Abstract
Osteoporosis is a common metabolic skeletal disorder characterized by decreased bone mass and deteriorated bone structure, leading to increased susceptibility to fractures. With aging population, osteoporotic fractures are of global health and socioeconomic importance. The three-dimensional microstructural information of the common osteoporosis-related fracture sites, including vertebra, femoral neck and distal radius, is a key for fully understanding osteoporosis pathogenesis and predicting the fracture risk. Low vertebral bone mineral density (BMD) is correlated with increased fracture of the spine. Vertebral BMD decreases from cervical to lumbar spine, with the lowest BMD at the third lumbar vertebra. Trabecular bone mass of the vertebrae is much lower than that of the peripheral bone. Cancellous bone of the vertebral body has a complex heterogeneous three-dimensional microstructure, with lower bone volume in the central and anterior superior regions. Trabecular bone quality is a key element to maintain the vertebral strength. The increased fragility of osteoporotic femoral neck is attributed to low cancellous bone volume and high compact porosity. Compared with age-matched controls, increased cortical porosity is observed at the femoral neck in osteoporotic fracture patients. Distal radius demonstrates spatial inhomogeneous characteristic in cortical microstructure. The medial region of the distal radius displays the highest cortical porosity compared with the lateral, anterior and posterior regions. Bone strength of the distal radius is mainly determined by cortical porosity, which deteriorates with advancing age.
Keywords: Osteoporosis, Fracture, Microstructure, Trabecular bone, Cortical bone, Vertebra, Femoral neck, Distal radius
Core tip: The most common sites of the osteoporotic fractures include the vertebra, femoral neck and distal radius, where the microstructural information is a key for fully understanding osteoporosis pathogenesis and improving the prediction of fracture risk. Vertebral strength is mostly preserved by trabecular bone, which is microstructurally inhomogeneous, with lower bone volume in the central and anterior superior regions. Increased fragility of osteoporotic femoral neck is attributed to low cancellous bone volume and high compact porosity. Distal radius shows significant variations in cortical porosity, which is the major element attributed to bone strength of the distal radius.
INTRODUCTION
Osteoporosis is a common metabolic skeletal disorder characterized by decreased bone mass and deteriorated bone structure, resulting in an increased susceptibility to fractures[1,2]. With the rapid growth in the elderly population, osteoporotic fracture is a global public health problem with enormous socioeconomic consequences[3]. Osteoporosis is estimated to affect more than 200 million people around the world. Osteoporosis leads to approximately 9 million new fractures annually, 1.4 million being in the vertebra, 1.7 million in the forearm and 1.6 million in the femoral neck[4]. A key characteristic of osteoporosis is fracture that occurs with little or no injury. Osteoporotic fractures might affect functioning of body movement, which can lead to disability, limit daily activities and affect the quality of life.
Osteoporosis can affect any bone in the body. However, osteoporotic fractures are some skeletal sites are more easily fractured than would normally be the case. However, osteoporotic fractures are more easily and more likely to occur at some special skeletal sites. Consistent with current clinical experience, the most common sites of fractures in osteoporotic patients include bones that are under certain strain as they bear body weight such as vertebra and femoral neck or take the stress when a person falls on an outstretched hand such as distal radius[5]. To prevent fractures is the major purpose of osteoporosis screening. When the external force applied to a bone exceeds its strength, a fracture would occur. The ability of a bone to tolerate loading depends on the quantity and quality of the bone. The intrinsic material properties of bone are bone mineral density (BMD), bone size, geometry, bone mineralization, microstructure and bone turnover[6].
The decline in BMD is related to decreased bone strength, increased bone fragility and elevated fracture risk. BMD is a major important predictor of subsequent osteoporotic fracture risk. Many techniques are available to determine BMD value. Low BMD is correlated with increased fracture risk[6]. Clinical studies demonstrate that BMD only accounts for bone strength partially and that there is a limitation of BMD measurements in evaluating fracture risk[7,8]. Recent studies show that bone microstructural information can detect early changes in osteoporotic process. Knowledge of bone microstructure is important to fully understanding the pathogenesis of osteoporotic fracture[9-11]. The microstructural properties of vertebra, femoral neck and distal radius are critical for predicting the fracture risk of these sites. Bone microstructure typically refers to histomorphometric parameters originally obtained from two-dimensional (2D) stained sections. The sample preparation process of this 2D approach is tedious and destructive. Bone structure is three-dimensional (3D). Owing to the substantially improved spatial resolution, it has been possible recently to analyze quantitatively 3D bone microarchitectural properties. Micro-computed tomography (CT) can provide excellent 3D spatial resolution of 10 m. A High-resolution peripheral quantitative CT (HR-pQCT) technique has been implemented on the XtremeCT scanner. The scanner provides 3D images with isotropic voxel size of 41 m or 82 m, the latter resulting in isotropic spatial resolution of about 130-150 m[12]. With these newly developed techniques, many studies have been carried out to investigate the variations of 3D cancellous bone microstructure, such as bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp). Cortical parameters such as BMD, thickness and porosity are also calculated[13]. All these parameters are important for evaluating bone quality (Table 1). This review article will discuss the bone microstructural parameters obtained from 3D work and newer technologies, especially the vertebra, femoral neck and distal radius, the common sites of the osteoporotic fractures according to the existing literature.
Table 1.
