Abstract
Positioning patients for spine surgery is pivotal for optimal operating conditions and operative-site exposure. During spine surgery, patients are placed in positions that are not physiologic and may lead to complications. Perioperative peripheral nerve injury (PPNI) and postoperative visual loss (POVL) are rare complications related to patient positioning during spine surgery that result in significant patient disability and functional loss. PPNI is usually due to stretch or compression of the peripheral nerve. PPNI may present as a brachial plexus injury or as an isolated injury of single nerve, most commonly the ulnar nerve. Understanding the etiology, mechanism and pattern of injury with each type of nerve injury is important for the prevention of PPNI. Intraoperative neuromonitoring has been used to detect peripheral nerve conduction abnormalities indicating peripheral nerve stress under general anesthesia and to guide modification of the upper extremity position to prevent PPNI. POVL usually results in permanent visual loss. Most cases are associated with prolonged spine procedures in the prone position under general anesthesia. The most common causes of POVL after spine surgery are ischemic optic neuropathy and central retinal artery occlusion. Posterior ischemic optic neuropathy is the most common cause of POVL after spine surgery. It is important for spine surgeons to be aware of POVL and to participate in safe, collaborative perioperative care of spine patients. Proper education of perioperative staff, combined with clear communication and collaboration while positioning patients in the operating room is the best and safest approach. The prevention of uncommon complications of spine surgery depends primarily on identifying high-risk patients, proper positioning and optimal intraoperative management of physiological parameters. Modification of risk factors extrinsic to the patient may help reduce the incidence of PPNI and POVL.
Keywords: Spine surgery, Complication, Position, Nerve injury, Visual loss
Core tip: Perioperative peripheral nerve injury (PPNI) and postoperative visual loss (POVL) are rare complications related to patient positioning during spine surgery. It is important for spine surgeons to be aware of PPNI and POVL to participate in safe, collaborative perioperative care of spine patients. Proper education of perioperative staff, combined with clear communication and collaboration while positioning patients in the operating room is the best and safest approach. The prevention of uncommon complications of spine surgery depends primarily on identifying high-risk patients, proper positioning and optimal intraoperative management of physiological parameters.
INTRODUCTION
Surgical procedures involving the spine are one of the commonly performed procedures worldwide. The number of spine fusion surgeries performed in the United States has increased steadily over the past two decades[1-4]. Positioning patients for spine surgery is pivotal for optimal operating conditions and operative-site exposure. During spine surgery, patients are placed in positions that are not physiologic, would not be tolerated for prolonged periods by the patient in the awake state, and may lead to complications. Although the incidence of complications associated with positioning patients for spine surgery is relatively low, position-related complications can be devastating and life changing to patients and their families. Understanding these uncommon complications and their etiology is pivotal to prevention, and necessary if one is to obtain a truly informed consent from the patient. In this review article we discuss two of the uncommon, less recognized complications related to patient positioning during spine surgery; perioperative peripheral nerve injury and postoperative visual loss (POVL).
PERIOPERATIVE NERVE INJURY
Perioperative peripheral nerve injury (PPNI) is a rare but important perioperative complication resulting in significant patient disability, functional loss and the potential for litigation[5,6]. The reported incidence of PPNI is 0.03%-0.1%[7,8]. The mechanism of perioperative peripheral nerve injury is not well understood[9]. In the American society of anesthesiologists (ASA) closed claims study, there is no apparent mechanism of injury in the majority of the nerve injury claims[6]. Neurosurgical and orthopedic surgical procedures have a significant association with perioperative peripheral nerve injury[7].
The normal reaction to increased loading of the peripheral nervous system (PNS) elements is progressively increasing muscle activity; this acts as a nociceptive mediated reflex to prevent further harmful elongation. But the use of muscle relaxants and inhaled anesthetics during general anesthesia may suppress this protective mechanism subjecting the PNS to greater elongation than would be tolerated in the normal awake state[10].
In an attempt to raise awareness and reduce the occurrence of PPNI, ASA formed a task force on the prevention of perioperative peripheral neuropathies. The task force published a practice advisory for the prevention of perioperative neuropathies in 2000 and 2011[11].
Anatomy and physiology of peripheral nerves
The PNS carries information to and from the central nervous system (CNS). The functional unit of the peripheral nerve system is the neuron. The neuron consists of a cell body, dendrites and a long axon. The cell body contains the cytoplasm and the nucleus. Dendrites are attached to the cell body and carry impulses to the cell. Axons are attached to the cell body and carry impulses away from the cell. Conduction of an impulse along a neuron progresses from the dendrite to the cell body to the axon. The axon of one neuron and the dendrite of the next neuron are connected through the synapse. The synapse is a gap where the dendrites of one neuron and the axon of the next neuron communicate via chemical transmitters. Portions of the cell body and the axon are covered by Schwan cells, which form myelin segments. Myelin is an insulating layer around the axons allowing quicker and more efficient impulse transmission.
The interior of all nerve cells is negatively charged with respect to the exterior of the cell. Once the action potential of the nerve cell reaches the threshold voltage, sodium channels in the region of the action potential open, allowing sodium to flow into the nerve cell and leading to complete depolarization of the membrane. The depolarization caused by sodium influx opens adjacent voltage-gated sodium channels in the membrane leading to depolarization. The repetition of this depolarization process creates a wave of depolarization along the nerve fiber known as the action potential.
The peripheral nerve is composed of multiple nerve fibers (axons) bundled together. The bundles of nerve fibers are bound together by connective tissue sheaths and form fascicles. The endoneurium is a connective tissue sheath containing blood capillaries (vasa nervorum) that supply nutrients and oxygen to the nerve tissues. The endoneurium secretes the endoneurial fluid which surrounds the axons. The fascicles are wrapped in a fibrous tissue, the perineurium. Epineurium is the fibrous sheath that covers the entire nerve (Figure 1). The extrinsic plexus of blood vessels present in the epineurium penetrate the perineurium to anastomose with the intrinsic circulation in the endoneurium.
Figure 1.
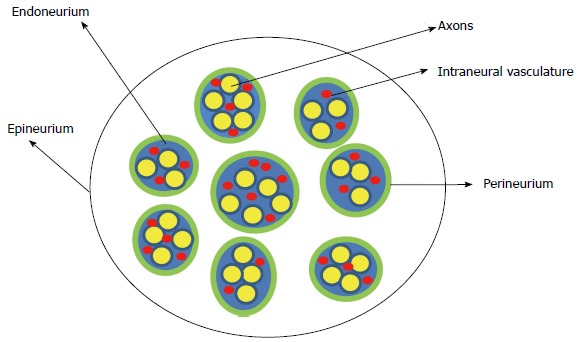
Schematic representation of the cross section of the peripheral nerve.
Tissue perfusion in the peripheral nerve is dependent on perfusion pressure. Perfusion pressure is defined as the difference between the mean arterial blood pressure and the internal pressure within nerve. In experimental animal models, high blood flow in the sciatic nerve was observed between mean blood pressures of 80-110 mmHg[12]. Acute hypotension was associated with a decrease in blood flow in the peripheral nerve[13]. Peripheral nerves lack vascular autoregulation[12-14]. Autoregulation is the intrinsic ability of an organ to maintain a constant blood flow despite changes in perfusion pressure. At mean blood pressures below 85 mmHg, there was marked decrease in the peripheral nerve blood flow[12]. A significant reduction in the blood flow to the nerve is required to affect the conduction of impulse in the nerve because blood flow to the peripheral nerve exceeds the metabolic requirements of the peripheral nerve by a significant margin[15]. Acute nerve ischemia leads to focal and generalized impairment of impulse conduction across the nerve that can be detected within 10 min of ischemia[16].
Mechanism of perioperative nerve injury
Direct trauma causing disruption and destruction of nerve fibers can lead to peripheral nerve dysfunction. Although direct trauma to peripheral nerves can be the cause of PPNI, it is not the cause in the majority of cases.
One of the main and crucial mechanisms of PPNI is ischemia of nerve fibers[17,18]. Slowing of nerve conduction due to ischemia of the nerve fibers is the hallmark of peripheral nerve injury. Focal demyelination may occur if local ischemia is prolonged, leading to sustained axonal damage[19-21]. Peripheral nerve studies in experimental animal model demonstrated that reperfusion injury after prolonged ischemia (3-7 h) results in endoneurial edema, conduction block, blood-nerve barrier disruption, intramyelinic edema and demyelination[22-24]. Ischemia leads to demyelination in rat sciatic nerve[25]. Focal nerve ischemia is an important pathologic mechanism in hyperesthesia, Wallerian degeneration and axonal injury in animal models[17]. Persistence of ischemia can lead to permanent peripheral nerve injury. Ischemia may be the final pathway of perioperative neuropathy[26-30]. The interdependence between ischemic and mechanical factors (stretch and compression) as a cause to nerve injury is well established, although incompletely understood.
Stretch of the peripheral nerve is one of the main mechanisms of peripheral nerve injury in perioperative patients[31]. During spine surgery, under general anesthesia, patients are frequently placed in positions that may stretch nerve fibers beyond their resting length. Overstretch of the nerve can lead to direct nerve damage via disruption of axons and vasa nervosum. Peripheral nerve injury occurs if nerves are stretched beyond 5%-15% of their resting length[32-34]. Stretch of the peripheral nerve leads to an increase in the intraneural pressure and compression of the intraneural capillaries and venules leading to a reduction in the perfusion pressure of the nerve fibers and ischemia[34,35]. Stretch may lead to reduction in the intraneural blood flow, leading to ischemia and endoneurial edema[34,36,37]. Stretch of the peripheral nerves has been shown to suppress axonal transport leading to changes in conduction characteristics[32,33,38,39].
Peripheral nerve compression is another related mechanism of PPNI[31]. Compression of peripheral nerve leads to damage of nerve fibers. Compression may lead to an increase in intraneural and extraneural pressures leading to a reduction in perfusion pressure; a reduction in the perfusion pressure leads to ischemia and slowing of conduction in the nerve fibers. Many operative positions during spine surgery subject peripheral nerves to compression.
Recent evidence suggests an inflammatory mechanism leading to perioperative ulnar nerve injury. Patients with persistent postoperative neuropathy had evidence of inflammatory reaction in peripheral nerves. Nerve biopsy of these patients revealed diffuse generalized microneuritis. Immunotherapy treatment with high-dose steroids resulted in significant improvement of ulnar neuropathy in these patients[40].
Risk factors for nerve injury
Certain drugs and chemicals may predispose patients to peripheral neuropathies[41]. Many conditions and medical diseases may render peripheral nerves more vulnerable to injury during the perioperative period[41]. Diseases affecting microvasculature, and anatomical differences, may contribute to nerve injury or render patients more susceptible to nerve injury. Hypertension, tobacco use, diabetes mellitus, general anesthesia, neurosurgical procedures and orthopedic surgery have been significantly associated with PPNI[7]. Advanced age has been linked to peripheral neuropathy after median sternotomy[42]. Hypovolemia, dehydration, hypotension, hypoxia, electrolyte disturbance and induced hypothermia have been associated with nerve injury[43]. The etiology of PPNI is multifactorial and involves patient predisposition, precipitating mechanical and physiologic factors.
Ulnar neuropathy
Ulnar neuropathy is the most common site of PPNI[8]. Ulnar nerve injuries comprised 28% of all anesthesia-related nerve injury malpractice claims[6]. Perioperative ulnar neuropathy occurred in 0.5% of surgical patients; primarily men between 50-75 years of age[44]. Ulnar neuropathy can lead to significant morbidity and loss of function. Ulnar nerve injury results in the inability to oppose or abduct the fifth finger and loss of sensation of the fourth and fifth fingers. Permanent injury will lead to a claw-like hand deformity due to atrophy of the intrinsic muscle of the hands. In one study, 3 out of 7 patients who developed perioperative ulnar neuropathy had permanent neuropathy with residual symptoms beyond 2 years[44]. Perioperative ulnar nerve injury has a delayed onset, most cases manifest within 2-7 d post-operatively (median 3 d)[5,19,44-49].
In a large retrospective review of ulnar neuropathy in anesthetized patients, the major complaints among patients with persistent ulnar neuropathy were their inability to grip tools and equipment due to loss of grip strength, discomfort and numbness. Perioperative ulnar neuropathy presented as sensory deficit in 47% of the cases while 53% of the deficits were mixed sensory and motor. Bilateral symptoms of ulnar neuropathy developed in 9% of the cases. Initial symptoms of were usually noted more than 24 h after the procedure, and appeared within 7 d in 90% of patients. Fewer than 10% of ulnar neuropathies were noted in the postoperative recovery unit. Fifty-three percent of patients with perioperative ulnar neuropathy who survived the first postoperative year regained complete sensory and motor functions and were asymptomatic. Six percent regained complete sensory and motor function but still complained of pain. At 1 year, 41% of patients had persistent deficits. Patients with sensory deficits had a better chance of complete recovery (80%) compared to patients with mixed motor and sensory deficits (35%)[19].
Patient related risk factors for perioperative ulnar nerve injury include male gender, older population, very thin and very obese patients, and prolonged postoperative immobilization[19]. The ulnar nerve may be susceptible to injury due to a pre-existing subclinical neuropathy. Pre-existing asymptomatic abnormal conduction in the contralateral ulnar nerve has been observed in patients who developed postoperative ulnar neuropathies[46]. Pre-existing subclinical neuropathy may manifest clinically in the perioperative period when patients are subject to certain predisposing factors[19,48,50]. Induced and prolonged hypotension has been associated with perioperative ulnar nerve injury[26,51,52]. Positioning during anesthesia has been related to ulnar neuropathy[52].
