Abstract
AIM: To evaluate the efficacy of a colonoscopy preparation that utilizes a reduced dose of sodium phosphate (NaP) and an adjunct.
METHODS: Sixty-two patients requiring screening colonoscopies were studied. Each patient was randomly allocated to receive either 50 NaP tablets (50 g) or 30 NaP tablets (30 g) with 10 mL of 0.75% sodium picosulfate for bowel preparation. NaP was administered at a rate of five tablets (5 g) or three tablets (3 g) every 15 min with 200 mL of water, beginning five to six hours before colonoscopy. The sodium picosulfate was administered with 200 mL of water on the night before the procedure. Both groups were compared in term of the efficacies of colonic cleansing, the time required for completion of the bowel preparation, and acceptability of the preparation.
RESULTS: Sixty patients (n = 30 for each group) were analyzed. The cleansing efficacy tended to be higher in the 30 g NaP plus sodium picosulfate group as assessed by the mean total Ottawa scale score (50 g NaP 6.70 ± 1. 42 vs 30 g NaP plus sodium picosulfate 6.17 ± 1.18 P = 0.072). The mean time for bowel preparation tended to be shorter in the 30 g NaP plus sodium picosulfate group (50 g NaP 189.9 ± 64.0 min vs 30 g NaP plus sodium picosulfate 161.8 ± 57.6 min, P = 0.065). There were no significant differences between the two groups in the acceptability of the preparations (50 g NaP 83.3% vs 30 g NaP plus sodium picosulfate 86.7%, P = 0.500). There were no adverse events related to bowel preparation in either of the groups.
CONCLUSION: The colonoscopy preparation that utilized 30 g NaP with sodium picosulfate was comparable to that utilizing 50 g NaP. This novel bowel preparation might be useful before colonoscopy.
Keywords: Bowel preparation, Colonoscopy, Colonoscopy preparation, Sodium phosphate, Sodium picosulfate
Core tip: Oral sodium phosphate (NaP) is used for bowel preparation before colonoscopy. It is desirable to reduce the dose of NaP due to the potential adverse events associated with NaP. In this study, we evaluated the efficacy of a colonoscopy preparation that utilized a reduced dose of NaP and an adjunct. This study demonstrated that 30 g NaP in combination with sodium picosulfate can be useful for bowel preparation prior to colonoscopy in Japanese populations. This report is the first to evaluate the efficacy of a bowel preparation using the minimally effective dose of NaP and an adjunct.
INTRODUCTION
The quality of bowel preparation influences the diagnostic accuracy of colonoscopy. Inadequate preparation negatively affects rates of polyp[1] and adenoma detection[2] during the procedure. The ideal preparation for colonoscopy would rapidly and reliably eliminate the colon of all fecal material without causing any gross or histological alternations of the colonic mucosa[3,4]. Additionally, the preparation should not cause any patient discomfort and should be safe.
Oral sodium phosphate (NaP), which draws water into the bowel lumen and stimulates peristalsis and evacuation, is used for bowel preparation prior to colonoscopy. Although this agent provides superior cleansing and is well-tolerated by most patients, there are concerns about its safety that are related to its osmotic action[5,6]. Moreover, recent reports[7-9] of renal injury associated with this agent have raised additional concerns. Given the mechanistic causes of the occurrence of adverse events, the use of reduced doses of NaP might decrease these potential risks. We hypothesized that the dose of NaP could be reduced if NaP is combined with an adjuvant colonic laxative for bowel preparation. Sodium picosulfate (SP) is a laxative that stimulates colonic movement and promotes evacuation. SP is often used as an adjunct to polyethylene glycol (PEG) for bowel preparation in Japan. In this study, we present a new method of bowel preparation prior to colonoscopy that utilizes a reduced dose of NaP in combination with SP, and we evaluated the efficacy of this method.
MATERIALS AND METHODS
This study was an investigator-blinded, randomized controlled trial. Outpatients visiting the health check-up center for screening colonoscopies were invited to participate in the study. Due to the potential for NaP preparations to induce fluid shifts and the recent results of the manufacturer’s post-marketing trial to assess the incidence of renal injury, patients with the following conditions were excluded: over 65 years of age with hypertension, ascites, renal insufficiency, congestive heart failure, and concurrent use of an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB). The study was designed in accordance with the Declaration of Helsinki and was approved by the ethics committee of our institute. Written informed consent was obtained from all patients.
