Abstract
Actinic prurigo is a photodermatosis that can affect the skin, conjunctiva and lips. It is caused by an abnormal reaction to sunlight and is more common in high-altitude living people, mainly in indigenous descendants. The diagnosis of actinic prurigo can be challenging, mainly when lip lesions are the only manifestation, which is not a common clinical presentation. The aim of this article is to report two cases of actinic prurigo showing only lip lesions. The patients were Afro-American and were unaware of possible Indian ancestry. Clinical exam, photographs, videoroscopy examination and biopsy were performed, and the diagnosis of actinic prurigo was established. Topical corticosteroid and lip balm with ultraviolet protection were prescribed with excellent results. The relevance of this report is to show that although some patients may not demonstrate the classical clinical presentation of actinic prurigo, the associated clinical and histological exams are determinants for the correct diagnosis and successful treatment of this disease.
Keywords: Actinic prurigo, Follicular cheilitis, Photodermatosis, High-altitude, Lip diseases
Core tip: The diagnosis of actinic prurigo can be challenging in the absence of classic clinical manifestations. Actinic prurigo is found in high-altitude living people, mainly in indigenous descendants. Disease onset is usually in childhood and rarely presents only on the lips. This study describes two rare cases from Rio de Janeiro city, Brazil, which is located at sea level. The patients were unaware of possible Indian ancestry. Moreover, actinic prurigo appeared in adulthood and lip lesions were the only manifestation. The associated clinical and histological exams are determinants for the correct diagnosis and successful treatment of this disease.
INTRODUCTION
Actinic prurigo (AP) is a type of photodermatosis, and is a rare familial inflammatory disease that primarily affects areas of skin exposed to the sun and can affect the lips and ocular conjunctiva (pseudopterygium formation)[1]. Pseudopterygium does not appear as a unique lesion in patients with AP, it is always preceded by skin and lip lesions, suggesting that this expression tends to appear later in the disease course. For this reason, the diagnosis of AP in its early stages is important to prevent subsequent complications[2]. AP of the lip, also known as follicular cheilitis, is mainly found on the vermillion of the lower lip. Lip lesions may appear early in the development of this disease and, consequently, its observation and accurate diagnosis can alert physicians or dentists to the possible development of other more severe lesions on the skin or conjunctiva[2]. AP occurs mainly in residents of high altitudes and affects ethnic groups, particularly in North and South America, who express major histocompatibility complex class I and II (HLA I and II), suggesting a genetic predisposition[3]. The aim of this article is to describe two cases of AP of the lips without the classical features of this disease (young age at onset, familial history, high-altitude living people, and an association with skin lesions).
CASE REPORT
Case one
A 63-year-old Afro-American woman presented to our Oral Diagnostic clinic complaining of lower lip lesions of 10 mo evolution, which had worsened in the last 6 mo. She was referred by two centers that had failed to establish the diagnosis. During physical exam, the lower lip showed edema, as well as multiple ulcers covered with yellowish crusts on the semimucosa (Figure 1A). The slightest touch or mouth opening resulted in significant bleeding, which, according to the patient was commonly observed. No alterations during intraoral examination were observed. The lesions were documented by clinical and videoroscopy images (Figure 1B) and were scraped for cytopathologic evaluation, which revealed moderate inflammation. Lip balm with ultraviolet (UV) protection was prescribed.
