Abstract
A 50-year-old male who underwent a HeartMate II left ventricular assist device placement for ischemic cardiomyopathy presented with discolored urine and hemolysis 3 mo after the operation. His hemolysis was thought to be due to thrombosis within the pump. Imaging studies were not able to visualize a left ventricular thrombus. Medical management with anticoagulation failed and he underwent surgery for a pump exchange. Intraoperatively, a firm thrombus was found within the pump of the HeartMate II, and the color of the urine changed dramatically from cola-colored to yellow which enabled us to confirm the diagnosis.
Keywords: Cardiac surgery, Hemolysis, Left ventricular assist device, Thrombosis
Core tip: Diagnosis of pump thrombosis is difficult, but the intraoperative change of the color of urine may be seen almost immediately after pump exchange. This report also highlights the technical aspect of replacing the HeartMate II pump, and we believe the images are educational for the readers.
INTRODUCTION
HeartMate II (Thoratec, Pleasanton, CA) is a continuous flow left ventricular assist device (LVAD), which has improved the quality of life and survival of patients who have end-stage heart failure refractory to medical therapy. The device is consisted of an inflow cannula in the left ventricle, axial flow pump, and an outflow graft to the ascending aorta. It is designed for long-term usage, either bridge to transplant or destination therapy. Since 2006, more than 10000 HeartMate II LVADs have been implanted, and 2500 LVADs have been implanted in 2013, according to the INTERMACS registry[1]. Consequently, the incidence of device-related complications, such as pump thrombosis, infection, bleeding, has increased, which often require re-admission and/or surgery[2]. Pump thrombosis is one of the common causes of hemolysis in patients with LVAD. Hemolysis related to the LVAD could be due to kinking of the outflow graft, malposition of the inflow cannula, or malfunction of the pump. We present a case of an LVAD thrombosis that presented with hemolysis and discolored urine 3 mo after the LVAD placement. The patient failed conservative medical management and underwent surgery for pump exchange. Thrombus was seen in the pump and the color of the urine changed dramatically after the pump was exchanged which enabled us to confirm the diagnosis of pump thrombosis.
CASE REPORT
A 50-year-old male with a history of axillo-bifemoral bypass for bilateral chronic iliac artery occlusive disease, ischemic cardiomyopathy with an ejection fraction of 20%, underwent placement of a HeartMate II LVAD as a bridge to cardiac transplantation. Preoperative hematology work-up disclosed no evidence of hypercoagulability. Heparin infusion was started on postoperative day 1 and warfarin was started on postoperative day 3. Heparin drip was maintained with a goal PTT level of 60 to 70 s until the INR reached 1.8. He was discharged on postoperative day 15 on aspirin 325 mg and warfarin with a target INR of 1.8 to 2.5. Upon discharge, the LVAD was set at 9200 rpm, giving a flow of 5.7 L/min, with a pulsatility index (the pulsatility of the flow through the pump) of 5.5 and pump power (a direct measurement of motor voltage and current) of 6.7 watts.
Three months later on his scheduled office visit, his lactate dehydrogenase (LDH) was found to be elevated to 1352 IU/L (Table 1). His baseline LDH level was between 400 and 500 IU/L. Interrogation of the pump parameters revealed several episodes of elevation in the pump power a few days before the office visit that had since resolved. It was thought to be due to a small pump thrombus that resolved spontaneously. Ten days later, he was admitted to the hospital due to discolored urine. Urine analysis showed strongly positive hemoglobin, very few red blood cells, and white blood cells. He denied any chest pain, shortness of breath, or edema. There were no signs of infection. Hemoglobin was 7.6 g/dL, plasma free hemoglobin was 52.0 mg/dL. The LDH, haptoglobin, AST were unable to be measured due to severe hemolysis. Other lab values included INR 1.7, serum creatinine 1.9 mg/dL (baseline 1.2 mg/dL), total bilirubin 1.1 mg/dL. Echocardiography showed severe biventricular dysfunction and opening of the aortic valve with every heartbeat. The velocity through the inflow cannula was 1.1 m/s. The LVAD parameters showed occasional pump power elevation over 9 watts. Heparin was initiated but there was no resolution of the discolored urine over three days. Although echocardiography and chest CT scan failed to demonstrate a thrombus, he was clinically diagnosed with pump thrombosis. Due to the persistently elevated creatinine and requirement of multiple blood transfusions for hemolytic anemia despite optimum medical therapy, the decision was made to proceed with pump exchange.
Table 1.