Protocol for the study of bowel preparations
All eligible patients were instructed to eat a low-fiber diet on the day before the colonoscopy and to abstain from food after 9 PM on the evening before the procedure. Each patient was randomly allocated to receive either 50 NaP tablets (50 g; Visiclear® ZERIA Pharmaceutical Co., Ltd., Tokyo, Japan) or 30 NaP tablets (30 g) plus 10 mL of 0.75% SP solution (Laxoberon® TEIJIN Pharmaceutical Co., Ltd., Tokyo, Japan) for bowel preparation. The randomization was conducted via the use of sealed envelopes with treatment allocations inside and by an investigator who was not involved in the colonoscopy procedure. NaP was administered at a rate of five tablets (5 g) or three tablets (3 g) every 15 min with 200 mL of water beginning five to six hours before the colonoscopy. SP was taken with 200 mL of water on the night before the procedure (Figure 1).
Figure 1.
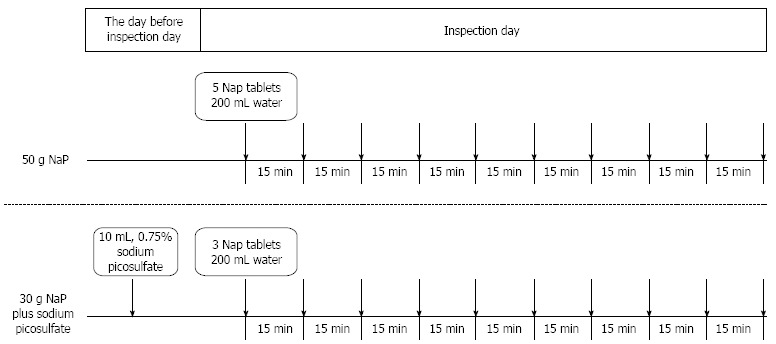
Study protocol for the bowel preparations. Sodium phosphate (NaP) was administered at a rate of five tablets (5 g) or three tablets (3 g) every 15 min with 200 mL of water beginning five to six hours before the colonoscopy. Sodium picosulfate was taken with 200 mL of water on the night before the procedure.
Evaluation of the preparations
The primary end point of this study was cleansing efficacy. The secondary outcomes included time for completion of the bowel preparation and acceptability of the preparation. Upon arriving to the health screening center, the patients submitted a compliance that detailed whether the drugs prescribed for bowel preparation had been taken properly, the time at which the NaP intake was initiated, and when the patients had clear stools. The patients were also asked to complete a written questionnaire to assess their overall impressions of the drugs used for bowel preparation on a 4-category Likert scale and yes or no answers regarding whether they experienced nausea, vomiting, bloating, abdominal pain or other symptoms. The efficacy of the colonic cleansing was graded using the Ottawa Bowel Preparation Scale (OBPS)[10] by a single endoscopist who was blinded to the doses of NaP. This scale uses scores for cleanliness of the recto-sigmoid colon, middle colon, and right colon that range from 0 to 4 (0 = excellent to 4 = inadequate). There is also a score for the overall volume of fluid that ranges from 0 to 2 (0 = small to 2 = large). The overall potential scores range from 0 (excellent preparation, no fluid) to 14 (inadequate in all segments with a large amount of fluid). The time for completion of the bowel preparation was defined as the time from the initiation of NaP until clear stools were noted. The acceptability of the bowel preparation was assessed by the patient’s overall impression on a four-category Likert scale: (1) acceptable; (2) relatively acceptable; (3) relatively unacceptable; and (4) unacceptable. Acceptability was defined as the rate of “acceptable” plus “relatively acceptable” responses. The groups were compared for the efficacies of the colonic cleansings, the times for the completion of the bowel preparations, and the acceptabilities of the preparations.
Statistical analysis
In this pilot study, the sample size was arbitrarily set at 30 patients per treatment arm to compare the two bowel preparation regimens. Continuous variables are reported as the mean ± SD, and categorical variables are presented as percentages. For the primary end point, a Mann-Whitney U-test was applied to compare the OBPS scores. For the secondary outcomes, Student’s t-test was applied to compare the times for the completion of the bowel preparations. The Fisher’s exact test was used to compare the acceptabilities of the preparations. Differences with P-values below 0.05 were considered statistically significant.
