosad resistance indicating that they are not the underlying mechanism of this phenomenon (Fig. 4).
Figure 4. Relationship between detoxification enzyme activity and LC50s for spinosad in spinosad-selected generations of the tomato borer Tuta absoluta.
Discussion
In countries managing invasive species and maintaining records of insecticide resistance development, insecticide use against the tomato borer can be described as waves of use of different (insecticide) groups. This is the case in Chile and Brazil, where the initial use of organophosphates, pyrethroids, and cartap was replaced by abamectin and subsequently by insect growth regulators as the main insecticide groups under use [10], [12]–[16], [19]–[21]. The main determinant in the replacement of an insecticide is the sequential development of insecticide resistance to the main insecticides being utilized at a given time, this phenomenon has evolved quickly in the tomato borer leading to control failures and relatively fast changes in the patterns of insecticide use [10], [12]–[17], [19]–[21]. Three insecticides are the main compounds currently being used against the tomato borer in Brazil, the spinosyn spinosad, the pyrrole chlorfenapyr, and the diamide chlorantraniliprole [18]–[21].
Quick reselection for resistance to insecticides has limited the range of available compounds for managing the tomato borer, increasing reliance on few molecules for this objective [20], . The concern of spreading insecticide resistant phenotypes of the tomato borer justifies the examination of the occurrence of insecticide resistance in this species. The new focus is on the few compounds under effective use, particularly in the likely centers of spread of the species. The emergence of spinosad resistance in South America is cause for concern, and the levels of resistance seemed to have increased quickly, but there is little information available beyond an initial survey [19]–[23]. The quick development of spinosad resistance in the region is suggestive of a highly inheritable (monogenic) trait, which was confirmed in our study.
Very high levels of spinosad resistance (>180,000-fold) were achieved within seven generations of selection from a field population already exhibiting resistance to spinosad [23]. The heritability of spinosad resistance proved high, with enough field variability to allow a quick selection for resistance, which has also been observed for spinosad resistance in the diamondback moth Plutella xylostella (L.) and the American serpentine leafminer Liriomyza trifolii (Burgess) [45], [46]. A monogenic pattern of inheritance is consistent with the fast selection and evolution of spinosad resistance and such autosomal monogenic inheritance was observed in the tomato borer, which was incompletely recessive. This inheritance seems to be the general pattern for spinosad resistance in insect pest species [47]–[49]. The simple inheritance and high heritability of spinosad resistance in the tomato borer reinforces the phytosanitary concerns of the quick dispersion of this pest species and the spread of insecticide resistant phenotypes or populations amenable to fast local selection for spinosad resistance [19], [31].
The potential cross-resistance to other insecticidal compounds is another issue of concern because it may further limit the management tools available against a pest species, particularly an invasive and very destructive species that is already difficult to control, such as the tomato borer [8]–[10], [19]. Cross-resistance in spinosad-resistant insect populations seems limited to related compounds [50], [51], as observed for the tomato borer where only cross-resistance to spinetoram, another spinosyn, was observed. This pattern of cross-resistance among spinosyns has been associated with altered target site sensitivity in the insect strains resistant to these compounds and their site of action [50]. This is consistent with our findings in the tomato borer, despite an earlier suggestion of the potential involvement of enhanced cytochrome P450-dependent monooxygenase activity in spinosad resistance in this species [22]. Initial correlational evidence of the potential involvement of enhanced esterase activity in spinosad resistance also suggested enhanced detoxification as a potential mechanism [23]. However, these earlier suggestions did not provide substantiated evidence for this possibility and our results did not support this hypothesis. Our results demonstrated altered target site sensitivity as the underlying mechanism of spinosad (and spinetoram) resistance in the tomato borer [50], [52].
