Abstract
Although injury and neuromuscular activation patterns may be common for all individuals, there are certain factors which differentiate neuromuscular activity responses between children, adults and elderly. The purpose of this study is to review recent evidence on age differences in neural activation and muscle balances around the knee when performing single joint movements. Particularly, current evidence indicates that there are some interesting similarities in the neuromuscular mechanisms by which children or the elderly differ compared with adults. Both children and elderly display a lower absolute muscle strength capacity than adults which cannot fully be explained by differences in muscle mass. Quadriceps activation failure is a common symptom of all knee injuries, irrespective of age but it is likely that its effect is more evident in children or adults. While one might expect that antagonist co-activation would differ between age categories, it appears that this is not the case. Although hamstring: quadriceps ratio levels are altered after knee injury, it is not clear whether this is an age specific response. Finally, evidence suggests that both children and the elderly display less stiffness of the quadriceps muscle-tendon unit than adults which affects their knee joint function.
Keywords: Knee stability; Knee joint; Stiffness; Electromyography; Strength imbalance; Aging, Co-activation; Age; Injuries
Core tip: Children and elderly display a lower absolute muscle strength capacity than young adults. This may be due to a higher quadriceps activation failure as well as a more compliant quadriceps muscle-tendon in children (probably due to maturation) and elderly (due to age effects on neuromuscular system) than adults which, in turn, leads to an altered strength capacity. In contrast, age differences in muscle co-activation are not age dependent. Current evidence precludes any conclusions on whether muscle strength balance ratios are age specific.
INTRODUCTION
The well documented benefits of physical activity and exercise for health include an increase in physical competency and psychosocial interaction as well as decreased health risks[1,2]. However, physical activity also carries a risk of injury[3,4].
The knee joint is one of the most common injured joints[5]. Alteration of normal neuromuscular function around the knee is considered as a significant contributor to injuries. For this reason, restoration of neuromuscular function represents a fundamental aim of post-injury rehabilitation.
Although injury and neuromuscular activation patterns may be common for all individuals, there are certain factors which differentiate neuromuscular activity responses between children, adults and elderly. The effects of growth and maturation on neuromuscular function have not been thoroughly investigated but there is evidence that children display different neuromuscular profiles compared with adults. It is also known that aging has a significant impact on the force generation capacity of the muscular system which is accompanied by changes in neuromuscular activation patterns. The purpose of this study is to review current and recent evidence on neural activation and muscle strength balances around the knee in children, adults and aged individuals. The main research question was whether there are similarities in neuromuscular interaction during single joint tests across the life span.
There are numerous techniques to evaluate neuromuscular function depending on the scope of assessment and the applied methodology. Evaluation may help in the understanding of the causes of knee injury, and aid in the development of more effective training and rehabilitation programs[6,7]. After providing a brief introduction on knee injury epidemiology, age differences in four different areas of neuromuscular function will be examined. First, the ability of the central nervous system to provide the essentially stimuli for muscular activation are examined. This is translated into quantification of the extent the central nervous system is able to activate the entire motor pool. Second, muscle co-activation which is defined as the simultaneous activity of various muscles acting around the knee will be examined. This is achieved mainly by comparing electromyography (EMG) signals of the antagonistic muscle groups of the knee. Third, muscle strength imbalances around the knee will be examined, mainly refereeing to the hamstrings (H) to quadriceps (Q) moment ratio (H:Q ratio) during isometric or isokinetic tests. Forth, factors related to the properties of the muscle-tendon units of the knee joint and their role for knee joint function will be presented. This study will focus on experimental evidence from single joint movements rather than multi-joint activities.
RESEARCH
A worldwide review of published work on neuromuscular interactions during single joint movements was conducted. Studies were selected for this review if they were written in English, they focused on neuro-muscular or musculo-tendinous strategies during knee joint tests. The literature search was performed from date of inception until end of November 2013 on the following electronic databases: Scopus (1995-2013), Web of Science (1970-2013), PubMed (1948-2013), Proquest, CINAHL, EBSCO, Embase, and Cochrane. The use of key words “knee”, “age-related”, “neuromuscular”, “children” “knee flexors”, “knee extensors”, “activation level”, “neural adaptation”, “ageing”, “muscle strength”, “antagonist”, “coactivation”, “co-contraction”, “ tendon stiffness” “injury mechanisms”. Studies excluded were non-English language papers, conference abstracts, research reports, personal correspondence. A total of 831 studies that met the inclusion criteria were assessed by two co-authors followed by blind assessment by a third co-author with respect to: (1) sample size; (2) reliability of measurement protocols; and (3) clear data presentation. Case studies or studies which did not report the reliability of their protocol or their data were not clearly presented were excluded from the analysis.
KNEE INJURY EPIDEMIOLOGY: A SHORT OVERVIEW ON AGE DIFFERENCES
The current literature on knee injuries is extensive and it cannot be fully presented in this review. Nevertheless, it is worthwhile to provide a brief overview on potential similarities and differences in knee injury profiles across lifespan.
Knee injuries are frequently seen in the everyday clinical practice of orthopaedic surgeons and general practitioners. In the general population, the incidence is suggested to be 11 cases per 1000 person-years[8]. In a recent study, Gage et al[9] examined 6664324 knee injuries and they found that individuals aged 15 to 24 years displayed the highest injury rate while children younger than 5 years had the lowest rate which is confirmed by similar studies in this area[8,10,11].
The most common injury is a knee sprain without clearly identifiable internal derangement, and the most common diagnoses are anterior cruciate ligament (ACL) tear (20.3%), medial meniscus tear (10.8%) and chondral lesion (10.6%)[10]. Other frequent diagnoses include acute patellar dislocation (22%) and collateral ligament tear (9%)[12].
There are various factors which have been considered to increase the risk for knee joint injury. In general, a higher age increases the risk of disabling knee injuries[13]. However, it appears that risk factors act in combination with other factors rather than individually. For example, higher age, obesity, and poor physical conditioning are frequently suggested to be risk factors for musculoskeletal injuries as a whole[13-15]. In another example, a higher age combined with higher weight increase the risk for deeper chondral lesions[15] as well as knee injuries in general[12]. The number of chondral lesions increases with age[16].
Systematic participation in sports and gender are additional factors which are also related to a higher injury risk. It is not surprising that current literature focuses primarily on young athletes[3,10,17-19]. For example, knee injuries are reported to account for 60% of high school sports-related surgeries[17,20]. Patellar dislocations typically occur in young adults during sports[21]. Risk factors for acute patellar dislocations are suggested to be higher height and weight[12,22]. Participation in sports, quadriceps muscle weakness, and female sex are associated with ACL tears[23-26] while all these factors acting in combination with older age increase the risk for meniscal tears[5,27]. Further, female athletes have been reported to be four to six times more likely to sustain a major knee injury[17,20].
Individuals 65 years and older sustained a higher proportion of injury due to stairs, ramps, landings, and floors (42.0%), compared to adults and children[9]. Furthermore, ageing is a well-defined risk factor for knee osteoarthritis, as the risk for osteoarthritis increases by 2 to 10 times in people between 30 and 60 years of age and even more for individuals above 60 years[28,29]. Knee arthritis is more common among men below the age of 50, while it is more frequent among women above this age[30]. Obesity and overweight are also known risk factors for knee osteoarthritis, due to mechanical overload of the knee joints[30-33]. Occupations requiring repetitive weight-lifting and squatting[34] as well as repetitive knee torsion[35] and knee bending have been associated with knee osteoarthritis.
To summarize, it appears that knee injury rates are higher in young adults than children and the elderly. Adults suffer mostly from ligamentous injuries chondral lesions and sprains, children display less serious injuries while arthritis represents a characteristic injury of older individuals. Knee injury risk factors, such as obesity, gender, body mass index and poor physical conditioning or systematic participation in sports contribute to injury, irrespective of age.
COMMON NEUROMUSCULAR MECHANISMS AROUND THE KNEE
Arthrogenic muscle inhibition
Knee injury or surgery or arthritis lead to weakness of the quadriceps muscle group[36-40]. One of the factors responsible for this atrophy is an on-going neural inhibition that prevents the quadriceps from being fully activated, a process known as arthrogenic muscle inhibition. This inhibition has been quantified using EMG or the interpolated twitch technique. In addition, activation failure can be induced by experimentally creating an effusion (via saline injection into the joint) which is typically seen after knee surgery[41].
Even early after injury, quadriceps weakness can be substantial, despite little time for atrophy[36]. Quadriceps EMG signal reduction ranges from 50% to 70% in the first few hours after meniscectomy; it then increases up to 80% for the next 3 d and it remains at high levels up to 15 d[42]. The reduction in the quadriceps EMG is somewhat lower after total knee arthroplasty reaching 30% in the first 4 wk after surgery[43]. Following ACL surgery, activation failure continues for approximately 6 mo[44,45] but it is gradually reduced to 6% deficit 18 mo after[46]. Similarly, total knee arthroplasty is followed by significant quadriceps inhibition up to 6 mo[47] and 24% decline 33 mo[47] after surgery.
The magnitude of quadriceps failure depends on the severity of joint damage, especially in individuals with ACL problems. For example, Urbach et al[48] found a lower central activation deficit in 30 patients with isolated rupture of the ACL compared with that displayed by patients with ACL rupture and accompanying joint damage. ACL rupture leads to a 3%-8% decline in quadriceps activation[36,49] while ACL rupture with simultaneous damage in other joint structures leads to a higher decline[48,50].
Central activation failure can also affect the uninvolved side[36,49-51]. Becker et al[51] showed that patients who underwent partial meniscectomy displayed a 20% failure in the injured side and 17% failure in the contralateral side. Similar results were reported for individuals who experienced an ACL injury[49] which led the authors to conclude that the difference between ACL injured patients and controls is due to a reduction in muscle size and activation failure. Chmielewski et al[36] also reported a decline in central muscle activation of 21% in both limbs post ACL-surgery[36]. Would this indicate a generalized activation failure and not solely a preferential one? The implication for testing and rehabilitation after knee surgery is that using strength measurements of the uninvolved limb as targets for rehabilitation of the involved limb may set lower strength targets than needed. In fact, Urbach et al[48] reported that due to contralateral deficits in central activation, the mean underestimation of the isometric muscle-force deficit ranged from 22% to 48%. Therefore, the validity of tests for the assessment of muscle function when using the uninjured side as reference was questioned. Others, however, did not find a quadriceps inhibition of the contralateral limb[52] proposing that rehabilitation protocols after knee joint injury should focus on ipsilateral and not bilateral neuromuscular and mechanical alterations that occur as a result of joint damage.
There are several factors which may contribute to activation failure such as swelling[53], pain[54], inflammation[55] and damage to joint receptors[56]. For example, activation failure may be due to swelling[53] and an associated increase in intraarticular pressure[57]. Since intraarticular pressure is higher towards knee extension, inhibition will be greater near extension rather than flexion[58]. For these reasons, in the acute stages after injury or surgery, isometric quadriceps exercises should be performed in 30 to 50° of knee flexion, where intraarticular pressure is the lowest[40].