RESULTS
A total of sixty-two patients were enrolled in the study from July 2012 to September 2013. One patient (30 g NaP plus SP group) was excluded from the study due to inadequate intake of NaP tablets. Another patient (50 g NaP group) completed the preparation but was not assessed for cleansing efficacy due to a previous surgery and was also excluded. Lastly, sixty patients (n = 30 for each group) were included in the analysis. There were no significant differences between the two groups in baseline characteristics, including gender, age, height, weight and body mass index (Table 1).
Table 1.
Baseline characteristics
| 50 g NaP (n = 30) | 30 g NaP plus sodium picosulfate (n = 30) | P value | |
| Man/female | 23/7 | 25/5 | 0.7471 |
| Age (yr) | 55.4 ± 9.4 | 55.9 ± 10.8 | 0.8292 |
| Height (m) | 1.66 ± 0.08 | 1.67 ± 0.09 | 0.5752 |
| Weight (kg) | 65.7 ± 10.8 | 66.7 ± 10.0 | 0.6952 |
| BMI (kg/m2) | 23.8 ± 2.6 | 23.9 ± 2.2 | 0.9292 |
Student’s t-test;
Fisher exact test. BMI: Body mass index; NaP: Sodium phosphate.
Efficacy of colonic cleansing
The mean total OBPS score of the 50 g NaP and 30 g NaP plus SP groups were 6.70 ± 1.42 and 6.17 ± 1.18 respectively. There was a trend toward greater cleansing efficacy in the 30 g NaP plus SP group. However, this difference was not statistically significant (P = 0.072). When the mean scores for each component of the OBPS (i.e., the scores for the recto-sigmoid colon, middle colon, right colon, and the volume of fluid) were analyzed, no significant differences between the two groups were found (Figure 2).
Figure 2.
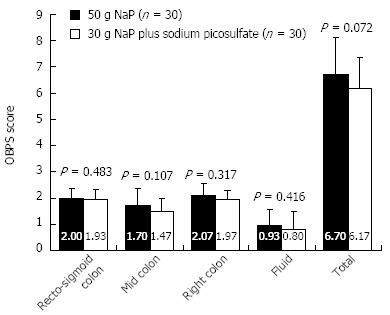
The mean Ottawa bowel preparation scale scores. There was a trend toward a lower mean total score in the 30 g NaP plus sodium picosulfate group compared to the 50 g NaP group, but this difference was not statistically significant (Mann-Whitney’s U-test, P = 0.072).
Times for the completion of the bowel preparation
The mean times for the completion of the bowel preparation in the 50 g NaP and 30 g NaP plus SP groups were 189.9 ± 64.0 min and 161.8 ± 57.6 min, respectively. There was a trend toward a shorter bowel preparation time in the 30 g NaP plus SP group. However, this difference was not statistically significant (P = 0.065, Figure 3).
Figure 3.
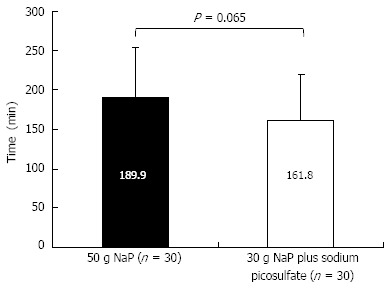
The mean time required for the completion of the bowel preparation. There was a trend toward a shorter bowel preparation time in the 30 g sodium phosphate (NaP) plus sodium picosulfate group compared to the 50 g NaP group, but this difference was not statistically significant (Student’s t-test, P = 0.065).
Acceptability of the preparations and adverse events
The patients’ overall impressions of the bowel preparations as rated on a four-category Likert scale are shown in Figure 4. The acceptabilities (i.e., the “acceptable” plus the “relatively acceptable” scores) of the preparation in the 50 g NaP and 30 g NaP plus SP groups were 83.3% and 86.7%, respectively. There was no significant difference between the two groups in the acceptabilities of the preparations (P = 0.500). The proportions of patients who reported symptoms after taking 50g NaP and 30 g NaP plus SP were 50% and 20%, respectively. There were no adverse events related to the bowel preparations in either of the groups.
Figure 4.
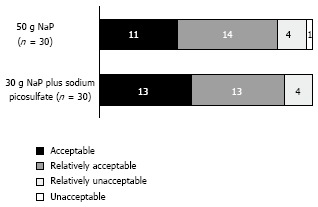
The patients’ overall impressions of the bowel preparations as assessed on a four-category Likert scale. There was no significant difference between the two groups in acceptabilities (i.e., the “acceptable” plus “relatively acceptable” scores) of the preparations (Fisher’s exact test, P = 0.500).