Spinosad resistance evolved quickly in the tomato borer under spinosad pressure reaching a high threshold of selection with over a level of 180,000-fold level of resistance, maintained with continued selection. However, the interruption of spinosad selection led to a quick erosion of spinosad resistance reestablishing the initial (reduced) levels of resistance after eight generations. This finding indicates that there is a fitness cost associated with insecticide resistance, which has been reported in different insect species and with different insecticides [46], [53]–[55]. The fitness disadvantage of spinosad resistant populations of the tomato borer without selection pressure by spinosyn applications allows for the potential of moderation as an insecticide resistance management strategy. In this case, the reduction of spinosad use for a few generations of the tomato borer (>10) will allow the eventual reestablishment of susceptibility to spinosad and the subsequent reuse of this insecticide in the area. Reselection for spinosad resistance is likely to be rapid based on what has been observed for other insecticides in the tomato borer and in other species [4]–[7], [20], [21], but insecticide rotation with compounds of different modes of action and detoxification should extend the field use of spinosyns against the tomato borer.
In summary, very high levels of spinosad resistance were quickly selected for in the tomato borer with a monogenic autosomal pattern of inheritance that was incompletely recessive. A cross-resistance spectrum to spinetoram, another spinosyn, was observed, which suggested that the likely resistance mechanism involved is an altered target site sensitivity, given that the activity of esterases and cytochrome P450-dependent monooxygenases were not associated with spinosad resistance in the tomato borer. Spinosad resistance was unstable without spinosad selection, suggesting that the suspension of spinosyn use against the tomato borer would be a useful component in spinosad resistance management for this species.
Spinosad use against this species in introduced areas should be carefully monitored to prevent rapid selection for high levels of resistance and the potential for its spread to new areas.
Acknowledgments
We would like to acknowledge the IRAC-BR and the affiliated agrochemical companies, who assisted in field collection of the insect populations and provided the insecticide formulations.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.
Funding Statement
The financial support provided by the National Council of Scientific and Technological Development (CNPq-484240/2011-0) (HAAS), CAPES Foundation (MRC), and the Insecticide Resistance Action Committee – Brazilian Section (IRAC-BR) (HAAS) was greatly appreciated and acknowledged here. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Olson LJ (2006) The economics of terrestrial invasive species: a review of the literature. Agric Resour Econ Rev 35: 178–194. [Google Scholar]
- 2. Haack RA, Herard F, Sun JH, Turgeon JJ (2010) Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective. Annu Rev Entomol 55: 521–546. [DOI] [PubMed] [Google Scholar]
- 3. Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Annu Rev Entomol 56: 375–399. [DOI] [PubMed] [Google Scholar]
- 4. Georghiou GP (1972) The evolution of resistance to pesticides. Annu Rev Ecol System 3: 133–168. [Google Scholar]
- 5. Brattsten LB, Holyoke CW, Leeper JR, Raffa KF (1986) Insecticide resistance: challenge to pest management and basic research. Science 231: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 6.Whalon ME, Mota-Sanchez D, Hollingworth RM (2008) Global Pesticide Resistance in Arthropods. CABI: Cambridge, MA, USA. 208.
- 7. Heckel DG (2012) Insecticide resistance after Silent Spring . Science 337: 1612–1614. [DOI] [PubMed] [Google Scholar]
- 8. Desneux N, Wajnberg E, Wyckhuys AG, Burgio G, Arpaia S, et al. (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83: 197–215. [Google Scholar]
- 9. Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84: 403–408. [Google Scholar]
- 10. Guedes RNC, Picanço MC (2012) The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. Bull OEPP/EPPO Bull 42: 211–216. [Google Scholar]
- 11.FAO [Food and Agriculture Organization of the United Nations] (2014) FAOSTAT. Available: http://faostat3.fao.org/faostat-gateway/go/to/download/Q/QC/E. Accessed 2014 Feb 5.
- 12. Salazar ER, Araya JE (1997) Detección de resistência a insecticidas em La polilla del tomate. Simiente 67: 8–22. [Google Scholar]
- 13. Siqueira HAA, Guedes RNC, Picanço MC (2000) Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric For Entomol 2: 147–153. [Google Scholar]
- 14. Siqueira HAA, Guedes RNC, Picanço MC (2000) Cartap resistance na synergism in populations of Tuta absoluta (Lep., Gelechiidae). J Appl Entomol 124: 233–238. [Google Scholar]
- 15. Salazar ER, Araya JE (2001) Respuesta de La polilla del tomate, Tuta absoluta (Meyrick), a insecticides em Arica. Agric Tecn 61: 429–435. [Google Scholar]
- 16. Siqueira HAA, Guedes RNC, Fragoso DB, Magalhães LC (2001) Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int J Pest Manag 47: 247–251. [Google Scholar]
- 17. Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop Entomol 34: 113–119. [Google Scholar]
- 18.MAPA [Ministério da Agricultura, Agropecuária e Abastecimento] (2014) AGROFIT: Sistema de Agrotóxicos Fitossanitários. Available: http://extranet.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 2014 Feb 6.
