Abstract
Patients with chronic hepatitis B are at significant risk for hepatocellular carcinoma (HCC). Globally, over half a million people each year are diagnosed with HCC, with marked geographical variations. Despite overwhelming evidence for a causal role of hepatitis B virus (HBV) infection in the development of HCC and a well-established relationship between high baseline hepatitis B viral load and cumulative risk of HCC, the molecular basis for this association has not been fully elucidated. In addition, a beneficial role for antiviral therapy in preventing the development of HCC has been difficult to establish. This review examines the biological and molecular mechanisms of HBV-related hepatocarcinogenesis, recent results on the effect of modern nucleos(t)ides on the rate of HCC development in high risk HBV cohorts and the potential mechanisms by which long-term antiviral therapy with potent inhibitors of HBV replication might reduce the risk of HCC in patients with chronic hepatitis B. Although evidence from randomized controlled trials shows the favourable effects of antiviral agents in achieving profound and durable suppression of HBV DNA levels while improving liver function and histology, robust evidence of other long-term clinical outcomes, such as prevention of HCC, are limited.
Keywords: Chronic hepatitis B, Entecavir, Hepatitis B virus, Hepatocellular carcinoma, Hepatocarcinogenesis, Nucleoside analogues, Risk reduction
Core tip: There is overwhelming evidence for the causal role of hepatitis B virus (HBV) infection in the development of hepatocellular carcinoma (HCC). However, evidence for the role of antiviral therapy in HCC prevention is inconclusive, in part due to the slow course of HCC development, which makes conducting outcome studies very challenging, while the effectiveness of modern antiviral agents in suppressing HBV means that untreated control group comparisons are ethically unacceptable. We review the impact of HBV treatment on the risk of HCC development, with special focus on emerging data for modern anti-HBV drugs such as entecavir and tenofovir.
INTRODUCTION
Worldwide, hepatocellular carcinoma (HCC) is diagnosed in over 500000 people each year[1]. Increasing age, male sex and chronic alcohol consumption are significant risk factors for the development of HCC. Although there is substantial geographical variation, the greatest burden of the disease is in East Asia, Eastern Europe and sub-Saharan Africa, where hepatitis B virus (HBV) infection is highly prevalent[1-4].
Globally, HBV infection is associated with approximately half of all cases of HCC, and almost all cases of HCC in children[1]. Chronic HBV infection may progress to cirrhosis and liver decompensation and, the majority (up to 80%) of patients with HBV-related HCC have underlying cirrhosis. The known risk factors for HBV-related HCC can be categorized into host factors, virus factors, and host-virus interactions. Host factors include male gender, Asian race, age older than 40 years, exposure to the mycotoxin aflatoxin, habitual smoking or alcohol consumption, and a family history of HCC[1-3,5,6]. Virus factors can include coinfection with hepatitis C virus (HCV) or hepatitis delta virus, pre-core (Pre-C) or basal core promoter mutations, high levels of HBV hepatocellular replication, and HBV genotype C. Host-virus interactions include the presence of cirrhosis, prolonged circulating hepatitis B surface antigen (HBsAg) and hepatitis Be antigen (HBeAg), and high levels of DNA-HBV and HBsAg.
Familial aggregation of risk for HCC has been well described in case-control studies in Asia[7-9]. In one study, the risk associated with having parents and/or siblings with HCC was evaluated in a large cohort of male HBV carriers, in a case-control study of HBV carriers with newly diagnosed HCC and HBV-positive subjects without HCC[9]. There was an increased risk for both HCC and cirrhosis for mothers and siblings but, of interest, not for fathers of case subjects[9]. For HCC, the adjusted odds ratios (ORs) according to kinship were 2.64 for mothers (95%CI: 1.60-4.34), 3.73 (2.64-5.27) for brothers, and 4.55 (2.22-9.31) for sisters, while the OR for fathers was only 1.36 (0.86-2.11). Overall, HBV carriers with a family history of HCC had an adjusted OR of 2.41 (95%CI: 1.47-3.95) for HCC if one relation was affected, rising to 5.55 (2.02-15.26) when two or more relations had HCC.