The main bone histomorphometric parameters and their significance
| Parameters | Meaning | Significance |
| BV/TV | Trabecular bone volume per total tissue volume | In osteoporotic patients, BV/TV significantly decreases, accompanied by low BMD |
| Tb.Th | Trabecular thickness | Trabeculae become thinner with the progression of osteoporosis for both women and men |
| Tb.N | Trabecular number | In osteoporotic patients, the decrease in Tb.N is usually greater in women than in men |
| Tb.Sp | Trabecular separation | Tb.Sp increases with the progression of osteoporosis |
| Co.Po | Cortical porosity | With the progression of osteoporosis, Co.Po increases, accompanied by low cortical BMD |
BV/TV: Bone volume fraction; Tb.Th: Trabecular thickness; Tb.N: Trabecular number; Tb.Sp: Trabecular separation; BMD: Bone mineral density.
VERTEBRA
The vertebrae are made up of 24 individual bones to bear the weight of the upper body and withstand substantial loads. Vertebral body is a thick oval segment of bone, composed of internal cancellous bone and a thin coating of compact bone. Intervertebral disc is a massive pad of fibrocartilage, which is firmly attached to vertebral body above and below, forming a flexible column. This lightweight structure contains a minimal amount of material in its structure. Cancellous bone of vertebral body is crucial for the function of the whole spinal column[14,15]. Osteoporotic fractures most often occur in the vertebrae. Approximately 700000 new vertebral fractures occur in the United States annually[16]. They are nearly twofold as common as other fractures, such as osteoporosis-related femoral neck and radial fractures. When osteoporosis is involved, a vertebral compression fracture generally is a patient’s earliest sign of a deteriorated skeleton from osteoporosis.
Cancellous bone of vertebra is metabolically more active than cortical bone and trabecular BMD may act as an initial predictor of spinal osteoporotic fracture[17]. There is a negative correlation between vertebral cancellous BMD and spinal fracture[14]. It is necessary to examine regional BMD separately in different levels, as osteoporosis-related spinal fractures occur frequently in the midthoracic region and thoracolumbar transitional area, as described by Wasnich[18]. Vertebral BMD can be explored using QCT-based BMD measurement approach[19-22]. Trabecular BMD of the cervical spine is significantly higher than that of the thoracic and lumbar one. Trabecular BMD of the first sacral vertebra is significantly higher than that of the lumbar vertebrae. In an age- and gender-stratified population-based non-invasive study, we examined trabecular volumetric BMD (vBMD) of thoracic and lumbar vertebrae[19-22]. Trabecular vBMD of vertebral body gradually decreased craniocaudally from the first thoracic (Th1) to third lumbar spine (L3) for both genders. Compared with Th1, vBMD at L3 declined around 30% (Figure 1). There was a very high correlation between adjacent vertebral BMD, though the BMD correlation became lower between vertebrae with increasing distance from each other. It might be suitable to use any vertebra for evaluating bone strength of spine. By using our knowledge available for BMD correlations, one can estimate the BMD of any vertebra, provided that one vertebral BMD is known.
Figure 1.
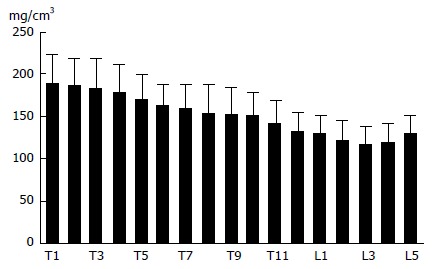
The trabecular bone mineral density of the thoracic and lumbar vertebrae[22]. The bone mineral density tends to decrease from the first thoracic to third lumbar vertebra.
The regional variation of vertebral microstructure has been examined extensively[23-25]. We studied 3D microstructure of L4 from Japanese cadaver donors by quantitative micro-CT and electron microscopic methods[25]. BV/TV and Tb.N of vertebral cancellous bone declined with advancing age. BV/TV decreased by 22%-24% from 60 to 90 years of age for both males and females. Age-dependent decreases of BV/TV were similar for males and females. Tb.N also decreased with age by 19% in males and 16% in females. Tb.Sp consistently increased with age. There was no significant decline of Tb.Th with advancing age. Thus, age-related decrease of BV/TV is mainly related to increased Tb.Sp and decreased Tb.N[17,23,25].
Cancellous bone of vertebra is complicated morphologically that contains numerous plate-like and rod-like trabeculae[24-27]. Trabecular plate-like or rod-like characteristic might be assessed by determining the structure model index (SMI). SMI is a crucial morphometric parameter which effects intensely on bone intrinsic properties. Vertebral cancellous bone has a more rod-like than plate-like structure. SMI of the vertebral cancellous bone increases by about 20% from 60 and 90 years of age. Vertebral trabeculae are gradually converted from plate-like to rod-like and consequently are more fragile and are especially prone to fracture. Cancellous connectivity density (Conn.D) is a basic characteristic of 3D network and is critical for the preservation of bone strength. When the amount of cancellous bone declines, the value of Conn.D would decrease correspondently, perhaps attributable to the small trabecular bone loss[25,26,28]. Vertebral trabecular Conn.D decreases significantly with advancing age. Age-dependent change of Conn.D is almost identical for males and females[25,28].
Determination of BMD locally is achievable using QCT owing to its high spatial resolution. However, clinical assessment is limited to just a few thin slices and QCT is commonly carried out in the central area of the vertebral body. As cancellous bone is heterogeneous microarchitecturally in the vertebral body[19,22,25], localization of low BMD value within the vertebral body is beneficial clinically and may play a role in clarifying pathophysiology of spinal osteoporosis-related fracture. QCT and micro-CT studies show that BV/TV is lower in central and anterior superior regions, compared with the posterior region of the vertebral body (Figure 2). The cancellous regional differences of the microarchitectural characteristic within the vertebrae is important for assessing the bone quality of vertebra and may also contribute to the pathogenesis of osteoporosis-related spinal fracture.