As stated above ulnar neuropathy occurs predominantly in men[5,19,47,53,54] with 70% of perioperative ulnar nerve injury cases occurring in males[19]. Anatomical differences may be responsible for this higher incidence of ulnar nerve injury. Studies of human male and female cadavers, showed that females have a significantly higher fat content (2-19 times) on the medial aspect of the elbow while men have a significantly larger tubercle of the coronoid process (1.5 times)[55]. Men have a thickened and more developed flexor retinaculum[8]. Men are more susceptible to direct pressure on unmyelinated ulnar nerve fibers than women[49].
The ulnar nerve has a superficial path along the medial epicondyle of the humerus[52]. The ulnar collateral artery and vein run in close proximity to the ulnar nerve and may be affected by external pressure leading to reduced perfusion, ischemia and nerve injury[30]. Compression of the ulnar nerve and its blood supply (the posterior ulnar collateral artery) at the area of the tubercle of the coronoid may lead to ischemia[55]. The ulnar nerve is relatively more sensitive to ischemia compared to median and radial nerves[27]. Experimental animal models demonstrated that the effects of compression on the ulnar nerve are potentiated by previous ischemia, even if the ischemia is of short duration[56]. The forearm position is a significant factor in determining pressure over the ulnar nerve at the elbow. Prielipp et al[30] investigated the relationship between forearm position and direct pressure on the elbow in awake normal volunteers, using a computerized pressure sensing mat. The study provided clear evidence that forearm supination significantly minimizes pressure over the ulnar nerve at the elbow (2 mmHg) compared with the neutral (69 mmHg) and prone (95 mmHg) forearm positions. Neutral forearm position resulted in significantly less pressure compared to the prone forearm position but more pressure compared to the supine forearm position. In the supine forearm position, the pressure over the ulnar nerve was low regardless of the degree of abduction of the arm at the shoulder. In the neutral forearm position, pressure over the ulnar nerve decreased as the arm was abducted between 30° and 90°. Pronation of the forearm produced the largest pressure over the ulnar nerve regardless of the abduction of the arm between 30° and 90°[30]. Extraneural pressures recorded along the path of the ulnar nerve in fresh cadaveric arms were significantly increased with elbow flexion beyond 90°. Concomitant shoulder abduction caused further increase in the pressure recorded at the post-condylar groove and the carpal tunnel[57].
Gelberman et al[58] investigated the relationship between the ulnar nerve and the cubital tunnel during flexion of the elbow in normal human cadavers. They observed a significant decrease in the cross-sectional area of the cubital tunnel coupled with an increase in the pressure within the cubital tunnel and ulnar nerve. Intraneural pressure of the ulnar increased significantly with the elbow flexed 70° or more. Extraneural pressure increased significantly when the elbow was flexed to 100° or more. The intraneural pressure was significantly increased at lesser degrees of flexion compared to the extraneural pressure. The authors conclude that the increase in the intraneural pressure of the ulnar nerve is not entirely due to extraneural compression. Dynamic changes in the cubital tunnel and the cross-section of the ulnar nerve contribute to the increased intraneural pressure with flexion. Compared with full extension, the mean area of the cubital tunnel in the subaponeurotic region decreased by 18% and 39% and the ulnar nerve mean area decreased by 24% and 50% with elbow flexed 90 and 135 degrees respectively. Intraneural and extraneural pressures within the cubital tunnel are lowest at approximately 45° of flexion[58]. Flexion of the elbow to 135° resulted in an 18% elongation of the ulnar nerve[59]. Elongation of peripheral nerve beyond 5%-15% of resting length can cause ischemia and nerve injury[32-34]. Stretch of the ulnar nerve by elevation of the shoulder, flexion of the elbow and dorsiflexion of the wrist caused a marked increase in the intraneural pressure[60].
Patel et al[61] assessed the morphologic changes in the ulnar nerve and cubital tunnel with elbow motion in fresh human cadavers using magnetic resonance imaging. During full extension the ulnar nerve appeared round in serial cross-sectional images and was surrounded by fat except at the inferior aspect of the medial epicondyle where the nerve was directly adjacent to bone. On flexion the nerve displaced the fat posteriorly and was relocated to a more anterior position in the cubital tunnel. On flexion the cross-section of the nerve progressively flattened from a round to an elliptical shape. With progressive flexion the course of the nerve changed from tortuous to more direct. With progressive elbow flexion the proximal cubital tunnel takes a wider and flatter appearance with the largest diameter changing from anteroposterior to mediolateral. The diameter of cubital tunnel in the subaponeurotic region decreased with progressive elbow flexion[61].
Although the proportion of nerve damage claims has not changed between the 2 ASA closed claims studies performed almost a decade apart, the pattern of nerve injury has changed. Compared to the ASA closed claims report published in 1990, the report published in 1999 showed a relative decrease in the incidence of ulnar nerve injury claims as a proportion of total nerve injury claims and a relative increase in spinal cord injury claims. However, the actual incidence and trend of nerve injury cannot be determined based on the closed claims data since it lacks a denominator. The closed claims project examines anesthesia-related malpractice claims; it does not present the nerve injury in population. In the ASA closed claims study, the mechanism of ulnar neuropathy was explicitly stated in only 9% of the claims[6].
Perioperative ulnar neuropathy is not confined to surgical patients. A prospective study of ulnar neuropathy in patients admitted to internal medicine services for nonsurgical conditions revealed that 0.2% of the patients developed new onset ulnar neuropathy while in hospital. Patients commonly rest in a supine position, flexing their elbows and resting their arms on their chest and abdomen. Elbow flexion may increase pressure on the ulnar nerve in the postcondyler groove of the humerus due to stretching of the cubital tunnel retinaculum. Forearm pronation may lead to external compression of the ulnar nerve[45]. It is therefore prudent to instruct patients to avoid prolonged flexion of the upper extremity on the abdomen and chest in the supine position.
Brachial plexus injury
Brachial plexus is the second most common site of PPNI accounting for 20% of all anesthesia-related nerve injury malpractice claims[6]. The reported incidence of brachial plexus injury in non-cardiac surgery is 0.02%[62]. The main mechanisms of brachial plexus injury are compression and stretch. The brachial plexus has a long course between the vertebra and the axillary fascia. Brachial plexus injury usually involves the upper nerve roots. Lower brachial nerve injuries are commonly associated with median sternotomy[43].
In the ASA closed claims project, patient positioning was responsible for 10% of brachial plexus malpractice claims. The use of shoulder braces and head-down position, arm malpositioning and prolonged neck extension were commonly identified mechanisms for brachial plexus injury[6]. The use of shoulder braces in Trendelenburg position may lead to compression of the brachial plexus between the clavicle and the first rib[8,63].
Brachial plexus injury is commonly due to overstretch of the brachial plexus[43]. Shoulder abduction greater than 90°, external rotation of the arm and posterior shoulder displacement can stretch the brachial plexus[43,64]. Downward tilting of the head and hyperabduction of the independent arm in the lateral position may stretch the brachial plexus and lead to brachial plexus injury[65]. Extension and lateral flexion of the head in the supine position may contribute to stretch of the brachial plexus on the contralateral side[43].
In the supine position, submaximal joint positions may stretch the brachial plexus to the extent it may affect physiologic processes in the peripheral nerve. Contralateral flexion of the cervical spine, lateral rotation of the shoulder combined with shoulder abduction and wrist extension may stress the brachial plexus. Elbow extension can cause substantial stress to the PNS. Simultaneous application of the different aforementioned components has a cumulative stressful impact on the brachial plexus. Individuals react differently to elongation of the peripheral nerve and individual variability increases as more components leading to stretch of the brachial plexus are added[10].
Median neuropathy
Median nerve injury is relatively rare and responsible for only 4% of all anesthesia-related nerve injury malpractice claims[6]. The median nerve may be injured during the insertion of an intravenous catheter in the antecubital fossa. However, stretch is the main mechanism of median nerve injury due to operative positioning.
Median neuropathy usually presents as a motor neuropathy with loss of the ability to oppose the first and fifth digits and decreased sensation over the palmar surface of the lateral three and half fingers. Median neuropathies do not resolve easily with most patient having sustained symptoms of motor dysfunction. Extension of the elbow may overstretch the median nerve leading to injury[11]. Muscular patients and patients with limited elbow extension range may be at risk for median nerve injury if the arm is fully extended under general anesthesia. The reduced range of extension in these patients may lead to similar contraction of median nerve making it more prone to overstretch[66]. Overextension of the elbow in the supine position to a point that is uncomfortable to the patient in the awake state should be avoided[11]. Wrist hyperextension for arterial line placement may lead to transient but significant impairment of the median nerve function. Prolonged hyperextension of the wrist may lead to slowing of nerve conduction and median nerve injury[67].
Radial neuropathy
Radial nerve injury is rare and accounts for just 3% of all anesthesia-related nerve injury malpractice claims[6]. The most common mechanism of radial nerve injury is direct compression at the spiral grove of the humerus. It may occur in the lateral position with abduction of the independent arm beyond 90° and suspension of the arm from a vertical screen support[68]. Direct compression by the overhead arm board at the mid-humerus may occur in the lateral position. Injury to the radial nerve results in wrist drop, inability to extend the metacarpophalangeal joint and inability to abduct the thumb with loss of sensation from the lateral and posterior arm, posterior forearm and a portion of the dorsal hand.
Intraoperative neuromonitoring
Intraoperative neuromonitoring is available in most institutions in the United States and is frequently used during spine surgery[69]. Commonly used intraoperative neuromonitoring modalities are somatosensory evoked potential (SSEP) and motor evoked potential. Neuromonitoring is primarily used to monitor the integrity of the spinal cord during spine surgery. However, SSEP monitoring has been used to detect peripheral nerve conduction abnormalities indicating peripheral nerve stress and impending injury during surgery under general anesthesia in variable intraoperative positions[70-86]. Conduction changes detected by SSEP may indicate position-related impending peripheral nerve injury. In a retrospective study of 1000 consecutive spine cases, position modification of the upper extremity lead to resolution of 92% of upper extremity SSEP changes[86]. Position modification strategies used in the review included correcting extreme elbow flexion and extension, decreasing shoulder abduction, releasing shoulder traction on tucked arms (caused by taping down the shoulder) and moving the upper extremity into the original position if the position had been modified. After position modification of the upper extremity and resolution of SSEP change, patients experienced no post-operative upper extremity peripheral nerve injury[86]. Significant SSEP change indicating impending upper extremity nerve injury is usually defined as reduction in amplitude of 50% or more and/or increase in latency of 10% or more[73,86]. Usually changes in both amplitude and latency are monitored and evaluated. Compared to latency, amplitude changes may be a more sensitive and valid measure of changes in nerve conduction[87,88]. Most SSEP components are mediated by large myelinated fibers. Some secondary peaks may be transmitted by smaller fibers. Potentials recorded from Erb’s point may be the most sensitive to ischemia[28].
Significant SSEP changes indicate abnormal conduction and impending nerve injury. If the changes persist for a prolonged period of time, permanent nerve injury may occur[30]. The use of SSEP to monitor extremity nerve function and guide position modification of the upper extremity into a more favorable position for the peripheral nerve may protect peripheral nerves from injury under general anesthesia. The incidence of position related significant upper extremity SSEP changes during spine surgery ranges from 1.8% to 15% depending on the operative position, patient group and type of spine surgery[79,83,86].
POSTOPERATIVE VISUAL LOSS
POVL is a rare but traumatic and devastating complication of spine surgery and general anesthesia. The reported prevalence rate of POVL after spine surgery is 0.0028%-0.2%[89-92]. The incidence of POVL associated with spine surgery in the prone position under general anesthesia has increased over the past several decades[93]. POVL usually results in permanent unilateral or bilateral visual loss. Most cases are associated with prolonged spine procedures in the prone position under general anesthesia. Posterior lumbar fusion and surgery for correction of scoliosis were associated with the highest rate of POVL[92]. POVL has been associated with instrumented spine surgery in the prone position[94]. The most common causes of POVL after spine surgery are ischemic optic neuropathy (ION) and central retinal artery (CRA) occlusion. ION is further classified into anterior ION (AION) and posterior ION (PION). PION is the most common cause of POVL after spine surgery. In 1999, the ASA committee on professional liability established the ASA POVL registry to identify predisposing factors and intraoperative risk factors. It is important for spine surgeons to be aware of POVL and to participate in safe, collaborative perioperative care of spine patients positioned in the prone position.
Anatomy and physiology of the optic nerve
The eye is a sphere that gathers and converts light information into neuronal signals. The wall of the globe has 3 layers; the outermost sclera (white of the eye), the middle uveal tract (contains the choroid) and the innermost layer (the retina). There are no blood vessels in the retina; the choroid layer, located posterior to the retina contains blood vessels and provides the retina with oxygen and nutrients. Retinal ganglion cells (RGC) in the retina are highly specialized neurons that produce neural signals when stimulated by light. Neural signals are transmitted to the brain along axons of RGC in the optic nerve (cranial nerve II).