- 19.Guedes RNC, Siqueira HAA (2012) The tomato borer Tuta absoluta: insecticide resistance and control failure. CAB Rev 7, no. 055. doi:10.1079/PAVSNNR20127055.
- 20. Silva GA, Picanço MC, Bacci L, Crespo ALB, Rosado JF, et al. (2011) Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta . Pest Manag Sci 67: 913–920. [DOI] [PubMed] [Google Scholar]
- 21. Gontijo PC, Picanço MC, Pereira EJG, Martins JC, Chediak M, et al. (2012) Spatial and temporal variation in the control failure likelihood of the tomato leaf miner, Tuta absoluta . Ann Appl Biol 162: 50–59. [Google Scholar]
- 22. Reyes M, Rocha K, Alarcón L, Siegwart M, Sauphanor B (2012) Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) to spinosad. Pestic Biochem Physiol 102: 45–50. [Google Scholar]
- 23.Campos MR (2013) Bases para o manejo da resistência de Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) a diamidas e espinosinas [Thesis]. Recife: Universidade Federal Rural de Pernambuco - UFRPE. 128 p. [Google Scholar]
- 24.Racke KD (2006) A reduced risk insecticide for organic agriculture: spinosad case study. In: Felsot AS, Racke KD, editors. Crop protection products for organic agriculture: environmental, health and efficacy assessment, vol. 947. Washington: ACS. 92–108.
- 25. Biondi A, Zappalà L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 8(9): e76548 doi:10.1371/journal.pone.0076548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puinean AM, Lansdell SJ, Collins T, Bielza P, Millar NS (2013) A nicotinic acetylcholine receptor transmembrane point mutation (G275E) associated with resistance to spinosad in Frankliniella occidentalis . J Neurochem 124: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suinaga FA, Casali VWD, Picanço M, Foster J (2004) Genetic divergence among tomato leafminer populations based on AFL analysis. Pesq Agropec Bras 39: 645–651. [Google Scholar]
- 28. Cifuentes D, Chynoweth R, Bielza P (2011) Genetic study of Mediterranean and South American populations of tomato leafminer Tuta absoluta (Povolny, 1994) (Lepidoptera: Gelechiidae) using ribosomal and mitochondrial markers. Pest Manag Sci 67: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 29. Roditakis R, Skarmoutsou C, Staurakaki M, Martínez-Aguirre M del R, García-Vidal L, et al. (2013) Determination of baseline susceptibility of European populations of Tuta absoluta (Meyrick) to indoxacarb and chlorantraniliprole using a novel dip bioassay method. Pest Manag Sci 69: 217–227. [DOI] [PubMed] [Google Scholar]
- 30. Roditakis R, Skarmoutsou C, Staurakaki M (2013) Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag Sci 69: 834–849. [DOI] [PubMed] [Google Scholar]
- 31. Haddi K, Berger M, Bielza P, Cifuentes D, Field LM, et al. (2012) Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomatoleaf miner (Tuta absoluta). Insect Biochem Mol Biol 42: 506–513. [DOI] [PubMed] [Google Scholar]
- 32.IRAC [Insecticide Resistance Action Committee] (2014) IRAC method no. 022: insecticide bioassay for Tuta absoluta Available: http://www.irac-online.org/wp-content/uploads/2009/09/Method_022_Tuta_.pdf. Accessed 2014 Feb 6.
- 33. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, et al. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 34. van Asperen K (1962) A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 8: 401–416. [Google Scholar]
- 35. Netter KJ, Seidel G (1964) An adaptively stimulated O-demethylating system in rat liver microssomes and its kinetic properties. J Pharmacol Exp Ther 146: 61–65. [PubMed] [Google Scholar]
- 36.LeOra-Software (2005) POLO-Plus, POLO for Windows computer program, ver. 2.0. Petaluma, CA: LeOra-Software.
