Abstract
Our understanding of psoriatic arthritis has evolved as new knowledge of the disease has emerged. However, the exact prevalence of psoriatic arthritis is unknown, and its pathogenesis has not been fully elucidated. Genetic, environmental, and immunologic factors have all been implicated in disease development. Early diagnosis and treatment have become primary objectives in clinical rheumatology. Psoriatic arthritis not only causes functional impairment, but also increases mortality risk of patients. The advent of new therapeutic agents capable of arresting the progression of joint damage is expected. However, early psoriatic arthritis assessment remains limited. The objectives of this article are to outline the epidemiology, diagnosis, and treatment of psoriatic arthritis and to suggest a paradigm for identifying early psoriatic arthritis patients.
Keywords: Arthritis, Psoriasis, Psoriatic arthritis, Spondyloarthritis
Core tip: Psoriatic arthritis, usually seronegative for rheumatoid factor, involves the inflammation of synovial tissue, entheses, skin. Clinical manifestation of psoriatic arthritis varies and is under-diagnosed in psoriasis patients. This article presented the epidemiology, diagnosis, and treatment of psoriatic arthritis and to suggest a paradigm for use in standard clinical practice.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic disease which involves the inflammation of synovial tissue, entheses, skin and usually seronegative for rheumatoid factor[1]. Spondyloarthritis complex includes ankylosing spondylitis, reactive arthritis, arthritis associated with inflammatory bowel disease, undifferentiated spondyloarthritis, and PsA[2,3]. PsA is belonged as one part of the spondyloarthritis complex. PsA patients have heterogeneous clinical presentations, with diverse articular and dermatological features and varied disease courses and outcomes. PsA was initially considered to be a mild disease, but in the past decade, 40%-60% of patients have developed erosive and deforming joint complications[4]. PsA-induced joint damaging complications not only lead to lower articular function and higher mortality but also affect patients’ ability to work and affect their social relationships[4]. The remission of PsA symptoms has been attributed to early diagnosis and treatment in recent studies[5,6]. However, PsA is underdiagnosed in psoriasis patients, which may be due to under-recognition of PsA symptoms and a lack of effective screening tools. The aims of this article were to present the epidemiology, diagnosis, and treatment of PsA and to suggest a paradigm for use in standard clinical practice.
DATA COLLECTION
We collected all of the articles published from January 2005 through October 2013 that described patients who were affected by PsA. By searching MEDLINE (National Library of Medicine, Bethesda, Maryland, United States), we used the key words “psoriatic arthritis” and “epidemiology of psoriatic arthritis” or “diagnosis of psoriatic arthritis” or “management of psoriatic arthritis” to obtain these articles. Articles that were not published in English, manuscripts without an abstract (which were assumed to not be original), and opinion articles were excluded from the review. The relevant information was extracted from the selecting articles and classified based on the following: PsA epidemiology, PsA diagnosis, PsA management, the setting of study, and the methodology of study.
The article searches were conducted from August 2013 to September 2013. Using the search terms previously described, a total of 853 papers were collected. All selected articles were reviewed by the authors and 109 articles were considered to be relevant. The study settings mostly located in European countries, the United States, Australia and Japan. The region that produced the most original information was Europe, which accounted for 35% of the articles. After analyzing the abstracts, we found that 85% of the studies were case reports, and 10% were retrospective. Additionally, 5% referenced other designs.
EPIDEMIOLOGY
It is difficult to determine the epidemiology of PsA due to the absence of universally accepted criteria for its diagnosis. The first classification criteria for PsA were proposed by Moll et al[7]. However, the pattern of disease may change over time and, therefore, is not useful for classification. The classification for psoriatic arthritis (CASPAR) criteria were developed in 2006 (Table 1)[7]. The CASPAR criteria are easier to use in epidemiologic studies. The specificity and sensitivity of these criteria are 98.7% and 91.4%, respectively[7].
Table 1.
The classification for psoriatic arthritis criteria[7] for diagnosing psoriatic arthritis-related inflammatory musculoskeletal disease (joint, spine or entheseal)
| Evidence of psoriasis (any of three) |
| Current1: Psoriatic skin or scalp disease present, as judged by a dermatologist or rheumatologist (score of 2) |
| Personal history: May be obtained from the patient, family doctor, dermatologist, or rheumatologist (score of 1) |
| Family history: In a first- or second-degree relative, according to patient report (score of 1) |
| Psoriatic nail dystrophy |
| Typical psoriatic Nail dystrophy, including onycholysis, pitting, and hyperkeratosis, observed on current physical examination (score of 1) |
| Negative rheumatoid factor |
| By any method except latex, but preferably by enzyme-linked immunosorbent assay or nephelometry, according to the local laboratory reference range (score of 1) |
| Dactylitis |
| Current: swelling of an entire digit (score of 1) |
| Personal history: recorded by a rheumatologist (score of 1) |
| Radiological evidence of juxta-articular new bone formation |
| Ill-defined ossification near the joint margins (but excluding osteophyte formation) on plain X-rays of the hand or foot (score of 1) |
Current: Psoriasis score of 2; others: 1. A PsA patient must have inflammatory articular disease with > 3 points from the following 5 categories.