The optic nerve is composed of about 1.2 million individual RGC axons and support cells. Axons of the RGC travel across the retina and converge near the center forming the optic nerve. This convergence of the RGC axons creates the blind spot of the eye, an area where no photoreceptors exist, only nerve fibers.
The optic nerve has a structure similar to the CNS tracts and is considered part of the CNS. In contrast to the ability of the mammalian PNS to regenerate axons after injury, mammalian CNS structural and functional regeneration after injury is minimal. Injury to the RGC usually results in lifelong visual loss due to the limited ability of RGC to regenerate their axons after optic nerve injury[95].
The blood supply of the eye comes from the ophthalmic artery, a branch of internal carotid artery. The CRA is a branch of the ophthalmic artery. The CRA penetrates the optic nerve superiorly and continues its course in the optic nerve to supply the retina. The ophthalmic artery gives rise to 1-5 posterior ciliary arteries. The posterior ciliary arteries give rise to short and long posterior ciliary arteries. The posterior ciliary arteries are end-arteries that provide blood supply to the head of the optic nerve and the retina.
The optic nerve can be divided into anterior and posterior portions based on differences in anatomy and blood supply[96]. The anterior portion (intraocular) of the optic nerve is that part of the optic nerve that lies anterior to the lamina cribrosa. The posterior (retrolaminar or intraorbital) portion of the optic nerve is the part of the optic nerve posterior to the lamina cribrosa. Lamina cribrosa is an elastic multilayered network of collagen fibers that insert into the scleral canal wall. The nerve fibers forming the optic nerve exit the eye posteriorly through the lamina cribrosa. The CRA and the central retinal vein pass through the lamina cribrosa to enter the optic disc.
The predominant cells in the anterior optic nerve are astrocytes, while microglial cells and oligodendrites are relatively more common in the posterior optic nerve. Unlike peripheral nerves, the posterior (retrolaminar) optic nerve is covered by all three meningeal layers; dura, arachnoid and pia matter.
The blood supply of the anterior optic nerve is derived from retinal arterioles, centripetal branches from the peripapillary choroid and short posterior ciliary arteries (Figure 2). The anterior optic nerve may receive blood from the intrascleral circle of Zinn and Haller when present.
Figure 2.
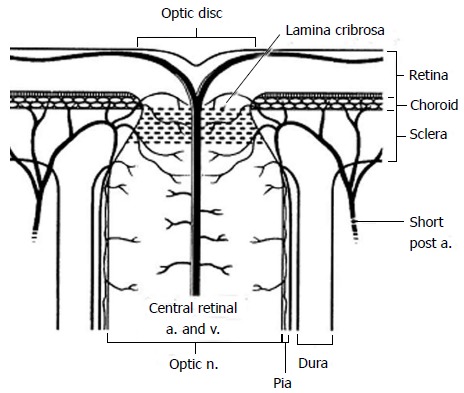
Diagram of the anterior optic nerve showing the arterial and small vessel supply to the choroid and optic nerve as it passes through the lamina cribrosa. Short post a: Short posterior ciliary artery; a: Artery; v: Vein; n: Nerve (Reprinted from Williams et al[140] with permission).
The blood supply of the posterior (retrolaminar) optic nerve is derived from two vascular systems; the centripetal (peripheral) system and centrifugal system (axial). The centripetal vasculature is the main and most consistent system supplying the posterior optic nerve. It is formed primarily of recurrent branches of the peripapillary choroid, and the circle of Zinn and Haller, with additional pial branches from the CRA and other orbital arteries. The centrifugal blood vascular system consists of a few branches of the CRA The centrifugal system is not always present and the number of branches is inconstant. To summarize, the main blood supply of the anterior optic nerve is derived from the short posterior ciliary arteries and the peripapillary choroid. The main blood supply to the posterior optic nerve is derived from recurrent branches of the peripapillary choroid and pial branches of the CRA.
It is important to realize there is not one universal pattern of blood supply for the optic nerve. There are many anatomic variations in vascular supply, which can lead to variable patterns of ischemia among individuals[97-100]. In most individuals, there are 2 to 3 posterior ciliary arteries; however in some the number may range from 1 to 5. It is also important to note that posterior ciliary arteries are end-arteries, and thus watershed zones exist between them[100]. Watershed areas, by definition, are areas that are at risk for decreased blood supply. Blood flow to the posterior optic nerve may be particularly vulnerable to ischemia because most of the arteries supplying the posterior optic nerve are end-arteries[98].
Blood flow to the optic nerve
The blood flow to the optic nerve head is dependent on perfusion pressure. Ocular perfusion pressure is the difference between mean arterial blood pressure and intraocular pressure (IOP) or venous pressure (whichever is higher)[101]. It is important to note that the mean arterial pressure determining optic nerve blood flow refers to that of the optic nerve vasculature and not the pressure in the brachial or radial arteries. Local arteriolar vasoconstriction may reduce perfusion to the optic nerve leading to ischemia despite a normal brachial blood pressure measurement. Thus three main factors determine optic nerve perfusion; vascular tone, arterial blood pressure and IOP.
Autoregulation
There is evidence that the optic nerve head autoregulates blood flow[102-105]. Autoregulation is achieved through alterations in resistance of the terminal arterioles. There are limitations to the degree to which arteriolar resistance can be altered to maintain perfusion. Autoregulation works within a range of mean arterial pressure, below or above which the local perfusion is dependent entirely on the difference between mean arterial blood pressure and intraocular or venous pressure. Factors leading to the breakdown of autoregulation of blood flow to the optic nerve include age, hypertension, uncontrolled blood pressure, diabetes mellitus, atherosclerosis, hypercholesterolemia, and vascular endothelial disorders[106-109]. A study of the blood flow to the optic nerve head using laser Doppler flowmetry in healthy volunteers demonstrated that blood flow was constant between ocular perfusion pressures of 56 to 80 mmHg. Not all patients have autoregulation of the blood flow to the optic nerve[105]. A study of autoregulation of the optic nerve in humans showed that 2 out 10 healthy young volunteers did not demonstrate autoregulation[104].
Arterial blood pressure
Arterial blood pressure is one of the main determinants of blood flow to the anterior and posterior optic nerve. There is a progressive fall in the blood pressure from the internal carotid artery, to the ophthalmic artery, to the posterior ciliary artery and then to the small branches supplying the optic nerve. The blood pressure in optic nerve may be half or less than that measured in the brachial artery[109]. Vascular changes such as atherosclerosis, vasospasm and vasculitis may lead to further decreases in blood flow to optic nerve. Critical drops in blood pressure below the lower limit of autoregulation will lead to a reduction in optic nerve blood flow. Hypotension resulting from antihypertensive medications or shock may lead to ischemia of the optic nerve and anterior ischemic optic neuropathy[110-113]. Nocturnal hypotension has been associated with glaucomatous visual loss[110,113,114]. Nocturnal arterial hypotension may be a key factor in the development of non-arteritic AION as more than 75% of these patients discover visual loss upon awakening in the morning[115]. Arterial hypertension can decrease blood flow to the optic nerve if it is outside the upper limit of autoregulation or there is an absence of autoregulation. In this setting a decrease in the blood flow is due to arteriolar vasoconstriction[109].
IOP
IOP is defined as the pressure exerted by the contents of the eye on its containing wall. Intraocular components like blood and aqueous humor can undergo significant volume changes that significantly alter IOP. External pressure on the eye globe can increase IOP by direct and indirect effects through volume changes of intraocular components. The normal IOP ranges from 10-20 mmHg with a diurnal variation of 2-3 mmHg. IOP decreases at night. IOP is a key determinant of blood flow to the optic nerve head. The blood flow to the optic nerve head is inversely proportionate to the IOP outside the range of autoregulation or if autoregulation is absent or defective. This effect may be intensified when coupled with hypotension or local vasospasm[116]. IOP may affect the blood flow to the anterior (intraocular) optic nerve. The effect IOP has on blood flow to the posterior (retrobulbar) optic nerve is unclear and probably of lesser significance.
Changes in arterial PCO2 tension can affect intraocular blood volume and IOP independent of hemodynamic changes[117-121]. The vascular resistance of the choroidal vessels varies directly with inhaled CO2[122]. High levels of PCO2 lead to intraocular vasodilation increasing intraocular blood volume and IOP.
The choroid is a vascular structure that contains the majority of the intraocular blood volume. Congestion of the choroid leads to an increase in the intraocular blood volume and IOP. The choroid is characterized by very high blood flow[123]. Most of the blood volume of the choroid is in the venules of the choroid. Venular filling of choroid depends on the pressure in the orbital veins[117]. Pressure in the orbital veins can be affected by body position[117,124,125]. Increases in orbital venous pressure may lead to an increase in IOP though choroid congestion. Trendelenburg position increases central venous pressure and may lead to an increase in IOP through congestion of the choroid. The choice of the operating room table and frame (Jackson table or Wilson frame) has no significant role in IOP increase caused by the prone position[126].
The majority of the aqueous humor outflow is passively drained into the episcleral veins[127,128]. This passive outflow process depends on the gradient between IOP and episcleral vein pressure (EVP). High IOP may reduce aqueous humor drainage while having minimal effect on production leading to an increase of the total aqueous humor volume[126]. The episcleral veins are valveless veins connected to the central venous circulation. Cephalad shift of blood and increase in central venous pressure (CVP) will increase the EVP[124,129]. A positive correlation exists between episcleral venous pressure and IOP.
Elevation in central venous pressure may reduce venous return from the eye leading to an increase in IOP. There is close correlation between CVP and IOP[117,118,130]. A parallel and instantaneous decrease in CVP and IOP was noticed with a change from Head-down (Trendelendburg) position to head-up (reverse Trendelenburg) position[117]. Factors that cause significant increase in CVP may lead to an increase in IOP. These include increased intrathoracic pressure, extreme neck flexion, dependent position of head relative to the heart and abdominal compression. The venous pressure may increase beyond IOP, and in such cases it becomes a key determinant of ocular perfusion pressure and blood flow to the optic nerve.
Effects of general anesthesia and surgical position
General anesthesia decreases IOP in the supine position[116,130,131]. IOP pressure has been shown to increase in anesthetized patients in the supine head-down (Trendelenburg) position[130,132]. Peak airway pressure, mean arterial blood pressure, duration of surgery and end-tidal CO2 are significant predictors of IOP in the anesthetized patient placed in the supine head-down position[132]. The prone position has been shown to increase IOP under general anesthesia in adult and pediatric patients[131,133]. IOP has been shown to increase in awake vertically inverted volunteers[134]. IOP has been shown to increase with elevated arterial carbon dioxide tension in anesthetized patients without eye disease[117]. Hyperventilation caused a rapid fall of IOP. IOP changes due to arterial carbon dioxide tension in anesthetized patients are presumably vascular in nature and are related to changes in the choroidal blood volume[117]. Intraoperative fluid balance may affect IOP. Acute oral water loading has been shown to significantly, though transiently, elevate IOP[135] while dehydration has been associated with significant reduction in IOP[136]. In the prone position, general anesthesia may lead to an increase in the intraocular blood volume by impairing autoregulation in the choroid circulation[137]. The IOP may become a critical factor in the perfusion of the anterior optic nerve in the presence of decreased hematocrit and mean arterial blood pressure[126]. Ozcan et al[126], showed that an increase in the IOP caused by the prone position in awake volunteers was ameliorated but not normalized by a 10° head-up (reverse Trendelenburg) position[126].
ION
ION is the most common reported cause of POVL after spine surgery[138-140]. Perioperative ischemic neuropathy is a multifactorial disease that is not well understood. ION presents as acute loss of vision or visual field defect. More than 50% of the cases present in ASA POVL registry had bilateral ION[141]. ION is divided into AION and PION. AION involves ischemia and infarction of the anterior optic nerve, while PION involves ischemia and infarction of the posterior optic nerve. It is uncommon for AION and PION to be present simultaneously. Usually ION presents as selective AION or PION, presumably due to different predisposing factors and differences in the blood supply to those portions of the nerve[141]. Hypertension, diabetes, obesity, hypotension, anemia, prone position, smoking, vascular disease, increased blood viscosity and abnormal anatomy have been associated with ION and perioperative visual loss[139,140,142-147]. Anemia, hypotension, peripheral vascular disease and blood transfusion were associated with ION after spine surgery[90,92]. ION has been associated with adverse effects of hypertensive medications and with sildenafil[110,148]. ION is more common in males[139,149]. The protective effect of estrogen in experimental animal models of cerebral ischemia has been established and may contribute to the lower incidence of ION in females[150]. Obesity, the use of the Wilson spinal frame, longer anesthetic duration and lower colloid use during intraoperative fluid administration have been associated with ION and POVL[149]. Most cases of ION occurred in relatively healthy individuals, further confirming the role interindividual anatomic and physiologic variations may play in the development of ION.
The association between hypotension, anemia and ION is unclear. Anemia and hypotension has been associated with ION[90,92]. However ION has been diagnosed in patients with a hematocrit nadir of 40% during spine surgery. In a retrospective case-control study by Myers et al[94], there was no difference in the lowest blood pressure between patients who developed POVL and those who did not. ION may occur in the absence of hypotension[139]. Although deliberate hypotension for spine cases has not been associated with POVL in previous studies, the studies lack power to detect a complication with a significantly low incidence like POVL[151,152]. ION may be due to a “compartment syndrome of the optic nerve”, a hypothesis related to increased venous pressure and interstitial fluid accumulation within the lamina cribrosa of the optic nerve (semi-rigid) or the bony optic canal[139].