The precise mechanism of this familial aggregation is unclear, but may in part be a result of a higher HBsAg carrier rate among mothers and siblings of HBV carriers compared with fathers, as a result of vertical transmission. Furthermore, although less well investigated, a family history of HCC also appears to increase HCC risk in Western populations[10].
There is a well-established relationship between cumulative risk of HBV-related HCC and baseline viral load (baseline serum HBV DNA). Elevated HBV DNA level is strongly predictive of HCC, independent of HBeAg status, serum alanine aminotransferase (ALT) level and cirrhosis, with a cumulative incidence rate of HCC at the end of the 13th year of follow up in a large prospective cohort study of 3653 subjects ranging from 1.30% for subjects with serum HBV DNA level of less than 300 copies/mL at study entry to 14.89% for an HBV DNA level of 106 copies/mL or greater at study entry[11]. A significant biological gradient of HCC risk in patients with higher baseline levels was also observed, independent of viral load achieved after treatment[11]. Subjects with similar HBV DNA levels at last follow-up but with higher viral loads at study entry had significantly higher risk of HCC than those with lower HBV DNA levels at study entry (Figure 1).
Figure 1.
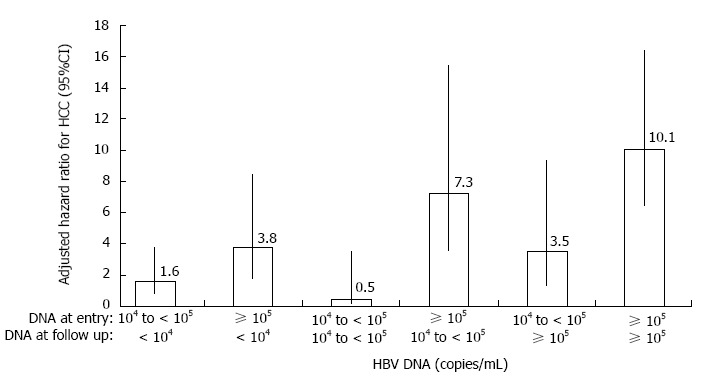
Adjusted hazard ratio for hepatocellular carcinoma by serum hepatitis B virus DNA levels at study entry and last follow-up. Data were adjusted for gender, age, cigarette smoking and alcohol consumption using Cox proportional hazards model. HCC: hepatocellular carcinoma; HBV: Hepatitis B virus. Data sourced from Chen et al[11].
These findings suggest the importance of close clinical monitoring for those with elevated serum HBV DNA, and that effective antiviral treatment may be valuable to lower the risk of HCC in patients with chronic HBV.
This article will review the current knowledge of the mechanisms of HBV-related hepatocarcinogenesis, examine the role of antiviral agents in reducing the risk of HCC, and discuss potential mechanisms for HCC risk reduction during long-term antiviral therapy.
LONG-TERM VIRAL SUPPRESSION AND LIVER-RELATED OUTCOMES IN CHRONIC HEPATITIS B
Long-term suppression of HBV is associated with substantial histological improvement and reversal of fibrosis or cirrhosis[12-15]. Strong correlations between viral load and histological grading, and between serum viral suppression and histological improvement have been observed[15], with indications that a greater than 1 log10 copies/mL change in median serum HBV DNA level will convert into a 2-point change in median histological grade. However, the direct contribution of antiviral treatment to the prevention of HBV-related HCC is less clear-cut. Interestingly, in HBeAg-negative patients, genotype B or C, low HBV-DNA and ALT levels and circulating HBsAg levels > 1000 IU/mL can predict hepatitis flares and progression[16].
The clinical benefit of first generation nucleoside analogues used in the treatment of HBV, such as lamivudine, is limited by the development of resistance and virological relapse after treatment cessation[12,17-19]. Entecavir and tenofovir dipivoxil are second generation nucleos(t)ide analogue reverse transcriptase inhibitors with potent activity against HBV and high genetic barrier to resistance[20-24]. Indeed, the cumulative annual incidence of resistance by year 6 of treatment may reach 76% (lamivudine) 29% (adefovir) and 25% (telbivudine), compared with 0%-1.2% for tenofovir dipivoxil and entecavir, respectively[25].