The mechanisms responsible for arthrogenic inhibition vary and include both central and peripheral nervous system. In a recent review, Rice et al[40] identified three spinal pathways which may affect arthrogenic inhibition. First, inhibition of group I nonreciprocal interneurons which receive inputs from tendon organs. Second, an enhanced flexion reflex that inhibits agonist activity and facilitates antagonist muscle activation[59]. Third, a deficit in the transmission of Ia input to the motoneuron pool, termed γ-loop dysfunction may be observed after ACL injury[60,61]. In addition, to the above spinal mechanisms, the role of corticomotor excitability as a contributor to activation failure was also examined. Interestingly, Heroux and Trenblay[62] reported a higher excitability of corticomotor projections targeting muscles in ACL deficient individuals. It has been proposed that this increase in corticospinal excitability may serve to counteract a-motoneuron inhibition by spinal reflex pathways[40].
In summary, athrogenic muscle inhibition represents a common symptom seen after many knee injuries. In many instances, clinicians consider reduced quadriceps strength as a result of muscle atrophy. However, the presence of inhibition after injury indicates that interventions employing only muscle strengthening exercises are not entirely appropriate to enhance neuro-muscular function. The use of techniques to increase quadriceps activation, such as electrical stimulation, has the potential to increase the effectiveness of rehabilitation programs.
Muscle co-activation
Neuromuscular function is not only related to the ability to recruit the entire motor unit pool of a certain muscle but also to the ability to achieve an optimal activation of all muscles acting around the knee. Muscle co-activation has been examined by comparing the surface electromyographic (EMG) signal of the involved muscles expressed as percentages of reference EMG values[63-67] or by using the EMG signals to calculate a co-contraction index[68]. Numerous studies have examined antagonist co-activation levels during various activities[69-72]. Antagonist co-activation of the hamstrings in most movements ranges from 5% to 10% and increases in more demanding activities such chair up and down exercises[69].
Early evidence indicated that hamstrings co-activation represents a reflex response to ACL loading which is also accompanied by quadriceps inhibition[64]. The presence of mechanoreceptor input provided by the cruciate ligaments have been confirmed in healthy individuals[73] but it is absent following surgical ACL reconstruction[74]. This was supported by several studies showing a higher hamstring EMG in ACL deficient patients during the impact phase of the side-step cutting manoeuvre[75], walking[76,77] or landing[78] although such patterns have not always been confirmed[79,80]. In addition, some studies have reported an earlier onset of muscle activity during the late stance phase of walking after ACL injury[76,77,79]. The increased and earlier hamstring and gastrocnemius activation in ACL deficient individuals aims to maintain the knee joint stable by preventing anterior subluxation as the ground reaction forces increase upon heel contact[76-77]. In addition, increased level of antagonist co-activation increases joint active stiffness[69]. This is also related with proprioception deficits often observed in ACL deficient knees[81].
More recent evidence indicates that non-contact ACL injuries are more likely when total hamstring pre-activation is much less than the corresponding quadriceps pre-activity during side cutting[82]. Furthermore, a higher hamstring coactivation near terminal knee extension was observed in ACL deficient individuals compared with uninjured individuals[83]. The observation that co-activation is found in both uninjured and injured individuals led Alkjaer et al[83] to suggest that antagonist co-activation is not only a reflex response but it may be modulated by central motor programming. Some evidence seems to support this statement[84,85], although, clearly more concrete evidence is necessary.
Using mathematically or EMG-driven models, research studies have estimated the antagonist moment in healthy subjects[86,87] and in ACL deficient subjects[83-84] as well as its effect on joint forces[73,86,88,89]. Isolated contraction of the quadriceps increases shear force between the tibia and the femur at the last 20° of knee extension which is partly counteracted by hamstring activation[86,88,89]. This results also in a wider pressure distribution along the articular surfaces of the joint and prevents early tissue damage and osteoarthritis[73] while it may reduce ACL strain at angles near full extension[90]. This notion is supported by modeling data by Yangawa et al[91], which confirms that coactivation of the hamstring muscles during isolated dynamic (isokinetic) knee extension effectively reduces anterior tibial translation. Further evidence seems to confirm these findings as a higher hamstring coactivation and moment near terminal knee extension was observed in ACL deficient individuals compared with uninjured individuals[83]. The elevated antagonist hamstring moment observed in the ACL deficient subjects may reflect a compensatory neuromuscular adaptation to counteract the increased laxity of the knee joint[83]. However, others have not found any difference in antagonist hamstring moment between ACL deficient, ACL reconstruction, and uninjured individuals[84]. Methodological issues in EMG - moment data treatment may account for these variations[83] which guarantees further research in this area.
Strength imbalances
Since neuromuscular activation is altered in knee pathological conditions, then changes in force generation capacity of the surrounding musculature may be observed. These are also accompanied by alterations in size of the muscle as a result of injury or subsequent immobilization. Muscular imbalances around the knee refer mainly to the relationship between absolute muscle strength developed by antagonistic muscle groups. The H:Q peak moment ratio takes into consideration the function of two opposing (agonist-antagonist) muscle groups and it represents the most frequent parameter used to estimate muscle strength balance[6,7,92].
The methods used to calculate the H:Q strength ratios vary. Early research studies have mainly examined the concentric H:Q ratios, frequently defined as “conventional” ratios[93,94]. A theoretical value of 0.6 of the ratio obtained frequently under isometric or slow isokinetic concentric tests is often considered as “normal”[95]. However, conventional ratios have been gradually been replaced by the “functional” ratios which involve the calculation of eccentric H: concentric Q (Hecc:Qcon) muscle strength ratio[6,7,92,93,96].
There has been a long debate on the usefulness of antagonist to agonist strength ratios as an injury predictor or as a target for restoring normal knee muscle function[97]. A methodological approach is to measure H:Q ratio in athletes in the pre-season period and follow this for the forthcoming seasons. It has been found that athletes with a Hcon:Qcon ratio closer to 1.0 may have a reduced risk of hamstrings strain[98]. Also, a Hcon : Qcon ratio closer to 1.0 in athletes with ACL injury has been suggested to reduce the risk of an anteriolateral subluxation of the tibia[99]. Croisier et al[100] identified a lower Hecc:Qcon ratio in players with a previous hamstring injury during the pre-season assessment and applied a rehabilitation program to restore the ratio into normal values. They then followed the players for 12 mo. Their results showed that none of the players experienced a re-injury. Further, epidemiological evidence in 462 players followed for one season showed a total of 35 hamstring injuries, most of which were experienced by players with lower Hcon:Qcon and Hecc:Qcon ratios[101]. Recently, Kim et al[95] found an association of lower than 0.6 of the Hcon:Qcon ratio at 60°/s and non-contact leg injuries in National College American Association athletes. In an almost parallel study, Fousekis et al[102] reported that professional soccer players with Hecc strength asymmetries were at greater risk of hamstring strain while players with Qecc strength and flexibility asymmetries were at greater risk of quadriceps strain.
Other studies have examined the ability of H:Q ratio to identify individuals with knee joint problems from uninjured ones. Early studies have identified[79,92] a significantly lower isokinetic Q moment in patients with ACL deficiency compared to healthy subjects while Hecc and Hcon moment deficits were not as significant. This is in line with later studies[103,104] who reported a higher H:Q ratio in subjects with ACL reconstruction[103,104] compared with uninjured individuals. Similar findings have been reported when comparing individuals with knee osteoarthritis with controls[105,106] which may indicate that compensation strategies with regards to antagonist to agonist muscle balances are more generic than solely ACL problems.
Knee related injuries may also be due to differences in strength between the two legs. Furthermore, strength levels of the unaffected limb frequently represent a reference value against which restoration of strength of the affected limb. Evidence on bilateral leg differences in soccer players is unclear as some studies have reported no differences[107] whereas others reported a 10% difference in both Q and H strength in favor of the non-dominant leg[108]. Others, however, have shown that bilateral leg differences exist only in the hamstrings but not in the quadriceps (players displayed weaker hamstrings in the dominant leg than the non-dominant one)[109,110]. The existence of muscle specific bilateral differences in strength led researchers to explore whether H:Q ratios differ between limbs. Again, there is some evidence that the non-dominant or non-preferred limb shows somewhat higher ratios than the dominant one but still this evidence is not always statistically significant[108,111] or differs between tested speeds[110]. However, a lower Hecc moment in the injured limb compared to the contralateral limb continues even after ACL reconstruction surgery[112]. It is not clear whether such deficits pre-existed or they were due to ACL injury or reconstruction.
Although functional ratios have been considered as better indicators of muscle balance, there is still not sufficient evidence supporting their use. A problem associated with the use of H:Q ratios is that they were assessed using peak force values during a maximum voluntary effort[113-115]. This raises two issues: (1) that injuries occur at a specific joint angle while the H:Q ratio is calculated using peak force values irrespective of joint angle. The value of calculating the H:Q ratio at a specific joint angle, the one which is closer to the injury mechanism of the specific knee structure would be higher[116] (Figure 1). Particularly, peak moment H/Q ratio ranges from 0.5 and 0.6[96,117] and increases near full knee extension exceeding values of 1.0[117,118]. This increase was attributed to a relative dominance of the H near full extension[118] in order to stabilize the knee joint when the strain on the ACL is the greatest[90]. The shift of Hecc/Qcon ratio at angles of knee extension was also attributed to a limitation in knee extensor motor unit recruitment at joint angles of greatest ACL strain[118]. Nevertheless, whether H:Q ratio at a specific joint angle can discriminate knee injured individuals from uninjured ones or to predict injury is still unclear; (2) during explosive movements, such as soccer match play situations, the time available to stabilize the knee joint is frequently very short (< 50 milliseconds)[119]. However, during a standard isometric test the peak force occurs within 400-500 ms from onset of contraction. This suggests that in most explosive movements there is no time available for maximum force generation. Thus, the relevance of using Hcon:Qcon and Hecc:Qcon based on peak values has been questioned[120]. In one of the first studies, Aagaard et al[115] proposed that rate of force development (RFD), defined as the rate of rise in force at the onset of contraction, may be a better index of neuromuscular activity around the knee. Based on these aspects, Zebis et al[120] have recently assessed the H:Q ratio using the RFD values obtained during maximum isometric contraction in twenty three soccer players. They reported that two female players who sustained an ACL injury had a normal H:Q peak force ratio but a low RFD H:Q ratio.
Figure 1.