PsA usually occurs in the age of 40 to 50 years old, and the disease may occur in young children and elderly patients as well[8]. Psoriasis vulgaris is the most common type of psoriasis with PsA[9]. A few proportion (4%-5%) of PSA cases are related to guttate and pustular psoriasis[10]. One to two percent of cases involve single nail without skin involvement[11]. Male-to-female ratio is from 0.7:1 to 2.1:1[11]. Approximately 10%-37% of patients have skin and joint disease simultaneously, and 6%-18% of patients have arthritis preceding psoriasis[12,13]. Environmental factors, including infection (such as streptococcus, human immunodeficiency virus), drug use, and joint trauma (mainly in children), are known to contribute to PsA[14,15]. Emotional stress plays an important role as a trigger for both skin and joint psoriasis[15]. However, the neuroimmunoendocrine mechanisms involved in this phenomenon have not been elucidated. One population-based study suggested that pregnancy and steroid use might trigger PsA in patients with psoriasis[16].
Table 2 shows the incidence and prevalence of PsA worldwide. There is substantial variability in the incidence and prevalence of PsA by country. The incidence of PsA varies from 0.1/100000 in Japan to 23.1/100000 in Finland[17-22]. The prevalence of PsA in Europe and America varies from 0.02%-0.42%[22-30]. The prevalence in Japan is approximately at 0.001%[31]. In China, the disease prevalence is 0.02%[32]. Indians were found to have the highest prevalence of PsA among the multiethnic population in Singapore[33].
Table 2.
Comparison of the incidence and prevalence of psoriatic arthritis among several countries
| Country | Incidence (1/100000) | Ref. | Prevalence | Ref. |
| Asia | ||||
| China | NA | 0.02% | [33] | |
| Japan | 0.1 | [17] | 0.001% | [32] |
| Europe | NA | NA | ||
| Greece | 3 | [20] | 0.17% | [29] |
| France | NA | 0.19% | [23] | |
| Italy | NA | 0.42% | [25] | |
| Germany | NA | 0.29% | [24] | |
| Finland | 23.1 | [18] | NA | |
| Sweden | 8 | [19] | 0.02%-0.25% | [30] |
| Iceland | NA | 0.14% | [28] | |
| Norway | NA | 0.2% | [27] | |
| Russia | NA | 0.3% | [31] | |
| Americas | ||||
| United States | 7.2 | [22] | 0.16% | [22] |
| Argentina | 6.3 | [92] | 0.07% | [21] |
| Mexico | NA | 0.02% | [26] | |
NA: Not available.
Collectively, compared to Americas and Europe, Asia has lower incidence and prevalence of PsA. The reasons for the difference of PsA morbidity in different areas are unclear. However, different case definitions and clinical settings in the studies may be one of the reasons.
DIAGNOSIS
Clinical manifestations
The clinical spectrum of PsA is diverse in nature; psoriatic patients might have axial skeleton disorders, nail changes, peripheral joint inflammation, entheses, tenosynovitis, or dactylitis. Each of these conditions can be found in isolation or in combination with others. The major clinical features of the disease are spondylitis (18%-46%), inflammatory neck pain (23%-39%), thoracic inflammatory pain (13%-21%), and axial symptoms (25%-50%)[8,34]. Most of patients with axial involvement can be no clinical symptoms and maintain their spinal mobility with no reduction in spinal flexion or chest expansion for more than 10 years [34,35].
Sacroiliitis is a common symptom among PsA patients[8,11,12,34,36]. Usually, it occur unilaterally and then become bilaterally in the following years. A study conducted in an Italian patient population using bone scans to detect active sacroiliitis found that the prevalence of sacroiliitis was 32%[37]. A multicenter study from the United States found that the prevalence of sacroiliitis was 78%[38]. It was found that one-third of PsA patients developed sacroiliitis after 5 years of illness and that half of patients developed sacroiliitis by 10 years[35]. Longer period of disease may be the cause of higher prevalence of sacroiliitis. Males have a three-fold greater risk of developing sacroiliitis than females have[11]. The onset time of PsA at younger age has higher risk to hip joint disease, however, there is no significant association between occurrence of enthesitis, dactylitis, and peripheral arthritis with the occurrence of hip joint disease[36].