A systematic review and meta-analysis found that entecavir, which is an acyclic guanosine nucleoside analogue, and tenofovir dipivoxil, an acyclic adenine nucleotide, are the most effective antiviral agents for the treatment of chronic hepatitis B[26]. In evaluations of lamivudine, pegylated interferon, adefovir, entecavir, telbivudine, and tenofovir, as monotherapies and combination therapies in treatment-naive individuals, entecavir and tenofovir dipivoxil consistently ranked in the top five treatments for surrogate outcomes, whereas entecavir was ranked first with regard to improving liver histology, and tenofovir dipivoxil was ranked first for inducing undetectable HBV DNA and normalizing ALT levels[26].
The long-term efficacy of entecavir was demonstrated in an open-label extension study following two phase 3 clinical studies, in which entecavir for a total duration of at least 3 years significantly improved liver histology, biochemical markers and fibrosis, accompanied by potent viral suppression in nucleoside-naïve, HBeAg-positive and HBeAg-negative patients with advanced fibrosis or cirrhosis[27]. Similarly, in an open-label extension study after two 48-wk phase 3 studies in patients with advanced fibrosis or cirrhosis, long-term suppression of HBV DNA during treatment with tenofovir dipivoxil for at least 5 years led to regression of fibrosis and cirrhosis[13]. In these studies, long-term maintenance of viral suppression with entecavir and tenofovir dipivoxil was feasible because of favourable safety profiles and the absence of virological rebound or genotypic resistance[14,27].
BIOLOGICAL AND MOLECULAR MECHANISMS OF HEPATOCARCINOGENESIS
While there is overwhelming epidemiological evidence for a causal role of chronic HBV infection in the development of hepatocellular carcinoma, the molecular mechanisms of HBV tumourigenesis remain incompletely understood, although it can be seen as a multi-factorial process involving both direct and indirect components, some of which may act synergistically. A summary of potential mechanisms for the development of HCC in patients with chronic HBV infection is shown in Figure 2. It has been proposed that insertional activation of cellular cancer-related genes by HBV DNA integration, induction of genetic instability by viral integration or by the regulatory protein HBx, and host DNA mutations due to high hepatocyte turnover, cytokine and growth factor release in the setting of chronic liver inflammation, hepatocyte injury, proliferating fibroblasts, and fibrosis/cirrhosis, may be mechanisms associated with HBV-induced carcinogenesis[28-32].
Figure 2.
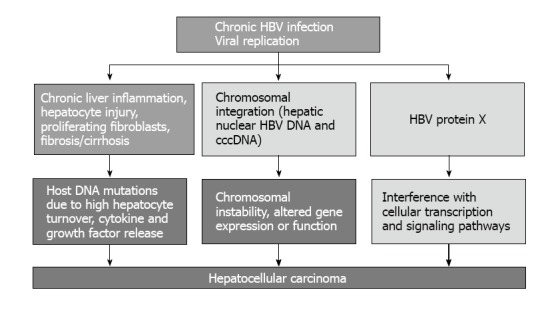
Mechanisms of chronic hepatitis B virus infection-related hepato-carcinogenesis[27-32]. cccDNA: Covalently closed circular DNA. HBV: Hepatitis B virus.
Among factors implicated in chronic HBV infection and hepatocarcinogenesis, HBx has an important role in activating HBV transcription and replication, and in the development of HCC, because it is involved in the activation of numerous signalling pathways and cellular promotors, activating the expression of genes involved in cell cycle control, oncogenesis, proliferation, inflammation and apoptosis[28-32]. HBx also modulates the transcriptional activity of CREB (cAMP responsive element-binding protein), which plays an essential role in liver metabolism and proliferation, and is associated with hepatocarcinogenesis[31].
A key mechanism for hepatocarcinogenesis is the integration of HBV DNA into the host genome and the formation of covalently closed circular DNA (cccDNA). This episomal form of viral DNA, which acts as a template for the transcription of viral genes and is responsible for the persistence of viral replication, is derived via a succession of biological steps following the transportation of relaxed HBV DNA into the nuclei of hepatocytes. Both cccDNA and HBV DNA sequences integrated into the host genome have transcriptional activity, resulting in synthesis of HBsAg[33].