DISCUSSION
PEG is an osmotically balanced electrolyte lavage solution that is widely used for bowel preparation prior to colonoscopy. PEG was introduced by Davis et al[11] in 1980. PEG passes through the bowel without net absorption or secretion, and significant fluid and electrolyte shifts are therefore avoided. The standard four-liter dosing regimen that is given the day before the procedure has been established as safe and effective[12-14]. However, poor compliance due to the salty taste, sulfate smell, and the large volume of solution required led to modifications of PEG preparation regimens[15,16].
NaP osmotically draws plasma water into the bowel lumen to promote colonic cleansing and is used as an alternative to PEG for bowel preparation prior to colonoscopy[5,6,17]. Oral NaP is available as an aqueous solution and in a tablet form. The tablet form of NaP was designed to improve the taste and limit the volume of liquid required. Phase III trials in which tablet NaP regimens were compared with four-liter PEG regimens demonstrated equal colon cleansing with fewer side effects of the NaP regimen[17]. Recent reviews and a meta-analysis have reported that NaP preparations are generally more effective and better tolerated than are PEG formulations[18,19].
The tablet preparation contains 1.5 g NaP and 0.5 g of inactive ingredients. One of the inactive tablet ingredients, microcrystalline cellulose (MCC), is thought to reduce visibility during colonoscopy. Consequently, new MCC-free preparation is now available[20,21]. The doses are 40 tablets (60 g) for the MCC-containing preparation and 32 tablets (48 g) for the MCC-free preparation[22]. Both preparations are divided into two doses that are administered at an interval of 10 to 12 h. All NaP regimens should be taken with a minimum of two liters of clear liquids. In Japan, a MCC-free tablet preparation that contains 1.0 g NaP is commercially available. The standard dose is 50 tablets (50 g), which are taken as five tablets every 15 min with 200 mL of water or green tea on the day of the procedure.
Because of its osmotic mechanism of action, NaP can result in potentially fatal fluid and electrolyte shifts, particularly in elderly patients and patients with bowel obstructions, small intestine disorders, renal or liver insufficiency, or congestive heart failure[23]. Additionally, a recent series of reports[7-9] described acute phosphate nephropathy followed by chronic renal insufficiency after taking NaP for bowel preparation. Nephrocalcinosis is the cause of renal injury and occurs when the concentration of phosphate increases, and calcium phosphate crystals are deposited in the renal tubules[9]. In a series of 21 patients who developed acute phosphate nephropathy, potential etiological factors included dehydration, increased age, hypertension, and concurrent use of an ACE inhibitor or ARB[8]. We conducted our study to examine the use of a preparation that involved a reduced dose of NaP because such a reduction might decrease the potential for adverse events.
Bowel preparations are typically judged by their efficacy, tolerability, and safety, and all three criteria have obvious clinical importance. The preparation time needed to cleanse the colon of fecal material is also an important factor for bowel preparations. In this pilot study, 30 g NaP plus SP tended to produce higher cleansing efficacy and a shorter bowel preparation time than did the 50 g NaP; however, these differences were not statistically significant. Additionally, this new bowel preparation method was acceptable to more than 85% of patients. These results indicate that the dose of NaP can be reduced to 30 g when it is combined with SP for bowel preparation.
In conclusion, although our study is a trial with a small number of cases from a single center, this report is the first to evaluate the efficacy of a bowel preparation that involves the minimally effective dose of NaP and an adjunct. This study demonstrated that 30 g NaP in combination with SP can be useful for bowel preparation prior to colonoscopy in Japanese populations.
COMMENTS
Background
Sodium phosphate (NaP) is a superior colonic cleanser and is well-tolerated; however, there are concerns about the potential to cause adverse events that include electrolyte shifts and renal injury.
Research frontiers
It is desirable to reduce the dose of NaP required for colonoscopy preparation.
Innovations and breakthroughs
In this study, a new method of colonoscopy preparation that utilizes a reduced dose of NaP and an adjunct was evaluated.
Applications
This study demonstrated that 30 g NaP in combination with sodium picosulfate (SP) can be useful for bowel preparation prior to colonoscopy.