PsA can simulate rheumatoid arthritis to involve the knee or a large joint with some small joints in fingers or toes (Figure 1)[39]. Polyarthritis is generally symmetrical and has dactylitis and enthesitis[37,39]. Oligoarthritis can be associated with dactylitis[37]. It is also found a shortening of the fingers with pencil-in-cup deformity[40].
Figure 1.
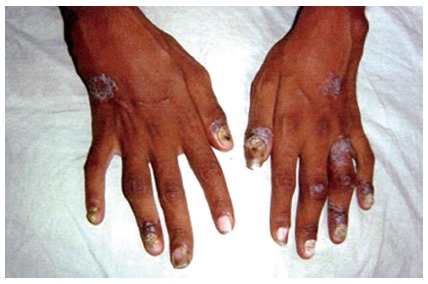
Peripheral hand joint involvement along with psoriatic skin lesion and nail changes. Reproduced with permission from Dhir et al[39].
Dactylitis was present in 32%-48% of patients with PsA in various studies[37,40-45]. Seventy-five percent of patients have toes with dactylitis and 50% of patients have multiple digits involved simultaneously[43]. The morbidity of dactylitis increases as the duration of disease prolongs[40-45].
Twenty-five to fifty three percent of PsA patients present enthesitis[36,44]. One study in Canada demonstrated that only 15% of patients had enthesitis at the beginning of treatment, but the incidence increased to 36% as the disease progressed[44]. The Achilles tendon, plantar fascia, and greater trochanter are the most common sites affected[44,45].
From 4% to 18% of patients with PsA are found to have acute anterior uveitis[8,46,47]. Uveitis is more common in PsA patients with the spondylitis, with or without peripheral joint involvement[9]. However, uveitis is uncommonly clinical presentation in Spain and Israel. The prevalence of uveitis among PsA patients in these areas is only 1%-3%[11,41].
Imaging findings
Radiography, ultrasonography, magnetic resonance imaging (MRI), computed tomography (CT), and bone scintigraphy[48] are imaging techniques for diagnosis of PsA. In recent years, MRI and ultrasonography are increasingly used for assessment of PsA, providing additional information of the pathogenesis of the disease.
The most characteristic radiological finding indicative of PsA is bone destruction and proliferation[49]. Figure 2 shows the characteristics of radiological findings of peripheral PsA: an asymmetrical distribution, distal interphalangeal joints involvement, periostitis, bone density preservation, bone ankylosis, and pencil-in-cup deformity[39]. Axial involvement includes paravertebral ossification, syndesmophytes, interspinous or anterior ligament calcification, apophysis, sclerosis, and asymmetrical sacroiliitis[49]. Cervical intervertebral discs may be narrowed, and ankylosis may be present with atlantoaxial fusion or subluxation[50,51]. Bone erosion and condyle osteolysis might be found in the temporomandibular joint[52].
Figure 2.
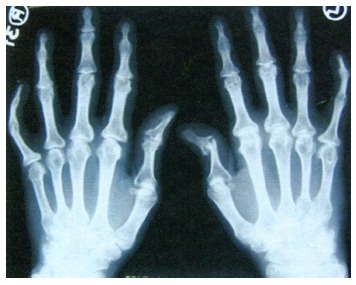
Ankylosis of distal interphalangeal joint on both hands, pencil in cup deformity in the first left interphalangeal joint on radiography. Reproduced with permission from Dhir et al[39].
Ultrasonography is a reliable method for investigating subclinical enthesopathy in the Achilles tendon and for confirming a diagnosis in symptomatic patients[53]. This method can be used to identify acute or degenerative tendinitis, rupture, peritendinitis, and retrocalcaneal or pre-Achilles bursitis[53]. Ultrasonography has been considered an important tool in the evaluation of PsA. Power Doppler ultrasonography is not only a useful tool to assess musculoskeletal and cutaneous involvement, but also a functional tool to monitor the efficacy of therapy and to guide steroid injections at the level of inflamed joints, tendon sheaths, and entheses[54].
MRI examination has improved our understanding of PsA by establishing that synovial inflammation is usually secondary to extrasynovial involvement, which helps to differentiate PsA from rheumatoid arthritis[55]. However, this diagnosis cannot always be precisely determined. The use of gadolinium contrast increases the odds of differentiation by calculating the relative enhancement and rate of early enhancement[55]. MRI has improved the quality of diagnosis and objective observation of the disease spectrum in PsA[55,56]. In addition, direct visualization of inflammation in the peripheral and axial joints and peripheral and axial entheses is the advantage of MRI. It may show the images among enthesitis, synovitis, and osteitis in PsA and support an spondyloarthritis (SpA) pattern of inflammation of enthuses, in which is the primary target of inflammation[56].