Figure 2.
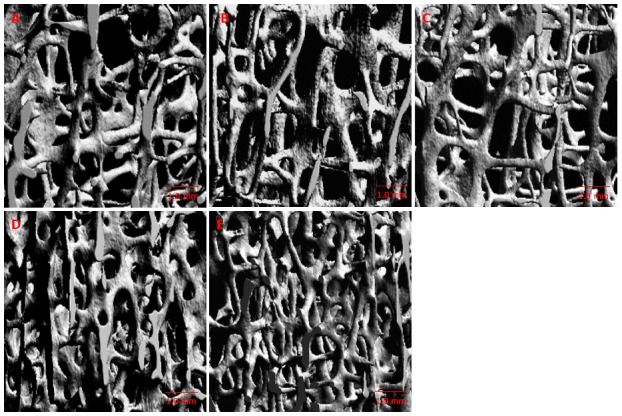
Micro-computed tomography image in different regions of vertebral body[25]. A: Anterosuperior; B: Anteroinferior; C: Central; D: Posterosuperior; E: Posteroinferior regions. The trabecular bone is lower in the anterosuperior and central regions.
By using scanning electron microscopy, it is easy to examine the trabecular resorption state, that is critical for cancellous structural integrity, possibly deciding if the bone strength is sustained or declined[25,29,30]. The decrease of spinal cancellous bone with advancing age is predominantly through trabecular perforation rather than trabecular general thinning[25,28]. It is demonstrated that osteoclasts resorb some perforated trabecular bone and the trabecular connectivity is destroyed. When the newly formed bone is insufficient adequately to replace missing bone, the trabecular connectivity will reduce and the bone will become more brittle and fragile[25,29,30]. Microcallus is a nodular aggregation of woven bone, which is often found predominantly on the thin vertical trabeculae (Figure 3A). Microcallus acts to preserve or repair a trabecula[25,31-33]. However, what triggers the microcallus formation is still subject to debate.
Figure 3.
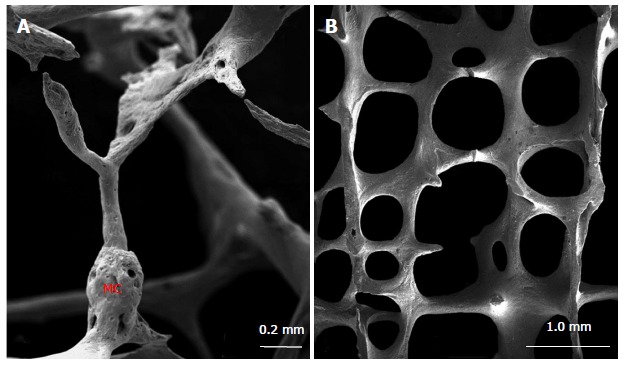
Scanning electron microscopic image of vertebral trabecular bone[25]. The microcallus (MC) is seen on the vertical trabecula (A). Tertical trabeculae are relatively thicker than the horizontal ones (B).
The conventional view is that a compressive load on vertebrae is mainly carried by the vertical trabeculae, whereas the horizontal trabeculae serve to prevent buckling of the vertical trabeculae[34,35]. This view is reinforced by finite element analyses of human vertebral bone specimens, which demonstrates that vertical trabeculae are more highly strained than horizontal ones under normal compressive loading, the[14,36]. Consequently, it is important to quantify the trabecular thickness as well as bone volume fraction for horizontal and vertical trabecular bone independently. Recently, a 3D approach was introduced to segment a trabecular network into vertical and horizontal trabeculae of the vertebral body[37-39]. Fields et al[37,38] found that vertical trabeculae played a particular important role for the compressive bone strength of vertebrae with low BMD and presumed that vertebral bone strength is better explained by the vertical trabecular bone volume fraction alone, than by the total trabecular bone volume fraction. The scanning electron microscopic images confirmed that the horizontal trabeculae were thinner, whereas the vertical ones were relatively thicker (Figure 3B). Both vertical and horizontal trabeculae decreased with age and vertical trabeculae were lost more rapidly in females than in males. Furthermore, the vertical as well as horizontal trabecular thickness were independent of age, however the ratio of horizontal/vertical trabecular thickness declined significantly with age suggesting a more pronounced thinning of horizontal trabeculae[39]. Age-related bone loss of trabecular elements results in compensatory hypertrophy of vertical trabeculae in females, but not in males[40].
Vertebral trabecular bone is inhomogeneous microstructurally. Age-dependent declines of BV/TV and Conn.D are similar in males and females. There are significant differences of some morphometric parameters between males and females. Age-dependent bone loss of vertebral trabeculae may be induced by elevated bone resorption activity. These findings elucidate the possible mechanisms of vertebral fractures[17,25].