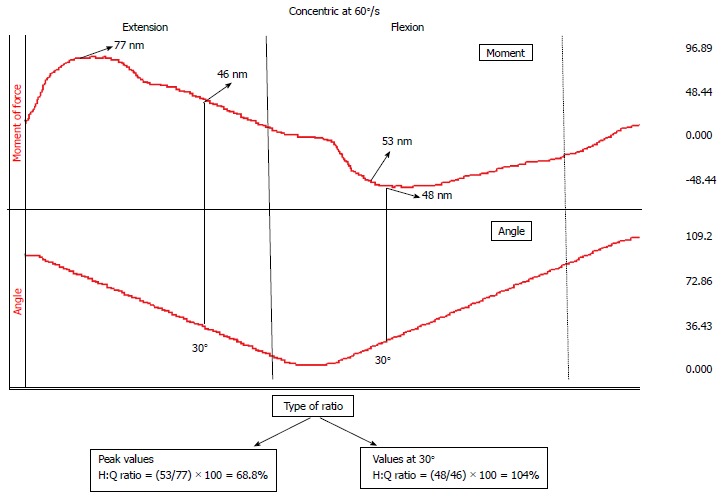
An example of different methods to calculate the hamstrings:quadriceps ratio. The raw data from moment of force (upper line) and angular position (lower line) as recorded from an isokinetic concentric knee extension-flexion trial. Using peak moment values results to a different ratio value compared with that obtained using values at a knee flexion angle of 30°. H: Hamstrings; Q: Quadriceps.
Gender differences
Male and female relative H:Q ratio profiles differ significantly during and following puberty[121]. Isokinetic dynamometer measurements show that male athletes demonstrate significantly greater hamstrings peak torques with increasing maturity, while peak hamstrings torque remains stable with increasing maturational stage in female athletes[121]. Thus, it appears that lower hamstrings strength and H:Q ratios of female athletes relative to males may be related to the development of neuromuscular imbalances associated with the onset on maturation. These neuromuscular imbalances may increase injury risk in pubertal and post pubertal female athletes[121,122]. In a thorough review, Hewett et al[123] analysed 23 research studies and reported that isokinetic H:Q ratios do not differ between genders at slow velocities. As angular velocity increases, males display higher H:Q ratio than females. The authors commented that this difference may be related to females’ decreased ability to dynamically control the knee joint during sports activities. However, more recent studies have reported an increase in both conventional and functional ratios in female athletes with increasing angular velocity[124,125], which is not in line with the above conclusion. This might be due to differences in the characteristics of the samples examined, as both these studies referred to trained female athletes whilst data examined by Hewett et al[123] included mainly sedentary or untrained individuals.
Gender differences in knee injury occurrence are also related to more global neuromuscular differences that lead to injury than solely H:Q ratios. Muscle co-activation can decrease the dynamic valgus motion of the knee, which potentially places the knee at increased risk of injury[123,126]. Individuals with chronic ACL deficiency showed lower internal/external rotation strength ratios than controls and acute ACL deficient subjects, indicating a compensatory mechanism developed by the patients to unload the ACL[103]. In contrast, ACL reconstruction patients showed fewer deficiencies compared with controls[103].
Gender differences in hamstring and quadriceps muscle co-activation have also been examined. Palmieri et al[127] reported that females displayed lower co-activation than males and that medial co-activation had a linear relationship with external knee abduction moment in females only. A higher knee abduction moment is considered as a risk factor for ACL injury[128]. Therefore, it appears that females display a greater risk for ACL injury than males. Similar results were reported by Rozzi et al[129] upon landing from a jump. There is no single explanation on why females display deficits only on lateral muscles and not on the medial part. There are suggestions that lateral muscles may co-activate more than the medial ones to resist internal rotation moments which may increase ACL loading[130]. However, more evidence is necessary.
NEUROMUSCULAR INTERACTIONS AROUND THE KNEE IN CHILDREN
Muscle strength increases during maturation, in terms of joint moment. This development is primary a consequence of hormonal changes which result in muscle mass augmentation (hypertrophy)[131], and in limb size increase (moment arm)[132]. However, differences in strength between children and adults cannot be fully explained by these parameters[133]. This designates the possible contribution of neuromuscular factors that could play a role in force deficit observed in children compared to adults. There are two main issues to mention regarding the neuromuscular aspect: Firstly, the level of central activation, i.e., to what extent the central nervous system is able to activate the entire motor pool, and secondly, the level of antagonist co-activation, which reduces the net amount of moment produced around the joint. Hence, strength gain observed during developmental ages could be partly attributed to neural adaptations. In addition to this, differences between children and adults in muscle tendon unit (MTU) architecture and stiffness might also play a role on the force development around the knee joint (Figure 2).
Figure 2.
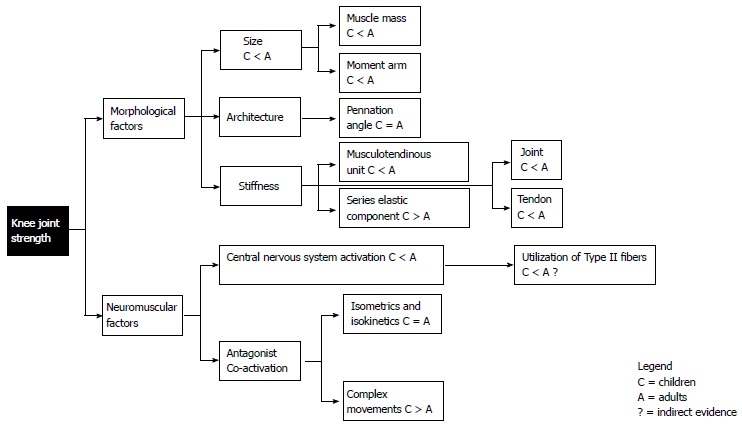
Schematic summary of comparison between children (C) and adults (A) regarding factors influencing knee joint strength.
Earlier studies have shown that the isokinetic strength normalized to cross sectional area (CSA) and thigh length is lower in 6-9 years old children compared to young adults[131]. The fact that this difference was more profound when the angular velocity was increasing reveals that muscle and limb size could not be the only factor affecting force production. This could be explained by findings supporting that children might have lower proportion of type-II muscle fibers[134], which have fast contractile properties. However, several studies revealed no significant differences in muscle-fiber composition between children and adults[135,136].
This raises the question whether children and adults possess similar proportion of muscle fibers types, but the former are not capable of fully recruit the fast ones. It has been shown that especially in large muscle groups, such as the quadriceps, children are incapable to fully recruit their motor units[137]. More recent studies using the twitch interpolation technique with magnetic or electric stimulation demonstrated that children activate their motor units in lesser extent than adults during knee extension[138], and this is particularly evident in girls when compared to women[139]. This finding observed in children could at least partially account for their force deficit compared to adults. Furthermore, assuming that the size principle is valid for children too (i.e., the higher the level of activation, the larger in size -and thus faster- motor units are recruited), it would be expected that children utilize in lesser extent type-II (fast) motor units compared to adults. This assumption is supported by experimental findings for the knee extensors, revealing that children have lower rate of torque development under isometric[140] and dynamic conditions[141].
Despite the simplicity of using the anatomical CSA for the estimation of muscle size of children and adults, the most appropriate measure is the physiological CSA, which accounts for the pennation angle and is calculated as the ratio of muscle volume to fascicle length[138]. However, no difference between 8-10 years old children and adults is observed in the pennation angle of all quadriceps heads[138]. To our knowledge, no respective data exist in the current literature, regarding the pennation angle for the hamstring muscles in children and adults. This piece of information could have important implications, since the pennation angle influences the shortening velocity of a muscle (and the force capacity of a muscle), and might affect the torque H:Q ratio at different contraction velocities.
Decreased torque H:Q ratio is an indicator for potential increased probability of lower extremity injury[142]. More particularly, it has been shown that collegiate athletes with isokinetic at 180 deg/s peak torque H:Q ratio less than 0.75 have higher incidence of injury[143]. According to cross-sectional studies, the isokinetic torque H:Q ratio at 60 deg/s remains unchanged from the age of 7 to 18 years, although the CSA H:Q ratio increases gradually after the age of 10 years[144]. On the other hand, post-pubescent athletes demonstrate a close correlation between the hamstrings and quadriceps CSA and the flexion and extension torque, respectively[145]. Furthermore, during puberty strength improvement of the knee flexors is diverged from the extensors, particularly for the females[121]. Although in males the hamstrings and quadriceps isokinetic peak torque increases proportionally during growth[107,121], in females the peak torque of hamstrings does not follow the improvement achieved in quadriceps[121]. This deficit in knee flexion torque observed in females results in a decreased torque H:Q ratio. Further gender specific imbalances are observed on the level of knee anterior/posterior and medial/lateral muscle activation[146] during dynamic multijoint tasks. Females activate their quadriceps more compared to males[147-150] and this could contribute to the decreased H:Q ratio in torque output. Furthermore, decreased medial to lateral quadriceps[151] and hamstrings[129] activation ratio observed in females, could increase valgus, and varus laxity. These observations regarding the imbalances in activation level and torque output of the thigh muscles could increase the risk for ACL injury because hamstrings function synergistically with the ACL, especially at knee joint angles less than 45 degrees[64].
A factor that could modify the torque H:Q ratio is the level of antagonist co-activation. However, no significant differences between children and adults have been observed[152,153]. Furthermore, in isometric contractions, the antagonist co-activation is even lower and still not significant between age groups[139,154]. On the other hand, co-activation is higher in children compared to adults when performing tasks involving multiple joints such as gait[155] and jumps[156,157]. This implies that movement coordination and learning factors might be an issue during developmental ages[133], considering that the process of maturation of the corticospinal tract in terms of conduction velocity is not complete until the age of 11 years[158] and that the pyramidal system attains full functionality during puberty[159].
Regarding the passive component of stiffness, Lebiedowska and Fisk[160] have shown that passive knee stiffness increases with stature, within an age range between 6 and 18 years. Furthermore, Kubo et al[161] measuring the tendon elongation of the vastus lateralis during isometric knee extension, concluded that the tendon of younger boys was more compliant than older boys and young men. In line with the idea that the MTU is more compliant in children, Asai et al[162] demonstrate that children had longer electromechanical delay compared to adults. This could also contribute to their reduced capacity to produce high rate of force development[140-141]. In contrast, series elastic component, quantified with quick-released movements in the knee extensors, revealed decreased stiffness with age[163]. The above differentiations in MTU stiffness between children and adults might influence the force/length relationship of the muscles acting around the knee joint. Stiffer MTU favors more direct force translation from the muscle to the bone[164], whereas the opposite situation requires greater shortening velocity of the contractile apparatus, in which children are inferior[140,141]. The concept of differences in MTU stiffness that are reflected to changes in the joint torque/angle relationship has been supported[165] but also questioned[139] in previous studies, and therefore requires further investigation. More particularly, Marginson et al[165] demonstrated that children demonstrate their maximal knee extension torque at more flexed joint angle (longer muscle) than adults, whereas O’ Brien et al[139] showed no difference in the optimal joint angle between children and adults.