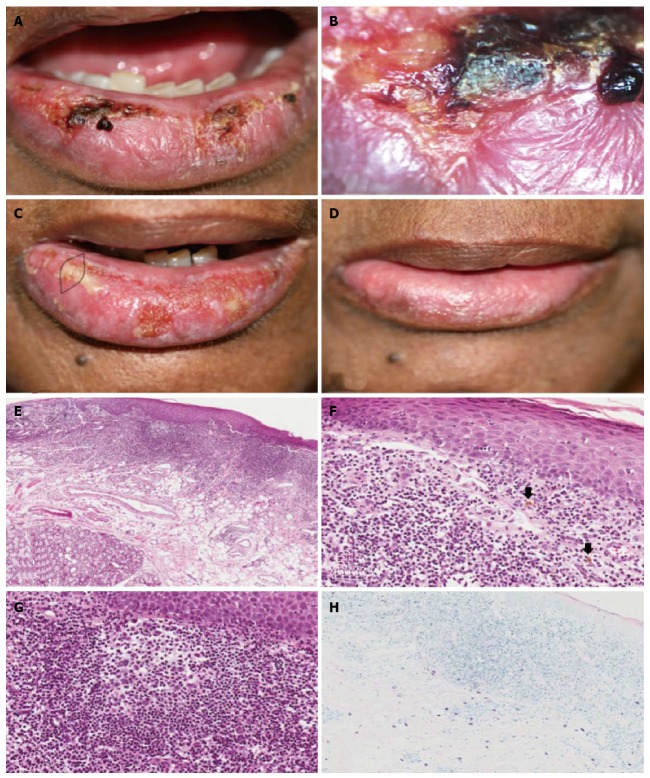
Figure 1.
Case 1. A: Clinical aspect at the first appointment, showing lower lip edema, ulcers and crusts; B: Videoroscopy image showing in detail the presence of ulcer and crust; C: Clinical aspect at the second appointment showing the area of biopsy; D: Clinical aspect one month after treatment, showing remission of the lip edema, ulcers and crusts; E: Histological aspects. Epithelial atrophy and intense diffuse lymphoplasmacytic inflammatory infiltrate extending deep into the fatty tissue (× 10, HE); F: Epithelium showing spongiosis, hydropic degeneration of the basal layer cells and lymphocytic exocytosis. In the connective tissue, lymphocytic inflammatory infiltrate and pigmentary incontinence (arrows) were observed (× 40, HE). G: Secondary lymphoid follicle (× 40, HE); H: Mast cells mainly in the deeper area of the connective tissue (× 20, Giemsa).
On the second visit, debridement of the lesions was performed, as well as a biopsy (the selected area was chosen by clinical and videoroscopy exam) (Figure 1C). The clinical diagnostic hypotheses were erythema multiforme and contact cheilitis. Microscopically (Figure 1E-H), the surface epithelium showed orthokeratosis, with some areas of parakeratosis, atrophy and areas of acanthosis, as well as basal layer degeneration and lymphocytic exocytosis. Ulceration was also present. The connective tissue exhibited pigmentary incontinence close to the overlying epithelium, dilated blood vessels, edema and intense and diffuse lymphocytic inflammatory infiltrate, with some plasma cells, extending deep into the fatty tissue. Some secondary lymphoid follicles were also present. Several mast cells were present predominantly in the deeper area of the connective tissue, mainly in the perivascular and perineural areas. Nonspecific chronic sialadenitis with ductal ectasia was also observed. There was no solar elastosis. The diagnosis of follicular cheilitis was established.
Following diagnosis, a combination of triamcinolone acetonide cream, neomycin sulfate, gramicidin and nystatin cream was prescribed three times a day. The patient was instructed to use gauze compresses with cold physiological saline and to continue using lip balm with UV protection. The patient was also referred to the dermatology and ophthalmology service for evaluation of signs and symptoms of AP. No ocular or skin lesions were observed.
Complete remission of the lip ulcers and crusts was observed after one month of treatment (Figure 1D). The patient was followed-up monthly for three months without evidence of recurrence. Two months after diagnosis and during the follow-up period, the patient reported she was of indigenous Brazilian descent. After the third consecutive monthly follow-up, the patient was followed-up every 4 mo to date (2 years after the first visit), and showed no lip lesions (Figure 1D). The patient did not develop any skin or ophthalmic lesions.