Highly porous cortical bone of the spine is very thin. Therefore, it is difficult to sort out the role of cortical bone, especially in aged individuals. It is difficult to determine the cortical thinness accurately with non-destructive methods. It is unclear whether the compact bone significantly contributes to biomechanical strength of whole vertebral bone. Cortical thickness of vertebral body ranges from 180 to 600 m, with a mean thickness of 380 m[40-43]. The compact bone of the cervical and lumbar vertebrae is relatively thicker than that of the thoracic one. The dorsal cortex is generally thinner than that of the ventral one. There is no significant gender difference in vertebral cortical thickness. There is a slight age-related decline in vertebral cortical thickness. Most studies highlight the importance of trabecular bone for maintaining bone strength of vertebrae, however recent studies indicate a crucial role of the cortical bone, especially in elderly individuals whose cancellous bone is lower[40-43].
FEMORAL NECK
Femoral neck has to bear high compressive and shear forces continually. These forces are approximately 1 ×body weight (BW) during standing, but they are much higher during physical activities[44]. Femoral neck fracture is generally induced by a fall, but may be caused by impact to the hip. When the bone becomes weak due to osteoporosis, only a slight external force is enough to make femoral neck more susceptible to fracture. This type of fracture is very serious and debilitating osteoporotic fracture. Osteoporosis-related femoral neck fractures are a major cause of mortality and morbidity in elderly people worldwide[45,46]. Gullberg et al[47] estimated that there were 1.25 million new femoral neck fractures occurred in the world annually and that the fracture number will increase by 310% in males and 240% in females by 2025. There have been many studies conducted to investigate the underlying causes of femoral neck fracture. It is suggested that 3D microstructures play a significant role in assessing the bone quality and provide compelling evidence to explain the bone strength[48-50].
The proximal femur was isolated by cutting at the base of femoral head and femoral neck. Cancellous bone specimen of 8 mm × 8 mm × 8 mm cube was prepared from the central part of femoral neck for quantitative micro-CT examination. Alterations of the femoral neck cancellous bone with advancing age include a decline in BV/TV and Tb.N, and an increase in Tb.Sp[49,51,52]. BV/TV decreases by around 20% from 60 to 90 years of age (Figure 4). Tb.N and Tb.Th decline, while Tb.Sp increases in males and females. The decrease of BV/TV with age is related to decreases in Tb.N and Tb.Th, and increases in Tb.Sp[49,50]. There are a few studies regarding SMI of femoral neck trabeculae[49,50,53]. It is found that SMI increases with age. Trabecular structure of the femoral neck becomes more rod-like with advancing age. Therefore it is more brittle and more likely to fracture. Conn.D decreases significantly with age[49,50]. When the trabecular bone volume fraction declines, Conn.D will decline concomitantly, probably because of small trabecular bone loss[25,49]. Ciarelli et al[48] examined 3D microarchitecture of femoral neck in hip fracture patients and nonfracture controls. There were more anisotropic 3D microstructures and relatively fewer cancellous elements transverse to the primary load axis in fracture cases. The changed 3D microstructures would be supposed to influence bone biomechanical characteristics. Relatively fewer transverse cancellous bones in fracture patients might produce diminished cross bracing and a high susceptibility to buckling of cancellous bone oriented along the loading axis, and the decreased resistance of transverse loads. This changed microstructure may distinguish between patients of high fracture risk and low fracture risk with identical trabecular bone volume[48].
Figure 4.
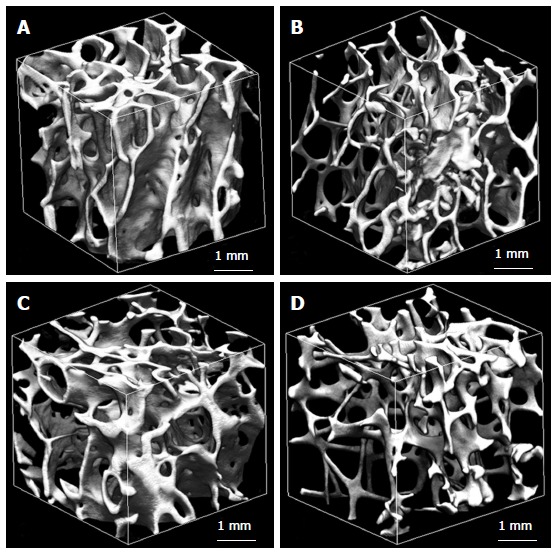
Trabecular microstructure of femoral neck from a man aged 62 years (A), a man aged 92 years (B), a woman aged 62 years (C), and a woman aged 92 years (D)[49]. The trabecular bone is higher in a man aged 62 years and is lower in a woman aged 92 years.
The femoral neck displays noted regional heterogeneity morphologically[49,54-56]. When the hip joint bears entire body weight vertically, compact bone of the inferior region is thicker than that of the superior region. Compact bone of the aged subjects is very thin in the upper region, while that of the lower region remain relatively thicker[54-56]. Cortical thickness of the superior posterior region decreases by 6.4% per decade in females between the ages 60 to 90 years. Similar but a significantly lesser effect is evident in males. The thinning of femoral cortex compromises the functional capacity of femoral neck to absorb energy independent of osteoporosis[49,55]. Cortical porosity (Ct.Po) of femoral neck varies from 5% to 13%[49,55-57]. With advancing age, the diameter of cortical pores increases and some pores adjacent to the endosteum coalesce, leaving the remnant cortexes that resemble to cancellous bone. The remained cortical bone close to the periosteum is kept with normal appearance including several enlarged pores. In elderly female individuals, enlarged cortical pores are present at the endosteal surface, as well as at the periosteal surface. Figure 5 indicates the age-dependent variations of cortical porosity in the inferior region of femoral neck. Cortical thickness (Ct.Th) declines by 3% to 5% and Ct.Po increases by 31% to 33% per decade between ages of 60 to 90 years[49]. The number of cortical pores has no marked age-related changes, whereas the diameter of cortical pore increases significantly with age[49,55-57]. Accordingly, increase of cortical porosity with advancing age is predominantly attributable to enlarged cortical pores. Compared with males, females have a greater Ct.Po and larger cortical pore. Consequently, in addition to age, gender is also an important factor to influence cortical porosity. With advancing age, especially in females several intracortical pores coalesce into a giant pores larger than 385 m[46,52,55]. The giant intracortical pore formation might have a pivotal function in the process of local cortical bone loss during aging.
