Abstract
The era of metal-on-metal (MoM) total hip arthroplasty has left the orthopaedic community with valuable insights and lessons on periprosthetic tissue reactions to metallic debris. Various terms have been used to describe the tissue reactions. Sometimes the nomenclature can be confusing. We present a review of the concepts introduced by Willert and Semlitsch in 1977, along with further developments made in the understanding of periprosthetic tissue reactions to metallic debris. We propose that periprosthetic tissue reactions be thought of as (1) gross (metallosis, necrosis, cyst formation and pseudotumour); (2) histological (macrophage-dominated, lymphocyte-dominated or mixed); and (3) molecular (expression of inflammatory mediators and cytokines such as interleukin-6 and tumor necrosis factor-alpha). Taper corrosion and modularity are discussed, along with future research directions to elucidate the antigen-presenting pathways and material-specific biomarkers which may allow early detection and intervention in a patient with adverse periprosthetic tissue reactions to metal wear debris.
Keywords: Periprosthetic tissue response, Metal-on-Metal, Total hip arthroplasty, Metal debris, Lymphocyte-dominated, Macrophage-dominated, Taper corrosion, Modularity
Core tip: Valuable lessons have been learnt from the era of metal-on-metal total hip arthroplasty. We present a review of the concepts introduced by Willert and Semlitsch in 1977, along with further developments made in the understanding of periprosthetic tissue reactions to metallic debris. We propose that periprosthetic tissue reactions be thought of as (1) gross (metallosis, necrosis, cyst formation and pseudotumour); (2) histological (macrophage-dominated, lymphocyte-dominated or mixed); and (3) molecular (expression of inflammatory mediators and cytokines such as interleukin-6 and tumor necrosis factor-alpha). Taper corrosion and modularity is discussed, along with future research directions in this area.
INTRODUCTION
Retrieval studies on failed metal-on-metal (MoM) total hip arthroplasties (THAs) have contributed significantly to the understanding of adverse local tissue reactions to metallic debris. The McKee Farrar and Ring implants used in the 1960s had MoM bearing surfaces[1-3]. Weber introduced the first second-generation MoM THA (cobalt-chrome alloy with a high carbon content)in 1988[4] .The success of large-diameter hip surface replacement further popularized MoM hip replacements[5-8]. Large-diameter MoM heads (36 mm diameter or larger), started being used in revision hip surgery and were later used in primary THAs. Registry data suggest that MoM devices have been implanted into over 60000 patients in England and Wales since 2003 and the figure is closer to a million in the United States[9,10].
Metal wear products in periprosthetic tissue may exist as particulate wear debris, metal ions in solution, metallo-protein complexes and byproducts of synergistic corrosion and wear processes (especially when modular interfaces are involved)[11,12]. Proteins present in body fluids and tissue can associate with metal particlulate debris especially those in the nanoscale range. These complexes can form haptens and there may exist interindividual variability in immunological threshold and response to these antigens[13,14]. Corrosion and wear at modular interfaces i.e., head-neck and neck-stem junction can contribute to the overall particle load[15-21].
Taper corrosion has also been recognized in metal-on-polyethylene THAs[19,21,22]. Kurtz and colleagues has studied a hundred femoral head-stem pairs. They have reported that by using a ceramic femoral head, cobalt and chrome fretting and corrosion from the modular head-neck taper can be decreased partially but it is difficult to eliminate it completely[23]. Metal particulate debris tends to be in the nanometre size range and MOM articulations generate approximately 1012-1014 particles per year[24]. Difficulties associated in isolating and characterizing these small nanometric particles suggest that the actual number of particles produced in vivo may be higher, taking into account also that intracellular corrosion of phagocytosed nanometric metal particles may occur[25,26].