Clearance of intrahepatic cccDNA and/or HBsAg is difficult to achieve but clinically meaningful endpoints for antiviral therapy in chronic hepatitis B, and may be associated with a decreased risk of developing HCC[33,34]. However, the exact role of antiviral treatment in preventing HBV-related HCC has been difficult to establish. Because of the slow biological evolution of HBV, longitudinal studies may necessitate continuation of antiviral treatment over decades, longer than most researchers or pharmaceutical companies can wait[2,35]. Furthermore, as modern antiviral agents are effective in suppressing viral replication[25,26,36-38], untreated control group comparisons are considered unethical and cannot be performed.
Recently, a large Taiwanese study showed that in HBsAg-positive patients, predictors of HCC included age, HBeAg status, HBV genotype, and ALT and HBV DNA levels, but not HBsAg levels; however, in a subgroup of HBeAg-negative patients with viral HBV-DNA < 2000 IU/mL, the risk of HCC significantly correlated with high HBsAg (≥ 1000 IU/mL), ALT and age, but not HBV-DNA[39].
ANTIVIRAL TREATMENT AND RISK OF HCC IN PATIENTS WITH CHRONIC HBV INFECTION
A number of systematic reviews and meta-analyses of the role of anti-HBV treatment in the prevention of HCC have been conducted[26,40-43], without conclusively demonstrating a beneficial impact on the preventing the development of HCC[2]. This is in part because of the inclusion of studies of older antiviral agents with limited antiviral potency and low genetic barriers, which are, therefore, associated with an increased risk of the development of HBV antiviral resistance mutations. In a recent electronic health records review of 2671 adults with chronic HBV infection enrolled in the Chronic Hepatitis Cohort Study, the adjusted hazard ratio (HR) for HCC risk in those receiving antiviral treatment was (HR = 0.39; 95%CI: 0.27-0.56; P < 0.001). In a subgroup analysis of patients with baseline laboratory data for serum fibrosis markers, antiviral treatment was associated with a lower risk of HCC after adjusting for cirrhosis markers of (adjusted HR, 0.24; 95%CI: 0.15-0.39; P < 0.001). In another subgroup analysis of patients with HBV DNA viral load data, in patients with HBV DNA > 20000 IU/mL, treated patients had a significantly lower risk of HCC compared with untreated patients[44].
In a recent meta-analysis of available randomized controlled trials, prospective cohort studies and case-control studies included 3433 treated patients and 4625 controls[42]. Antiviral treatment was shown to modestly reduce the incidence of HCC in patients with established cirrhosis, but there was no reduction in non-cirrhotic patients. A recent critical review[40] found that potent and persistent suppression of HBV viral load was more effectively maintained with nucleoside analogues than with other antivirals, leading to reversal of fibrosis and cirrhosis, and indications of a reduction in the incidence of HCC. However, this cannot be taken as high level evidence, as no direct data relating to entecavir and tenofovir dipivoxil were available in this analysis. Of five studies of oral antiviral agents included in the review (2036 patients treated with nucleoside analogues), all except one were retrospective, and most of were with lamivudine or adefovir, older agents[40]. However, all studies showed some reduction in HCC. The only randomized trial included in the systematic review was published in 2004, and showed that lamivudine reduced the incidence of cirrhosis and HCC in patients with chronic hepatitis B and advanced cirrhosis[18]. Ten studies of interferon-α showed inconsistent results, in part because interferon-α was associated with only moderate suppression of HBV DNA. However, recent evidence from two phase 3 clinical trials presented at the 2013 Annual Meeting of the European Association for the Study of the Liver (EASL) suggests that the observed incidence of HCC is lower than expected in patients with chronic hepatitis B treated with tenofovir dipivoxil[45]. The incidence of HCC was lower than predicted (as assessed by the REACH-B risk model), with a measurable effect in non-cirrhotic patients after 2 years, reaching a 55% reduction at 6 years of treatment (P = 0.05)[45]. Tenofovir dipivoxil had less effect in patients with cirrhosis.
The evidence base for the effect of entecavir on HCC risk is somewhat stronger than that for tenofovir dipivoxil, and will therefore be addressed separately in a subsequent section.