Awake volunteers positioned in the prone position demonstrated a significant increase (20 mmHg) in the IOP after 8 min compared to the supine position (14.1 mmHg)[153]. Cheng et al[131] investigated the effect of prone positioning on IOP in 20 anesthetized patients having spine surgery. Patients with preexisting eye disease or previous eye surgery were not included. Patients were positioned in the prone position with their heads in pinned head-holder in a neutral position with neck flexion limited to less than 15° from horizontal. Mean arterial pressure was kept within 20% of awake values and end–tidal carbon dioxide level was maintained at 30-35 mmHg. IOP was measured at baseline and 5 times throughout the procedure. Two measurements of the IOP were made in the prone position; before incision and after the conclusion of the surgery. The IOP in the prone position before incision was significantly higher (27 mmHg) than both supine anesthetized and awake (baseline). The IOP was significantly higher (40 mmHg) in the prone position after the conclusion of surgery compared to all previous measurements. The mean duration in the prone position before the second measurement was 320 min. The authors concluded that IOP increased significantly in the anesthetized patient in the prone position and the magnitude of this increase is related to the amount of time spent in that position. Increases in IOPmay lead to reductions in ocular perfusion pressure despite normal systemic blood pressure[131].
Lee et al[139] analyzed 93 spine cases with POVL from the ASA POVL registry. Ischemic optic neuropathy was the cause of visual loss in 89% of cases. PION was the most common cause of optic neuropathy occurring in 56 of 83 ION cases. Nineteen patients were diagnosed with AION and 8 patients had unspecified ION. Compared to cases of CRAO, ION cases occurred more often in males (72%) undergoing elective surgery (96%). Most patients were relatively healthy with no preoperative history of glaucoma. Most cases of ION occurred with spine fusion and instrumentation involving more than one vertebral level in the thoracic, lumbar or sacral spine. All but 2 patients were positioned prone. The mean anesthetic duration was 9.8 h with 84% of cases lasting 6 h or longer. The mean prone duration was 7.7 h. Eighty-two percent of cases had an EBL of 1 liter or more. Only one patient in 83 showed signs of periocular trauma. Bilateral ION was documented in 66% of ION cases with a median onset time for reporting symptoms of 15 h. ION can occur without compression of the globe as 16 patients who developed ION were placed in Mayfield pins. Key findings of the review were the higher incidence of ION in males, the association of ION with an EBL of 1000 mL or greater, and a duration of surgery of 6 h or longer. The authors recommend discussing the risk of POVL with patients undergoing lengthy spine surgery in the prone position[139].
Shen et al[89] investigated the prevalence of POVL in the United States over a 10-year period from 1996 to 2005 using The Nationwide Inpatient Sample. The prevalence rate for POVL was 0.03% after spinal fusion and 0.0086% after laminectomy without fusion. Age, male gender, anemia, and posterior approach for surgery were associated with significantly higher odds of developing POVL. Patients younger than 18 years had the highest prevalence rate (0.35%). The prevalence rate of POVL was 0.05% in the posterior approach compared to 0.006% in the anterior approach. Men had 1.3 time higher odds ratio of visual loss, and twice the odds ratio for developing ION compared to women. Contrary to POVL after cardiac surgery, existing co-morbidities were not associated with greater odds for developing POVL and ION with spine fusion surgery[89].
Holy et al[138] performed a retrospective matched case-control study to determine the incidence and risk factors of ION in a single institution. The reported incidence of documented ION after spine surgery was 0.36%. The majority of cases (75%) of ION patients after spine surgery had PION. The majority (94%) of the patients with ION in all surgical procedures (including spine surgery) were men. The authors found no difference in hematocrit levels or blood pressure values or the use vasopressor between cases and controls[138].
Grant et al[147] investigated the effect of prolonged prone positioning on ocular parameters in 10 volunteers. The authors demonstrated a progressive increase in the IOP, choroid layer thickness and retrobulbar diameter of the optic nerve in the prone position compared to supine position over 5 h. The peak increase for most parameters was at 5 h in the prone position. Compared to the prone horizontal position, a 4° reverse Trendelendburg prone position had minimal effect on these changes. With elevation of the head of the stretcher 30° in the supine position, all parameters retuned to baseline after 30 min. In the prone position, the optic nerve diameter showed a significant increase in diameter without significant difference between horizontal and 4° Trendelenburg. Choroid layer thickness showed mild improvement (reduction) with 4° Trendelenburg position. The authors related the increase in the prone diameter of retrobulbar optic nerve to a dependent increase in subarachnoid fluid or venous congestion rather than intrinsic swelling[147].
AION
AION is associated with spine surgery and is the most common cause of ION associated with open heart surgery[138]. AION results from ischemia of the anterior (intraocular) optic nerve presumably due to occlusion of the posterior ciliary circulation[154]. AION is painless and usually irreversible[141]. High cholesterol, smoking, high fibrinogen levels[154], diabetes[155], nocturnal arterial hypotension[110] and lack of autoregulation[104] have been associated with occurrence of non-arteritic spontaneous AION. Interindividual variation in the blood supply to the anterior optic nerve may predispose patients to ischemia in watershed zones leading to AION[99]. Variability in the severity of the visual loss associated with AION may be due to variation in the blood supply resulting in various ischemic effects[140,156]. An increase in IOP may play a role in reduced perfusion to the anterior (intraocular) optic nerve. AION has been associated with increased blood viscosity. An increase in blood viscosity may reduce perfusion pressure leading to ischemia of the anterior portion of the optic nerve. Sickle cell disease and polycythemia may be associated with AION presumably due to increased blood viscosity and decreased perfusion of the optic nerve in certain individuals[141,157]. AION may occur due to reduced oxygen carrying capacity and transport, as in the case anemia and hemorrhage[158,159]. Patients with a small optic disc are at higher risk of developing AION[160,161].
In AION the optic disc is initially swollen. Early swelling of the optic disc is a key differentiating point from PION. Over months the swelling gradually evolves into optic atrophy[138,141,156,162-164]. Splinter hemorrhages around the optic disc may be present[165]. Visual defects most commonly occur in the inferior half of the visual field[145,166,167].
PION
PION is the most common type of ION after spine surgery and is the most common cause of POVL associated with spine surgery[139,141,162]. PION occurs due to ischemia of the retrobulbar (intraorbital) optic nerve. Reported risk factors associated with PION include; prone position, prolonged spine surgery, systemic hypertension, intraoperative hypotension, anemia, diabetes, smoking and coronary artery disease[142].
In contrast to anterior ION, optic nerve swelling is absent on ophthalmoscopic examination. Later in the course of PION, the atrophy of the posterior optic nerve fibers will involve the anterior optic nerve head resulting in a pale and atrophic optic disc. The etiology of PION is multifactorial[140,141]. Severe anemia and hypotension in predisposed individuals placed in the prone position for prolonged periods of time are reported to be more likely causes of PION rather than occlusive vascular disease[141]. Interindividual variability and inconsistency in the blood supply to the posterior (retrobulbar) optic nerve plays a role in the development of postoperative PION[140,168]. PION have been associated with surgery, trauma and gastrointestinal bleeding in which severe anemia and hypotension occurred[91,94,140,169,170]. Prognosis is usually poorer with PION compared to AION[171].
Arterial infarction of the retrobulbar optic nerve due to ischemia is primarily due to decreased oxygen delivery. Decreased oxygen delivery may be due to a decrease in arterial perfusion pressure, increased resistance to blood flow or a reduction in oxygen carrying capacity[147]. The prone position may contribute to increased orbital venous pressure or venous congestion which may contribute to a decrease in arterial perfusion pressure and venous infarct respectively. One of the postulated mechanisms for PION is venous infarct. Venous infarct is a venoarteriolar response caused by secondary constriction in small arterioles in response to venous congestion[172,173].
Gill et al[141] reviewed 7 studies representing 102 cases of POVL associated with spine surgery. PION was the most common cause of POVL. Patients who developed POVL after spine surgery had an age range of 46 to 53 years and at least one co-morbidity. Median operative time ranged from 385 to 410 min while the average blood loss ranged from 3.5 to 4.3 L. There was no visual improvement in the majority of cases. The authors concluded that an acute anemic state may have additive or synergistic effects in predisposed patient with certain comorbidities leading to the visual loss associated with spine surgery[141].
Enlargement of the superior ophthalmic veins with bilateral PION after prolonged spine surgery has been reported in a 55 years old male. Magnetic resonance imaging revealed significant enlargement of the superior ophthalmic veins 19 h after the surgery that resolved 5 mo after the surgery. Enlargement of the superior ophthalmic veins indicate the role of orbital venous pressure in the development of PION associated with surgery in the prone position[174]. Prolonged prone positioning has been shown to increase the diameter of the retrobulbar optic nerve possibly due venous congestion[147].
Central retinal artery occlusion
Central retinal artery occlusion (CRAO) may be caused by direct pressure on the globe, emboli or low retinal perfusion pressure[141]. Pressure on the eye globe increases IOP and has been associated with POVL [91,175,176]. The use of a horseshoe headrest for spine surgery in the prone position has been associated with CRAO and POVL[176,177]. Analysis of the spine cases with POVL showed that CRAO was present in 10 of the 93 cases[139]. The mean age for patients with CRAO was 46 years. Horseshoe headrests were used in 3 cases. Mayfield pins were not used in any of the cases with CRAO. Median estimated blood loss and mean anesthetic duration were significantly less in CRAO cases compared to patients with ION. All cases of CRAO were unilateral. Periocular trauma was documented in 7 of the 10 cases of CRAO. Risk factors for CRAO differ considerably from those of ION. Cases of CRAO after spine surgery have not been associated with degree of blood loss, anemia, bilateral loss of vision, or duration of the prone position, indicating a different etiology than ION[139]. Ophthalmologic examination shows pale, edematous retina, fibrin or cholesterol emboli and cherry-red spot on the fovea. Optic atrophy occurs in half the patient with CRAO[140].
POSITIONING PATIENTS FOR SPINE SURGERY
Awareness of the potential rare complications of patient positioning during spine surgery is essential for improved care and reducing the likelihood of occurrence of such complications. Complete prevention of PPNI and POVL is unrealistic because of the multifactorial etiology of the complications and lack of clear, definitive knowledge regarding etiology. Proper education of perioperative staff, combined with clear communication and collaboration while positioning patients in the operating room is the best and safest approach. The prevention of uncommon complications of spine surgery depends primarily on identifying high-risk patients, proper positioning and optimal intraoperative management of physiological parameters. Modification of risk factors extrinsic to the patient may help reduce the incidence of perioperative peripheral nerve injury and POVL.
Identifying high risk patients
High-risk patients for PPNI are usually middle aged males, with extreme body habitus. Prolonged hospitalization is a risk factor for the development of perioperative ulnar neuropathy. Certain operative positions used during spine surgery may create risks for loss of nerve function of the upper extremity. The prone position has been linked to claims of nerve injury[5]. Patients placed in the prone surrender (superman) position and lateral decubitus position had a significantly higher incidence of position-related impending upper extremity nerve injury compared to patients positioned in the supine arms tucked, supine arms out, and prone arms tucked positions[86]. Patients with a previous history of upper extremity peripheral nerve injury should be considered at increased risk of developing PPNI.
High-risk patients for POVL are those expected to undergo prolonged procedures on multiple vertebral levels in the prone position with a significant anticipated blood loss. The ASA task force for the prevention of POVL considers a surgery prolonged when it exceeds 6.5 h and significant blood loss when the patient’s blood loss exceeds 44.7% of estimated blood volume[178]. It is advisable to discuss POVL with these patients when obtaining informed consent. It is also important to inform patients about the multifactorial etiology of POVL, the lack of clear understanding of the etiology, anatomical differences between individuals and the very low incidence of this rare, but devastating complication. Consideration should be given to staging surgery in high-risk patients, as this may reduce the risk of POVL[178]. However, the decision to stage spine surgery for high-risk patient should be individualized and weighed against other perioperative risks.
Proper positioning
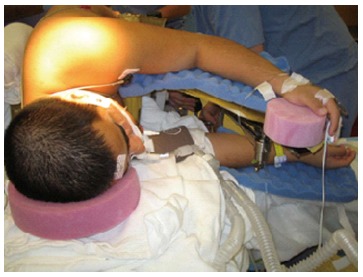
The prone surrender position: In the prone surrender (superman) position, injury can occur along the entire length of the brachial plexus. Patients placed in the prone surrender(superman) position had a significantly higher incidence of position-related impending upper extremity nerve injury detected by SSEP compared to patients positioned in the supine arms tucked, supine arms out and prone arms tucked positions[86]. Stretch is the main mechanism of injury. If the head is directed away from the arm this can stretch the brachial plexus, therefore lateral neck rotation should be avoided. Although patients may comfortably tolerate arm abduction greater than 90° in the prone surrender position[11], it is advisable to limit the shoulder abducted to less than 90° to avoid overstretch of the brachial plexus. Depression of the shoulder girdle should be avoided. The longitudinal axis of the forearm should be parallel to the longitudinal axis of patient to avoid outward rotation of the arm. Extreme elbow flexion should be avoided. However, in the prone position the range of motion for the elbow extension and flexion is limited. The forearm should be placed in a neutral position to minimize the direct pressure on the ulnar nerve at the elbow (Figure 3). The forearm should be at or below the table mattress surface. The elbow and the inner aspect of the upper arm should be padded with foam to avoid direct pressure on the nerves. Prolonged overextension of wrist over the wrist board placed for arterial lines should be avoided as it may stretch the median nerve. The head of the humerus may compress the neurovascular bundle in the axilla leading to nerve damage[179]. In a steep, prone Trendelenburg position the brachial plexus may be compressed between the clavicle and the first rib especially with use of shoulder braces. Vigilance and frequent checking of patient positioning is important. The use of SSEP helps to detect impending upper extremity peripheral nerve injury and guide position modification of the upper extremity.