Figure 5.
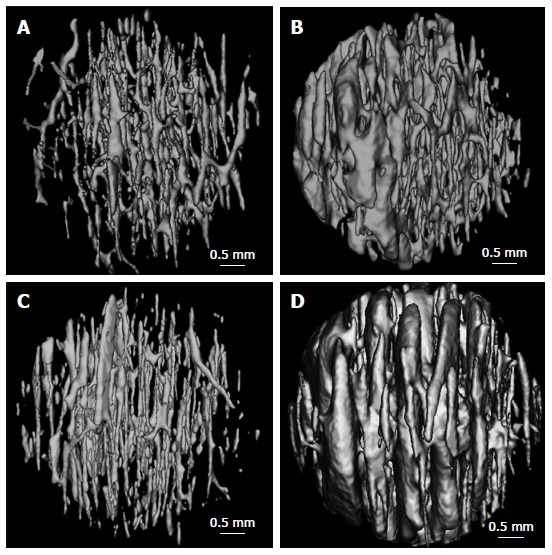
Three D reconstructed images of the canal networks in the inferior femoral neck cortex from a man aged 62 years (A), a man aged 92 years (B), a woman aged 62 years (C), and a woman aged 92 years (D)[49]. There are more enlarged canals in the 92 years than those of the 62 years.
Osteoporotic fractures of femoral neck are considered to be caused by both cancellous bone loss and compact bone thinning. The relative contribution of compact bone and cancellous bone to whole bone strength of the femoral neck is still poorly understood. It has been shown that an increase in Ct.Po is the most noticeable age-dependent change of femoral neck. The decline in BV/TV with age is more apparent than that of Ct.Th. There is a statistically significant negative correlation between BV/TV and Ct.Po. Ct.Th and BV/TV are lower, and Ct.Po is higher in females, when compares with males. The above results might be used as reference for racial comparison with age and gender, and contribute to the pathogenesis of osteoporosis-related fracture at the femoral neck[49,55,58].
DISTAL RADIUS
Distal radius fractures are very common in osteoporosis patients[59]. The most common cause of the distal radial fracture is a fall on the outstretched hand in people with normal or low bone mineral density[60]. When people fall from standing position, the sudden external force can cause fracture of the distal radius. However, the severity of fall required to cause radial fracture in osteoporotic patients is much less than the subjects with normal BMD, because of the greater skeletal fragility.
Population-based cross-sectional studies by HR-pQCT imaging technique uncovered that BV/TV of the radial cancellous bone declines by 26% in males and 27% in females from 60 to 90 years of age[61]. Trabecular bone volume of distal radius remains relatively stable until midlife and thereafter decreases[61-63]. Trabecular bone volume is higher in males than in females of the same age. Age-dependent decreases in the trabecular BV/TV and BMD are similar for males and females from 20 to 90 years of age[61-63]. There is a different microstructural basis for the decline of cancellous bone volume with advancing age between males and females. Gender difference of cancellous bone loss with age is present at the distal radius. Decreases of Tb.N and increases of Tb.Sp are observed in females, whereas in males the decrease of BV/TV is primarily caused by trabecular thinning, leading to a substantial decline in Tb.Th and unchanged Tb.N[61-63].
Recent studies highlight the importance of the cortical microstructure in the maintenance of the radial strength[62,64]. Cortical bone at the distal radius can be analyzed structurally with HR-pQCT method[63]. Cortical porosity significantly increased with age. Cortical porosity parameters of the distal radius provided an important decade-wise discrimination for females in their fifties and sixties[62,63,65]. Cortical vBMD is dramatically decreased in older women than in younger women[66,67]. There is no significant alteration in the cortical vBMD with age in males. As compared with younger subjects, older men and women have elevated values of Ct.Po and cortical pore diameter. Bone strength of distal radial cortex strongly correlated inversely with Ct.Po, which has a major impact on bone quality[63-65]. Age-dependent increase of Ct.Po in females is more than twice as high as in males. Cortical bones have a tendency to become thinning more with age in females than in males. Compared with males, females have lower bone strength of the distal radius. The gender difference is perhaps attributable higher cortical porosity in females.
As compared with young subjects, older women and men had significantly worse microstructure of cortical bone, including increased Ct.Po, but generally similar trabecular bone parameters of the distal radius. The main effect of age independent of BMD is on cortical morphometric parameters[62]. The spatial inhomogeneous characteristic in cortical porosity is particularly noticeable at the distal radius. The anterior region exhibits the lowest Ct.Po, while the medial region shows the highest. Ct.Po is more than twofold higher in the medial region than in the anterior region. Ct.Th is lowest in the lateral region and highest in the anterior and posterior regions. Ct.BMD is lowest in the lateral region and highest in the posterior region. Increased Ct.Po is investigated in the medial region of the distal radius, which is adjacent to the ulna[66]. Assessment of region-dependent cortical parameters is critical for evaluating therapeutic effect and for understanding osteoporosis and its related fracture. Histomorphometric changes of the cortical bone display significant deficits in cortical structure at the distal radius with age as an important base for osteoporotic fracture mechanism[54,67]. Collectively, these findings suggest that cortical porosity is a crucial element of bone strength that deteriorates with advancing age.