EVIDENCE FOR HCC RISK REDUCTION WITH ENTECAVIR
Although the major goals for therapy in chronic hepatitis B are to delay or prevent progressive liver disease and the development of cirrhosis and HCC[2], as yet no definitive evidence from randomized controlled trials has shown that antiviral therapy delays or prevents the development of HCC. However, there are a number of recent studies analyzing a potential beneficial impact of entecavir on the development of HCC.
A case-control study that followed a large cohort of Japanese patients with HBV for more than 5 years, compared 472 patients treated with entecavir with a historical cohort without treatment as a control group (n = 1143)[6]. The use of a propensity matching score, applied to match patients from both groups with the same baseline covariates of risk for HCC, minimized study biases. A total of 316 patients in each group (control and entecavir) were matched for comparison. The median follow-up was 3.3 years in the entecavir group and 7.6 years in the historical control group (P < 0.001). The cumulative rates of HCC at 5 years were 3.7% in the entecavir group and 13.7% in controls (P < 0.001), showing that entecavir significantly reduced the 5-year risk of developing HCC in treatment-naïve patients, compared with control (adjusted HR = 0.37, 95%CI: 0.15-0.91; P = 0.030). After multivariate analysis, age, alcohol consumption, pre-existing cirrhosis, HBeAg positive status and platelet count lower than 150000/mL were associated with risk of HCC development. Only entecavir was significantly associated with a reduction of HCC incidence (HR = 0.23, P = 0.001). The mutation resistance to drug was 0.8% (4/472) in the entecavir group. The reduction was greater in patients with cirrhosis, and was higher than that observed with a propensity score matched lamivudine cohort[6]. To assess the impact of entecavir treatment further, the authors applied several established risk models to three studies that utilized HCC risk scales, based on established risk factors for HCC[6].
Entecavir treatment significantly reduced the risk of HCC development in patients with high risk according to risk scores in the Yang et al[46] (P = 0.006) and Yuen studies et al[47] (P = 0.002), but not in low score patients. Likewise, in the Wong study[48], patients with a high risk score had a significant reduction in risk of developing HCC (P < 0.01), whereas there was a borderline significance in those with intermediate risk (P = 0.062) and no reduction in low risk patients.
Furthermore, entecavir may reduce the risk of HCC recurrence in patients with chronic hepatitis B. In a longitudinal study in patients with newly-diagnosed HCC treated with curative percutaneous radiofrequency ablation (RFA), entecavir administration significantly reduced the incidence of new HCC lesions, compared with patients who did not received treatment after RFA[49]. The risk of HCC recurrence was significantly lower in entecavir recipients than in nucleoside-naïve patients (OR = 0.077, P = 0.016), as well as in those treated with another nucleoside analogue (OR = 0.145, P = 0.012). Even when cases of marginal recurrence and recurrence within 6 months of initial treatment were excluded, eliminating the possibility of residual tumour or missed tumour at initial diagnosis, the risk of HCC recurrence was still significantly lower in the entecavir group than in nucleoside-naïve patients (OR = 0.198, P = 0.004).
However, in a recent “real life” multicentre Italian study, patients with cirrhosis were still at risk of developing HCC over time, despite profound and durable viral suppression with entecavir. A total of 418 nucleoside-naïve patients with HBV received entecavir for up to 66 mo in the study[50]. All patients achieved undetectable HBV DNA by year 5, regardless of baseline histology or HBeAg status; 62% achieved HBeAg seroconversion and the HBsAg loss rate was 33%[50]. Clinical decompensation did not occur during follow-up among the 164 patients with cirrhosis, indicating that entecavir was effective in preventing the progression of cirrhosis. Nevertheless, despite long-term viral suppression and successful prevention of decompensation of cirrhosis, the cumulative incidence of HCC in cirrhotic patients was still 14% at year 5 (2.8% per year). This suggests that some cellular clones of pre-malignant cells may have already developed before treatment was initiated, and emphasizes the importance of ongoing surveillance for HCC, particularly in patients with cirrhosis. Reviewing the evidence for a multistep model for the process of hepatocarcinogenesis, YN Park[35] concluded that dysplastic lesions consisting of microscopic dysplastic foci and macroscopic dysplastic nodules may be precursor lesions of HCC. Early detection of precursor lesions may be important in identifying patients at higher risk of developing HCC and, together with diagnosing early HCC, may improve long-term survival for patients with chronic hepatitis B by allowing early initiation of effective antiviral therapy.