Figure 3.
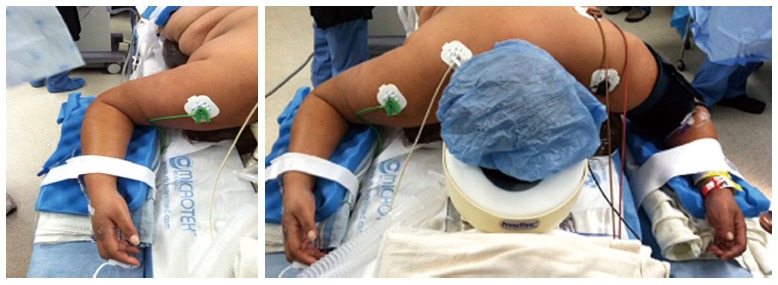
Positioning patient in the prone surrender (superman) position. The head should be in neutral position on foam supporting head frame (e.g., proneview®) to avoid any direct pressure to the eye. The shoulders should be abducted less than 90°, lateral rotation of the upper arm and extreme elbow flexion should be avoided. The forearm should be positioned in the neutral position to minimize direct pressure on the ulnar nerve in the elbow. Soft foam padding should be placed under the elbows and between the inner upper around the gel rolls (or supporting frame) supporting the body. The level of the forearm should be at or below the mattress surface.
The prone position is a known risk factor for POVL[139]. When patients are placed in the prone position, direct pressure on the eye must be avoided as it may cause CRAO[178]. The horseshoe head rest has been associated with CVAO and POVL in the prone position and therefore should be avoided if possible. Head positioning in Mayfield pins avoids direct pressure on the eye globe (Figure 4). Another choice is using foam positioning devices for the head, like the proneview®. The proneview® consists of a foam cushion in a plastic frame that supports the face without applying pressure on the eyes, nose or mouth and a mirror that allows frequent examination of the eye and facial structures (Figure 5). The use of the Wilson spinal frame has been associated with ION and POVL[149]. High-risk patients should be positioned with the head above the heart when possible. This will help reduce venous congestion in the eye and orbit and hopefully avoid an increase in the IOP and intraorbital pressure. The head should be in a neutral forward position when possible avoiding significant neck flexion, extension, lateral flexion or rotation[178].
Figure 4.
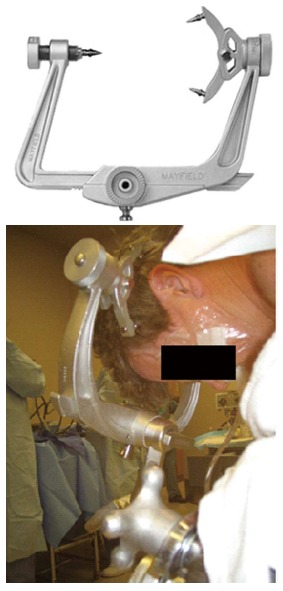
Mayfield (pinned) head holder.
Figure 5.

The proneview® allows prone positioning without any pressure on the facial structures. The mirror provided allows frequent checking of facial structures in the prone position.
The lateral decubitus position: The lateral decubitus position is used less frequently than the prone position for spine surgery. Patients placed in the lateral decubitus position had a significantly higher incidence of position-related impending upper extremity nerve injury detected by SSEP compared to patients positioned in the supine arms tucked, supine arms out and prone arms tucked positions[86]. In the lateral decubitus position compression is the main mechanism of peripheral nerve injury of the dependent brachial plexus. The brachial plexus may be compressed between the thorax and the humeral head[43]. The use of chest roll (also known as axillary roll) may help reduce the brachial plexus injury at this compression point. It is important to apply the chest roll under the chest and not in the axilla (Figure 6). Placing the roll in the axilla will increase the pressure on the brachial plexus in the axilla predisposing the patient to nerve injury. In the lateral decubitus position, there is increased pressure under the dependent shoulder. The average pressure under the dependent shoulder in the lateral position is 66 mmHg (and can exceed 100 mmHg). The pressure under the dependent shoulder decreased to 20 mmHg when the chest wall was elevated using an inflatable chest roll. The pressure further decreased to 12 mmHg when the head was supported by a second inflatable pillow to allow straightening of the cervical spine avoiding lateral angulation of the cervical spine. Patients placed in the lateral decubitus position had an average lateral angulation of neck of 14 degrees. After applying an inflatable chest roll, the average lateral angulation of the neck significantly increased to 20°. When the neck was brought into alignment by inflating a second pillow under the head, the lateral neck angulation decreased significantly to 4°. Using inflatable pillows beneath the dependent chest was associated with significantly less pressure beneath the dependent shoulder and chest compared to a 1000 mL intravenous fluid bag or gel-pads. Prolonged lateralization of the cervical spine can stretch the brachial plexus on the nondependent side[180]. Pronation of the forearm, shoulder abduction more than 90°, extreme elbow flexion and extension should be avoided in the nondependent arm. The nondependent arm rest should be positioned in a way that maintain the arm horizontal and at the same level of shoulder joint (Figure 7). Excessive elevation of the nondependent arm at a level higher than the shoulder joint can overstretch the brachial plexus and predispose the patient to radial nerve injury in the nondependent arm.
Figure 6.
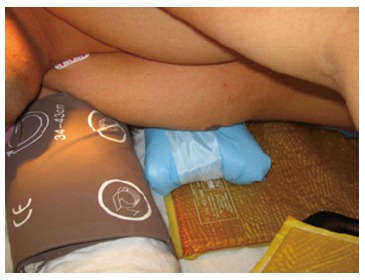
Proper placement of chest roll under the dependent chest in the lateral decubitus position. The chest roll should not be placed under the dependent axilla.
Figure 7.
Positioning the upper extremity in the lateral decubitus position. The shoulder abduction more than 90°, extreme elbow flexion and forearm pronation should be avoided in the nondependent arm. The nondependent and dependent elbows should be padded with foam. Placing foam or blankets under the dependent hand and foreram to avoid full extension may reduce the likelihood of median nerve injury. Head and neck should be in neutral forward position avoiding neck flexion extension, lateral rotation and lateral flexion.
POVL has been associated with spine surgery in the lateral decubitus position[139]. Asymmetric bilateral PION with significant involvement of the dependent eye has been reported after spine surgery in the lateral decubitus position[181]. Compression of the dependent eye should be avoided. Neutral forward position of the neck should be maintained to optimize venous drainage from the eye and the orbit. High-risk patients should be positioned with the head above the heart when possible[178].
Supine and prone arms tucked positions: Patients are placed in the supine arms tucked and prone arms tucked position for anterior and posterior cervical spine fusion surgeries respectively. The incidence of impending position-related upper extremity nerve injury detected by SSEP changes are 1.8% and 2.1% for the supine arms tucked and prone arms tucked positions respectively[86]. In both positions, it is important to position the forearm in a neutral position while padding the elbow with foam pad. The neck should be maintained in the neutral forward position whenever possible. In the prone arms tucked position, the use of a horseshoe head rest should be avoided. The use of the Mayfield pinned head holder is preferable to avoid direct external pressure on the eye. High-risk patients should be positioned with the head above the heart when possible[178].
Intraoperative management of physiological parameters
Ischemic times and thresholds that may lead to clinical perioperative injury of the peripheral nerve and the optic nerve are not documented in humans. With the lack of this knowledge, it is advisable to optimize physiologic parameters by maintaining them close to patient’s baseline values, especially in high-risk cases. Physiologic parameters determining oxygen delivery to the peripheral nerve and the optic nerve may have additive or synergistic effect in predisposed patients placed in challenging operative positions for prolonged periods. Maintaining physiologic mean arterial blood pressure parameters, avoiding severe anemia and venous congestion are important aspects of intraoperative management that may improve oxygen delivery to areas at risk.
Although patient predisposition and intraoperative positioning are usually the risk factors associated with peripheral nerve injury, hypotension and anemia can affect oxygen delivery to the peripheral nerve especially in the presence of stretch or compression. Mean arterial blood pressure has been identified as an independent predictor of upper extremity neurapraxia detected by SSEP in the prone surrender position[182]. The extent and duration of hypotension, and anemia that may cause PPNI in predisposed individuals is not documented.
The ASA task force on the prevention of POVL believes that the use of deliberate hypotension during spine surgery has not been shown to be associated with the development of perioperative visual loss; however, it is advisable to avoid deliberate hypotension in high-risk patients (e.g., with preoperative chronic hypertension). If deliberate hypotension will be used in patients without preoperative hypertension, the blood pressure should be maintained on average within 24% of baseline MAP or with a minimum systolic BP of 84 mmHg. Central venous pressure monitoring should be considered in high-risk cases. Colloids should be used with crystalloids in patients with substantial blood loss. Hemoglobin should be monitored periodically in high-risk cases with significant blood loss. There is no documented lower level of hemoglobin that would eliminate the risk of POVL[178].
Until we have a better understanding of the effects of hypotension and anemia on PPNI and POVL it is advisable to maintain intraoperative mean arterial blood pressure and hemoglobin levels close to preoperative levels in patients at high-risk for PPNI and POVL.