CONCLUSION
Osteoporosis is a skeletal disorder with a decreased bone mass and a deteriorated bone microstructure, resulting in reduced bone strength, elevated bone fragility and increased fracture risk. Bone microstructural properties can detect early alterations in bone fragility process and are an important predictor of bone strength. The changes of bone microstructure with osteoporosis in the axial and peripheral bone are complex. Cancellous and compact bone work effectively together to preserve biomechanical competence of the skeleton. Cancellous bone microstructure is crucial to preserve bone quality of the axial skeleton, while cortical bone is critically important for maintaining skeletal integrity, especially at the appendicular sites where the cortical bone is a major contributor to bone strength[64]. The bone strength of vertebra is preserved predominantly by cancellous bone. Trabecular bone mass of vertebra is much lower than that of the peripheral bone. Trabecular bone of vertebral body has a complex heterogeneous microstructure, with reduced BMD in the central and anterior superior regions. Elevated fragility of femoral neck in osteoporotic subjects is attributed to decreased cancellous bone volume and increased compact porosity. The main microstructural characteristic of cortical bone is cortical porosity, which is significantly higher at femoral neck in osteoporotic fracture patients than that of the controls[68]. Distal radius demonstrates obvious differences in cortical microstructure. The medial region of the distal radius has the highest Ct.Po compared with the lateral, anterior and posterior regions. Cortical porosity of the distal radius plays an important role in maintaining local bone quality that deteriorates with advancing age. There has been remarkable progress in our understanding of the pathophysiology of osteoporosis and its related fracture. However, greater effort is needed to elucidate precise mechanism of the bone fragility at the common sites of osteoporotic fractures.
Footnotes
P- Reviewer: Brufsky A, Barzilay JI S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc. 2008;56:935–941. doi: 10.1111/j.1532-5415.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 2.Pietschmann P, Rauner M, Sipos W, Kerschan-Schindl K. Osteoporosis: an age-related and gender-specific disease--a mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- 3.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 5.Warriner AH, Patkar NM, Curtis JR, Delzell E, Gary L, Kilgore M, Saag K. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64:46–53. doi: 10.1016/j.jclinepi.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 7.Genant HK, Engelke K, Prevrhal S. Advanced CT bone imaging in osteoporosis. Rheumatology (Oxford) 2008;47 Suppl 4:iv9–i16. doi: 10.1093/rheumatology/ken180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouxsein ML. Technology insight: noninvasive assessment of bone strength in osteoporosis. Nat Clin Pract Rheumatol. 2008;4:310–318. doi: 10.1038/ncprheum0798. [DOI] [PubMed] [Google Scholar]
- 9.Brandi ML. Microarchitecture, the key to bone quality. Rheumatology (Oxford) 2009;48 Suppl 4:iv3–iv8. doi: 10.1093/rheumatology/kep273. [DOI] [PubMed] [Google Scholar]
- 10.Gabet Y, Bab I. Microarchitectural changes in the aging skeleton. Curr Osteoporos Rep. 2011;9:177–183. doi: 10.1007/s11914-011-0072-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Zhou X, Fujita H, Onozuka M, Kubo KY. Age-related changes in trabecular and cortical bone microstructure. Int J Endocrinol. 2013;2013:213234. doi: 10.1155/2013/213234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burghardt AJ, Pialat JB, Kazakia GJ, Boutroy S, Engelke K, Patsch JM, Valentinitsch A, Liu D, Szabo E, Bogado CE, et al. Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2013;28:524–536. doi: 10.1002/jbmr.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell P, McHugh PE, O’Mahoney D. Vertebral osteoporosis and trabecular bone quality. Ann Biomed Eng. 2007;35:170–189. doi: 10.1007/s10439-006-9239-9. [DOI] [PubMed] [Google Scholar]
- 15.Ruyssen-Witrand A, Gossec L, Kolta S, Dougados M, Roux C. Vertebral dimensions as risk factor of vertebral fracture in osteoporotic patients: a systematic literature review. Osteoporos Int. 2007;18:1271–1278. doi: 10.1007/s00198-007-0356-6. [DOI] [PubMed] [Google Scholar]
- 16.Siemionow K, Lieberman IH. Vertebral augmentation in osteoporosis and bone metastasis. Curr Opin Support Palliat Care. 2007;1:323–327. doi: 10.1097/SPC.0b013e3282f33714. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Hayashi T, Zhou X, Fujita H, Onozuka M, Kubo KY. Sophisticated imaging technology in the assessment of osteoporosis risk. In: Dionyssiotis Y. Osteoporosis. InTech, Rijeka, Croatia. 2012:181–194. [Google Scholar]
- 18.Wasnich RD. Vertebral fracture epidemiology. Bone. 1996;18:179S–183S. doi: 10.1016/8756-3282(95)00499-8. [DOI] [PubMed] [Google Scholar]
- 19.Bouxsein ML, Melton LJ, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, et al. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT. J Bone Miner Res. 2006;21:1475–1482. doi: 10.1359/jbmr.060606. [DOI] [PubMed] [Google Scholar]