Case two
A 58-year-old Afro-American woman, presented to our Oral Diagnostic clinic complaining of a painful lesion on the lower lip of four years evolution. Physical examination showed the presence of a yellowish crust of 1.3 cm × 0.8 cm, on the left side, which was easily seen during the examination, revealing an ulcerated area. The lips were swollen and dry (Figure 2A). The lesions were documented by clinical and videoroscopy images (Figure 2B) and were scraped for cytopathologic evaluation, which revealed moderate inflammation. No alterations were observed during the intraoral examination. Lip balm with UV protection was prescribed. On the second visit, a biopsy was performed (the selected area was chosen by clinical and videoroscopy exam). The diagnostic hypotheses were erythema multiforme and acute actinic cheilitis. Microscopically (Figure 2C and D), the lesion was covered by stratified ortokeratinized squamous epithelium showing atrophy, ulceration, spongiosis and hydropic degeneration of the basal layer. The underlying connective tissue showed pigmentary incontinence close to the overlying epithelium, dilated blood vessels with areas of intense inflammatory infiltrate, mainly composed of lymphoplasmacytic cells, and the formation of well-formed secondary lymphoid follicles. Mast cells were also observed between the lymphocytes and plasma cells. The inflammatory infiltration extended deep into the fatty tissue. There was no solar elastosis. The diagnosis of follicular cheilitis was established.
Figure 2.
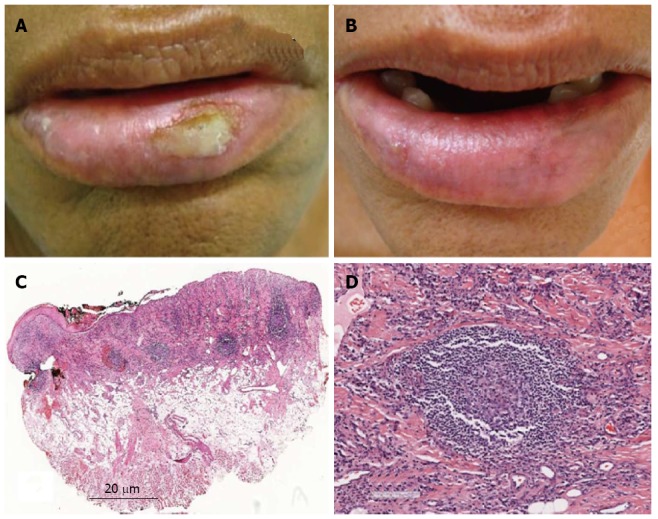
Case 2. A: Clinical aspect at the first appointment, showing lower lip edema, dryness and ulcer on the left side of the semimucosa; B: Clinical aspect at the second appointment, showing remission of the ulceration on the left side, and only a small ulcer on the right side of the semimucosa; C: Histological aspects. Lower power view showing epithelial atrophy and ulceration. In the connective tissue, an intense, diffuse inflammatory infiltrate extending deep into the fatty tissue, with some lymphoid follicles was observed (HE); D: Secondary lymphoid follicle (HE).
The patient was followed-up (one month after the first visit) and showed remission of the ulceration on the left side, with only a small ulcer on the right side of the lip (Figure 2B). She was referred for dermatological and ophthalmological evaluation and asked to return to our clinic one month later. The patient did not return.
A search of the medical literature was performed by two authors separately, using Pubmed, Lilacs, Scielo and Cochrane databases, without year and language restriction, using the terms: (1) prurigo AND actinic; and (2) follicular AND cheilitis. The last search was performed in November 2013. A paper considered eligible for inclusion on review had to include a case report or a study with at least one case under the name “actinic prurigo” or “follicular cheilitis” and with lip lesions as the only manifestation (Table 1). Only two papers satisfied the criteria: Vega-Memije et al[2] and Mounsdon et al[4]. In the study by Vega-Memije et al[2], 116 patients presented with actinic prurigo cheilitis; of these, 74 (63.8%) were female, aged from 9 to 82 years (mean, 27.8 years). Ninety-nine percent of the patients lived in areas more than 1000 m above sea level and only one case was from a geographic area below this altitude. AP cheilitis was the only manifestation of the disease in 32 (27.6%) patients. Mounsdon et al[4] described two North American Indians, one man and one woman, who showed only lip lesions, however, there was no information on their place of residence. In addition, a thesis describing a study of 43 patients with actinic prurigo of the lips was found in Google Scholar[5]. Although this study was carried out in Brazil, it was a retrospective analysis of patients resident in Mexico, where this disease is very common. In 17 (39.54%) cases, the lesion was located only on the lips. To make comparative analyses with the cases presented in our paper, 16 patients in this study were included; one was excluded because the age of the patient was not provided. Patient age ranged from 11 to 63 (mean 26 years). Information on where the patients lived was not provided (Table 2).