- 37.Robertson JL, Russell RM, Preisler HK, Savin NE (2007) Bioassays with Arthropods, 2nd ed. Boca Raton: CRC. 224 p. [Google Scholar]
- 38.Hartl DL (2000) A primer of population genetics, 3rd ed. Sunderland: Sinauer. 221 p. [Google Scholar]
- 39.Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th ed. Longman: New York. 480 p. [Google Scholar]
- 40. Stone BF (1968) A formula for determining degree of dominance in cases of moonofactorial inheritance of resistance to chemicals. Bull WHO 38: 325–326. [PMC free article] [PubMed] [Google Scholar]
- 41. Tabashnik BE, Cushing NL, Finson N, Johnson MW (1990) Field development of resistance to Bacillus thunringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 83: 1671–1676. [Google Scholar]
- 42.Sokal R, Rohlf F (2012) Biometry, 4th ed. New York: Freeman. 937 p. [Google Scholar]
- 43. Lande R (1981) The minimum number of genes contributing to quantitative variation between and within populations. Genetics 99: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SAS Institute (2008) SAS/STAT user’s version. Cary, NC: SAS Institute.
- 45. Ferguson JS (2004) Development and stability of insecticide resistance in the leafminer Liriomyza trifolii (Diptera: Agromyzidae) to cyromazine, abamectin, and spinosad. J Econ Entomol 97: 112–119. [DOI] [PubMed] [Google Scholar]
- 46. Sayyed AH, Ferre J, Wright DJ (2000) Mode of inheritance and stability of resistance to Bacillus thunringiensis var kurstaki in a diamondback moth (Plutella xylostella) population from Malaysia. Pest Manag Sci 56: 743–748. [Google Scholar]
- 47. Zhao JZ, Li YX, Collins HL, Gusukuma-Minuto L, Mau RF, et al. (2002) Monitoring and characterization of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad. J Econ Entomol 95: 430–436. [DOI] [PubMed] [Google Scholar]
- 48. Sayyed AH, Saeed S, Noor-ul-ane M, Crickmore N (2008) Genetic, biochemical, and physiological characterization of sponisad resistance in Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 101: 1658–1666. [DOI] [PubMed] [Google Scholar]
- 49. Sparks TC, Dripps JE, Watson GB, Paroonagian D (2012) Resistance and cross-resistance to the spinosyns – a review and analysis. Pestic Biochem Physiol 102: 1–10. [Google Scholar]
- 50.Salgado VL, Sparks TC (2005) The spinosyns: chemistry, biochemistry, mode of action, and resistance. In: Lawrence IG, Kostas I, Sarjeet SG, editors. Comprehenisve molecular insect science, vol 6. Amsterdam: Elsevier. 137–173.
- 51. Watson GB, Chouinard SW, Cook KR, Geng C, Gifford JM, et al. (2010) A spinosyn-sensitive Drosophila melanogaster nicotinic acetylcholine receptor identified through chemically induced target site resistance, resistance gene identification, and heterologous expression. Insect Biochem Mol Biol 40: 376–384. [DOI] [PubMed] [Google Scholar]
- 52. Salgado VL, Saar R (2004) Desensitizing and non-desensitizing subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J Insect Physiol 50: 867–879. [DOI] [PubMed] [Google Scholar]
- 53. Wang D, Qiu X, Wang H, Qiao K, Wang K (2010) Reduced fitness associated with spinosad resistance in Helicoverpa armigera . Phytoparasitica 38: 103–110. [Google Scholar]
- 54. Coustau C, Chevillon C, ffrench-Constant R (2000) Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol Evol 15: 378–383. [DOI] [PubMed] [Google Scholar]
- 55. Guedes RNC, Oliveira EE, Guedes NMP, Ribeiro B, Serrão JE (2006) Cost and mitigation of insecticide resistance in the maize weevil, Sitophilus zeamais . Physiol Entomol 31: 30–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.