As there is stronger evidence that entecavir reduces the risk of developing HCC in patients with associated risk factors such as older age, gender, high HBV viral load, cirrhosis, fibrosis, liver laboratory markers, and core promoter mutations, targeting HCC prophylaxis with entecavir to high-risk patients with chronic hepatitis B may be a rational therapeutic approach. However, this suggestion should be supported by appropriately designed trials.
POTENTIAL MECHANISMS OF HCC RISK REDUCTION IN PATIENTS ON LONG-TERM ANTIVIRAL TREATMENT
In addition to the robust relationship between higher baseline viral load and cumulative risk of HBV-related HCC, several other mechanisms may contribute to reducing HCC risk.
As cirrhosis is in itself a risk factor for HCC development[1,5], the reversal of cirrhosis associated with long-term HBV viral suppression by effective antiviral therapy may, at least in part, decrease the risk for HCC development. Patients treated with entecavir in two phase 3 studies in nucleoside-naïve patients with HBeAg-positive and HBeAg-negative disease, respectively, and who subsequently were treated in a long-term extension study, underwent liver biopsy after at least 3 years of treatment. Improvement in liver histology was observed in 96% of patients, including all patients with advanced fibrosis or cirrhosis at the phase 3 baseline[27].
Tenofovir dipivoxil also improved liver histology at week 240 of treatment in an open-label extension study following two 48-week phase 3 trials in which patients received tenofovir dipivoxil plus adefovir[14]. At the time of a repeat liver biopsy, 73% of HBeAg-positive patients and 85% of HBeAg-negative patients had normal serum levels of ALT, accompanied by profound viral suppression. A total of 87% of patients had histological improvement, including reversal of cirrhosis in 74% of those with cirrhosis at baseline[14].
These findings of biochemical and histological improvement with entecavir and tenofovir dipivoxil may partly explain a reduction of HCC development in high risk patients.
Inhibition of the intracellular recycling pathway leading to a decrease in levels of intrahepatic cccDNA has been observed during long-term viral suppression, and depletion of cccDNA occurs by hepatocyte turnover as a result of loss by natural liver cell division and/or cell death during injury/regeneration cycles[33,51]. Currently, determination of cccDNA is not feasible by non-invasive means as a liver biopsy is required, and it has been proposed that serum HBsAg quantification may be used as a surrogate marker for cccDNA levels[2,33]. However, a recent study showed that, despite profound HBV DNA reduction, HBsAg and cccDNA decline was small on a short-term basis (1 year), and the magnitude of HBsAg reduction did not correlate with cccDNA[34].
Overall, these results suggest that even when HBV DNA intermediates are suppressed by nucleoside analogues, HBV may still replenish cccDNA by preferentially transporting the viral genome back to the hepatocyte nucleus instead of being enveloped and exocytosed to peripheral blood[34].
If clearance of cccDNA might contribute to decreased risk of HCC[23,33], it is likely that long-term therapy is needed to eliminate intrahepatic cccDNA, and it is therefore interesting that 48 wk of treatment with entecavir has very recently been shown to result in significantly greater reductions from baseline hepatic HBV cccDNA levels, as well as total hepatic HBV DNA, than lamivudine[52]. In this, the ETV-022 trial, cccDNA reduction was related to lower baseline serum HBV DNA and lower baseline necroinflammation. In addition, greater reduction of cccDNA at week 48 was associated with a higher on-treatment reduction in HBV DNA, Knodell necroinflammatory score and serum ALT, as well as higher HBeAg clearance[52].
CONCLUSION
Although there is overwhelming evidence of the causal role of HBV infection in the development of hepatocarcinogenesis, the evidence for the role of long-term antiviral therapy in the prevention of HCC in patients with chronic hepatitis B is modest. The limited evidence may, in part, be related to the difficulties of conducting longitudinal outcome studies, as HCC develops slowly, necessitating very long-term follow-up studies, and the effectiveness of modern antiviral agents in suppressing viral replication means that untreated control group comparisons are not considered ethically acceptable. However, there is persuasive evidence that entecavir reduces the risk of developing HBV-related HCC, particularly in high-risk patients. Entecavir also lowers the risk of recurrence after radiofrequency ablation of HCC. As HCC development is rare in low-risk patients, longer follow-up durations are needed to fully assess the potential effect of entecavir on preventing the development of HCC in patients with HBV infection.