It is apparent that the function of the knee depends on multiple factors, which are influenced during the developmental ages. Despite this complexity, O’Brien et al[138] concluded that children’s and adults’ specific tension (the ratio between muscle strength and size) of the quadriceps is the same, taking into account differences in physiological cross sectional area, moment arm, level of activation, and co-activation. This implies that the muscle tissue is qualitatively very similar in children and adults. It is concluded that regardless of structural differences in muscle size, moment arm-joint angle relationship, central voluntary activation, H:Q ratio, and muscle-tendon stiffness, children’s neuromuscular system is highly adaptive, although further systematic research with longitudinal studies are required to improve our understanding on the effects of growth and development in the force and power output of children.
NEUROMUSCULAR INTERACTIONS AROUND THE KNEE IN THE ELDERLY
The aging process is associated with a significant decline in muscle strength (dynapenia) and strength development that might be caused by alterations of skeletal muscle properties as well as by neural modulations[166,167]. Regarding the knee joint, the reported age-related decrease in the measured isometric muscle force/moment of the knee extensors ranges from 19% to 38% when comparing groups of similar physical activity level[166,168-173] (Table 1). Even greater differences (50% or more) have been reported for people in their ninth decade and beyond[166]. When comparing the specific tension of the knee extensors between young and old women, a reduction of 17% during isometric contraction has been reported[174] (Table 1).
Table 1.
Information provided by cited articles about age-related reduction in muscle force
| Ref. | Age-related reduction in muscle force/torque | Age of participants, yr | Testing condition | Physical activity level |
| Baroni et al[171] | 30%-36% 40%-53% | y: 30 ± 6 o: 69 ± 5 yr | Isometric KE Concentric KE (60-360º/s) | No systematic training No systematic training |
| Laudani et al[173] | 36.9% | y: 28 ± 2 o: 70 ± 3 | Isometric KE | Sedentary adults |
| Karamanidis et al[169] | 21% 18.9% | y: 21-32 o: 60-69 | Isometric KE Isometric KE | Endurance runners Not active |
| Mademli et al[170] | 28% | y: 30 ± 7 o: 65 ± 3 | Isometric KE | Physically active |
| Savelberg et al[172] | 33% 43% | y: 23 ± 2 o: 65 ± 3 | Isometric KE Isometric KF | Active runners Active runners |
| Macaluso et al[174] | 17% 30% | y: 23 ± 6 o: 70 ± 2 | Isometric KE Isometric KF | Active Active |
| Frontera et al[195] | 15.5%-22% 17%-23% | 12-yr longitudinal study, initial mean age 65 ± 2 | Isokinetic KF (60 and 240º/s) Isokinetic KE (60 and240º/s) | Healthy Healthy |
KE: Knee extension; KF: Knee flexion; Y: Young; O: Old.
The age-related decline in muscle strength is gender specific, with men losing almost twice as much strength as women[175]. Nevertheless, in absolute values, older women demonstrate significantly lower strength than men[176,177], which can be explained predominantly by their higher fat mass[176]. Indeed, when investigating the decline in muscle quality of the knee flexors and extensors, i.e., peak torque per unit of muscle mass, it was found that the rate of the decline was the same for both genders[178]. The higher proportion of body fat in women may put them at significant biomechanical disadvantage for greater disability in old age[176]. It seems that due to their gender-related lower average strength, old women may be at greater risk than old men of becoming impaired in certain motor tasks[177].
Furthermore, when measuring knee extensor moments at different knee angle positions, the percentage loss of muscle strength was different at the different positions[168,179]. Karamanidis et al[168] found that the aging process revealed a clear reduction in maximal knee extension moment at intermediate knee joint angles (140° and 110°), but there was virtually no age effect at more extended (160° and 170°) or flexed (80°) knee joint positions. The authors proposed among other, two potential explanations for this phenomenon: (1) The discrepancy in the age-related reduction in muscle strength within the quadriceps muscles, with greater decline in Vastii (monoarticular) than in rectus femoris (biarticular) muscle[172]. It has been reported that, while the moment-knee-joint angle relationship of the Vastii muscles described by a parabolic curve having its vertex (maximum value) between 100° and 120°, the rectus femoris demonstrates a rather flat joint-moment-length curve[172]. Thus, it is possible that the relative contribution of the rectus femoris to the total knee extension moment is higher at more extended or flexed knee joint positions[172], where no age-related effect on quadriceps muscle strength was found; and (2) The modulation of the EMG activity. In their study, Karamanidis et al[168] found that older adults have an increased quadriceps femoris EMG activity at more extended (160° and 170°) as well as at moreflexed (80°) knee joint angles in comparison to younger adults. This was not the case at intermediate knee joint angles (110° and 140°).
Knee flexors have been reported to demonstrate similar decline as knee extensors due to the aging process[166]. Nevertheless, Ogawa et al[180] found no significant change in muscle volumes and average CSA for the hamstring muscles between young and old adults, whereas quadriceps muscle volume and average CSA were 20% and 16% lower, respectively. This resulted to greater age-related decline in the specific tension for the knee flexors compared to knee extensors (Table 1)[174,180]. In contrast to the knee extensors, for the knee flexors the strength reduction is mainly caused by deterioration of the biarticular muscles, and not of the monoarticular muscles[172]. Furthermore, for the knee flexors, the age-related reduction of joint moment is almost invariant to joint angle[172], something that does not hold for the knee extensors, as already mentioned above.
Age-related muscle weakness is associated with the well described decline of skeletal muscle mass. Yet, more recent studies have shown that this relationship is less robust than once believed[167]. Goodpaster et al[175], when measuring knee extensor strength by isokinetic dynamometry, found that although the loss of muscle mass is associated with the decline in strength of older adults, this strength decline is much more rapid than the concomitant loss of muscle mass. Moreover, they reported that maintaining or gaining muscle mass does not prevent aging-associated reduction in muscle strength. Furthermore, there are age-related alterations in torque production capability that are not explained by a reduction in muscle mass, including reduced specific tension and slower rate of isometric torque production (expressed relative to peak torque)[167]. The altered neuromuscular activation is another critical component of the weakness observed in senescence[167].
Nevertheless, the studies focusing on the underlying neuromuscular mechanisms of age-related reduction in knee extensors force generation capacity are limited. Moreover, the reported results are partially conflicting, especially the ones concerning alterations in neural drive to the quadriceps muscle. While some studies find greater activation deficit in the elderly, compared to young adults[181,182], other studies do not find any significant differences between young and old in the ability to activate the knee extensor muscles to a high degree (93%-96%)[183-186]. Harridge et al[187] found that very old adults (85-97 years) demonstrated significant impairment in central activation, with mean knee extensor voluntary activation level of only 81% (range: 69%-93%)[187]. This outcome suggests that deficits in the neural drive essentially contribute to the weakness of the knee extensor muscles observed in very old age[188]. On the contrary, Miller et al[189] found that the ability to activate the quadriceps muscle was generally very high, and there was no significant difference between older (96%) and younger (98%) subjects. The study was conducted on 20 moderately active older subjects (mean age 75 years) and 12 younger (mean age 25 years). The above described inconsistency in reported findings may be primarily related to methodological limitations and differences in the techniques used to estimate muscles voluntary activation[181], as well as to different physical condition of participants[188]. Mau-Moeller et al[181] estimated the neural drive to the knee extensor muscles during maximal isometric contractions by means of both interpolated twitch technique and the root mean square of the EMG signal normalized to maximal M wave[181]. Both techniques led to the same outcome, i.e., there was an age-related decline in the neural drive to the muscle which resulted in muscle weakness. Regarding the knee flexor muscles, to our knowledge there is no study investigating their voluntary activation.
Another neuromuscular mechanism of age-related reduction in knee extensors force generation capacity, regards the age-related changes in antagonistic muscle coactivation. The mechanical opposition to the agonist action can contribute to the reduced exerted moment at the knee joint. Studies investigating the effect of aging on the coactivation during knee extension are limited and their findings lack of consensus. Laudani et al[173] found that old (mean, 70 years) and young (mean, 28 years) adults with similar physical activity level do not demonstrate significant difference in the coactivation during maximum isometric contractions (26.2% ± 22.8% vs 29.6% ± 20.5%). The increased standard deviation in their measured values indicates high intra-group variability, assigning to coactivation a rather person-dependent instead of age-related nature. Regarding dynamic contractions, no association was found between normalized antagonist activation and velocity, indicating that changes in coactivation cannot be responsible for age-related deficit in force production[190]. On the contrary, Tracy et al[191] found that old subjects (mean, 71.5 years) exhibited during submaximal isometric and anisometric contractions, greater coactivation of antagonist muscle compared to young ones (mean, 22 years). Similar findings have been reported for measurements over women during isometric knee extension contraction[174]. Furthermore, there is a highly determinant effect of coactivation on the capacity to produce isometric force on a short period of time[192]. However, significantly higher antagonistic coactivation was only found during contraction of the knee extensors and not during knee flexion[174]. During knee flexion, the co-contraction of knee extensors was found to be significantly lower for both old and young adults[173].
The transfer of force between the muscular and skeletal systems may be affected by age-related changes in muscle architecture, as well as in the length and compliance of tendons[167]. An age-related reduction in vastus lateralis tendon and aponeurosis stiffness has been reported[168-170] (Figure 3). Thus, the greater compliance of the aged tendon and aponeurosis can influence the force-length and force-velocity relationship of the muscle (contractile element) and consequently its force generating potential[193]. The result is a more deteriorate function of the knee extensor muscles in the older population.
Figure 3.
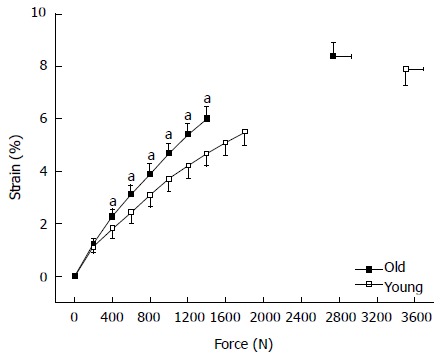
Strain-force curves of the vastus lateralis tendon and aponeurosis. The strain values at every 200 N and at maximum calculated tendon force during maximal voluntary isometric knee flexion contraction are displayed. The curves end at 1400 N for the old adults and at 1800 for the young ones, these values correspond to the maximum common force achieved by all subjects in either group, old and young adults. Y: Young (n = 12); O: Old adults (n = 14); Means and SEM; Age effect: (aP < 0.05) vs young.
The above mentioned age-related alterations in neuromuscular interactions around the knee joint lead to differences in the way old adults perform activities of daily living. For example, when older adults descend and ascend stairs and ramps, they demonstrate an altered control strategy compare to young adults, causing a redistribution of the mechanical load at the tibiofemoral joint[194]. This has effects on the initiation and progression of knee osteoarthritis in the elderly, which in turn makes movement even more difficult[194].
CONCLUSION
In this review, we attempted to provide a global view of the neuromuscular mechanisms associated with knee joint injuries across lifespan. It is certain that neuromuscular strategies and mechanisms differ between children, adults and the elderly. However, there are some interesting similarities in the mechanisms by which children or elderly differ compared with adults.