Footnotes
P- Reviewer: Aota Y, Knutsen G S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30:1441–1445; discussion 1446-1447. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 2.Oglesby M, Fineberg SJ, Patel AA, Pelton MA, Singh K. Epidemiological trends in cervical spine surgery for degenerative diseases between 2002 and 2009. Spine (Phila Pa 1976) 2013;38:1226–1232. doi: 10.1097/BRS.0b013e31828be75d. [DOI] [PubMed] [Google Scholar]
- 3.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976) 2006;31:2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroll DA, Caplan RA, Posner K, Ward RJ, Cheney FW. Nerve injury associated with anesthesia. Anesthesiology. 1990;73:202–207. doi: 10.1097/00000542-199008000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cheney FW, Domino KB, Caplan RA, Posner KL. Nerve injury associated with anesthesia: a closed claims analysis. Anesthesiology. 1999;90:1062–1069. doi: 10.1097/00000542-199904000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Welch MB, Brummett CM, Welch TD, Tremper KK, Shanks AM, Guglani P, Mashour GA. Perioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology. 2009;111:490–497. doi: 10.1097/ALN.0b013e3181af61cb. [DOI] [PubMed] [Google Scholar]
- 8.Cassoria L, Lee JW. Patient positioning in anesthesia. In: Miller RD, ed , editors. Miller’s Anesthesia, 7th edition. Philadelphia: Elsevier; 2009. pp. 1151–1170. [Google Scholar]
- 9.Caplan RA. Will we ever understand perioperative neuropathy? A fresh approach offers hope and insight. Anesthesiology. 1999;91:335–336. doi: 10.1097/00000542-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Coppieters MW, Van de Velde M, Stappaerts KH. Positioning in anesthesiology: toward a better understanding of stretch-induced perioperative neuropathies. Anesthesiology. 2002;97:75–81. doi: 10.1097/00000542-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Anesthesiologists Task Force on Prevention of Perioperative Peripheral Neuropathies. Practice advisory for the prevention of perioperative peripheral neuropathies: an updated report by the American Society of Anesthesiologists Task Force on prevention of perioperative peripheral neuropathies. Anesthesiology. 2011;114:741–754. doi: 10.1097/ALN.0b013e3181fcbff3. [DOI] [PubMed] [Google Scholar]
- 12.Smith DR, Kobrine AI, Rizzoli HV. Absence of autoregulation in peripheral nerve blood flow. J Neurol Sci. 1977;33:347–352. doi: 10.1016/0022-510x(77)90132-0. [DOI] [PubMed] [Google Scholar]
- 13.McManis PG, Schmelzer JD, Zollman PJ, Low PA. Blood flow and autoregulation in somatic and autonomic ganglia. Comparison with sciatic nerve. Brain. 1997;120(Pt 3):445–449. doi: 10.1093/brain/120.3.445. [DOI] [PubMed] [Google Scholar]
- 14.Sundqvist T, Oberg PA, Rapoport SI. Blood flow in rat sciatic nerve during hypotension. Exp Neurol. 1985;90:139–148. doi: 10.1016/0014-4886(85)90047-0. [DOI] [PubMed] [Google Scholar]
- 15.Low PA, Tuck RR. Effects of changes of blood pressure, respiratory acidosis and hypoxia on blood flow in the sciatic nerve of the rat. J Physiol. 1984;347:513–524. doi: 10.1113/jphysiol.1984.sp015079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry GJ, Cornblath DR, Brown MJ. Transient conduction block following acute peripheral nerve ischemia. Muscle Nerve. 1985;8:409–412. doi: 10.1002/mus.880080510. [DOI] [PubMed] [Google Scholar]
- 17.Myers RR, Yamamoto T, Yaksh TL, Powell HC. The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology. 1993;78:308–316. doi: 10.1097/00000542-199302000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Bonner SM, Pridie AK. Sciatic nerve palsy following uneventful sciatic nerve block. Anaesthesia. 1997;52:1205–1207. doi: 10.1111/j.1365-2044.1997.258-az0396.x. [DOI] [PubMed] [Google Scholar]
- 19.Warner MA, Warner ME, Martin JT. Ulnar neuropathy. Incidence, outcome, and risk factors in sedated or anesthetized patients. Anesthesiology. 1994;81:1332–1340. [PubMed] [Google Scholar]
- 20.Sunderland S. The intraneural topography of the radial, median and ulnar nerves. Brain. 1945;68:243–299. doi: 10.1093/brain/68.4.243. [DOI] [PubMed] [Google Scholar]
- 21.Aguayo A, Nair CP, Midgley R. Experimental progressive compression neuropathy in the rabbit. Histologic and electrophysiologic studies. Arch Neurol. 1971;24:358–364. doi: 10.1001/archneur.1971.00480340090010. [DOI] [PubMed] [Google Scholar]
- 22.Nukada H, McMorran PD. Perivascular demyelination and intramyelinic oedema in reperfusion nerve injury. J Anat. 1994;185:259–266. [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelzer JD, Zochodne DW, Low PA. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci USA. 1989;86:1639–1642. doi: 10.1073/pnas.86.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mäkitie J, Teräväinen H. Peripheral nerve injury and recovery after temporary ischemia. Acta Neuropathol. 1977;37:55–63. doi: 10.1007/BF00684541. [DOI] [PubMed] [Google Scholar]
- 25.Nukada H, Powell HC, Myers RR. Perineurial window: demyelination in nonherniated endoneurium with reduced nerve blood flow. J Neuropathol Exp Neurol. 1992;51:523–530. doi: 10.1097/00005072-199209000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Swenson JD, Bull DA. Postoperative ulnar neuropathy associated with prolonged ischemia in the upper extremity during coronary artery bypass surgery. Anesth Analg. 1997;85:1275–1277. doi: 10.1097/00000539-199712000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Swenson JD, Hutchinson DT, Bromberg M, Pace NL. Rapid onset of ulnar nerve dysfunction during transient occlusion of the brachial artery. Anesth Analg. 1998;87:677–680. doi: 10.1097/00000539-199809000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T, Muroga T, Kimura J. Tourniquet-induced ischemia and somatosensory evoked potentials. Neurology. 1981;31:1524–1529. doi: 10.1212/wnl.31.12.1524. [DOI] [PubMed] [Google Scholar]
- 29.Kozu H, Tamura E, Parry GJ. Endoneurial blood supply to peripheral nerves is not uniform. J Neurol Sci. 1992;111:204–208. doi: 10.1016/0022-510x(92)90070-2. [DOI] [PubMed] [Google Scholar]
- 30.Prielipp RC, Morell RC, Walker FO, Santos CC, Bennett J, Butterworth J. Ulnar nerve pressure: influence of arm position and relationship to somatosensory evoked potentials. Anesthesiology. 1999;91:345–354. doi: 10.1097/00000542-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Winfree CJ, Kline DG. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63:5–18; discussion 18. doi: 10.1016/j.surneu.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Wall EJ, Massie JB, Kwan MK, Rydevik BL, Myers RR, Garfin SR. Experimental stretch neuropathy. Changes in nerve conduction under tension. J Bone Joint Surg Br. 1992;74:126–129. doi: 10.1302/0301-620X.74B1.1732240. [DOI] [PubMed] [Google Scholar]
- 33.Tanoue M, Yamaga M, Ide J, Takagi K. Acute stretching of peripheral nerves inhibits retrograde axonal transport. J Hand Surg Br. 1996;21:358–363. doi: 10.1016/s0266-7681(05)80203-7. [DOI] [PubMed] [Google Scholar]
- 34.Ogata K, Naito M. Blood flow of peripheral nerve effects of dissection, stretching and compression. J Hand Surg Br. 1986;11:10–14. doi: 10.1016/0266-7681(86)90003-3. [DOI] [PubMed] [Google Scholar]
- 35.Brown R, Pedowitz R, Rydevik B, Woo S, Hargens A, Massie J, Kwan M, Garfin SR. Effects of acute graded strain on efferent conduction properties in the rabbit tibial nerve. Clin Orthop Relat Res. 1993;(296):288–294. [PubMed] [Google Scholar]
- 36.Lundborg G. Intraneural microcirculation. Orthop Clin North Am. 1988;19:1–12. [PubMed] [Google Scholar]
- 37.Rydevik BL, Kwan MK, Myers RR, Brown RA, Triggs KJ, Woo SL, Garfin SR. An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res. 1990;8:694–701. doi: 10.1002/jor.1100080511. [DOI] [PubMed] [Google Scholar]
- 38.Koike H. The disturbance of the fast axonal transport of protein by passive stretching of an axon in Aplysia. J Physiol. 1987;390:489–500. doi: 10.1113/jphysiol.1987.sp016713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwan MK, Wall EJ, Massie J, Garfin SR. Strain, stress and stretch of peripheral nerve. Rabbit experiments in vitro and in vivo. Acta Orthop Scand. 1992;63:267–272. doi: 10.3109/17453679209154780. [DOI] [PubMed] [Google Scholar]
- 40.Staff NP, Engelstad J, Klein CJ, Amrami KK, Spinner RJ, Dyck PJ, Warner MA, Warner ME, Dyck PJ. Post-surgical inflammatory neuropathy. Brain. 2010;133:2866–2880. doi: 10.1093/brain/awq252. [DOI] [PubMed] [Google Scholar]
- 41.Prielipp RC, Morell RC, Butterworth J. Ulnar nerve injury and perioperative arm positioning. Anesthesiol Clin North America. 2002;20:589–603. doi: 10.1016/s0889-8537(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 42.Sharma AD, Parmley CL, Sreeram G, Grocott HP. Peripheral nerve injuries during cardiac surgery: risk factors, diagnosis, prognosis, and prevention. Anesth Analg. 2000;91:1358–1369. doi: 10.1097/00000539-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Sawyer RJ, Richmond MN, Hickey JD, Jarrratt JA. Peripheral nerve injuries associated with anaesthesia. Anaesthesia. 2000;55:980–991. doi: 10.1046/j.1365-2044.2000.01614.x. [DOI] [PubMed] [Google Scholar]
- 44.Warner MA, Warner DO, Matsumoto JY, Harper CM, Schroeder DR, Maxson PM. Ulnar neuropathy in surgical patients. Anesthesiology. 1999;90:54–59. doi: 10.1097/00000542-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Warner MA, Warner DO, Harper CM, Schroeder DR, Maxson PM. Ulnar neuropathy in medical patients. Anesthesiology. 2000;92:613–615. doi: 10.1097/00000542-200002000-00047. [DOI] [PubMed] [Google Scholar]
- 46.Alvine FG, Schurrer ME. Postoperative ulnar-nerve palsy. Are there predisposing factors? J Bone Joint Surg Am. 1987;69:255–259. [PubMed] [Google Scholar]
- 47.Perreault L, Drolet P, Farny J. Ulnar nerve palsy at the elbow after general anaesthesia. Can J Anaesth. 1992;39:499–503. doi: 10.1007/BF03008716. [DOI] [PubMed] [Google Scholar]
- 48.Stoelting RK. Postoperative ulnar nerve palsy--is it a preventable complication? Anesth Analg. 1993;76:7–9. doi: 10.1213/00000539-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Morell RC, Prielipp RC, Harwood TN, James RL, Butterworth JF. Men are more susceptible than women to direct pressure on unmyelinated ulnar nerve fibers. Anesth Analg. 2003;97:1183–1188, table of contents. doi: 10.1213/01.ANE.0000078822.97549.F7. [DOI] [PubMed] [Google Scholar]
- 50.Casscells CD, Lindsey RW, Ebersole J, Li B. Ulnar neuropathy after median sternotomy. Clin Orthop Relat Res. 1993;(291):259–265. [PubMed] [Google Scholar]
- 51.Jones HD. Ulnar nerve damage following general anaesthetic. A case possibly related to diabetes mellitus. Anaesthesia. 1967;22:471–475. doi: 10.1111/j.1365-2044.1967.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 52.Britt BA, Gordon RA. Peripheral nerve injuries associated with anaesthesia. Can Anaesth Soc J. 1964;11:514–536. doi: 10.1007/BF03005094. [DOI] [PubMed] [Google Scholar]
- 53.Cameron MG, Stewart OJ. Ulnar nerve injury associated with anaesthesia. Can Anaesth Soc J. 1975;22:253–264. doi: 10.1007/BF03004834. [DOI] [PubMed] [Google Scholar]
- 54.Wadsworth TG. The external compression syndrome of the ulnar nerve at the cubital tunnel. Clin Orthop Relat Res. 1977;(124):189–204. [PubMed] [Google Scholar]
- 55.Contreras MG, Warner MA, Charboneau WJ, Cahill DR. Anatomy of the ulnar nerve at the elbow: potential relationship of acute ulnar neuropathy to gender differences. Clin Anat. 1998;11:372–378. doi: 10.1002/(SICI)1098-2353(1998)11:6<372::AID-CA2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 56.Ogata K, Shimon S, Owen J, Manske PR. Effects of compression and devascularisation on ulnar nerve function. A quantitative study of regional blood flow and nerve conduction in monkeys. J Hand Surg Br. 1991;16:104–108. doi: 10.1016/0266-7681(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 57.Macnicol MF. Extraneural pressures affecting the ulnar nerve at the elbow. Hand. 1982;14:5–11. doi: 10.1016/s0072-968x(82)80030-2. [DOI] [PubMed] [Google Scholar]
- 58.Gelberman RH, Yamaguchi K, Hollstien SB, Winn SS, Heidenreich FP, Bindra RR, Hsieh P, Silva MJ. Changes in interstitial pressure and cross-sectional area of the cubital tunnel and of the ulnar nerve with flexion of the elbow. An experimental study in human cadavera. J Bone Joint Surg Am. 1998;80:492–501. doi: 10.2106/00004623-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Schuind FA, Goldschmidt D, Bastin C, Burny F. A biomechanical study of the ulnar nerve at the elbow. J Hand Surg Br. 1995;20:623–627. doi: 10.1016/s0266-7681(05)80124-x. [DOI] [PubMed] [Google Scholar]
- 60.Pechan J, Julis I. The pressure measurement in the ulnar nerve. A contribution to the pathophysiology of the cubital tunnel syndrome. J Biomech. 1975;8:75–79. doi: 10.1016/0021-9290(75)90045-7. [DOI] [PubMed] [Google Scholar]
- 61.Patel VV, Heidenreich FP, Bindra RR, Yamaguchi K, Gelberman RH. Morphologic changes in the ulnar nerve at the elbow with flexion and extension: a magnetic resonance imaging study with 3-dimensional reconstruction. J Shoulder Elbow Surg. 1998;7:368–374. doi: 10.1016/s1058-2746(98)90025-8. [DOI] [PubMed] [Google Scholar]