Terminology
SP is often used as an adjunct to polyethylene glycol for bowel preparation in Japan.
Peer review
This is an interesting research article about a common clinical problem related to bowel cleaning prior to colonoscopy examinations. The study was well designed, and its results were clearly demonstrated.
Footnotes
P- Reviewer: Nagata K, Venkatachalam RV, Su SB S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 2.Thomas-Gibson S, Rogers P, Cooper S, Man R, Rutter MD, Suzuki N, Swain D, Thuraisingam A, Atkin W. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy. 2006;38:456–460. doi: 10.1055/s-2006-925259. [DOI] [PubMed] [Google Scholar]
- 3.Mamula P, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Kwon RS, Rodriguez SA, et al. Colonoscopy preparation. Gastrointest Endosc. 2009;69:1201–1209. doi: 10.1016/j.gie.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Gastrointest Endosc. 2006;63:894–909. doi: 10.1016/j.gie.2006.03.918. [DOI] [PubMed] [Google Scholar]
- 5.Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointest Endosc. 2002;56:895–902. doi: 10.1067/mge.2002.129522. [DOI] [PubMed] [Google Scholar]
- 6.Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422–427. [PubMed] [Google Scholar]
- 7.Sica DA, Carl D, Zfass AM. Acute phosphate nephropathy--an emerging issue. Am J Gastroenterol. 2007;102:1844–1847. doi: 10.1111/j.1572-0241.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 9.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006–1007. doi: 10.1056/NEJM200309043491020. [DOI] [PubMed] [Google Scholar]
- 10.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–486. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 11.Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78:991–995. [PubMed] [Google Scholar]
- 12.DiPalma JA, Brady CE, Stewart DL, Karlin DA, McKinney MK, Clement DJ, Coleman TW, Pierson WP. Comparison of colon cleansing methods in preparation for colonoscopy. Gastroenterology. 1984;86:856–860. [PubMed] [Google Scholar]
- 13.Ernstoff JJ, Howard DA, Marshall JB, Jumshyd A, McCullough AJ. A randomized blinded clinical trial of a rapid colonic lavage solution (Golytely) compared with standard preparation for colonoscopy and barium enema. Gastroenterology. 1983;84:1512–1516. [PubMed] [Google Scholar]
- 14.Thomas G, Brozinsky S, Isenberg JI. Patient acceptance and effectiveness of a balanced lavage solution (Golytely) versus the standard preparation for colonoscopy. Gastroenterology. 1982;82:435–437. [PubMed] [Google Scholar]
- 15.DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc. 1990;36:285–289. doi: 10.1016/s0016-5107(90)71025-5. [DOI] [PubMed] [Google Scholar]
- 16.Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum. 1994;37:229–233; discussion 233-234. doi: 10.1007/BF02048160. [DOI] [PubMed] [Google Scholar]
- 17.Kastenberg D, Chasen R, Choudhary C, Riff D, Steinberg S, Weiss E, Wruble L. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001;54:705–713. doi: 10.1067/mge.2001.119733. [DOI] [PubMed] [Google Scholar]
- 18.Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373–384. doi: 10.1111/j.1365-2036.2006.03212.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. 2006;8:247–258. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 20.Rex DK, Schwartz H, Goldstein M, Popp J, Katz S, Barish C, Karlstadt RG, Rose M, Walker K, Lottes S, et al. Safety and colon-cleansing efficacy of a new residue-free formulation of sodium phosphate tablets. Am J Gastroenterol. 2006;101:2594–2604. doi: 10.1111/j.1572-0241.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 21.Wruble L, Demicco M, Medoff J, Safdi A, Bernstein J, Dalke D, Rose M, Karlstadt RG, Ettinger N, Zhang B. Residue-free sodium phosphate tablets (OsmoPrep) versus Visicol for colon cleansing: a randomized, investigator-blinded trial. Gastrointest Endosc. 2007;65:660–670. doi: 10.1016/j.gie.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK. Dosing considerations in the use of sodium phosphate bowel preparations for colonoscopy. Ann Pharmacother. 2007;41:1466–1475. doi: 10.1345/aph.1K206. [DOI] [PubMed] [Google Scholar]
- 23.Curran MP, Plosker GL. Oral sodium phosphate solution: a review of its use as a colorectal cleanser. Drugs. 2004;64:1697–1714. doi: 10.2165/00003495-200464150-00009. [DOI] [PubMed] [Google Scholar]