Both children and elderly display a lower absolute muscle strength capacity than adults. This deficit may be due to a lower muscle mass (especially of the quadriceps) displayed by children and elderly, obviously for different reasons. The effects of a lower muscle mass are more evident in older individuals. However, when variations in muscle mass are taken into consideration, there are still differences between different age categories.
Quadriceps activation failure is a common symptom of all knee injuries, irrespective of age. However, for those individuals who have a lower quadriceps strength capacity, it is reasonable to suggest that functional impairment will also be higher. If we assume that knee injury conditions (swelling or pain and inflammation) are constant amongst different age groups, an initial difference in the ability to recruit the entire motor unit pool of the muscle would also contribute to a higher impairment after injury. Our review indicates that this is the case for both children (probably due to maturation) and elderly (due to age effects on neuromuscular system).
Another factor which might have affected the impaired ability to produce maximum muscle strength is a higher antagonist co-activation. Although co-activation levels may contribute to a high joint stability and stiffness, it appears that co-activation levels do not differ between children and adults or between elderly and adults, at least during isolated (static or dynamic) joint strength testing conditions. This indicates that it is the reduced muscle mass and central activation of the agonist muscles rather than higher co-activation by the antagonists that contributes to age related differences in absolute strength. It follows, that this particular neuromuscular mechanism, central or peripheral, is not age specific.
While extensive research has examined the strength balance around the knee through the H:Q ratio, there is a marked difference in the amount of research performed in adults compared to that performed in children and the elderly. Nevertheless, it appears that H:Q ratio levels are altered after knee injury mainly as a result of a lower quadriceps muscle strength. Current evidence does not indicate whether H:Q ratio differs between different age groups. Sparse data indicate that hamstring muscle strength tends to be relatively less affected by age compared with quadriceps muscle strength, but this is only a speculation.
It appears that stiffness of the muscle-tendon units around the knee differs between age groups. Interestingly, there is a common pattern regarding age variations in muscle-tendon stiffness: both children and the elderly display less stiffness of the quadriceps MTU than adults. While in children this may be due to sexual maturation and in elderly due to deterioration of tissue, it could be suggested that the main characteristic is similar: both children and elderly show a more compliant muscle-tendon unit. It seems that tendons adaptations follow muscle’s force capacity. Muscle force determines the strain of tendon cells, i.e., the higher the force applied to tendon the higher its deformation. There is evidence that strain of tendon cells is an important regulator for the homeostasis of connective tissues. The resulted more compliant tendon in children and elderly affects both the force-length and force-velocity relationship of their muscles and, in turn, leads to an altered strength capacity.
Finally, an interesting question is whether age-related differences in neuromuscular strategies around the knee depend on gender. There have been no studies that specifically addressed such a question. Neverthless, current evidence indicates that females display a higher injury rate than males. Such variation is observed from an early age where, there is evidence that muscle strength and co-activation profiles may place girls to a greater injury risk than boys. Similar results are also reported for older individuals, where additional factors, such as body mass, also contribute to gender variations.
Footnotes
P- Reviewer: Eric Y, Kumar P, Louboutin JP, Laudner K, Rudroff T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- 2.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 3.Louw QA, Manilall J, Grimmer KA. Epidemiology of knee injuries among adolescents: a systematic review. Br J Sports Med. 2008;42:2–10. doi: 10.1136/bjsm.2007.035360. [DOI] [PubMed] [Google Scholar]
- 4.Moustaki M, Pitsos N, Dalamaga M, Dessypris N, Petridou E. Home and leisure activities and childhood knee injuries. Injury. 2005;36:644–650. doi: 10.1016/j.injury.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Baker P, Reading I, Cooper C, Coggon D. Knee disorders in the general population and their relation to occupation. Occup Environ Med. 2003;60:794–797. doi: 10.1136/oem.60.10.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellis E, Baltzopoulos V. Isokinetic eccentric exercise. Sports Med. 1995;19:202–222. doi: 10.2165/00007256-199519030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Coombs R, Garbutt G. Developments in the use of the hamstring/quadriceps ratio for the assessment of muscle balance. J Sports Sci Med. 2002;1:56–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Kannus P, Järvinen M. Incidence of knee injuries and the need for further care. A one-year prospective follow-up study. J Sports Med Phys Fitness. 1989;29:321–325. [PubMed] [Google Scholar]
- 9.Gage BE, McIlvain NM, Collins CL, Fields SK, Comstock RD. Epidemiology of 6.6 million knee injuries presenting to United States emergency departments from 1999 through 2008. Acad Emerg Med. 2012;19:378–385. doi: 10.1111/j.1553-2712.2012.01315.x. [DOI] [PubMed] [Google Scholar]
- 10.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13:184–188. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Steinbrück K. [Epidemiology of sports injuries--25-year-analysis of sports orthopedic-traumatologic ambulatory care] Sportverletz Sportschaden. 1999;13:38–52. doi: 10.1055/s-2007-993313. [DOI] [PubMed] [Google Scholar]
- 12.Kuikka PI, Pihlajamäki HK, Mattila VM. Knee injuries related to sports in young adult males during military service - incidence and risk factors. Scand J Med Sci Sports. 2013;23:281–287. doi: 10.1111/j.1600-0838.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 13.Sulsky SI, Mundt KA, Bigelow C, Amoroso PJ. Case-control study of discharge from the U.S. Army for disabling occupational knee injury: the role of gender, race/ethnicity, and age. Am J Prev Med. 2000;18:103–111. doi: 10.1016/s0749-3797(99)00175-0. [DOI] [PubMed] [Google Scholar]
- 14.Heir T, Eide G. Age, body composition, aerobic fitness and health condition as risk factors for musculoskeletal injuries in conscripts. Scand J Med Sci Sports. 1996;6:222–227. doi: 10.1111/j.1600-0838.1996.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 15.Eskelinen AP, Visuri T, Larni HM, Ritsilä V. Primary cartilage lesions of the knee joint in young male adults. Overweight as a predisposing factor. An arthroscopic study. Scand J Surg. 2004;93:229–233. doi: 10.1177/145749690409300311. [DOI] [PubMed] [Google Scholar]
- 16.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 17.Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36:1116–1122. doi: 10.1177/0363546508314400. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin N Am. 2007;18:417–438, viii. doi: 10.1016/j.pmr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Silvers HJ, Mandelbaum BR. Prevention of anterior cruciate ligament injury in the female athlete. Br J Sports Med. 2007;41 Suppl 1:i52–i59. doi: 10.1136/bjsm.2007.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell JW, Barber-Foss KD. Injury patterns in selected high school sports: a review of the 1995-1997 seasons. J Athl Train. 1999;34:277–284. [PMC free article] [PubMed] [Google Scholar]
- 21.Atkin DM, Fithian DC, Marangi KS, Stone ML, Dobson BE, Mendelsohn C. Characteristics of patients with primary acute lateral patellar dislocation and their recovery within the first 6 months of injury. Am J Sports Med. 2000;28:472–479. doi: 10.1177/03635465000280040601. [DOI] [PubMed] [Google Scholar]
- 22.Sillanpää P, Mattila VM, Iivonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008;40:606–611. doi: 10.1249/MSS.0b013e318160740f. [DOI] [PubMed] [Google Scholar]
- 23.Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am J Sports Med. 2001;29:58–66. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- 24.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31:831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 25.Parkkari J, Pasanen K, Mattila VM, Kannus P, Rimpelä A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42:422–426. doi: 10.1136/bjsm.2008.046185. [DOI] [PubMed] [Google Scholar]
- 26.Bodor M. Quadriceps protects the anterior cruciate ligament. J Orthop Res. 2001;19:629–633. doi: 10.1016/S0736-0266(01)00050-X. [DOI] [PubMed] [Google Scholar]
- 27.Baker P, Coggon D, Reading I, Barrett D, McLaren M, Cooper C. Sports injury, occupational physical activity, joint laxity, and meniscal damage. J Rheumatol. 2002;29:557–563. [PubMed] [Google Scholar]
- 28.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 30.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 31.Reijman M, Pols HA, Bergink AP, Hazes JM, Belo JN, Lievense AM, Bierma-Zeinstra SM. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66:158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manninen P, Riihimäki H, Heliövaara M, Mäkelä P. Overweight, gender and knee osteoarthritis. Int J Obes Relat Metab Disord. 1996;20:595–597. [PubMed] [Google Scholar]
- 33.Guillemin F. Describing the epidemiology of rheumatic diseases: methodological aspects. Curr Opin Rheumatol. 2012;24:187–192. doi: 10.1097/BOR.0b013e32834ff314. [DOI] [PubMed] [Google Scholar]
- 34.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB, Jordan JM. Associations of occupational tasks with knee and hip osteoarthritis: the Johnston County Osteoarthritis Project. J Rheumatol. 2010;37:842–850. doi: 10.3899/jrheum.090302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sangha O. Epidemiology of rheumatic diseases. Rheumatology (Oxford) 2000;39 Suppl 2:3–12. doi: 10.1093/rheumatology/39.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 36.Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Baker KR, Xu L, Zhang YQ, Nevitt M, Niu J, Aliabadi P, Yu W, Felson D. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese - The Beijing Osteoarthritis Study. Arthritis and Rheumatism. 2004;50:1815–1821. doi: 10.1002/art.20261. [DOI] [PubMed] [Google Scholar]