CT is another useful tool for diagnosis of PsA. CT plays a limited role in the diagnosis of peripheral joints, however, it may be useful in assessing spine disease[57]. The sensitivity of CT in the detection of erosions of sacroiliac joint is similar to that of MRI, but MRI is more effective in monitoring synovial inflammation. The specificity of bone scintigraphy for diagnosis of PsA has improved when supplanted with ultrasonography and MRI techniques[57].
Taken together, conventional radiography, ultrasonography, and MRI have similar diagnostic efficacy in the assessment of joint space width[48]. Radiology is less sensitive than ultrasonography and MRI in the assessment of other features of joint inflammation[48]. Radiography allows the detailed analysis of morphostructural and blood flow changes in multiple psoriasis-affected sites (skin, joints, tendons, entheses, and nails)[58]. Ultrasonography with power Doppler has shown that psoriasis patients without PsA more commonly exhibit synovitis and enthesopathy than do patients with other skin diseases. Additionally, ultrasonography has shown a significant prevalence of musculoskeletal asymptomatic involvement (3.2% synovitis and 11.6% enthesopathy)[59]. MRI is more sensitive in detecting small erosions and enthesitis[48].
TREATMENT
The basic goals of PsA treatment are helping patient to alleviate from the suffering of the disease, to preserve the joint structure, to improve patients’ physical activities, and to reduce the risk of mortality. As a rule, all PsA patients must be informed of the characteristics of the disease and given psychological counseling and physiotherapy.
Corticosteroids
Mild forms of the disease may respond to nonsteroidal inflammatory agents, which are occasionally given in combination with intra-articular glucocorticoid injections[60]. Intra-articular corticosteroids may represent a therapeutic option in cases of mono- or oligoarticular joint involvement in PsA. The systemic use of corticosteroids is not recommended due to a lack of evidence regarding its efficacy and due to the risk of severe adverse events and relapse of skin psoriasis upon discontinuation[60].
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed as an initial therapy for both peripheral and axial disease[60]. For example, according to the measurement by the American College of Rheumatology Responders Index 20 (ACR20), the treatment of PsA patients with celecoxib at a dose of 200 or 400 mg over two weeks increased their rates of clinical response by 21% and 11%, respectively[61]. However, there was no difference in response between patients treated with celecoxib and untreated patients after 12 wk[61]. Treatment with NSAIDs represents an option for the short-term symptomatic treatment of PsA[60,62-64].
Conventional disease-modifying antirheumatic drugs
Disease-modifying antirheumatic drugs (DMARDs) include methotrexate, oral and parenteral gold, cyclosporine, leflunomide, azathioprine and 6-mercaptopurine, antimalarial agents, D-penicillamine, colchicines, retinoids, photochemotherapy, somatostatin, and sulfasalazine[60]. Moderate to severe forms of the disease are initially treated with the same therapy as in the mild form of the disease, but with the addition of DMARDs[61]. The efficacy of methotrexate in the treatment of PsA is controversial; although this drug is occasionally used in combination with NSAIDs, its use should be carefully monitored due to the possibility of hepatotoxicity[60,63,64]. Cyclosporine is an efficacious option for the treatment of PsA, and its results may be potentiated by combination with adalimumab. Leflunomide may be used in the treatment of PsA but should be carefully monitored due to its hepatotoxicity. Sulfasalazine can be used in PsA to afford pain relief[63,64].
Anti-tumor necrosis factor agents
Table 3 summarizes the current biological therapies for the treatment of moderate to severe psoriasis and PsA[63-65]. Adult patients who have had moderate to severe active PsA (at least three swollen and painful joints) for more than six months and those with psoriatic skin lesions or a history of psoriasis and an intolerance to NSAIDs or DMARDs over three months, whether combined or not combined with methotrexate, are the indications for the use of anti-tumor necrosis factor (TNF) agents (e.g., infliximab, etanercept, adalimumab, and golimumab)[63,64]. Although it is difficult to quantify the occurrence of adverse effects, there are no statistically significant differences in the safety profiles among the various anti-TNF drugs using for treatment of PsA[63,64].