- 20.Singer K, Edmondston S, Day R, Breidahl P, Price R. Prediction of thoracic and lumbar vertebral body compressive strength: correlations with bone mineral density and vertebral region. Bone. 1995;17:167–174. doi: 10.1016/s8756-3282(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 21.Yoganandan N, Pintar FA, Stemper BD, Baisden JL, Aktay R, Shender BS, Paskoff G, Laud P. Trabecular bone density of male human cervical and lumbar vertebrae. Bone. 2006;39:336–344. doi: 10.1016/j.bone.2006.01.160. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Chen H, Miyamoto K, Zhou X, Hara T, Yokoyama R, Kanematsu M, Hoshi H, Fujita H. Analysis of bone mineral density distribution at trabecular bones in thoracic and lumbar vertebrae using X-ray CT images. J Bone Miner Metab. 2011;29:174–185. doi: 10.1007/s00774-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 23.Gong H, Zhang M, Yeung HY, Qin L. Regional variations in microstructural properties of vertebral trabeculae with aging. J Bone Miner Metab. 2005;23:174–180. doi: 10.1007/s00774-004-0557-4. [DOI] [PubMed] [Google Scholar]
- 24.Hulme PA, Boyd SK, Ferguson SJ. Regional variation in vertebral bone morphology and its contribution to vertebral fracture strength. Bone. 2007;41:946–957. doi: 10.1016/j.bone.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Shoumura S, Emura S, Bunai Y. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos Int. 2008;19:1473–1483. doi: 10.1007/s00198-008-0593-3. [DOI] [PubMed] [Google Scholar]
- 26.Eckstein F, Fischbeck M, Kuhn V, Link TM, Priemel M, Lochmüller EM. Determinants and heterogeneity of mechanical competence throughout the thoracolumbar spine of elderly women and men. Bone. 2004;35:364–374. doi: 10.1016/j.bone.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Grote HJ, Amling M, Vogel M, Hahn M, Pösl M, Delling G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone. 1995;16:301–308. doi: 10.1016/8756-3282(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen JS, Ebbesen EN, Mosekilde LI. Age-related differences between thinning of horizontal and vertical trabeculae in human lumbar bone as assessed by a new computerized method. Bone. 2002;31:136–142. doi: 10.1016/s8756-3282(02)00801-3. [DOI] [PubMed] [Google Scholar]
- 29.Jayasinghe JA, Jones SJ, Boyde A. Scanning electron microscopy of human lumbar vertebral trabecular bone surfaces. Virchows Arch A Pathol Anat Histopathol. 1993;422:25–34. doi: 10.1007/BF01605129. [DOI] [PubMed] [Google Scholar]
- 30.Mosekilde L. Vertebral structure and strength in vivo and in vitro. Calcif Tissue Int. 1993;53 Suppl 1:S121–S15; discussion S121-S15. doi: 10.1007/BF01673420. [DOI] [PubMed] [Google Scholar]
- 31.Hahn M, Vogel M, Amling M, Ritzel H, Delling G. Microcallus formations of the cancellous bone: a quantitative analysis of the human spine. J Bone Miner Res. 1995;10:1410–1416. doi: 10.1002/jbmr.5650100919. [DOI] [PubMed] [Google Scholar]
- 32.Cheng XG, Nicholson PH, Lowet G, Boonen S, Sun Y, Rüegsegger P, Müller R, Dequeker J. Prevalence of trabecular microcallus formation in the vertebral body and the femoral neck. Calcif Tissue Int. 1997;60:479–484. doi: 10.1007/s002239900266. [DOI] [PubMed] [Google Scholar]
- 33.Banse X, Devogelaer JP, Holmyard D, Grynpas M. Vertebral cancellous bone turn-over: microcallus and bridges in backscatter electron microscopy. Micron. 2005;36:710–714. doi: 10.1016/j.micron.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Bell GH, Dunbar O, Beck JS, Gibb A. Variations in strength of vertebrae with age and their relation to osteoporosis. Calcif Tissue Res. 1967;1:75–86. doi: 10.1007/BF02008077. [DOI] [PubMed] [Google Scholar]
- 35.Jensen KS, Mosekilde L, Mosekilde L. A model of vertebral trabecular bone architecture and its mechanical properties. Bone. 1990;11:417–423. doi: 10.1016/8756-3282(90)90137-n. [DOI] [PubMed] [Google Scholar]
- 36.Homminga J, Van-Rietbergen B, Lochmüller EM, Weinans H, Eckstein F, Huiskes R. The osteoporotic vertebral structure is well adapted to the loads of daily life, but not to infrequent “error” loads. Bone. 2004;34:510–516. doi: 10.1016/j.bone.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Fields AJ, Lee GL, Liu XS, Jekir MG, Guo XE, Keaveny TM. Influence of vertical trabeculae on the compressive strength of the human vertebra. J Bone Miner Res. 2011;26:263–269. doi: 10.1002/jbmr.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fields AJ, Nawathe S, Eswaran SK, Jekir MG, Adams MF, Papadopoulos P, Keaveny TM. Vertebral fragility and structural redundancy. J Bone Miner Res. 2012;27:2152–2158. doi: 10.1002/jbmr.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomsen JS, Niklassen AS, Ebbesen EN, Brüel A. Age-related changes of vertical and horizontal lumbar vertebral trabecular 3D bone microstructure is different in women and men. Bone. 2013;57:47–55. doi: 10.1016/j.bone.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 40.Ritzel H, Amling M, Pösl M, Hahn M, Delling G. The thickness of human vertebral cortical bone and its changes in aging and osteoporosis: a histomorphometric analysis of the complete spinal column from thirty-seven autopsy specimens. J Bone Miner Res. 1997;12:89–95. doi: 10.1359/jbmr.1997.12.1.89. [DOI] [PubMed] [Google Scholar]