Laboratory values
| Outpatient (1 mo prior to admission) | Outpatient (1 wk prior to admission) | Admission | Post pump exchange (POD 7) | |
| White blood cells (B/L) | 6.5 | 6.7 | 8 | 10.7 |
| Hemoglobin (g/dL) | 10.8 | 8.4 | 7.6 | 11.6 |
| Hematocrit | 35.1% | 27.9% | 24.5% | 36.4% |
| Platelets (B/L) | 158 | 139 | 164 | 162 |
| Reticulocytes | 7.4% | 2.8% | ||
| Na (mEq/L) | 139 | 136 | 131 | 137 |
| K (mEq/L) | 4 | 4.7 | 4.9 | 3.8 |
| BUN (mg/dL) | 17 | 16 | 28 | 26 |
| Creatinine (mg/dL) | 1.2 | 1.4 | 1.9 | 1.5 |
| Total bilirubin (mg/dL) | 0.4 | 0.5 | 1.1 | 0.9 |
| Aspartate aminotransferase (IU/L) | 40 | 60 | 1 | 34 |
| Lactate dehydrogenase (IU/L) | 707 | 1382 | 1 | 527 |
| Plasma free hemoglobin (mg/dL) | 52 | 6.4 | ||
| Haptoglobin (mg/dL) | 1 | |||
| Urine color | Light red | Yellow | ||
| Red blood cell in urine (/HPF) | 1 | < 1 |
Unable to be obtained due to hemolysis.
After resternotomy, cardiopulmonary bypass (CPB) was established with ascending aorta and right femoral vein cannulation, as the femoral arteries were not able to be cannulated due to iliac artery occlusions. To gain access to the inflow portion of the LVAD, a left subcostal incision was added and the body of the HeartMate II pump was removed by unscrewing the inflow- and outflow- connections. It was replaced with a new HeartMate II pump (Figure 1). There was no thrombus in the inflow cannula or outflow conduit. Intraoperative inspection of the original LVAD interior demonstrated a firm thrombus along the inlet stator (Figure 2). The urine color was tea-colored before CPB (Figure 3A). It changed to reddish upon cessation of flow from the original LVAD and institution of CPB (Figure 3B). Following initiation of the new LVAD flow and discontinuation of CPB, it changed to a yellow color (Figure 3C). Postoperative recovery was steady and his renal function recovered with clear urine (Table 1). Anticoagulation therapy consisted of intravenous heparin with overlapping warfarin (INR 2.5 to 3.5), aspirin 325 mg, and clopidogrel 75 mg. He was discharged home on postoperative day 8. The patient was symptom free afterwards, and underwent heart transplant 2 mo later.
Figure 1.
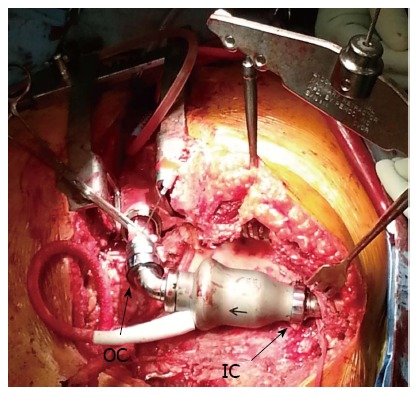
Intraoperative photo after the replacement of the pump. IC: Inflow connection; OC: Outflow connection.
Figure 2.
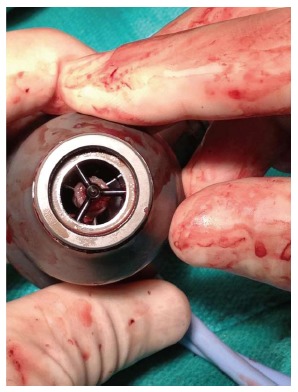
Inspection of the pump revealed a firm thrombus along the inlet stator.
Figure 3.
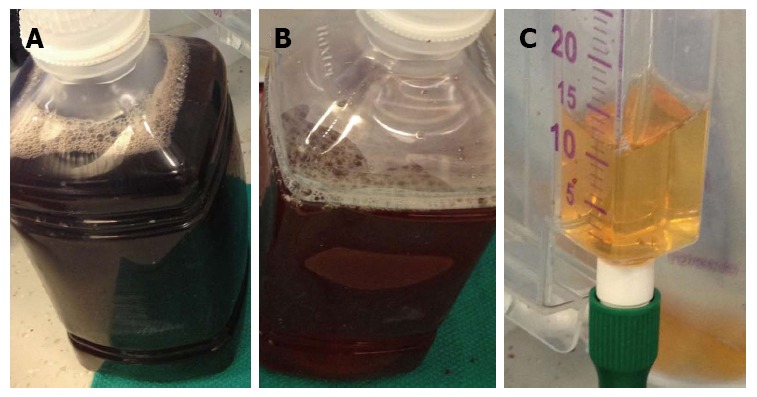
Urine color. A: Before CPB; B: After CPB and cessation of the old pump; C: After pump replacement. CPB: Cardiopulmonary bypass.