Table 3.
| Drug | Treatment |
| Anti-TNF | |
| Adalimumab | PsA: 40 mg sc every other week. Psoriasis: 80 mg sc at week 0, 40 mg sc every other week thereafter |
| Etanercept | PsA: 25 mg sc twice per week. Psoriasis: 50 mg sc twice weekly for 3 mo, 50 mg/wk thereafter |
| Golimumab | PsA: 50 mg sc every month |
| Infliximab | PsA and psoriasis: 5 mg/kg at week 0, 2, and 6, every 8 wk thereafter |
| Anti-IL-17 | |
| Brodalumab | In clinical trials |
| Ixekizumab | In clinical trials |
| Secukinumab | In clinical trials |
| Anti-IL-12/IL-23 | |
| Briakinumab | In clinical trials |
| Ustekinumab | Psoriasis: 45 mg (weight < 100 kg) or 90 mg (weight > 100 kg) sc at wk 0 and 4, followed by 45 mg or 90 mg every 12 wk |
| Anti-T cell activation | |
| Alefacept | Psoriasis: 15 mg IM weekly for 12 wk |
PsA: Psoriatic arthritis; SC: Subcutaneous injection; IM: Intramuscular injection; TNF: Tumor necrosis factor; IL: Interleukin.
CONCLUSION
The incidence and prevalence of PsA vary worldwide. The incidence and prevalence of PsA in Asia are lower than in North American and European countries. Early diagnosis and treatment for PsA improve patient’s outcomes. PsA is underdiagnosed among psoriasis patients. Physicians should be alert the possibility of PsA when a patient with preexisting psoriasis has arthritis. If needed, counsel a rheumatologist for help. The treatment of PsA should be considered all aspects of the disease, including clinical manifestations, mental problems, and maintenance of articular function.
Footnotes
P- Reviewer: Chen GS, La Montagna G S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
References
- 1.Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64 Suppl 2:ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill T, Silman AJ. Psoriatic arthritis. Historical background and epidemiology. Baillieres Clin Rheumatol. 1994;8:245–261. doi: 10.1016/s0950-3579(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 4.Slobodin G, Rosner I, Rozenbaum M, Boulman N, Kessel A, Toubi E. Psoriatic arthropathy: where now? Isr Med Assoc J. 2009;11:430–434. [PubMed] [Google Scholar]
- 5.Helliwell PS, Mease PJ, FitzGerald O, Taylor WJ, van der Heijde D. Peripheral spondyloarthritis and psoriatic arthritis; overlaps and distinctions: a report from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1446–1449. doi: 10.3899/jrheum.130460. [DOI] [PubMed] [Google Scholar]
- 6.Palmer D, El Miedany Y. Early psoriatic arthritis: facing the challenge. Br J Nurs. 2013;22:1014–1020. doi: 10.12968/bjon.2013.22.17.1014. [DOI] [PubMed] [Google Scholar]
- 7.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 8.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)--an analysis of 220 patients. Q J Med. 1987;62:127–141. [PubMed] [Google Scholar]
- 9.Jones SM, Armas JB, Cohen MG, Lovell CR, Evison G, McHugh NJ. Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br J Rheumatol. 1994;33:834–839. doi: 10.1093/rheumatology/33.9.834. [DOI] [PubMed] [Google Scholar]
- 10.Rajendran CP, Ledge SG, Rani KP, Madhavan R. Psoriatic arthritis. J Assoc Physicians India. 2003;51:1065–1068. [PubMed] [Google Scholar]
- 11.Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol. 1991;30:245–250. doi: 10.1093/rheumatology/30.4.245. [DOI] [PubMed] [Google Scholar]
- 12.Madland TM, Apalset EM, Johannessen AE, Rossebö B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in Western Norway. J Rheumatol. 2005;32:1918–1922. [PubMed] [Google Scholar]
- 13.Nossent JC, Gran JT. Epidemiological and clinical characteristics of psoriatic arthritis in northern Norway. Scand J Rheumatol. 2009;38:251–255. doi: 10.1080/03009740802609558. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Farewell VT, Kopciuk KA, Cook RJ. HLA markers and progression in psoriatic arthritis. J Rheumatol. 1998;25:730–733. [PubMed] [Google Scholar]
- 15.Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol. 2005;52:1–19. doi: 10.1016/j.jaad.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Thumboo J, Uramoto K, Shbeeb MI, O’Fallon WM, Crowson CS, Gibson LE, Michet CJ, Gabriel SE. Risk factors for the development of psoriatic arthritis: a population based nested case control study. J Rheumatol. 2002;29:757–762. [PubMed] [Google Scholar]