- 38.Lynch AD, Logerstedt DS, Axe MJ, Snyder-Mackler L. Quadriceps activation failure after anterior cruciate ligament rupture is not mediated by knee joint effusion. J Orthop Sports Phys Ther. 2012;42:502–510. doi: 10.2519/jospt.2012.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87:1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice DA, McNair PJ. Quadriceps Arthrogenic Muscle Inhibition: Neural Mechanisms and Treatment Perspectives. Semin Arthritis Rheum. 2010;40:250–266. doi: 10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps Activation Following Knee Injuries: A Systematic Review. J Athl Train. 2010;45:87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shakespeare DT, Stokes M, Sherman KP, Young A. Reflex inhibition of the quadriceps after minesctomy - lack of association with pain. Clin Physiol. 1985;5:137–144. doi: 10.1111/j.1475-097x.1985.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 43.Machner A, Pap G, Awiszus F. Evaluation of quadriceps strength and voluntary activation after unicompartmental arthroplasty for medial osteoarthritis of the knee. J Orthop Res. 2002;20:108–111. doi: 10.1016/S0736-0266(01)00068-7. [DOI] [PubMed] [Google Scholar]
- 44.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Suter E, Herzog W, Bray RC. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech. 1998;13:314–319. doi: 10.1016/s0268-0033(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 46.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris - A prospective twitch interpolation study. BJJ. 2001;83B:1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 47.Berth A, Urbach D, Awiszus F. Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:1432–1436. doi: 10.1053/apmr.2002.34829. [DOI] [PubMed] [Google Scholar]
- 48.Urbach D, Awiszus F. Impaired ability of voluntary quadriceps activation bilaterally interferes with function testing after knee injuries. A twitch interpolation study. Int J Sports Med. 2002;23:231–236. doi: 10.1055/s-2002-29074. [DOI] [PubMed] [Google Scholar]
- 49.Urbach D, Nebelung W, Weiler HT, Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31:1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthitic knees. Rheumatology. 1993;32:127–131. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- 51.Becker R, Berth A, Nehring M, Awiszus F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J Orthop Res. 2004;22:768–773. doi: 10.1016/j.orthres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Palmieri RM, Ingersoll CD, Edwards JE, Hoffman MA, Stone MB, Babington JP, Cordova ML, Krause BA. Arthrogenic muscle inhibition is not present in the limb contralateral to a simulated knee joint effusion. American PM&R. 2003;82:910–916. doi: 10.1097/01.PHM.0000098045.04883.02. [DOI] [PubMed] [Google Scholar]
- 53.Reeves ND, Maffulli N. A case highlighting the influence of knee joint effusion on muscle inhibition and size. Nat Clin Pract Rheumatol. 2008;4:153–158. doi: 10.1038/ncprheum0709. [DOI] [PubMed] [Google Scholar]
- 54.Arvidsson I, Eriksson E, Knutsson E, Arner S. Reduction of pain inhibition on voluntary muscle activation by epidural analgesia. Orthopedics. 1986;9:1415–1419. doi: 10.3928/0147-7447-19861001-13. [DOI] [PubMed] [Google Scholar]
- 55.Fahrer H, Rentsch HU, Gerber NJ, Beyeler C, Hess CW, Grunig B. Knee effusion and reflex inhibition of the quadriceps - a bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–638. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- 56.Hurley MV. The effects of joint damage on muscle function, proprioception and rehabilitation. Man Ther. 1997;2:11–17. doi: 10.1054/math.1997.0281. [DOI] [PubMed] [Google Scholar]
- 57.Jensen K, Graf BK. The effects of knee effusion on quadriceps strength and knee intraarticular pressure. Arthroscopy. 1993;9:52–56. doi: 10.1016/s0749-8063(05)80343-3. [DOI] [PubMed] [Google Scholar]
- 58.Jones DW, Jones DA, Newham DJ. Chronic knee effusion and aspiration - the effect on quadriceps inhibition. Rheumatology. 1987;26:370–374. doi: 10.1093/rheumatology/26.5.370. [DOI] [PubMed] [Google Scholar]
- 59.Leroux A, Belanger M, Boucher JP. Pain effect on monosynaptic and polysynaptic reflex inhibition. Arch Phys Med Rehabil. 1995;76:576–582. doi: 10.1016/s0003-9993(95)80514-1. [DOI] [PubMed] [Google Scholar]
- 60.Konishi Y, Fukubayashi T, Takeshita D. Possible mechanism of quadriceps femoris weakness in patients with ruptured anterior cruciate ligament. Med Sci Sports Exerc. 2002;34:1414–1418. doi: 10.1097/00005768-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Konishi Y, Konishi H, Fukubayashi T. Gamma loop dysfunction in quadriceps on the contralateral side in patients with ruptured ACL. Med Sci Sports Exerc. 2003;35:897–900. doi: 10.1249/01.MSS.0000069754.07541.D2. [DOI] [PubMed] [Google Scholar]
- 62.Heroux ME, Trenblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. KSSTA. 2006;14:823–833. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 63.Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sports Med. 1998;25:37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- 64.Solomonow M, Baratta R, Zhou BH, Shoji H, Bose W, Beck C, D’Ambrosia R. The synergistic action of the anterior cruciate ligament and thigh muscles in maintaining joint stability. Am J Sports Med. 1987;15:207–213. doi: 10.1177/036354658701500302. [DOI] [PubMed] [Google Scholar]
- 65.Knutson L, Soderberg G, Ballantyne B, Clarke W. A study of various normalization procedures for within day electromyographic data. J Electromyogr Kinesiol. 1994;4:47–59. doi: 10.1016/1050-6411(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 66.Viitasalo JT, Salo A, Lahtinen J. Neuromuscular functioning of athletes and non-athletes in the drop jump. Eur J Appl Physiol Occup Physiol. 1998;78:432–440. doi: 10.1007/s004210050442. [DOI] [PubMed] [Google Scholar]
- 67.Bobbert MF, Huijing PA, van Ingen Schenau GJ. Drop jumping. II. The influence of dropping height on the biomechanics of drop jumping. Med Sci Sports Exerc. 1987;19:339–346. [PubMed] [Google Scholar]
- 68.Kellis E, Arabatzi F, Papadopoulos C. Muscle co-activation around the knee in drop jumping using the co-contraction index. J Electromyogr Kinesiol. 2003;13:229–238. doi: 10.1016/s1050-6411(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 69.Richards CL. EMG activity level comparisons in quadriceps and hamstrings in five dynamic activities. In: Winter DA, Norman RW, Wells RP, Hayes KC, Patla AE, et al., editors. Biomechanics IX-A. Champaign, IL: Human Kinetics; 1985. pp. 313–317. [Google Scholar]
- 70.Zimmermann CL, Cook TM, Bravard MS, Hansen MM, Honomichl RT, Karns ST, Lammers MA, Steele SA, Yunker LK, Zebrowski RM. Effects of stair-stepping exercise direction and cadence on EMG activity of selected lower extremity muscle groups. J Orthop Sports Phys Ther. 1994;19:173–180. doi: 10.2519/jospt.1994.19.3.173. [DOI] [PubMed] [Google Scholar]
- 71.Brask B, Lueke RH, Soderberg GL. Electromyographic analysis of selected muscles during the lateral step-up exercise. Phys Ther. 1984;64:324–329. doi: 10.1093/ptj/64.3.324. [DOI] [PubMed] [Google Scholar]
- 72.Gryzlo SM, Patek RM, Pink M, Perry J. Electromyographic analysis of knee rehabilitation exercises. J Orthop Sports Phys Ther. 1994;20:36–43. doi: 10.2519/jospt.1994.20.1.36. [DOI] [PubMed] [Google Scholar]
- 73.Baratta R, Solomonow M, Zhou BH, Letson D, Chuinard R, D’Ambrosia R. Muscular coactivation. The role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16:113–122. doi: 10.1177/036354658801600205. [DOI] [PubMed] [Google Scholar]
- 74.Krogsgaard MR, Fischer-Rasmussen T, Dyhre-Poulsen P. Absence of sensory function in the reconstructed anterior cruciate ligament. J Electromyogr Kinesiol. 2011;21:82–86. doi: 10.1016/j.jelekin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Branch TP, Hunter R, Donath M. Dynamic EMG analysis of anterior cruciate deficient legs with and without bracing during cutting. Am J Sports Med. 1989;17:35–41. doi: 10.1177/036354658901700106. [DOI] [PubMed] [Google Scholar]
- 76.Kålund S, Sinkjaer T, Arendt-Nielsen L, Simonsen O. Altered timing of hamstring muscle action in anterior cruciate ligament deficient patients. Am J Sports Med. 1990;18:245–248. doi: 10.1177/036354659001800304. [DOI] [PubMed] [Google Scholar]
- 77.Sinkjær T, Arendt-Nielsen L. Knee stability and muscle coordination in patients with anterior cruciate ligament injuries: An electromyographic approach. J Electromyogr Kinesiol. 1991;1:209–217. doi: 10.1016/1050-6411(91)90036-5. [DOI] [PubMed] [Google Scholar]
- 78.McNair PJ, Marshall RN. Landing characteristics in subjects with normal and anterior cruciate ligament deficient knee joints. Arch Phys Med Rehabil. 1994;75:584–589. [PubMed] [Google Scholar]
- 79.Tibone JE, Antich TJ, Fanton GS, Moynes DR, Perry J. Functional analysis of anterior cruciate ligament instability. Am J Sports Med. 1986;14:276–284. doi: 10.1177/036354658601400406. [DOI] [PubMed] [Google Scholar]
- 80.Carlson S, Nordstrand A. The coordination of the knee muscles in some voluntary movements and in the gait in cases with and without knee injuries. A. cta Chir Scandinavica. 1968;134:423–426. [PubMed] [Google Scholar]
- 81.Beard DJ, Kyberd PJ, Fergusson CM, Dodd CA. Proprioception after rupture of the anterior cruciate ligament. An objective indication of the need for surgery? J Bone Joint Surg Br. 1993;75:311–315. doi: 10.1302/0301-620X.75B2.8444956. [DOI] [PubMed] [Google Scholar]
- 82.Zebis MK, Andersen LL, Bencke J, Kjaer M, Aagaard P. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am J Sports Med. 2009;37:1967–1973. doi: 10.1177/0363546509335000. [DOI] [PubMed] [Google Scholar]
- 83.Alkjær T, Simonsen EB, Magnusson SP, Dyhre-Poulsen P, Aagaard P. Antagonist muscle moment is increased in ACL deficient subjects during maximal dynamic knee extension. Knee. 2012;19:633–639. doi: 10.1016/j.knee.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Bryant AL, Creaby MW, Newton RU, Steele JR. Hamstring antagonist torque generated in vivo following ACL rupture and ACL reconstruction. Knee. 2010;17:287–290. doi: 10.1016/j.knee.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Grabiner MD, Koh TJ, Miller GF. Further evidence against a direct automatic neuromotor link between the ACL and hamstrings. Med Sci Sports Exerc. 1992;24:1075–1079. [PubMed] [Google Scholar]
- 86.Kellis E, Baltzopoulos V. The effects of the antagonist muscle force on intersegmental loading during isokinetic efforts of the knee extensors. J Biomech. 1999;32:19–25. doi: 10.1016/s0021-9290(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 87.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Bojsen-Møller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10:58–67. doi: 10.1034/j.1600-0838.2000.010002058.x. [DOI] [PubMed] [Google Scholar]
- 88.O’Connor JJ. Can muscle co-contraction protect knee ligaments after injury or repair? J Bone Joint Surg Br. 1993;75:41–48. doi: 10.1302/0301-620X.75B1.8421032. [DOI] [PubMed] [Google Scholar]
- 89.Yasuda K, Sasaki T. Exercise after anterior cruciate ligament reconstruction. The force exerted on the tibia by the separate isometric contractions of the quadriceps or the hamstrings. Clin Orthop Relat Res. 1987;(220):275–283. [PubMed] [Google Scholar]