- 62.Cooper DE, Jenkins RS, Bready L, Rockwood CA. The prevention of injuries of the brachial plexus secondary to malposition of the patient during surgery. Clin Orthop Relat Res. 1988;(228):33–41. [PubMed] [Google Scholar]
- 63.Warner MA. Patient positioning and nerve injury. Anesthesiology clinics of North America. 1996;14:561–572. [Google Scholar]
- 64.Dhvner KG. Nerve Injuries Following Operations: A Survey of Cases Occurring During a Six-Year Period. Anesthesiology. 1950;11:289–293. [Google Scholar]
- 65.Ngamprasertwong P, Phupong V, Uerpairojkit K. Brachial plexus injury related to improper positioning during general anesthesia. J Anesth. 2004;18:132–134. doi: 10.1007/s00540-003-0220-6. [DOI] [PubMed] [Google Scholar]
- 66.Warner MA. Patient positioning. In: Barash PG, Cullen BF, Stoelting RK eds, editors. Clinical anesthesia 5th ed. Phildelphia: Lippincot Williams & Wilkins; 2006. pp. 643–667. [Google Scholar]
- 67.Chowet AL, Lopez JR, Brock-Utne JG, Jaffe RA. Wrist hyperextension leads to median nerve conduction block: implications for intra-arterial catheter placement. Anesthesiology. 2004;100:287–291. doi: 10.1097/00000542-200402000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Nicholson MJ, Eversole UH. Nerve injuries incident to anesthesia and operation. Anesth Analg. 1957;36:19–32. [PubMed] [Google Scholar]
- 69.Blinder J, El-Mansouri M, Connelly NR. Survey of anesthesia practice in spine surgery patients in the United States. J Anesth. 2003;17:8–12. doi: 10.1007/s005400300002. [DOI] [PubMed] [Google Scholar]
- 70.Mahla ME, Long DM, McKennett J, Green C, McPherson RW. Detection of brachial plexus dysfunction by somatosensory evoked potential monitoring--a report of two cases. Anesthesiology. 1984;60:248–252. doi: 10.1097/00000542-198403000-00019. [DOI] [PubMed] [Google Scholar]
- 71.Pitman MI, Nainzadeh N, Ergas E, Springer S. The use of somatosensory evoked potentials for detection of neuropraxia during shoulder arthroscopy. Arthroscopy. 1988;4:250–255. doi: 10.1016/s0749-8063(88)80039-2. [DOI] [PubMed] [Google Scholar]
- 72.Hickey C, Gugino LD, Aglio LS, Mark JB, Son SL, Maddi R. Intraoperative somatosensory evoked potential monitoring predicts peripheral nerve injury during cardiac surgery. Anesthesiology. 1993;78:29–35. doi: 10.1097/00000542-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Jellish WS, Martucci J, Blakeman B, Hudson E. Somatosensory evoked potential monitoring of the brachial plexus to predict nerve injury during internal mammary artery harvest: intraoperative comparisons of the Rultract and Pittman sternal retractors. J Cardiothorac Vasc Anesth. 1994;8:398–403. doi: 10.1016/1053-0770(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 74.Jones SC, Fernau R, Woeltjen BL. Use of somatosensory evoked potentials to detect peripheral ischemia and potential injury resulting from positioning of the surgical patient: case reports and discussion. Spine J. 2004;4:360–362. doi: 10.1016/j.spinee.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz DM, Sestokas AK, Hilibrand AS, Vaccaro AR, Bose B, Li M, Albert TJ. Neurophysiological identification of position-induced neurologic injury during anterior cervical spine surgery. J Clin Monit Comput. 2006;20:437–444. doi: 10.1007/s10877-006-9032-1. [DOI] [PubMed] [Google Scholar]
- 76.Bethune AJ, Houlden DA, Smith TS, Yee AJ, Midha R, Singrakhia M. Generalized peripheral nerve failure during thoracic spine surgery: a case report. J Clin Monit Comput. 2007;21:41–47. doi: 10.1007/s10877-006-9056-6. [DOI] [PubMed] [Google Scholar]
- 77.Anastasian ZH, Ramnath B, Komotar RJ, Bruce JN, Sisti MB, Gallo EJ, Emerson RG, Heyer EJ. Evoked potential monitoring identifies possible neurological injury during positioning for craniotomy. Anesth Analg. 2009;109:817–821. doi: 10.1213/ane.0b013e3181b086bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung I, Glow JA, Dimopoulos V, Walid MS, Smisson HF, Johnston KW, Robinson JS, Grigorian AA. Upper-limb somatosensory evoked potential monitoring in lumbosacral spine surgery: a prognostic marker for position-related ulnar nerve injury. Spine J. 2009;9:287–295. doi: 10.1016/j.spinee.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Schwartz DM, Drummond DS, Hahn M, Ecker ML, Dormans JP. Prevention of positional brachial plexopathy during surgical correction of scoliosis. J Spinal Disord. 2000;13:178–182. doi: 10.1097/00002517-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 80.Jahangiri FR, Holmberg A, Vega-Bermudez F, Arlet V. Preventing position-related brachial plexus injury with intraoperative somatosensory evoked potentials and transcranial electrical motor evoked potentials during anterior cervical spine surgery. Am J Electroneurodiagnostic Technol. 2011;51:198–205. [PubMed] [Google Scholar]
- 81.Davis SF, Abdel Khalek M, Giles J, Fox C, Lirette L, Kandil E. Detection and prevention of impending brachial plexus injury secondary to arm positioning using ulnar nerve somatosensory evoked potentials during transaxillary approach for thyroid lobectomy. Am J Electroneurodiagnostic Technol. 2011;51:274–279. [PubMed] [Google Scholar]
- 82.Jellish WS, Sherazee G, Patel J, Cunanan R, Steele J, Garibashvilli K, Baldwin M, Anderson D, Leonetti JP. Somatosensory evoked potentials help prevent positioning-related brachial plexus injury during skull base surgery. Otolaryngol Head Neck Surg. 2013;149:168–173. doi: 10.1177/0194599813482878. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien MF, Lenke LG, Bridwell KH, Padberg A, Stokes M. Evoked potential monitoring of the upper extremities during thoracic and lumbar spinal deformity surgery: a prospective study. J Spinal Disord. 1994;7:277–284. [PubMed] [Google Scholar]
- 84.Makarov MR, Delgado MR, Birch JG, Samchukov ML. Monitoring peripheral nerve function during external fixation of upper extremities. J Pediatr Orthop. 1997;17:663–667. doi: 10.1097/00004694-199709000-00017. [DOI] [PubMed] [Google Scholar]
- 85.Ohashi N, Furutani K, Ishii H, Baba H. Perioperative brachial plexus injury caused by hyperabduction of the upper extremity in a patient with Ehlers-Danlos syndrome in the prone position. Masui. 2012;61:626–628. [PubMed] [Google Scholar]
- 86.Kamel IR, Drum ET, Koch SA, Whitten JA, Gaughan JP, Barnette RE, Wendling WW. The use of somatosensory evoked potentials to determine the relationship between patient positioning and impending upper extremity nerve injury during spine surgery: a retrospective analysis. Anesth Analg. 2006;102:1538–1542. doi: 10.1213/01.ane.0000198666.11523.d6. [DOI] [PubMed] [Google Scholar]
- 87.Forbes HJ, Allen PW, Waller CS, Jones SJ, Edgar MA, Webb PJ, Ransford AO. Spinal cord monitoring in scoliosis surgery. Experience with 1168 cases. J Bone Joint Surg Br. 1991;73:487–491. doi: 10.1302/0301-620X.73B3.1670455. [DOI] [PubMed] [Google Scholar]
- 88.Albanese SA, Spadaro JA, Lubicky JP, Henderson NA. Somatosensory cortical evoked potential changes after deformity correction. Spine (Phila Pa 1976) 1991;16:S371–S374. [PubMed] [Google Scholar]
- 89.Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109:1534–1545. doi: 10.1213/ane.0b013e3181b0500b. [DOI] [PubMed] [Google Scholar]
- 90.Chang SH, Miller NR. The incidence of vision loss due to perioperative ischemic optic neuropathy associated with spine surgery: the Johns Hopkins Hospital Experience. Spine (Phila Pa 1976) 2005;30:1299–1302. doi: 10.1097/01.brs.0000163884.11476.25. [DOI] [PubMed] [Google Scholar]
- 91.Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997;22:1319–1324. doi: 10.1097/00007632-199706150-00008. [DOI] [PubMed] [Google Scholar]
- 92.Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976) 2008;33:1491–1496. doi: 10.1097/BRS.0b013e318175d1bf. [DOI] [PubMed] [Google Scholar]
- 93.Stambough JL, Dolan D, Werner R, Godfrey E. Ophthalmologic complications associated with prone positioning in spine surgery. J Am Acad Orthop Surg. 2007;15:156–165. doi: 10.5435/00124635-200703000-00005. [DOI] [PubMed] [Google Scholar]
- 94.Myers MA, Hamilton SR, Bogosian AJ, Smith CH, Wagner TA. Visual loss as a complication of spine surgery. A review of 37 cases. Spine (Phila Pa 1976) 1997;22:1325–1329. doi: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 95.Benowitz LI, Yin Y. Optic nerve regeneration. Arch Ophthalmol. 2010;128:1059–1064. doi: 10.1001/archophthalmol.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hayreh SS, Revie IH, Edwards J. Vasogenic origin of visual field defects and optic nerve changes in glaucoma. Br J Ophthalmol. 1970;54:461–472. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayreh SS. The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res. 2001;20:563–593. doi: 10.1016/s1350-9462(01)00004-0. [DOI] [PubMed] [Google Scholar]
- 99.Hayreh SS. Inter-individual variation in blood supply of the optic nerve head. Its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985;59:217–246. doi: 10.1007/BF00159262. [DOI] [PubMed] [Google Scholar]
- 100.Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye (Lond) 1990;4(Pt 2):273–289. doi: 10.1038/eye.1990.39. [DOI] [PubMed] [Google Scholar]
- 101.Hayreh SS. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4:251–263. [PubMed] [Google Scholar]
- 102.Hayreh SS. Blood flow in the optic nerve head and factors that may influence it. Prog Retin Eye Res. 2001;20:595–624. doi: 10.1016/s1350-9462(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 103.Weinstein JM, Duckrow RB, Beard D, Brennan RW. Regional optic nerve blood flow and its autoregulation. Invest Ophthalmol Vis Sci. 1983;24:1559–1565. [PubMed] [Google Scholar]
- 104.Pillunat LE, Anderson DR, Knighton RW, Joos KM, Feuer WJ. Autoregulation of human optic nerve head circulation in response to increased intraocular pressure. Exp Eye Res. 1997;64:737–744. doi: 10.1006/exer.1996.0263. [DOI] [PubMed] [Google Scholar]
- 105.Movaffaghy A, Chamot SR, Petrig BL, Riva CE. Blood flow in the human optic nerve head during isometric exercise. Exp Eye Res. 1998;67:561–568. doi: 10.1006/exer.1998.0556. [DOI] [PubMed] [Google Scholar]
- 106.Hayreh SS, Servais GE, Virdi PS. Fundus lesions in malignant hypertension. IV. Focal intraretinal periarteriolar transudates. Ophthalmology. 1986;93:60–73. doi: 10.1016/s0161-6420(86)33774-6. [DOI] [PubMed] [Google Scholar]
- 107.Hayreh SS, Bill A, Sperber GO. Effects of high intraocular pressure on the glucose metabolism in the retina and optic nerve in old atherosclerotic monkeys. Graefes Arch Clin Exp Ophthalmol. 1994;232:745–752. doi: 10.1007/BF00184278. [DOI] [PubMed] [Google Scholar]
- 108.Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: a new concept in ophthalmology? Surv Ophthalmol. 1994;39:123–132. doi: 10.1016/0039-6257(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 109.Hayreh SS. Pathophysiology of glaucomatous optic neuropthy: Role of optic nerve had vascular insufficiency. JCGP. 2008;2:6–17. [Google Scholar]
- 110.Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96. doi: 10.1159/000027399. [DOI] [PubMed] [Google Scholar]
- 111.Hayreh SS. The role of age and cardiovascular disease in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43 Suppl 1:S27–S42. doi: 10.1016/s0039-6257(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 112.Hayreh SS. Anterior ischemic optic neuropathy. VIII. Clinical features and pathogenesis of post-hemorrhagic amaurosis. Ophthalmology. 1987;94:1488–1502. doi: 10.1016/s0161-6420(87)33273-7. [DOI] [PubMed] [Google Scholar]
- 113.Graham SL, Drance SM, Wijsman K, Douglas GR, Mikelberg FS. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–69. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 114.Béchetoille A, Bresson-Dumont H. Diurnal and nocturnal blood pressure drops in patients with focal ischemic glaucoma. Graefes Arch Clin Exp Ophthalmol. 1994;232:675–679. doi: 10.1007/BF00171383. [DOI] [PubMed] [Google Scholar]
- 115.Hayreh SS, Podhajsky PA, Zimmerman B. Nonarteritic anterior ischemic optic neuropathy: time of onset of visual loss. Am J Ophthalmol. 1997;124:641–647. doi: 10.1016/s0002-9394(14)70902-x. [DOI] [PubMed] [Google Scholar]
- 116.Murphy DF. Anesthesia and intraocular pressure. Anesth Analg. 1985;64:520–530. [PubMed] [Google Scholar]
- 117.Hvidberg A, Kessing SV, Fernandes A. Effect of changes in PCO2 and body positions on intraocular pressure during general anaesthesia. Acta Ophthalmol (Copenh) 1981;59:465–475. doi: 10.1111/j.1755-3768.1981.tb08331.x. [DOI] [PubMed] [Google Scholar]