- 17.Uppal SS, Abraham M, Chowdhury RI, Kumari R, Pathan EM, Al Rashed A. Ankylosing spondylitis and undifferentiated spondyloarthritis in Kuwait: a comparison between Arabs and South Asians. Clin Rheumatol. 2006;25:219–224. doi: 10.1007/s10067-005-1162-1. [DOI] [PubMed] [Google Scholar]
- 18.Savolainen E, Kaipiainen-Seppänen O, Kröger L, Luosujärvi R. Total incidence and distribution of inflammatory joint diseases in a defined population: results from the Kuopio 2000 arthritis survey. J Rheumatol. 2003;30:2460–2468. [PubMed] [Google Scholar]
- 19.Söderlin MK, Börjesson O, Kautiainen H, Skogh T, Leirisalo-Repo M. Annual incidence of inflammatory joint diseases in a population based study in southern Sweden. Ann Rheum Dis. 2002;61:911–915. doi: 10.1136/ard.61.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alamanos Y, Papadopoulos NG, Voulgari PV, Siozos C, Psychos DN, Tympanidou M, Drosos AA. Epidemiology of psoriatic arthritis in northwest Greece, 1982-2001. J Rheumatol. 2003;30:2641–2644. [PubMed] [Google Scholar]
- 21.Soriano ER, Rosa J, Velozo E, Schpilberg M, Imamura PM, Diaz J, Catoggio LJ. Incidence and prevalence of psoriatic arthritis in Buenos Aires, Argentina: a 6-year health management organization-based study. Rheumatology (Oxford) 2011;50:729–734. doi: 10.1093/rheumatology/keq369. [DOI] [PubMed] [Google Scholar]
- 22.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. 2009;36:361–367. doi: 10.3899/jrheum.080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saraux A, Guillemin F, Guggenbuhl P, Roux CH, Fardellone P, Le Bihan E, Cantagrel A, Chary-Valckenaere I, Euller-Ziegler L, Flipo RM, et al. Prevalence of spondyloarthropathies in France: 2001. Ann Rheum Dis. 2005;64:1431–1435. doi: 10.1136/ard.2004.029207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun J, Bollow M, Remlinger G, Eggens U, Rudwaleit M, Distler A, Sieper J. Prevalence of spondylarthropathies in HLAB27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.De Angelis R, Salaffi F, Grassi W. Prevalence of spondyloarthropathies in an Italian population sample: a regional community-based study. Scand J Rheumatol. 2007;36:14–21. doi: 10.1080/03009740600904243. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Nemegyei J, Peláez-Ballestas I, Sanin LH, Cardiel MH, Ramirez-Angulo A, Goycochea-Robles MV. Prevalence of musculoskeletal pain and rheumatic diseases in the southeastern region of Mexico. A COPCORD-based community survey. J Rheumatol Suppl. 2011;86:21–25. doi: 10.3899/jrheum.100954. [DOI] [PubMed] [Google Scholar]
- 27.Love TJ, Gudbjornsson B, Gudjonsson JE, Valdimarsson H. Psoriatic arthritis in Reykjavik, Iceland: prevalence, demographics, and disease course. J Rheumatol. 2007;34:2082–2088. [PubMed] [Google Scholar]
- 28.Trontzas P, Andrianakos A, Miyakis S, Pantelidou K, Vafiadou E, Garantziotou V, Voudouris C. Seronegative spondyloarthropathies in Greece: a population-based study of prevalence, clinical pattern, and management. The ESORDIG study. Clin Rheumatol. 2005;24:583–589. doi: 10.1007/s10067-005-1106-9. [DOI] [PubMed] [Google Scholar]
- 29.Haglund E, Bremander AB, Petersson IF, Strömbeck B, Bergman S, Jacobsson LT, Turkiewicz A, Geborek P, Englund M. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann Rheum Dis. 2011;70:943–948. doi: 10.1136/ard.2010.141598. [DOI] [PubMed] [Google Scholar]
- 30.Alexeeva L, Krylov M, Vturin V, Mylov N, Erdesz S, Benevolenskaya L. Prevalence of spondyloarthropathies and HLA-B27 in the native population of Chukotka, Russia. J Rheumatol. 1994;21:2298–2300. [PubMed] [Google Scholar]
- 31.Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, Makino H, Shibata T, Shingu M, Sakou T, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. 2001;28:554–559. [PubMed] [Google Scholar]
- 32.Liao ZT, Pan YF, Huang JL, Huang F, Chi WJ, Zhang KX, Lin ZM, Wu YQ, He WZ, Wu J, et al. An epidemiological survey of low back pain and axial spondyloarthritis in a Chinese Han population. Scand J Rheumatol. 2009;38:455–459. doi: 10.3109/03009740902978085. [DOI] [PubMed] [Google Scholar]