TISSUE REACTIONS TO WEAR PRODUCTS
Willert et al[27] in 1977, described the tissue reactions of the articular capsule to wear products of artificial joint prostheses. In their landmark article, they reported the development of a foreign-body reaction (consisting of macrophages and foreign-body giant cells) to wear debris. This foreign-body reaction takes place in the neocapsule and, depending on its magnitude, may lead to the formation of granulation tissue, which may subsequently cause scarring and decrease joint mobility. They went on to discuss the concept of an “equilibrium state”, which is achieved when the periprosthetic lymph vessels are effectively clearing the wear debris at the rate of debris production (Figure 1). If the periprosthetic lymph channels are overwhelmed, excess wear debris then spills over via the surrounding tissue into the implant-bone interface, mainly trabecular bone and marrow. Additionally, effusions into the joint space become enriched with wear products. The increase of intracapsular pressure due to muscular activity and compression not only increases local bone resorption[28] but also introduces dissociation of the interface membranes and implant surfaces. We now know this as the “effective joint space” as described by Schmalzried and colleagues in 1992[29]. Joint fluid helps to transport wear particles to new sites, resulting in activation of osteoclasts and inhibition of osteoblasts via molecular signaling pathways involving a host of inflammatory mediators. This phenomenon has also been called “particle disease”[30,31]. The “threshold” of the periprosthetic lymphatics to effectively clear wear debris is subject to interindividual variability as well as on the volume of wear (e.g., high rates of UHMWPE wear). This phenomenon may partially explain why some people develop adverse tissue reactions and early osteolysis (Figure 2) in response to metal debris whilst others seem to have a mild or no reaction, assuming all other factors being equal. Since then, research efforts have focused on the types of tissue reactions, immunological and molecular pathways involved. These pathways are still not well-understood, though some light has been shed on the types of tissue reactions to particulate wear debris.
Figure 1.
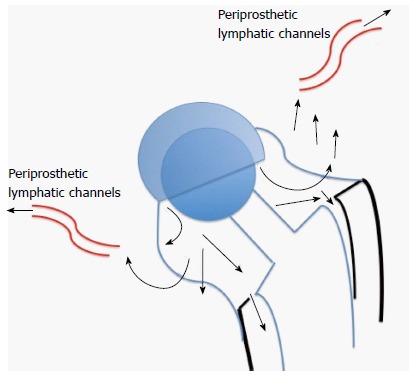
The Willert-Semlitsch concept of clearance of wear debris by periprosthetic lymph channels. If production of wear debris exceeds the ability of the lymph channels to clear it, the debris then “spills” over into the effective joint space and initiates osteolytic pathways.
Figure 2.
Plain radiograph showing early, progressive osteolysis in response to metallic wear debris.
ADVERSE TISSUE REACTIONS IN MOM THAS
Adverse tissue reactions may be systemic or local. Higher serum and solid organ metal ion levels may theoretically have carcinogenic and teratogenic potential. Various terms have been coined to describe the adverse local tissue reactions seen in MoM THA and the nomenclature is debatable. Essentially, adverse local tissue reaction (ALTR) encompasses all types of adverse local tissue reactions to debris, whereas adverse reaction to metallic debris (ARMD) and aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) represent more specific descriptions. For clarity of thought, it may be useful to think about local periprosthetic tissue reactions at the gross, histological and molecular levels.
GROSS TISSUE REACTIONS
Gross intraoperative findings in revision operations for failed aseptic metal-metal hip replacements range from metallosis, large joint effusions, necrosis and pseudotumours[32-46]. “Metallosis” comprises local damage and changes in tissue characteristics provoked by a metallic foreign body in the host with (1) direct (by pressure, destruction or displacement of tissues); (2) collateral (by chemical reactions with body fluids, electrolytic processes with direct galvanic impairment of cellular activity and impregnation of host tissue with ionizing metallic particulate matter; and (3) the resulting biologic reactions of the adjacent tissues”[47]. A pseudotumour is defined as a granulomatous lesion or a destructive cystic lesion, neither infective nor neoplastic, that is at least 5 cm in size, has developed in the vicinity of the total joint replacement (with or without communication with the joint), and resembles a tumour[48].
HISTOLOGY: MACROPHAGE-DOMINATED AND LYMPHOCYTE-DOMINATED REACTIONS
Histologically, to avoid confusion associated with the nomenclature, we differentiate the predominant cellular responses into a macrophage-dominated type and a lymphocyte-dominated type. Other features which may be seen are fibrin exudation and necrosis. The lymphocyte based tissue response differs from macrophage dominated tissue response as the former is adaptive and displays “memory”. The lymphocyte dominated tissue response may resemble a type IV delayed hypersensitivity reaction. This type of tissue reaction can lead to development of early aseptic loosening and progressive osteolysis in patients with MoM total hip arthroplasty. This phenomenon may also be seen in the context of corrosion and wear at modular interfaces in non-MoM THA[49-54]. The two responses may co-exist and research efforts are being channeled into identifying the factors which are responsible for the predominant type of tissue response.
We analyzed tissue response, serum and periprosthetic tissue metal content among a cohort of 28 small-diameter MoM THAs and found that the overall metal content in the periprosthetic tissues correlated with type of tissue response. Serum metal content did not predict type of tissue response (Table 1)[54].
Table 1.