Table 1.
Results of the literature search for “actinic prurigo” or “follicular cheilitis” of the lip
| Actinic prurigo | Follicular cheilitis | Eligible paper1 | |
| Pubmed | 143 | 9 | 2 |
| Lilacs | 25 | 0 | 0 |
| Scielo | 3 | 0 | 0 |
| Cochrane | 7 | 1 | 0 |
A paper considered eligible for inclusion in the review had to include a case report or a study with at least one case under the name “actinic prurigo” or “follicular cheilitis”, and show lip lesions as the only manifestation.
Table 2.
Data from patients with actinic prurigo, with only lip lesions
DISCUSSION
Photodermatoses form an important group of skin diseases, which can be disabling to the patient, and represent a challenge in diagnosis and treatment[6]. Although dark skin has larger quantities of melanin compared to white skin, which gives greater protection against the sun’s rays, photodermatoses are common in dark-skinned people[7]. AP is an example of a photodermatosis that affects mostly Mestizos in the Americas. This is the result of miscegenation between Europeans and Indians, which prevails in Mexico, Guatemala, Honduras, Colombia, Ecuador, Peru, Bolivia, and Argentina, and in some indigenous communities in North America and Canada[8-10]. AP usually begins in childhood, around 4-5 years old[5], although it can manifest at any age, affecting more women than men (2:1), and in some cases with familial history[11].
The severity of the disease is altitude-dependent, presumably because of the sustained intensity of sun exposure. It is believed that this is the reason why AP is found mostly in regions with altitude above 1000 m[3]. These data make our cases interesting, as both patients lived in Brazil, in cities at sea level, and did not report being indigenous descendants during anamnesis, did not have a positive familial history, and showed the first signs and symptoms in adulthood.
AP lesions are mainly found in sun-exposed areas[3,12,13]. Lips and conjunctiva can also be affected[3,12]. Nevertheless, in Asians, conjunctivitis and cheilitis are not common[14]. The patients presented in this paper showed lip lesions as the only manifestation of AP. Although there are few reports and studies in the literature regarding patients with AP showing only lip lesions, this may occur in up 40% of cases[5]. In cases of AP with lip lesions as the only manifestation it is more difficult to establish an accurate diagnosis, which should alert clinicians to the possibility of the development of other more severe lesions, such as skin or conjunctival lesions. Therefore, it is important to refer these patients for ophthalmological and dermatological evaluation.
AP lip lesions are characterized by swelling, peeling, cracking, crusting, itching, exudation, and secondary ulceration[3,12]. Cheilitis intensity is variable. In the acute phase, yellow crusts adhered to the surface are observed, whereas in the chronic phase, the lesions are covered with dry scales, and the course is generally prolonged, with relapses worsened by constant sun exposure[2,8,5].
During the evaluation of our patients, we used videoroscopy which enabled better visualization of the lip lesions. As both patients showed extensive lesions, the choice of the biopsy area was difficult and videoroscopy was used to help choose the best biopsy area. The lesions were similar to those of AP lip lesions described in the literature.
Clinical differential diagnoses regarding AP include actinic cheilitis, frictional contact cheilitis and granulomatous cheilitis[5]. In the present cases, we also considered the possibility of acute actinic cheilitis, which was later rejected due the evolution time and because the patients did not report intense sunlight exposure. The other clinical diagnoses were erythema multiforme, which was rejected due to the course of the lesions, and contact cheilitis, but we were unable to identify a substance which could cause the lip lesions, especially over such a long time. Although several clinical factors associated with follicular cheilitis were not observed in the present cases, the clinical exam associated with the histopathological diagnosis was a determinant in establishing the final diagnosis.
Studies in the literature define the histopathological pattern of AP lip lesions as showing acanthosis, spongiosis and basal layer hydropic degeneration[2]. Areas of ulceration may also be seen. Edema, dilated and congested vessels, with dense predominantly lymphocytic inflammatory infiltrate, which may contain lymphoid follicles and eosinophils are also seen in the connective tissue[2,4,12]. Furthermore, some studies report that discrete exocytosis in the basal epithelium and pigmentary incontinence in the sub epithelial connective tissue may be observed[2]. The presence of lymphoid follicles is considered by some authors to be a pathognomonic feature of AP and this is the reason why the term follicular cheilitis is used[12]. Mast cells and macrophages may be found in the inflammatory infiltrate[5]. The histopathological findings in our cases are consistent with the description in the literature. The identification of lymphoid follicles in both cases was important in establishing the diagnosis.