- 41.Roux JP, Wegrzyn J, Arlot ME, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML. Contribution of trabecular and cortical components to biomechanical behavior of human vertebrae: an ex vivo study. J Bone Miner Res. 2010;25:356–361. doi: 10.1359/jbmr.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eswaran SK, Gupta A, Adams MF, Keaveny TM. Cortical and trabecular load sharing in the human vertebral body. J Bone Miner Res. 2006;21:307–314. doi: 10.1359/jbmr.2006.21.2.307. [DOI] [PubMed] [Google Scholar]
- 43.Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML. Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res. 2011;26:974–983. doi: 10.1002/jbmr.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotz JC, Cheal EJ, Hayes WC. Stress distributions within the proximal femur during gait and falls: implications for osteoporotic fracture. Osteoporos Int. 1995;5:252–261. doi: 10.1007/BF01774015. [DOI] [PubMed] [Google Scholar]
- 45.Dubey A, Koval KJ, Zuckerman JD. Hip fracture epidemiology: a review. Am J Orthop (Belle Mead NJ) 1999;28:497–506. [PubMed] [Google Scholar]
- 46.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 47.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 48.Ciarelli TE, Fyhrie DP, Schaffler MB, Goldstein SA. Variations in three-dimensional cancellous bone architecture of the proximal femur in female hip fractures and in controls. J Bone Miner Res. 2000;15:32–40. doi: 10.1359/jbmr.2000.15.1.32. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Zhou X, Shoumura S, Emura S, Bunai Y. Age- and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos Int. 2010;21:627–636. doi: 10.1007/s00198-009-0993-z. [DOI] [PubMed] [Google Scholar]
- 50.Cui WQ, Won YY, Baek MH, Lee DH, Chung YS, Hur JH, Ma YZ. Age-and region-dependent changes in three-dimensional microstructural properties of proximal femoral trabeculae. Osteoporos Int. 2008;19:1579–1587. doi: 10.1007/s00198-008-0601-7. [DOI] [PubMed] [Google Scholar]
- 51.Eckstein F, Matsuura M, Kuhn V, Priemel M, Müller R, Link TM, Lochmüller EM. Sex differences of human trabecular bone microstructure in aging are site-dependent. J Bone Miner Res. 2007;22:817–824. doi: 10.1359/jbmr.070301. [DOI] [PubMed] [Google Scholar]
- 52.Lochmüller EM, Matsuura M, Bauer J, Hitzl W, Link TM, Müller R, Eckstein F. Site-specific deterioration of trabecular bone architecture in men and women with advancing age. J Bone Miner Res. 2008;23:1964–1973. doi: 10.1359/jbmr.080709. [DOI] [PubMed] [Google Scholar]
- 53.Stauber M, Müller R. Age-related changes in trabecular bone microstructures: global and local morphometry. Osteoporos Int. 2006;17:616–626. doi: 10.1007/s00198-005-0025-6. [DOI] [PubMed] [Google Scholar]
- 54.Poole KE, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N, Reeve J. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res. 2010;25:482–491. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 55.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 56.Bousson V, Peyrin F, Bergot C, Hausard M, Sautet A, Laredo JD. Cortical bone in the human femoral neck: three-dimensional appearance and porosity using synchrotron radiation. J Bone Miner Res. 2004;19:794–801. doi: 10.1359/JBMR.040124. [DOI] [PubMed] [Google Scholar]
- 57.Chappard C, Bensalah S, Olivier C, Gouttenoire PJ, Marchadier A, Benhamou C, Peyrin F. 3D characterization of pores in the cortical bone of human femur in the elderly at different locations as determined by synchrotron micro-computed tomography images. Osteoporos Int. 2013;24:1023–1033. doi: 10.1007/s00198-012-2044-4. [DOI] [PubMed] [Google Scholar]
- 58.Jordan GR, Loveridge N, Bell KL, Power J, Rushton N, Reeve J. Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone. 2000;26:305–313. doi: 10.1016/s8756-3282(99)00272-0. [DOI] [PubMed] [Google Scholar]
- 59.Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28:113–125. doi: 10.1016/j.hcl.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diamantopoulos AP, Rohde G, Johnsrud I, Skoie IM, Hochberg M, Haugeberg G. The epidemiology of low- and high-energy distal radius fracture in middle-aged and elderly men and women in Southern Norway. PLoS One. 2012;7:e43367. doi: 10.1371/journal.pone.0043367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21:124–131. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res. 2012;27:637–644. doi: 10.1002/jbmr.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011;26:50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- 64.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–993. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 66.Kazakia GJ, Nirody JA, Bernstein G, Sode M, Burghardt AJ, Majumdar S. Age- and gender-related differences in cortical geometry and microstructure: Improved sensitivity by regional analysis. Bone. 2013;52:623–631. doi: 10.1016/j.bone.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kazakia GJ, Burghardt AJ, Link TM, Majumdar S. Variations in morphological and biomechanical indices at the distal radius in subjects with identical BMD. J Biomech. 2011;44:257–266. doi: 10.1016/j.jbiomech.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell KL, Loveridge N, Power J, Garrahan N, Meggitt BF, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24:57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]