Tissue metal content, but not serum metal content has a positive correlation with type of periprosthetic tissue response in a series of 28 small diameter metal-on-metal total hip arthroplasties
| Cobalt, mg/L | Chrome, mg/L | Nickel, mg/L | |
| Tissue metal content | |||
| Macrophage-dominated | 17.25 | 21 | 22.5 |
| Lymphocyte-dominated | 13.41 | 21.92 | 8.41 |
| Serum metal content | |||
| Macrophage-dominated | 0.3 | 2 | 0.6 |
| Lymphocyte-dominated | 45.2 | 163.6 | 1.6 |
Twenty-seven patients (28 hips) who were revised from second-generation small-diameter MoM bearing couples (Sikomet®, 0.08% carbon content) to ceramic-on-ultra high molecular weight polyethylene (UHMWPE) (8 hips), metal-on-UHMWPE- (19 hips), or ceramic-on-ceramic (1 hip). The duration of implantation was 54 to 86 mo with a mean of 66 mo. The Cobalt, Chromium, and Nickel content of the periprosthetic tissue was in the range of 1.4 to 4604.0 μg/g. The tissues with a dominant lymphocytic response had a higher mean metal content as compared to macrophage dominant response i.e., 222.2 ± 52.9 μg/g and 3.0 ± 0.9 μg/g respectively (P = 0.001). The content of nickel in the tissue was similar in both groups but the amount of cobalt was approximately hundred and fifty times higher in the lymphocyte-dominant group. Figure 3 illustrates the typical lymphocyte-dominated tissue response seen in a small-diameter MoM THA and phagocytosed intracellular metal particles from retrieved tissues in large diameter MoM THA.
Figure 3.
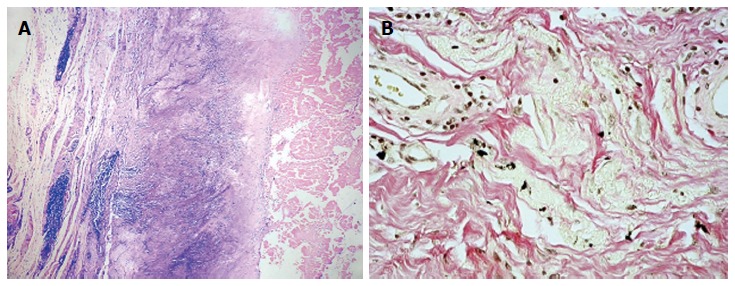
Pathologic figure. A: Diffuse and perivascular lymphocytic infiltration (lymphocyte-dominated tissue response) seen in retrieved periprosthetic tissues from small-diameter metal-on-metal (MoM) total hip arthroplasties (THAs). Haematoxylin-eosin, magnification, × 10; B: Intracellular metal particles seen in retrieval tissues from large-diameter MoM THA. These particles may undergo intracellular corrosion. Haematoxylin-eosin, magnification, × 40.
Head size may be another factor which drives the predominant type of tissue response in one direction or another. Bosker et al[55] has described that the MoM hip replacements with large heads had higher rates of pseudotumour development. The incidence of pseudotumour formation was 38.5% in this study at a mean follow-up of 3.6 years. In their cohort, patients with higher serum metal levels quadrupled their risk of forming pseudotumors. Langton et al[56] described an ALVAL type of tissue reactionin failed ASR hips. Kawakita et al[57] has described a case of histologically proven pseudotumour following a large diameter MoM hip arthroplasty. The patient developed unilateral leg edema secondary to a pelvic mass (pseudotumour) 14 mo after hip replacement surgery. Corrosion at the head-neck interface in large diameter MoM THA[17,18] may be contributory to their failure and possibly lead to different profile of wear debris in the periprosthetic tissues. This is presented in more detail in the subsequent section on modularity and taper corrosion.
MOLECULAR PATHWAYS
Molecular pathways leading to early aseptic loosening among MoM implants are not well understood either. A variety of inflammatory mediators such as interleukin-6 (IL-6), prostaglandin E2 (PGE2) and tumor necrosis factor-alpha (TNF-α) have been shown to be expressed by monocytic cells in periprosthetic tissue of failed joint arthroplasties[58,59]. Caicedo and colleagues suggested that soluble ions more than particulate cobalt-alloy implant debris induce monocyte co-stimulatory molecule expression and release of proinflammatory cytokines which contribute to metal-induced lymphocyte reactivity[60]. Tuan et al[61] observed that many pro-osteoclastic inflammatory cytokines not only promote osteoclastogenesis but also interfere with osteogenesis led by osteoprogenitor cells. Lin et al[62] investigated the suppression of chronic inflammation by inhibiting NF-κB activity as a strategy to combat wear particle induced periprosthetic osteolysis. Ren and colleagues from the University of Kansas group previously reported that VEGF inhibitor treatment prevented UHMWPE particle-induced inflammatory osteolysis[63]. Most of these inflammatory chemokines are upregulated in MoM implant failures, periprosthetic tissue affected by osteolysis due to polyethylene wear debris as well as other disease states involving chronic inflammation and even malignancy (e.g., multiple myeloma) and are not specific to the inciting agent or material[64]. The common end-point for each of these pathways is osteoclast activation and bone resorption[65,66], leading to implant loosening and revision surgery. Future research efforts should be channeled towards identifying a molecular marker which is material-specific i.e., is upregulated by the presence of metallic wear debris but not affected by polymeric wear debris and infection.