In summary, there is emerging evidence suggesting that treatment with entecavir or tenofovir dipivoxil (though less extensive than with entecavir) significantly reduces, but does not completely eliminate, HCC risk in patients with HBV-associated cirrhosis.
ACKNOWLEDGEMENTS
We thank Ray Hill, an independent medical writer, who provided medical writing support on behalf of inScience Communications, Springer Healthcare.
Footnotes
Supported by Bristol-Myers Squibb
P- Reviewer: Toshikuni N S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49:S4–S12. doi: 10.1002/hep.22946. [DOI] [PubMed] [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.Rossi C, Shrier I, Marshall L, Cnossen S, Schwartzman K, Klein MB, Schwarzer G, Greenaway C. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One. 2012;7:e44611. doi: 10.1371/journal.pone.0044611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Jiang Q, Zhou X, Ding B, Wang R, Zhao G, Chen Y. HBV infection and familial aggregation of liver cancer: an analysis of case-control family study. Cancer Causes Control. 2004;15:845–850. doi: 10.1023/B:CACO.0000043435.59195.3c. [DOI] [PubMed] [Google Scholar]
- 8.Park CH, Jeong SH, Yim HW, Kim JD, Bae SH, Choi JY, Yoon SK. Family history influences the early onset of hepatocellular carcinoma. World J Gastroenterol. 2012;18:2661–2667. doi: 10.3748/wjg.v18.i21.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu MW, Chang HC, Liaw YF, Lin SM, Lee SD, Liu CJ, Chen PJ, Hsiao TJ, Lee PH, Chen CJ. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst. 2000;92:1159–1164. doi: 10.1093/jnci/92.14.1159. [DOI] [PubMed] [Google Scholar]
- 10.Turati F, Edefonti V, Talamini R, Ferraroni M, Malvezzi M, Bravi F, Franceschi S, Montella M, Polesel J, Zucchetto A, et al. Family history of liver cancer and hepatocellular carcinoma. Hepatology. 2012;55:1416–1425. doi: 10.1002/hep.24794. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF. Impact of therapy on the outcome of chronic hepatitis B. Liver Int. 2013;33 Suppl 1:111–115. doi: 10.1111/liv.12057. [DOI] [PubMed] [Google Scholar]
- 13.Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274–276. doi: 10.1016/j.cgh.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 15.Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37:1309–1319. doi: 10.1053/jhep.2003.50208. [DOI] [PubMed] [Google Scholar]
- 16.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Hsu CA, Kuo SF, Liu CH, Chen PJ, et al. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology. 2013;57:441–450. doi: 10.1002/hep.26041. [DOI] [PubMed] [Google Scholar]
- 17.Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z, et al. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344–352. doi: 10.1111/j.1365-2036.2011.04738.x. [DOI] [PubMed] [Google Scholar]
- 18.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 19.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 20.Jenh AM, Pham PA. Tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Expert Rev Anti Infect Ther. 2010;8:1079–1092. doi: 10.1586/eri.10.91. [DOI] [PubMed] [Google Scholar]
- 21.Buti M, Homs M. Tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Expert Rev Gastroenterol Hepatol. 2012;6:413–421. doi: 10.1586/egh.12.19. [DOI] [PubMed] [Google Scholar]
- 22.Scott LJ, Keating GM. Entecavir: a review of its use in chronic hepatitis B. Drugs. 2009;69:1003–1033. doi: 10.2165/00003495-200969080-00005. [DOI] [PubMed] [Google Scholar]
- 23.Perry CM, Simpson D. Tenofovir disoproxil fumarate: in chronic hepatitis B. Drugs. 2009;69:2245–2256. doi: 10.2165/10482940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Keating GM. Entecavir: a review of its use in the treatment of chronic hepatitis B in patients with decompensated liver disease. Drugs. 2011;71:2511–2529. doi: 10.2165/11208510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Petersen J, Buti M. Considerations for the long-term treatment of chronic hepatitis B with nucleos(t)ide analogs. Expert Rev Gastroenterol Hepatol. 2012;6:683–693; quiz 694. doi: 10.1586/egh.12.52. [DOI] [PubMed] [Google Scholar]