No solar elastosis was found in the AP lesions, which facilitates the differential diagnosis from actinic cheilitis[2,4,5,12]. It is necessary to differentiate AP from polymorphic light eruption, which is clinically similar, but microscopically does not show lymphocytic infiltrate with lymphoid follicles[12].
With regard to the treatment of AP, as a general measure, it is recommended to reduce sun exposure, use protective clothing including hats, and sunscreen. However, these measures are not sufficient to treat AP. There is evidence that AP is an autoimmune disease, and therefore immunosuppressive drugs produce good results[3]. Treatment of AP varies according to the severity and extent of the lesions, and includes topical and systemic corticosteroids to reduce the inflammation and itching of active lesions, antibiotics for secondary infections, antihistamines, antimalarials and thalidomide, which have been shown to be the most effective drugs for the treatment of AP[12,15-18].
AP prognosis is not good, despite several treatment options, the lesions may have a chronic course and are difficult to control if patients live in sunny areas, are occupationally exposed to the sun or live in high altitudes[19]. In case 1, the patient responded well to treatment with a topical corticosteroid and prevention measures; she had no lesions up to the last follow-up (14 mo after diagnosis). The patient in case 2 was treated only with prevention measures (including the use of lip balm with UV protection). In the follow-up, one month after diagnosis, the lesions disappeared, but she did not return for her follow-up appointment.
AP is a well-known disease, occurring mainly in Mestizos, living in high altitudes with onset during childhood. The cases presented here were a challenge to diagnose as the clinical characteristics were different from the classical manifestations of AP: the lesions began in adulthood, the patients lived at sea level and did not report, at least during the interview, being indigenous descendants, and neither reported having a familial history of alterations. In these cases, without skin lesions, the diagnosis of AP in the early stages is important, as it can alert the clinician to the possible development of other more severe lesions, and, thus, referring the patients for an ophthalmologic and dermatologic evaluation is mandatory.
COMMENTS
Case characteristics
This paper reports two cases of actinic prurigo in which the lower lips were the only sites of involvement.
Clinical diagnosis
The relevance of these cases is that, although some important aspects do not follow the classical features of actinic prurigo, the associated clinical and histological exams can be determinants of the correct diagnosis and successful treatment.
Imaging diagnosis
Clinical exam, photographs, videoroscopy examination and biopsy were performed, and the diagnosis of actinic prurigo was established.
Peer review
It is an interesting case, it is well written.
Footnotes
Supported by CNPq
P- Reviewer: Chong WS, Ozyigit MT, Torres-álvarez MB S- Editor: Wen LL L- Editor: Webster JR E- Editor: Lu YJ
References
- 1.Maga-a M, Domínguez R, Vázquez R, González N, Cazarín J. Prurigo solar en la ni-ez: manifestaciones cutáneas, oculares y labiales; Actinic prurigo in childhood: cutaneous, ocular and labial manifestations. Bol méd Hosp Infant Méx junho de. 1999;56:326–331. [Google Scholar]
- 2.Vega-Memije ME, Mosqueda-Taylor A, Irigoyen-Camacho ME, Hojyo-Tomoka MT, Domínguez-Soto L. Actinic prurigo cheilitis: clinicopathologic analysis and therapeutic results in 116 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:83–91. doi: 10.1067/moe.2002.123539. [DOI] [PubMed] [Google Scholar]