- 118.Cooper RL, Beale DG, Constable IJ, Grose GC. Continual monitoring of intraocular pressure: effect of central venous pressure, respiration, and eye movements on continual recordings of intraocular pressure in the rabbit, dog, and man. Br J Ophthalmol. 1979;63:799–804. doi: 10.1136/bjo.63.12.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adams AP, Freedman A, Henville JD. Normocapnic anaesthesia for intraocular surgery. Br J Ophthalmol. 1979;63:204–210. doi: 10.1136/bjo.63.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samuel JR, Beaugié A. Effect of carbon dioxide on the intraocular pressure in man during general anaesthesia. Br J Ophthalmol. 1974;58:62–67. doi: 10.1136/bjo.58.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spalter HF, Teneick re, nahas gg. effect of hypercapnia on retinal vessel size at constant intracranial pressure. Am J Ophthalmol. 1964;57:741–745. doi: 10.1016/0002-9394(64)92218-4. [DOI] [PubMed] [Google Scholar]
- 122.Friedman E, Chandra SR. Choroidal blood flow. 3. Effects of oxygen and carbon dioxide. Arch Ophthalmol. 1972;87:70–71. doi: 10.1001/archopht.1972.01000020072015. [DOI] [PubMed] [Google Scholar]
- 123.Weiter JJ, Shiyoung R. Anatomy and physiology: retinal and choroidal circulation. In: Yanoff M, ed , editors. Opthalmology. St. Louis: Mosby; 1999. pp. 1–4. [Google Scholar]
- 124.Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987;103:523–526. doi: 10.1016/s0002-9394(14)74275-8. [DOI] [PubMed] [Google Scholar]
- 125.Kaskel D, Müller-Breitenkamp R, Wilmans I, Rudolf H, Jessen K. [The influence of changes in body position on intraocular pressure, episcleral venous pressure, and blood pressure (author’s transl)] Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;208:217–228. doi: 10.1007/BF00419376. [DOI] [PubMed] [Google Scholar]
- 126.Ozcan MS, Praetel C, Bhatti MT, Gravenstein N, Mahla ME, Seubert CN. The effect of body inclination during prone positioning on intraocular pressure in awake volunteers: a comparison of two operating tables. Anesth Analg. 2004;99:1152–118, table of contents. doi: 10.1213/01.ANE.0000130851.37039.50. [DOI] [PubMed] [Google Scholar]
- 127.Mäepea O, Bill A. The pressures in the episcleral veins, Schlemm’s canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989;49:645–663. doi: 10.1016/s0014-4835(89)80060-0. [DOI] [PubMed] [Google Scholar]
- 128.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- 129.Smith TJ, Lewis J. Effect of inverted body position intraocular pressure. Am J Ophthalmol. 1985;99:617–618. doi: 10.1016/s0002-9394(14)77989-9. [DOI] [PubMed] [Google Scholar]
- 130.Tsamparlakis J, Casey TA, Howell W, Edridge A. Dependence of intraocular pressure on induced hypotension and posture during surgical anaesthesia. Trans Ophthalmol Soc U K. 1980;100:521–526. [PubMed] [Google Scholar]
- 131.Cheng MA, Todorov A, Tempelhoff R, McHugh T, Crowder CM, Lauryssen C. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology. 2001;95:1351–1355. doi: 10.1097/00000542-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 132.Awad H, Santilli S, Ohr M, Roth A, Yan W, Fernandez S, Roth S, Patel V. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–478. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 133.Szmuk P, Steiner JW, Pop RB, You J, Weakley DR, Swift DM, Sessler DI. Intraocular pressure in pediatric patients during prone surgery. Anesth Analg. 2013;116:1309–1313. doi: 10.1213/ANE.0b013e31828d3730. [DOI] [PubMed] [Google Scholar]
- 134.Draeger J, Hanke K. Postural variations of intraocular pressure--preflight experiments for the D1-mission. Ophthalmic Res. 1986;18:55–60. doi: 10.1159/000265415. [DOI] [PubMed] [Google Scholar]
- 135.Brucculeri M, Hammel T, Harris A, Malinovsky V, Martin B. Regulation of intraocular pressure after water drinking. J Glaucoma. 1999;8:111–116. [PubMed] [Google Scholar]
- 136.Martin B, Harris A, Hammel T, Malinovsky V. Mechanism of exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1999;40:1011–1015. [PubMed] [Google Scholar]
- 137.Hyams SW, Friedman Z, Neumann E. Elevated intraocular pressure in the prone position. A new provocative test for angle-closure glaucoma. Am J Ophthalmol. 1968;66:661–672. doi: 10.1016/0002-9394(68)91287-7. [DOI] [PubMed] [Google Scholar]
- 138.Holy SE, Tsai JH, McAllister RK, Smith KH. Perioperative ischemic optic neuropathy: a case control analysis of 126,666 surgical procedures at a single institution. Anesthesiology. 2009;110:246–253. doi: 10.1097/ALN.0b013e318194b238. [DOI] [PubMed] [Google Scholar]
- 139.Lee LA, Roth S, Posner KL, Cheney FW, Caplan RA, Newman NJ, Domino KB. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652–69; quiz 867-868. doi: 10.1097/00000542-200610000-00007. [DOI] [PubMed] [Google Scholar]
- 140.Williams EL, Hart WM, Tempelhoff R. Postoperative ischemic optic neuropathy. Anesth Analg. 1995;80:1018–1029. doi: 10.1097/00000539-199505000-00029. [DOI] [PubMed] [Google Scholar]
- 141.Gill B, Heavner JE. Postoperative visual loss associated with spine surgery. Eur Spine J. 2006;15:479–484. doi: 10.1007/s00586-005-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katz DM, Trobe JD, Cornblath WT, Kline LB. Ischemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol. 1994;112:925–931. doi: 10.1001/archopht.1994.01090190073024. [DOI] [PubMed] [Google Scholar]
- 143.Feit RH, Tomsak RL, Ellenberger C. Structural factors in the pathogenesis of ischemic optic neuropathy. Am J Ophthalmol. 1984;98:105–108. doi: 10.1016/0002-9394(84)90196-x. [DOI] [PubMed] [Google Scholar]
- 144.Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994;80:222–226. doi: 10.1097/00000542-199401000-00033. [DOI] [PubMed] [Google Scholar]
- 145.Boghen DR, Glaser JS. Ischaemic optic neuropathy. The clinical profile and history. Brain. 1975;98:689–708. doi: 10.1093/brain/98.4.689. [DOI] [PubMed] [Google Scholar]
- 146.Beri M, Klugman MR, Kohler JA, Hayreh SS. Anterior ischemic optic neuropathy. VII. Incidence of bilaterality and various influencing factors. Ophthalmology. 1987;94:1020–1028. doi: 10.1016/s0161-6420(87)33350-0. [DOI] [PubMed] [Google Scholar]
- 147.Grant GP, Szirth BC, Bennett HL, Huang SS, Thaker RS, Heary RF, Turbin RE. Effects of prone and reverse trendelenburg positioning on ocular parameters. Anesthesiology. 2010;112:57–65. doi: 10.1097/ALN.0b013e3181c294e1. [DOI] [PubMed] [Google Scholar]
- 148.Pomeranz HD, Bhavsar AR. Nonarteritic ischemic optic neuropathy developing soon after use of sildenafil (viagra): a report of seven new cases. J Neuroophthalmol. 2005;25:9–13. doi: 10.1097/00041327-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 149.Lee LA. Perioperative visual loss and anesthetic management. Curr Opin Anaesthesiol. 2013;26:375–381. doi: 10.1097/ACO.0b013e328360dcd9. [DOI] [PubMed] [Google Scholar]
- 150.Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malcolm-Smith NA, McMaster MJ. The use of induced hypotension to control bleeding during posterior fusion for scoliosis. J Bone Joint Surg Br. 1983;65:255–258. doi: 10.1302/0301-620X.65B3.6841391. [DOI] [PubMed] [Google Scholar]
- 152.Patel NJ, Patel BS, Paskin S, Laufer S. Induced moderate hypotensive anesthesia for spinal fusion and Harrington-rod instrumentation. J Bone Joint Surg Am. 1985;67:1384–1387. [PubMed] [Google Scholar]
- 153.Lam AK, Douthwaite WA. Does the change of anterior chamber depth or/and episcleral venous pressure cause intraocular pressure change in postural variation? Optom Vis Sci. 1997;74:664–667. doi: 10.1097/00006324-199708000-00028. [DOI] [PubMed] [Google Scholar]
- 154.Talks SJ, Chong NH, Gibson JM, Dodson PM. Fibrinogen, cholesterol and smoking as risk factors for non-arteritic anterior ischaemic optic neuropathy. Eye (Lond) 1995;9(Pt 1):85–88. doi: 10.1038/eye.1995.13. [DOI] [PubMed] [Google Scholar]
- 155.Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy. A case-control study of potential risk factors. Arch Ophthalmol. 1997;115:1403–1407. doi: 10.1001/archopht.1997.01100160573008. [DOI] [PubMed] [Google Scholar]
- 156.Hayreh SS. Anterior ischemic optic neuropathy. V. Optic disc edema an early sign. Arch Ophthalmol. 1981;99:1030–1040. doi: 10.1001/archopht.1981.03930011030010. [DOI] [PubMed] [Google Scholar]
- 157.Slavin ML, Barondes MJ. Ischemic optic neuropathy in sickle cell disease. Am J Ophthalmol. 1988;105:212–213. doi: 10.1016/0002-9394(88)90191-2. [DOI] [PubMed] [Google Scholar]
- 158.Laibovitz RA. Reversible ischemic optic neuropathy in severe anemia. Tex Med. 1982;78:56–58. [PubMed] [Google Scholar]
- 159.Ballen PH, Fox MJ, Weissman GS. Ischemic optic neuropathy secondary to intestinal hemorrhage. Ann Ophthalmol. 1985;17:486–488. [PubMed] [Google Scholar]
- 160.Jonas JB, Gusek GC, Naumann GO. Anterior ischemic optic neuropathy: nonarteritic form in small and giant cell arteritis in normal sized optic discs. Int Ophthalmol. 1988;12:119–125. doi: 10.1007/BF00137137. [DOI] [PubMed] [Google Scholar]
- 161.Mansour AM, Shoch D, Logani S. Optic disk size in ischemic optic neuropathy. Am J Ophthalmol. 1988;106:587–589. doi: 10.1016/0002-9394(88)90591-0. [DOI] [PubMed] [Google Scholar]
- 162.Roth S. Postoperative visual loss. Anesthesia. In: Miller RD, ed , editors. Miller’s Anesthesia, 7th ed. Philadelphia: Elsevier; 2009. pp. 2821–2841. [Google Scholar]
- 163.Hayreh SS. Anterior ischaemic optic neuropathy. III. Treatment, prophylaxis, and differential diagnosis. Br J Ophthalmol. 1974;58:981–989. doi: 10.1136/bjo.58.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wilson JF, Freeman SB, Breene DP. Anterior ischemic optic neuropathy causing blindness in the head and neck surgery patient. Arch Otolaryngol Head Neck Surg. 1991;117:1304–1306. doi: 10.1001/archotol.1991.01870230120021. [DOI] [PubMed] [Google Scholar]
- 165.Hayreh SS. Anterior ischaemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol. 1974;58:964–980. doi: 10.1136/bjo.58.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96:478–483. doi: 10.1016/s0002-9394(14)77911-5. [DOI] [PubMed] [Google Scholar]
- 167.Quigley HA, Miller NR, Green WR. The pattern of optic nerve fiber loss in anterior ischemic optic neuropathy. Am J Ophthalmol. 1985;100:769–776. doi: 10.1016/s0002-9394(14)73365-3. [DOI] [PubMed] [Google Scholar]
- 168.Beck RW, Servais GE, Hayreh SS. Anterior ischemic optic neuropathy. IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94:1503–1508. [PubMed] [Google Scholar]
- 169.Hayreh SS. Posterior ischemic optic neuropathy. Ophthalmologica. 1981;182:29–41. doi: 10.1159/000309085. [DOI] [PubMed] [Google Scholar]
- 170.Johnson MW, Kincaid MC, Trobe JD. Bilateral retrobulbar optic nerve infarctions after blood loss and hypotension. A clinicopathologic case study. Ophthalmology. 1987;94:1577–1584. doi: 10.1016/s0161-6420(87)33236-1. [DOI] [PubMed] [Google Scholar]
- 171.Dunker S, Hsu HY, Sebag J, Sadun AA. Perioperative risk factors for posterior ischemic optic neuropathy. J Am Coll Surg. 2002;194:705–710. doi: 10.1016/s1072-7515(02)01210-3. [DOI] [PubMed] [Google Scholar]
- 172.Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50:15–26. doi: 10.1016/j.survophthal.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 173.Levin LA, Danesh-Meyer HV. Hypothesis: a venous etiology for nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2008;126:1582–1585. doi: 10.1001/archopht.126.11.1582. [DOI] [PubMed] [Google Scholar]
- 174.Reddy A, Foroozan R, Edmond JC, Hinckley LK. Dilated superior ophthalmic veins and posterior ischemic optic neuropathy after prolonged spine surgery. J Neuroophthalmol. 2008;28:327–328. doi: 10.1097/WNO.0b013e318175c86c. [DOI] [PubMed] [Google Scholar]
- 175.Givner I, Jaffe N. Occlusion of the central retinal artery following anesthesia. Arch Ophthal. 1950;43:197–201. doi: 10.1001/archopht.1950.00910010204001. [DOI] [PubMed] [Google Scholar]
- 176.Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976) 1993;18:1226–1228. doi: 10.1097/00007632-199307000-00017. [DOI] [PubMed] [Google Scholar]
- 177.Bekar A, Türeyen K, Aksoy K. Unilateral blindness due to patient positioning during cervical syringomyelia surgery: unilateral blindness after prone position. J Neurosurg Anesthesiol. 1996;8:227–229. doi: 10.1097/00008506-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 178.American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Practice advisory for perioperative visual loss associated with spine surgery: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology. 2012;116:274–285. doi: 10.1097/ALN.0b013e31823c104d. [DOI] [PubMed] [Google Scholar]
- 179.Martin JT. The ventral dscubitus (prone) positions. In: Martin JT, Warner MA, eds , editors. Positioning in anesthesia and surgery, 3rd ed. Philadelphia: W. B Saunders; 1997. pp. 144–195. [Google Scholar]
- 180.Gonzalez Della Valle A, Salonia-Ruzo P, Peterson MG, Salvati EA, Sharrock NE. Inflatable pillows as axillary support devices during surgery performed in the lateral decubitus position under epidural anesthesia. Anesth Analg. 2001;93:1338–1343. doi: 10.1097/00000539-200111000-00062. [DOI] [PubMed] [Google Scholar]
- 181.Heitz JW, Audu PB. Asymmetric postoperative visual loss after spine surgery in the lateral decubitus position. Br J Anaesth. 2008;101:380–382. doi: 10.1093/bja/aen163. [DOI] [PubMed] [Google Scholar]
- 182.Kamel IR, Patel D, Gaughan JP, Damiano T, Barnette RE. Identifying Predictors of Upper Extremity Neurapraxia in the Prone “Surrender” Position Using SSEP (Abstract) Anesthesiology. 2008;109:A1018. [Google Scholar]