- 26.Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DK, Pham B, Ungar WJ, Einarson TR, Heathcote EJ, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139:1218–1229. doi: 10.1053/j.gastro.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 27.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 28.Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13:74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652–1656. doi: 10.3748/wjg.14.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 32.Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4853–4857. doi: 10.3748/wjg.v17.i44.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoulim F, Testoni B, Lebossé F. Kinetics of intrahepatic covalently closed circular DNA and serum hepatitis B surface antigen during antiviral therapy for chronic hepatitis B: lessons from experimental and clinical studies. Clin Gastroenterol Hepatol. 2013;11:1011–1013. doi: 10.1016/j.cgh.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Wong DK, Seto WK, Fung J, Ip P, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol. 2013;11:1004–1010.e1. doi: 10.1016/j.cgh.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Park YN. Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med. 2011;135:704–715. doi: 10.5858/2010-0524-RA.1. [DOI] [PubMed] [Google Scholar]
- 36.Lam YF, Yuen MF, Seto WK, Lai CL. Current Antiviral Therapy of Chronic Hepatitis B: Efficacy and Safety. Curr Hepat Rep. 2011;10:235–243. doi: 10.1007/s11901-011-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–1075. doi: 10.1002/hep.21627. [DOI] [PubMed] [Google Scholar]
- 38.Ayoub WS, Keeffe EB. Review article: current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2011;34:1145–1158. doi: 10.1111/j.1365-2036.2011.04869.x. [DOI] [PubMed] [Google Scholar]
- 39.Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140–1149.e3; quiz e13-e14. doi: 10.1053/j.gastro.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57:399–408. doi: 10.1002/hep.25937. [DOI] [PubMed] [Google Scholar]
- 41.Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Thiele M, Gluud LL, Dahl EK, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma and mortality in chronic hepatitis B: systematic review and meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 44.Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V, Boscarino JA, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim W, Berg T, Loomba R, Schall RA, Dinh P, Yee L, Martins E, Flaherty J, Gurel S, Buti M. Long term tenofovir disoproxil fumarate (TDF) therapy and the risk of hepatocellular carcinoma [Abstract]; 48th Annual Meeting of the European Association for the Study of the Liver (EASL 2013); 2013 April 24-28; Amsterdam, The Netherlands. 2013. p. S19. [Google Scholar]
- 46.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 47.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 48.Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660–1665. doi: 10.1200/JCO.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 49.Lee D, Lee J-H, Cho Y, Lee YB, Kwon JH, Yu SJ, Kim YJ, Yoon J-H, Lee H-S, Kim CY. Entecavir treatment significantly reduces the risk of hepatocellular carcinoma recurrence in patients with chronic hepatitis B [Abstract]; 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9-13; Boston, MA, USA. Wiley-Blackwell 111 River St, Hoboken 07030-5774, NJ USA: 2012. pp. 371A–371A. [Google Scholar]
- 50.Lampertico P, Soffredini R, Vigano M, Minola E, Cologni G, Rizzi M, Zaltron S, Vavassori A, Carosi G, Angeli E. Entecavir treatment for NUC na?ve, field practice patients with chronic hepatitis B: excellent viral suppression and safety profile over 5 years of treatment; 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); 2012 November 9-13; Boston, MA, USA. Wiley-Blackwell 111 River St, Hoboken 07030-5774, NJ USA: 2012. pp. 370A–371A. [Google Scholar]
- 51.Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11:909–916. [PubMed] [Google Scholar]
- 52.Bowden S, Locarnini SA, Chang TT, Chao Y-C, Han KH, Gish RG, de Man R, Llamoso C, Tang H. Impact of Entecavir versus lamivudine on hepatic covalently closed-circular DNA and total hepatic HBV DNA in nucleoside-naïve HBeAg positive chronic hepatitis B patients [Poster]; 48th EASL The International Liver Congress; 2013 April 24; Amsterdam, The Netherlands. 2013. [Google Scholar]