- 90.Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renström PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- 91.Yanagawa T, Shelburne K, Serpas F, Pandy M. Effect of hamstrings muscle action on stability of the ACL-deficient knee in isokinetic extension exercise. Clin Biomech (Bristol, Avon) 2002;17:705–712. doi: 10.1016/s0268-0033(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 92.Dvir Z, Eger G, Halperin N, Shklar A. Thigh muscle activity and anterior cruciate ligament insufficiency. Clin Biomech (Bristol, Avon) 1989;4:87–91. doi: 10.1016/0268-0033(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 93.Baltzopoulos V, Brodie DA. Isokinetic dynamometry. Applications and limitations. Sports Med. 1989;8:101–116. doi: 10.2165/00007256-198908020-00003. [DOI] [PubMed] [Google Scholar]
- 94.Knapik JJ, Wright JE, Mawdsley RH, Braun JM. Isokinetic, isometric and isotonic strength relationships. Arch Phys Med Rehabil. 1983;64:77–80. [PubMed] [Google Scholar]
- 95.Kim D, Hong J. Hamstring to quadriceps strength ratio and noncontact leg injuries: A prospective study during one season. Isokinet Exerc Sci. 2011;19:1–6. [Google Scholar]
- 96.Aagaard P, Simonsen EB, Magnusson SP, Larsson B, Dyhre-Poulsen P. A new concept for isokinetic hamstring: quadriceps muscle strength ratio. Am J Sports Med. 1998;26:231–237. doi: 10.1177/03635465980260021201. [DOI] [PubMed] [Google Scholar]
- 97.Hoskins W, Pollard H. Hamstring injury management--Part 2: Treatment. Man Ther. 2005;10:180–190. doi: 10.1016/j.math.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Orchard J, Marsden J, Lord S, Garlick D. Preseason hamstring muscle weakness associated with hamstring muscle injury in Australian footballers. Am J Sports Med. 1997;25:81–85. doi: 10.1177/036354659702500116. [DOI] [PubMed] [Google Scholar]
- 99.Li RC, Maffulli N, Hsu YC, Chan KM. Isokinetic strength of the quadriceps and hamstrings and functional ability of anterior cruciate deficient knees in recreational athletes. Br J Sports Med. 1996;30:161–164. doi: 10.1136/bjsm.30.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Croisier JL, Forthomme B, Namurois MH, Vanderthommen M, Crielaard JM. Hamstring muscle strain recurrence and strength performance disorders. Am J Sports Med. 2002;30:199–203. doi: 10.1177/03635465020300020901. [DOI] [PubMed] [Google Scholar]
- 101.Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36:1469–1475. doi: 10.1177/0363546508316764. [DOI] [PubMed] [Google Scholar]
- 102.Fousekis K, Tsepis E, Poulmedis P, Athanasopoulos S, Vagenas G. Intrinsic risk factors of non-contact quadriceps and hamstring strains in soccer: a prospective study of 100 professional players. Br J Sports Med. 2011;45:709–714. doi: 10.1136/bjsm.2010.077560. [DOI] [PubMed] [Google Scholar]
- 103.Zhang LQ, Nuber GW, Bowen MK, Koh JL, Butler JP. Multiaxis muscle strength in ACL deficient and reconstructed knees: compensatory mechanism. Med Sci Sports Exerc. 2002;34:2–8. doi: 10.1097/00005768-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 104.Patel RR, Hurwitz DE, Bush-Joseph CA, Bach BR, Andriacchi TP. Comparison of clinical and dynamic knee function in patients with anterior cruciate ligament deficiency. Am J Sports Med. 2003;31:68–74. doi: 10.1177/03635465030310012301. [DOI] [PubMed] [Google Scholar]
- 105.Hortobágyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004;51:562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 106.Patsika G, Kellis E, Kofotolis N, Salonikidis K, Amiridis IG. Synergetic and antagonist muscle strength and activity in women with knee osteoarthritis. J Geriatr Phys Ther. 2014;37:17–23. doi: 10.1519/JPT.0b013e31828fccc1. [DOI] [PubMed] [Google Scholar]
- 107.Kellis S, Kellis E, Manou V, Gerodimos V. Prediction of knee extensor and flexor isokinetic strength in young male soccer players. J Orthop Sports Phys Ther. 2000;30:693–701. doi: 10.2519/jospt.2000.30.11.693. [DOI] [PubMed] [Google Scholar]
- 108.Daneshjoo A, Rahnama N, Mokhtar AH, Yusof A. Bilateral and unilateral asymmetries of isokinetic strength and flexibility in male young professional soccer players. J Hum Kinet. 2013;36:45–53. doi: 10.2478/hukin-2013-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tourny-Chollet C, Leroy D, Eger HL, Beuret-Blanquart F. Isokinetic knee muscle strength of soccer players according to their position. Isokinet Exerc Sci. 2000;8:187–193. [Google Scholar]
- 110.Cheung RT, Smith AW, Wong del P. H: q ratios and bilateral leg strength in college field and court sports players. J Hum Kinet. 2012;33:63–71. doi: 10.2478/v10078-012-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brito J, Fernandes L, Seabra A, Soares JM, Krustrup P, Rebelo A. Isokinetic strength effects of FIFA’s “The 11 ” injury prevention training programme. Isokinet Exerc Sci. 2010;18:211–215. [Google Scholar]
- 112.Osternig LR, Caster BL, James CR. Contralateral hamstring (biceps femoris) coactivation patterns and anterior cruciate ligament dysfunction. Med Sci Sports Exerc. 1995;27:805–808. [PubMed] [Google Scholar]
- 113.de Abreu Camarda SR, Denadai BS. Does muscle imbalance affect fatigue after soccer specific intermittent protocol? J Sci Med Sport. 2012;15:355–360. doi: 10.1016/j.jsams.2011.11.257. [DOI] [PubMed] [Google Scholar]
- 114.Kannus P. Isokinetic evaluation of muscular performance: implications for muscle testing and rehabilitation. Int J Sports Med. 1994;15 Suppl 1:S11–S18. doi: 10.1055/s-2007-1021104. [DOI] [PubMed] [Google Scholar]
- 115.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol (1985) 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- 116.Kellis E, Katis A. Quantification of functional knee flexor to extensor moment ratio using isokinetics and electromyography. J Athl Train. 2007;42:477–485. [PMC free article] [PubMed] [Google Scholar]
- 117.Aagaard P, Simonsen EB, Trolle M, Bangsbo J, Klausen K. Isokinetic hamstring/quadriceps strength ratio: influence from joint angular velocity, gravity correction and contraction mode. Acta Physiol Scand. 1995;154:421–427. doi: 10.1111/j.1748-1716.1995.tb09927.x. [DOI] [PubMed] [Google Scholar]
- 118.Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Hamstring and quadriceps strength balance in normal and hamstring anterior cruciate ligament-reconstructed subjects. Clin J Sport Med. 2004;14:274–280. doi: 10.1097/00042752-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 119.Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35:359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 120.Zebis MK, Andersen LL, Ellingsgaard H, Aagaard P. Rapid hamstring/quadriceps force capacity in male vs. female elite soccer players. J Strength Cond Res. 2011;25:1989–1993. doi: 10.1519/JSC.0b013e3181e501a6. [DOI] [PubMed] [Google Scholar]
- 121.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86-A:1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 122.Hewett TE, Myer GD, Ford KR, Heidt RS, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33:492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 123.Hewett TE, Myer GD, Zazulak BT. Hamstrings to quadriceps peak torque ratios diverge between sexes with increasing isokinetic angular velocity. J Sci Med Sport. 2008;11:452–459. doi: 10.1016/j.jsams.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Andrade Mdos S, de Lira CA, Vancini RL, de Almeida AA, Benedito-Silva AA, da Silva AC. Profiling the isokinetic shoulder rotator muscle strength in 13- to 36-year-old male and female handball players. Phys Ther Sport. 2013;14:246–252. doi: 10.1016/j.ptsp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 125.Jenkins ND, Hawkey MJ, Costa PB, Fiddler RE, Thompson BJ, Ryan ED, Smith D, Sobolewski EJ, Conchola EC, Akehi K, et al. Functional hamstrings: quadriceps ratios in elite women’s soccer players. J Sports Sci. 2013;31:612–617. doi: 10.1080/02640414.2012.742958. [DOI] [PubMed] [Google Scholar]
- 126.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 127.Palmieri-Smith RM, McLean SG, Ashton-Miller JA, Wojtys EM. Association of quadriceps and hamstrings cocontraction patterns with knee joint loading. J Athl Train. 2009;44:256–263. doi: 10.4085/1062-6050-44.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McLean SG, Fellin RE, Suedekum N, Calabrese G, Passerallo A, Joy S. Impact of fatigue on gender-based high-risk landing strategies. Med Sci Sports Exerc. 2007;39:502–514. doi: 10.1249/mss.0b013e3180d47f0. [DOI] [PubMed] [Google Scholar]
- 129.Rozzi SL, Lephart SM, Gear WS, Fu FH. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27:312–319. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 130.Besier TF, Lloyd DG, Ackland TR. Muscle activation strategies at the knee during running and cutting maneuvers. Med Sci Sports Exerc. 2003;35:119–127. doi: 10.1097/00005768-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 131.Kanehisa H, Ikegawa S, Tsunoda N, Fukunaga T. Strength and cross-sectional area of knee extensor muscles in children. Eur J Appl Physiol Occup Physiol. 1994;68:402–405. doi: 10.1007/BF00843736. [DOI] [PubMed] [Google Scholar]
- 132.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. Moment arms of the knee extensor mechanism in children and adults. J Anat. 2009;215:198–205. doi: 10.1111/j.1469-7580.2009.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Praagh E, Doré E. Short-term muscle power during growth and maturation. Sports Med. 2002;32:701–728. doi: 10.2165/00007256-200232110-00003. [DOI] [PubMed] [Google Scholar]
- 134.Sjöström M, Lexell J, Downham DY. Differences in fiber number and fiber type proportion within fascicles. A quantitative morphological study of whole vastus lateralis muscle from childhood to old age. Anat Rec. 1992;234:183–189. doi: 10.1002/ar.1092340205. [DOI] [PubMed] [Google Scholar]
- 135.Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types. 4. Children’s biopsies. Neurology. 1969;19:591–605. doi: 10.1212/wnl.19.6.591. [DOI] [PubMed] [Google Scholar]
- 136.Bell RD, MacDougall JD, Billeter R, Howald H. Muscle fiber types and morphometric analysis of skeletal msucle in six-year-old children. Med Sci Sports Exerc. 1980;12:28–31. [PubMed] [Google Scholar]
- 137.Ramsay JA, Blimkie CJ, Smith K, Garner S, MacDougall JD, Sale DG. Strength training effects in prepubescent boys. Med Sci Sports Exerc. 1990;22:605–614. doi: 10.1249/00005768-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 138.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. In vivo measurements of muscle specific tension in adults and children. Exp Physiol. 2010;95:202–210. doi: 10.1113/expphysiol.2009.048967. [DOI] [PubMed] [Google Scholar]
- 139.O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN. The effects of agonist and antagonist muscle activation on the knee extension moment-angle relationship in adults and children. Eur J Appl Physiol. 2009;106:849–856. doi: 10.1007/s00421-009-1088-4. [DOI] [PubMed] [Google Scholar]
- 140.Dotan R, Mitchell C, Cohen R, Gabriel D, Klentrou P, Falk B. Child-adult differences in the kinetics of torque development. J Sports Sci. 2013;31:945–953. doi: 10.1080/02640414.2012.757343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paasuke M, Ereline J, Gapeyeva H. Knee extensor muscle strength and vertical jumping performance characteristics in pre- and post-pubertal boys. Pediatr Exerc Sci. 2001;13:60–69. [Google Scholar]