- 33.Thumboo J, Tham SN, Tay YK, Chee T, Mow B, Chia HP, Boey ML. Patterns of psoriatic arthritis in Orientals. J Rheumatol. 1997;24:1949–1953. [PubMed] [Google Scholar]
- 34.Veale D, Rogers S, Fitzgerald O. Classification of clinical subsets in psoriatic arthritis. Br J Rheumatol. 1994;33:133–138. doi: 10.1093/rheumatology/33.2.133. [DOI] [PubMed] [Google Scholar]
- 35.Chandran V, Barrett J, Schentag CT, Farewell VT, Gladman DD. Axial psoriatic arthritis: update on a longterm prospective study. J Rheumatol. 2009;36:2744–2750. doi: 10.3899/jrheum.090412. [DOI] [PubMed] [Google Scholar]
- 36.Michet CJ, Mason TG, Mazlumzadeh M. Hip joint disease in psoriatic arthritis: risk factors and natural history. Ann Rheum Dis. 2005;64:1068–1070. doi: 10.1136/ard.2004.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Punzi L, Pianon M, Rossini P, Schiavon F, Gambari PF. Clinical and laboratory manifestations of elderly onset psoriatic arthritis: a comparison with younger onset disease. Ann Rheum Dis. 1999;58:226–229. doi: 10.1136/ard.58.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battistone MJ, Manaster BJ, Reda DJ, Clegg DO. The prevalence of sacroilitis in psoriatic arthritis: new perspectives from a large, multicenter cohort. A Department of Veterans Affairs Cooperative Study. Skeletal Radiol. 1999;28:196–201. doi: 10.1007/s002560050500. [DOI] [PubMed] [Google Scholar]
- 39.Dhir V, Aggarwal A. Psoriatic arthritis: a critical review. Clin Rev Allergy Immunol. 2013;44:141–148. doi: 10.1007/s12016-012-8302-6. [DOI] [PubMed] [Google Scholar]
- 40.Helliwell PS, Porter G, Taylor WJ. Polyarticular psoriatic arthritis is more like oligoarticular psoriatic arthritis, than rheumatoid arthritis. Ann Rheum Dis. 2007;66:113–117. doi: 10.1136/ard.2006.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zisman D, Eder L, Elias M, Laor A, Bitterman H, Rozenbaum M, Feld J, Rimar D, Rosner I. Clinical and demographic characteristics of patients with psoriatic arthritis in northern Israel. Rheumatol Int. 2012;32:595–600. doi: 10.1007/s00296-010-1673-1. [DOI] [PubMed] [Google Scholar]
- 42.Williamson L, Dalbeth N, Dockerty JL, Gee BC, Weatherall R, Wordsworth BP. Extended report: nail disease in psoriatic arthritis--clinically important, potentially treatable and often overlooked. Rheumatology (Oxford) 2004;43:790–794. doi: 10.1093/rheumatology/keh198. [DOI] [PubMed] [Google Scholar]
- 43.Brockbank JE, Stein M, Schentag CT, Gladman DD. Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis. 2005;64:188–190. doi: 10.1136/ard.2003.018184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gladman DD, Chandran V. Observational cohort studies: lessons learnt from the University of Toronto Psoriatic Arthritis Program. Rheumatology (Oxford) 2011;50:25–31. doi: 10.1093/rheumatology/keq262. [DOI] [PubMed] [Google Scholar]
- 45.Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O. Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol. 1999;26:1746–1751. [PubMed] [Google Scholar]
- 46.Lambert JR, Wright V. Eye inflammation in psoriatic arthritis. Ann Rheum Dis. 1976;35:354–356. doi: 10.1136/ard.35.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Queiro R, Belzunegui J, González C, De DJ, Sarasqueta C, Torre JC, Figueroa M. Clinically asymptomatic axial disease in psoriatic spondyloarthropathy. A retrospective study. Clin Rheumatol. 2002;21:10–13. doi: 10.1007/s100670200003. [DOI] [PubMed] [Google Scholar]
- 48.Sankowski AJ, Lebkowska UM, Cwikła J, Walecka I, Walecki J. The comparison of efficacy of different imaging techniques (conventional radiography, ultrasonography, magnetic resonance) in assessment of wrist joints and metacarpophalangeal joints in patients with psoriatic arthritis. Pol J Radiol. 2013;78:18–29. doi: 10.12659/PJR.883764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Möller B, Bonel H, Rotzetter M, Villiger PM, Ziswiler HR. Measuring finger joint cartilage by ultrasound as a promising alternative to conventional radiograph imaging. Arthritis Rheum. 2009;61:435–441. doi: 10.1002/art.24424. [DOI] [PubMed] [Google Scholar]