TAPER CORROSION AND MODULARITY
Modular interfaces in joint replacement surgery perhaps represent a double-edged sword. Modularity has, beyond doubt, made the technical complexity of surgical operations (particularly revisions) much easier but has also introduced a new set of problems for the revision surgeon - problems associated with the release of corrosion and wear debris from these interfaces. The cone-taper (head-neck) interface and neck-stem interface (when modular necks are used) in THA surgery represent two potential interfaces for a crevice environment and mechanically assisted corrosion leading to instability.
Collier et al[67,68] were one of the pioneer groups who studied the head-neck or cone-taper interface. They reported corrosion at the head-neck junction in a cohort of THAs which had dissimilar metal alloys in the head and neck but not in endoprosthetic components made from similar metals. This has since been shown to not be the case, with many cases of marked corrosion reported at the head-neck of same alloy systems. Willert et al[53] observed that a protective passivation layer of an alloy may prevent corrosion until micromotion sets in and abrades this layer. The current understanding of this process is termed mechanically-assisted crevice corrosion.
Gill et al[19] reported corrosion at the neck-stem junction as an important source of debris leading to pseudotumour formation. Higgs et al[16] studied 134 heads and 60 stems (41 modular necks) of 8 different bearing designs (5 manufacturers) and concluded that dissimilar alloy pairing, larger head sizes, increased medio-lateral offsets and longer neck moment arms were all associated with increased taper damage at the modular interfaces. Cook et al[22] have reported pseudotumour formation due to tribocorrosion at the taper interface of large diameter metal-on-polyethylene modular total hip replacements. Cooper’s group reported the occurrence of adverse local tissue reactions (ALTR) similar to those seen in MoM THAs and corrosion at the head-neck junction in ten patients with a metal-on-polyethylene total hip prostheses, from three different manufacturers[21].
We have reported the occurrence of corrosion and instability at the cone-taper interface, tissue metal content and element analysis of periprosthetic wear debris and type of tissue response (macrophage-dominated vs lymphocyte dominated) among 2 cohorts of failed MoM total hip arthroplasties (THA’s)[17,18,54]. The first cohort consisted of 27 patients (28 hips) with small-diameter MoM bearing couples (Sikomet®, 0.08% carbon content) as described above. The second cohort consisted of 110 patients who had 114 revisions of large-diameter head MoM THAs (LDH® head (Zimmer Inc, Warsaw, IN, United States) and a DUROM® hip cup (Zimmer Inc, Warsaw, IN, United States).The head size ranged from 46-58 mm. The duration of implantation was 26 to 68 mo with a mean of 46 mo. All implants were revised to ceramic-on-polyethylene articulating couples. Among the first cohort of small diameter MoM THA’s, there was no evidence of corrosion or instability at the cone-taper interface of the retrieved implants intraoperatively. In contrast, we have reported corrosion at the cone-taper interface as being a significant mode of failure in large-diameter MoM hip arthroplasties[18] . Out of 114 revisions of large-diameter MoM THA’s, 107 (94%) had evidence of corrosion and instability at the head-neck interface. One hundred six (93%) of the 114 hips had joint effusions and tissues with a grayish necrotic appearance were found around the implants, respectively. Intraoperatively, in 94% (n = 107), the cones and the tapers were unstable and showed a black color suggestive of corrosion. Interestingly, only 9 cases in this series had a lymphocyte-dominated tissue response and all other cases had a foreign-body type, macrophage-dominated tissue response. Element analysis with Inductive-Coupled Plasma Mass Spectrometry (ICPMS) showed a very different profile of wear debris with titanium or iron predominating, suggestive of abrasive wear from the neck taper.
Goldberg et al[69] reported that the combination of dissimilar alloys, metallurgical condition of the alloys, implantation time, and flexural rigidity of the femoral neck were predictors of corrosion of the neck and head. Implantation time, lateral offset, femoral stem modularity, and dissimilar alloys have been implicated as predictors of taper corrosion in a recent multicenter retrieval study[16]. The emergence of this phenomenon in non-MoM THAs certainly brings to light the reality of the problem and we recommend that modularity should be used with a hint of caution.