- 3.Mesa AMS. Prurigo actínico em la ninez. Dermatol Pediatr Lat. 2005;3:193–200. [Google Scholar]
- 4.Mounsdon T, Kratochvil F, Auclair P, Neale J, Lee L. Actinic prurigo of the lower lip. Review of the literature and report of five cases. Oral Surg Oral Med Oral Pathol. 1988;65:327–332. doi: 10.1016/0030-4220(88)90117-x. [DOI] [PubMed] [Google Scholar]
- 5.Rizo VHT. Estudo clínicopatológico e imunoistoquímico de prurigo actínico de lábio [dissertation] [Piracicaba]: Universidade Estadual de Campinas;; 2009. [Google Scholar]
- 6.Gambichler T, Al-Muhammadi R, Boms S. Immunologically mediated photodermatoses: diagnosis and treatment. Am J Clin Dermatol. 2009;10:169–180. doi: 10.2165/00128071-200910030-00003. [DOI] [PubMed] [Google Scholar]
- 7.Sharma VK, Sahni K, Wadhwani AR. Photodermatoses in pigmented skin. Photochem Photobiol Sci. 2013;12:65–77. doi: 10.1039/c2pp25182e. [DOI] [PubMed] [Google Scholar]
- 8.Hojyo-Tomoka T, Vega-Memije E, Granados J, Flores O, Cortés-Franco R, Teixeira F, Domínguez-Soto L. Actinic prurigo: an update. Int J Dermatol. 1995;34:380–384. doi: 10.1111/j.1365-4362.1995.tb04435.x. [DOI] [PubMed] [Google Scholar]
- 9.Hojyo-Tomoka MT, Cortés-Franco R, Domínguez-Soto L, Vega-Memije E, Teixeira F, Reyes M. Follicular cheilitis. Am J Dermatopathol. 1996;18:330–331. doi: 10.1097/00000372-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Duran MM, Bernal J. HLA typing in actinic prurigo. J Am Acad Dermatol. 1992;26:658. doi: 10.1016/s0190-9622(08)80803-1. [DOI] [PubMed] [Google Scholar]
- 11.Arrese JE, Dominguez-Soto L, Hojyo-Tomoka MT, Vega-Memije E, Cortés-Franco R, Guevara E, Piérard GE. Effectors of inflammation in actinic prurigo. J Am Acad Dermatol. 2001;44:957–961. doi: 10.1067/mjd.2001.113477. [DOI] [PubMed] [Google Scholar]
- 12.Hojyo-Tomoka MT, Vega-Memije ME, Cortes-Franco R, Domínguez-Soto L. Diagnosis and treatment of actinic prurigo. Dermatol Ther. 2003;16:40–44. doi: 10.1046/j.1529-8019.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 13.Lane PR, Hogan DJ, Martel MJ, Reeder B, Irvine J. Actinic prurigo: clinical features and prognosis. J Am Acad Dermatol. 1992;26:683–692. doi: 10.1016/0190-9622(92)70093-u. [DOI] [PubMed] [Google Scholar]
- 14.Ker KJ, Chong WS, Theng CT. Clinical characteristics of adult-onset actinic prurigo in Asians: a case series. Indian J Dermatol Venereol Leprol. 2013;79:783–788. doi: 10.4103/0378-6323.120726. [DOI] [PubMed] [Google Scholar]
- 15.Domínguez-Soto L, Hojyo-Tomoka MT, Vega-Memije E, Cortés-Franco R, Waxtein L, Guevara E. Photodermatoses in tropical countries. Clin Dermatol. 1999;17:237–243; discussion 105-106. doi: 10.1016/s0738-081x(99)00015-2. [DOI] [PubMed] [Google Scholar]
- 16.Ng JC, Foley PA, Crouch RB, Baker CS. A case of severe actinic prurigo successfully treated with thalidomide. Australas J Dermatol. 2001;42:192–195. doi: 10.1046/j.1440-0960.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 17.Crouch R, Foley P, Baker C. Actinic prurigo: a retrospective analysis of 21 cases referred to an Australian photobiology clinic. Australas J Dermatol. 2002;43:128–132. [PubMed] [Google Scholar]
- 18.Ross G, Foley P, Baker C. Actinic prurigo. Photodermatol Photoimmunol Photomed. 2008;24:272–275. doi: 10.1111/j.1600-0781.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 19.Akaraphanth R, Sindhavananda J, Gritiyarangsan P. Adult-onset actinic prurigo in Thailand. Photodermatol Photoimmunol Photomed. 2007;23:234–237. doi: 10.1111/j.1600-0781.2007.00316.x. [DOI] [PubMed] [Google Scholar]