- 50.Backhaus M, Kamradt T, Sandrock D, Loreck D, Fritz J, Wolf KJ, Raber H, Hamm B, Burmester GR, Bollow M. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 1999;42:1232–1245. doi: 10.1002/1529-0131(199906)42:6<1232::AID-ANR21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Porter GG. Psoriatic arthritis. Plain radiology and other imaging techniques. Baillieres Clin Rheumatol. 1994;8:465–482. doi: 10.1016/s0950-3579(94)80029-4. [DOI] [PubMed] [Google Scholar]
- 52.Könönen M. Radiographic changes in the condyle of the temporomandibular joint in psoriatic arthritis. Acta Radiol. 1987;28:185–188. [PubMed] [Google Scholar]
- 53.De Simone C, Guerriero C, Giampetruzzi AR, Costantini M, Di Gregorio F, Amerio P. Achilles tendinitis in psoriasis: clinical and sonographic findings. J Am Acad Dermatol. 2003;49:217–222. doi: 10.1067/s0190-9622(03)00904-6. [DOI] [PubMed] [Google Scholar]
- 54.Spannow AH, Pfeiffer-Jensen M, Andersen NT, Herlin T, Stenbøg E. Ultrasonographic measurements of joint cartilage thickness in healthy children: age- and sex-related standard reference values. J Rheumatol. 2010;37:2595–2601. doi: 10.3899/jrheum.100101. [DOI] [PubMed] [Google Scholar]
- 55.Schwenzer NF, Kötter I, Henes JC, Schraml C, Fritz J, Claussen CD, Horger M. The role of dynamic contrast-enhanced MRI in the differential diagnosis of psoriatic and rheumatoid arthritis. AJR Am J Roentgenol. 2010;194:715–720. doi: 10.2214/AJR.09.2671. [DOI] [PubMed] [Google Scholar]
- 56.Schoellnast H, Deutschmann HA, Hermann J, Schaffler GJ, Reittner P, Kammerhuber F, Szolar DH, Preidler KW. Psoriatic arthritis and rheumatoid arthritis: findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187:351–357. doi: 10.2214/AJR.04.1798. [DOI] [PubMed] [Google Scholar]
- 57.Coates LC, Hodgson R, Conaghan PG, Freeston JE. MRI and ultrasonography for diagnosis and monitoring of psoriatic arthritis. Best Pract Res Clin Rheumatol. 2012;26:805–822. doi: 10.1016/j.berh.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez M, Filippucci E, De Angelis R, Filosa G, Kane D, Grassi W. A sonographic spectrum of psoriatic arthritis: “the five targets”. Clin Rheumatol. 2010;29:133–142. doi: 10.1007/s10067-009-1292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naredo E, Möller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E, Mayordomo L, Moragues C, Uson J, de Agustín JJ, et al. High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 2011;50:1838–1848. doi: 10.1093/rheumatology/ker078. [DOI] [PubMed] [Google Scholar]
- 60.Nash P, Clegg DO. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann Rheum Dis. 2005;64 Suppl 2:ii74–ii77. doi: 10.1136/ard.2004.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kivitz AJ, Espinoza LR, Sherrer YR, Liu-Dumaw M, West CR. A comparison of the efficacy and safety of celecoxib 200 mg and celecoxib 400 mg once daily in treating the signs and symptoms of psoriatic arthritis. Semin Arthritis Rheum. 2007;37:164–173. doi: 10.1016/j.semarthrit.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Coates LC, Tillett W, Chandler D, Helliwell PS, Korendowych E, Kyle S, McInnes IB, Oliver S, Ormerod A, Smith C, et al. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology (Oxford) 2013;52:1754–1757. doi: 10.1093/rheumatology/ket187. [DOI] [PubMed] [Google Scholar]
- 63.Carneiro S, Azevedo VF, Bonfiglioli R, Ranza R, Gonçalves CR, Keiserman M, Meirelles Ede S, Pinheiro Mde M, Ximenes AC, Bernardo W, et al. Recommendations for the management and treatment of psoriatic arthritis. Rev Bras Reumatol. 2013;53:227–241. [PubMed] [Google Scholar]
- 64.Ash Z, Gaujoux-Viala C, Gossec L, Hensor EM, FitzGerald O, Winthrop K, van der Heijde D, Emery P, Smolen JS, Marzo-Ortega H. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis. 2012;71:319–326. doi: 10.1136/ard.2011.150995. [DOI] [PubMed] [Google Scholar]
- 65.Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: a focus on apremilast. Drug Des Devel Ther. 2013;7:201–210. doi: 10.2147/DDDT.S32713. [DOI] [PMC free article] [PubMed] [Google Scholar]