- 142.Myer GD, Ford KR, Hewett TE. Rationale and Clinical Techniques for Anterior Cruciate Ligament Injury Prevention Among Female Athletes. J Athl Train. 2004;39:352–364. [PMC free article] [PubMed] [Google Scholar]
- 143.Knapik JJ, Bauman CL, Jones BH, Harris JM, Vaughan L. Preseason strength and flexibility imbalances associated with athletic injuries in female collegiate athletes. Am J Sports Med. 1991;19:76–81. doi: 10.1177/036354659101900113. [DOI] [PubMed] [Google Scholar]
- 144.Kanehisa H, Yata H, Ikegawa S, Fukunaga T. A cross-sectional study of the size and strength of the lower leg muscles during growth. Eur J Appl Physiol Occup Physiol. 1995;72:150–156. doi: 10.1007/BF00964130. [DOI] [PubMed] [Google Scholar]
- 145.Hoshikawa Y, Muramatsu M, Iida T, Uchiyama A, Nakajima Y, Kanehisa H. Event-related differences in the cross-sectional areas and torque generation capabilities of quadriceps femoris and hamstrings in male high school athletes. J Physiol Anthropol. 2010;29:13–21. doi: 10.2114/jpa2.29.13. [DOI] [PubMed] [Google Scholar]
- 146.Hewett TE, Zazulak BT, Myer GD, Ford KR. A review of electromyographic activation levels, timing differences, and increased anterior cruciate ligament injury incidence in female athletes. Br J Sports Med. 2005;39:347–350. doi: 10.1136/bjsm.2005.018572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Malinzak R, Colby S, Kirkendall D, Yu B, Garrett W. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech. 2001;16:438–445. doi: 10.1016/s0268-0033(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 148.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes. Decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24:765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 149.Wojtys EM, Huston LJ, Taylor PD, Bastian SD. Neuromuscular adaptations in isokinetic, isotonic, and agility training programs. Am J Sports Med. 1996;24:187–192. doi: 10.1177/036354659602400212. [DOI] [PubMed] [Google Scholar]
- 150.Zazulak BT, Ponce PL, Straub SJ, Medvecky MJ, Avedisian L, Hewett TE. Gender comparison of hip muscle activity during single-leg landing. J Orthop Sports Phys Ther. 2005;35:292–299. doi: 10.2519/jospt.2005.35.5.292. [DOI] [PubMed] [Google Scholar]
- 151.Myer GD, Ford KR, Hewett TE. The effects of gender on quadriceps muscle activation strategies during a maneuver that mimics a high ACL injury risk position. J Electromyogr Kinesiol. 2005;15:181–189. doi: 10.1016/j.jelekin.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 152.Bassa E, Patikas D, Kotzamanidis C. Activation of antagonist knee muscles during isokinetic efforts in prepubertal and adult males. Pediatr Exerc Sci. 2005;17:171–181. [Google Scholar]
- 153.Kellis E, Unnithan VB. Co-activation of vastus lateralis and biceps femoris muscles in pubertal children and adults. Eur J Appl Physiol Occup Physiol. 1999;79:504–511. doi: 10.1007/s004210050545. [DOI] [PubMed] [Google Scholar]
- 154.Cohen R, Mitchell C, Dotan R, Gabriel D, Klentrou P, Falk B. Do neuromuscular adaptations occur in endurance-trained boys and men? Appl Physiol Nutr Metab. 2010;35:471–479. doi: 10.1139/H10-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frost G, Dowling J, Dyson K, Bar-Or O. Cocontraction in three age groups of children during treadmill locomotion. J Electromyogr Kinesiol. 1997;7:179–186. doi: 10.1016/s1050-6411(97)84626-3. [DOI] [PubMed] [Google Scholar]
- 156.Lazaridis SN, Bassa EI, Patikas D, Hatzikotoulas K, Lazaridis FK, Kotzamanidis CM. Biomechanical comparison in different jumping tasks between untrained boys and men. Pediatr Exerc Sci. 2013;25:101–113. doi: 10.1123/pes.25.1.101. [DOI] [PubMed] [Google Scholar]
- 157.Lazaridis S, Bassa E, Patikas D, Giakas G, Gollhofer A, Kotzamanidis C. Neuromuscular differences between prepubescents boys and adult men during drop jump. Eur J Appl Physiol. 2010;110:67–74. doi: 10.1007/s00421-010-1452-4. [DOI] [PubMed] [Google Scholar]
- 158.Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Åstrand PO. Children and adolescents: performane, measurements, education. In: Coudert J, Van Praagh E, eds , et al., editors. Pediatric work physiology XVI: children and exercise. Paris: Masson; 1992. pp. 3–7. [Google Scholar]
- 160.Lebiedowska MK, Fisk JR. Passive dynamics of the knee joint in healthy children and children affected by spastic paresis. Clin Biomech (Bristol, Avon) 1999;14:653–660. doi: 10.1016/s0268-0033(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 161.Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001;22:138–143. doi: 10.1055/s-2001-11337. [DOI] [PubMed] [Google Scholar]
- 162.Asai H, Aoki J. Force development of dynamic and static contractions in children and adults. Int J Sports Med. 1996;17:170–174. doi: 10.1055/s-2007-972827. [DOI] [PubMed] [Google Scholar]
- 163.Cornu C, Goubel F, Fardeau M. Stiffness of knee extensors in Duchenne muscular dystrophy. Muscle Nerve. 1998;21:1772–1774. doi: 10.1002/(sici)1097-4598(199812)21:12<1772::aid-mus21>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 164.Wilson GJ, Murphy AJ, Pryor JF. Musculotendinous stiffness: its relationship to eccentric, isometric, and concentric performance. J Appl Physiol (1985) 1994;76:2714–2719. doi: 10.1152/jappl.1994.76.6.2714. [DOI] [PubMed] [Google Scholar]
- 165.Marginson V, Eston R. The relationship between torque and joint angle during knee extension in boys and men. J Sports Sci. 2001;19:875–880. doi: 10.1080/026404101753113822. [DOI] [PubMed] [Google Scholar]
- 166.Doherty TJ. Invited Review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 167.Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67:41–47. doi: 10.1093/gerona/glr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol. 2005;208:3907–3923. doi: 10.1242/jeb.01830. [DOI] [PubMed] [Google Scholar]
- 169.Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech. 2006;39:406–417. doi: 10.1016/j.jbiomech.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 170.Mademli L, Arampatzis A, Walsh M. Age-related effect of static and cyclic loadings on the strain-force curve of the vastus lateralis tendon and aponeurosis. J Biomech Eng. 2008;130:011007. doi: 10.1115/1.2838036. [DOI] [PubMed] [Google Scholar]
- 171.Baroni BM, Geremia JM, Rodrigues R, Borges MK, Jinha A, Herzog W, Vaz MA. Functional and morphological adaptations to aging in knee extensor muscles of physically active men. J Appl Biomech. 2013;29:535–542. doi: 10.1123/jab.29.5.535. [DOI] [PubMed] [Google Scholar]
- 172.Savelberg HH, Meijer K. The effect of age and joint angle on the proportionality of extensor and flexor strength at the knee joint. J Gerontol A Biol Sci Med Sci. 2004;59:1120–1128. doi: 10.1093/gerona/59.11.1120. [DOI] [PubMed] [Google Scholar]
- 173.Laudani L, Vannozzi G, Sawacha Z, della Croce U, Cereatti A, Macaluso A. Association between physical activity levels and physiological factors underlying mobility in young, middle-aged and older individuals living in a city district. PLoS One. 2013;8:e74227. doi: 10.1371/journal.pone.0074227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- 175.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 176.Tseng LA, Delmonico MJ, Visser M, Boudreau RM, Goodpaster BH, Schwartz AV, Simonsick EM, Satterfield S, Harris T, Newman AB. Body composition explains sex differential in physical performance among older adults. J Gerontol A Biol Sci Med Sci. 2014;69:93–100. doi: 10.1093/gerona/glt027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rantanen T, Era P, Heikkinen E. Maximal isometric knee extension strength and stair-mounting ability in 75- and 80-year-old men and women. Scand J Rehabil Med. 1996;28:89–93. [PubMed] [Google Scholar]
- 178.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol (1985) 1999;86:188–194. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 179.Samuel D, Rowe PJ. Effect of ageing on isometric strength through joint range at knee and hip joints in three age groups of older adults. Gerontology. 2009;55:621–629. doi: 10.1159/000236043. [DOI] [PubMed] [Google Scholar]
- 180.Ogawa M, Yasuda T, Abe T. Component characteristics of thigh muscle volume in young and older healthy men. Clin Physiol Funct Imaging. 2012;32:89–93. doi: 10.1111/j.1475-097X.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 181.Mau-Moeller A, Behrens M, Lindner T, Bader R, Bruhn S. Age-related changes in neuromuscular function of the quadriceps muscle in physically active adults. J Electromyogr Kinesiol. 2013;23:640–648. doi: 10.1016/j.jelekin.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 182.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- 183.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 184.Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11:405–412. doi: 10.1016/s1050-6411(01)00023-2. [DOI] [PubMed] [Google Scholar]
- 185.Cannon J, Kay D, Tarpenning KM, Marino FE. Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct I. 2007;27:91–100. doi: 10.1111/j.1475-097X.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 186.Suetta C, Aagaard P, Magnusson SP, Andersen LL, Sipilä S, Rosted A, Jakobsen AK, Duus B, Kjaer M. Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: effects of unilateral long-term disuse due to hip-osteoarthritis. J Appl Physiol (1985) 2007;102:942–948. doi: 10.1152/japplphysiol.00067.2006. [DOI] [PubMed] [Google Scholar]
- 187.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 188.Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–199. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miller M, Flansbjer UB, Downham D, Lexell J. Superimposed electrical stimulation: assessment of voluntary activation and perceived discomfort in healthy, moderately active older and younger women and men. Am J Phys Med Rehabil. 2006;85:945–950. doi: 10.1097/01.phm.0000247648.62957.19. [DOI] [PubMed] [Google Scholar]
- 190.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985) 2002;92:1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 192.Pereira MP, Gonçalves M. Muscular coactivation (CA) around the knee reduces power production in elderly women. Arch Gerontol Geriatr. 2011;52:317–321. doi: 10.1016/j.archger.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 193.Arampatzis A, Mademli L. Tendon. In: Mooren FC, ed , et al., editors. Encyclopedia of Exercise Medicine in Health and Disease. Berlin-Heidelberg: Springer-Verlag; 2012. pp. 843–849. [Google Scholar]
- 194.Karamanidis K, Arampatzis A. Altered control strategy between leading and trailing leg increases knee adduction moment in the elderly while descending stairs. J Biomech. 2011;44:706–711. doi: 10.1016/j.jbiomech.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 195.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol (1985) 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]