DISCUSSION
LVAD therapy requires a balance between anticoagulation and hemostasis to prevent the complications of bleeding and thrombosis. There are many anticoagulation regimens to achieve this goal, and most combine inhibition of the clotting cascade with warfarin and at least one antiplatelet agent. The optimal anticoagulation/anti-platelet strategy remains elusive because of the heterogeneity in the reaction between the biological components (i.e., blood) and artificial surfaces (i.e., LVAD) as well as the variability in the responsiveness to anticoagulants and anti-platelet medications[3]. As a result of this imperfect coexistence between “man” and “machine”, the leading causes of LVAD readmissions include bleeding and thrombosis. Thrombosis of the LVAD is a potentially lethal complication which occurs in 2% to 3% of the patients who receive the HeartMate II LVAD and the incidence is reported to be increasing[4-6]. Patients typically present with elevated pump power, heat over the pump, heart failure and signs of hemolysis. Echocardiography may show opening of the aortic valve due to inadequate decompression of the left ventricle (LV) and increased LV end-diastolic diameter. Serial recording of LV end-diastolic diameter while increasing the pump speeds may diagnose pump thrombus or other flow obstructions[7]. However, there have been reports of pump thrombosis with normal echo and pump parameters as well[8,9]. Hemolysis may be the only sign of thrombosis, although hemolysis may be due to various reasons, such as kinking of the outflow graft, malposition of the inflow cannula or the pump itself (high shear stress, etc.)[8,9]. The diagnostic challenge is that pump thrombus may not be visualized with contrast CT scan or echocardiography, due to artifacts caused by the metallic housing of the LVAD[8]. In our case, we were able to confirm that the hemolysis was due to pump thrombosis by intraoperative inspection of the removed pump and the resolution of the urine after pump exchange.
Change of urine color is easily noticeable to patients and should be promptly addressed as a sign of possible pump thrombosis. In the current era of non-pulsatile LVAD therapy, it is likely that the risk of pump thrombosis and hemolysis will remain, and LVAD exchange may be necessary in cases that are refractory to medical management. Fortunately, the modular nature of LVAD technology allows for pump exchange with a reasonable degree of safety; the mortality is reported to be 6% to 7%[10,11]. In contrast, medical management; adding anti-platelet agents such as dipyridamole or clopidogrel, increasing the dose of aspirin and/or increasing the target PT-INR for anticoagulation, resulted in a 48.2% mortality in the following six months after the diagnosis of pump thrombosis[6].
In conclusion, thrombosis during LVAD therapy is a potentially life-threatening complication requiring prompt diagnosis and management. We presented a report of LVAD thrombosis causing hemolysis and discoloration of the urine that resolved promptly after the pump exchange. The diagnosis is challenging, but we were able to confirm the diagnosis by intraoperative inspection of the pump and the prompt resolution of the discolored urine.
COMMENTS
Case characteristics
A 50-year-old-male with a history of HeartMate II implantation presented with discolored urine.
Clinical diagnosis
He denied any chest pain, shortness of breath, or edema.
Differential diagnosis
Discolored urine and the lab values suggesting hemolysis, occasional pump power spikes were thought to be due to pump thrombosis.
Laboratory diagnosis
Hemoglobin 7.6 g/dL; plasma free hemoglobin 52.0 mg/dL; PT-INR 1.7; serum creatinine 1.9 mg/dL; total bilirubin 1.1 mg/dL. The lactate dehydrogenase, haptoglobin, AST were not able to be measured due to severe hemolysis.
Imaging diagnosis
Echocardiography showed severe biventricular dysfunction and opening of the aortic valve with every heartbeat.
Treatment
The patient underwent pump exchange.
Related reports
Medical management resulted in a 48.2% mortality in the following six months after the diagnosis of pump thrombosis.
Experiences and lessons
The diagnosis of pump thrombosis is challenging, but the authors were able to confirm the diagnosis by intraoperative inspection of the pump and the prompt resolution of the discolored urine.
Peer review
The manuscript describes frequent complication of left ventricular assist device. The manuscript is well written and has a good structure with excellent images.
Footnotes
P- Reviewer: Rodriguez-Castro KI, Said SAM, Shah R S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.INTERMACS. Quarterly Statistical Report, 2013 4th Quarter. Available from: http: //www.uab.edu/medicine/intermacs/research/statistical-summaries.
- 2.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 3.Rossi M, Serraino GF, Jiritano F, Renzulli A. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg. 2012;15:733–740. doi: 10.1093/icvts/ivs297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, Frazier OH, Heatley G, Farrar DJ, John R. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–887. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 5.John R, Kamdar F, Liao K, Colvin-Adams M, Miller L, Joyce L, Boyle A. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008;136:1318–1323. doi: 10.1016/j.jtcvs.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 6.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 7.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764–1775. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer AL, Kuehn C, Weidemann J, Malehsa D, Bara C, Fischer S, Haverich A, Strüber M. Thrombus formation in a HeartMate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008;135:203–204. doi: 10.1016/j.jtcvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Bhamidipati CM, Ailawadi G, Bergin J, Kern JA. Early thrombus in a HeartMate II left ventricular assist device: a potential cause of hemolysis and diagnostic dilemma. J Thorac Cardiovasc Surg. 2010;140:e7–e8. doi: 10.1016/j.jtcvs.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moazami N, Milano CA, John R, Sun B, Adamson RM, Pagani FD, Smedira N, Slaughter MS, Farrar DJ, Frazier OH. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg. 2013;95:500–505. doi: 10.1016/j.athoracsur.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Ota T, Yerebakan H, Akashi H, Takayama H, Uriel N, Colombo PC, Jorde UP, Naka Y. Continuous-flow left ventricular assist device exchange: clinical outcomes. J Heart Lung Transplant. 2014;33:65–70. doi: 10.1016/j.healun.2013.07.003. [DOI] [PubMed] [Google Scholar]