CONCLUSION
MoM total hip arthroplasties and their failures have given the orthopedic community valuable insights into periprosthetic adverse tissue reactions. Further research needs to be directed towards the immunological mechanisms, antigen-presenting and molecular pathways responsible for these adverse tissue reactions. Identification of material-specific biomarkers will potentially allow early diagnosis of adverse tissue reactions and facilitate early intervention in these patients.
ACKNOWLEDGMENTS
Dr med. Hans-Georg Willert, Professor, to whom this work is dedicated, passed away on September 25th, 2006.
Footnotes
P- Reviewer: Cooper HJ S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Brown SR, Davies WA, DeHeer DH, Swanson AB. Long-term survival of McKee-Farrar total hip prostheses. Clin Orthop Relat Res. 2002;(402):157–163. doi: 10.1097/00003086-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Howie DW, McCalden RW, Nawana NS, Costi K, Pearcy MJ, Subramanian C. The long-term wear of retrieved McKee-Farrar metal-on-metal total hip prostheses. J Arthroplasty. 2005;20:350–357. doi: 10.1016/j.arth.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Bryant MJ, Mollan RA, Nixon JR. Survivorship analysis of the Ring hip arthroplasty. J Arthroplasty. 1991;6 Suppl:S5–10. doi: 10.1016/s0883-5403(08)80049-6. [DOI] [PubMed] [Google Scholar]
- 4.Weber BG. [Metal-metal total prosthesis of the hip joint: back to the future] Z Orthop Ihre Grenzgeb. 1992;130:306–309. doi: 10.1055/s-2008-1039623. [DOI] [PubMed] [Google Scholar]
- 5.Hing C, Back D, Shimmin A. Hip resurfacing: indications, results, and conclusions. Instr Course Lect. 2007;56:171–178. [PubMed] [Google Scholar]
- 6.Su EP, Su SL. Metal-on-metal surface replacement: a triumph of hope over reason: opposes. Orthopedics. 2011;34:e442–e444. doi: 10.3928/01477447-20110714-22. [DOI] [PubMed] [Google Scholar]
- 7.Grecula MJ. Resurfacing arthroplasty in osteonecrosis of the hip. Orthop Clin North Am. 2005;36:231–242, x. doi: 10.1016/j.ocl.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Amstutz HC, Le Duff MJ. Background of metal-on-metal resurfacing. Proc Inst Mech Eng H. 2006;220:85–94. doi: 10.1243/095441105X69088. [DOI] [PubMed] [Google Scholar]
- 9.Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet. 2012;379:1199–1204. doi: 10.1016/S0140-6736(12)60353-5. [DOI] [PubMed] [Google Scholar]
- 10.Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410. doi: 10.1136/bmj.e1410. [DOI] [PubMed] [Google Scholar]
- 11.Catelas I, Wimmer MA. New insights into wear and biological effects of metal-on-metal bearings. J Bone Joint Surg Am. 2011;93 Suppl 2:76–83. doi: 10.2106/JBJS.J.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs JJ, Hallab NJ, Skipor AK, Urban RM. Metal degradation products: a cause for concern in metal-metal bearings? Clin Orthop Relat Res. 2003;(417):139–147. doi: 10.1097/01.blo.0000096810.78689.62. [DOI] [PubMed] [Google Scholar]
- 13.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Orthopaedic implant related metal toxicity in terms of human lymphocyte reactivity to metal-protein complexes produced from cobalt-base and titanium-base implant alloy degradation. Mol Cell Biochem. 2001;222:127–136. [PubMed] [Google Scholar]
- 14.Tkaczyk C, Huk OL, Mwale F, Antoniou J, Zukor DJ, Petit A, Tabrizian M. Investigation of the binding of Cr(III) complexes to bovine and human serum proteins: a proteomic approach. J Biomed Mater Res A. 2010;94:214–222. doi: 10.1002/jbm.a.32700. [DOI] [PubMed] [Google Scholar]
- 15.Matthies AK, Racasan R, Bills P, Blunt L, Cro S, Panagiotidou A, Blunn G, Skinner J, Hart AJ. Material loss at the taper junction of retrieved large head metal-on-metal total hip replacements. J Orthop Res. 2013;31:1677–1685. doi: 10.1002/jor.22431. [DOI] [PubMed] [Google Scholar]
- 16.Higgs GB, Hanzlik JA, MacDonald DW, Gilbert JL, Rimnac CM, Kurtz SM. Is increased modularity associated with increased fretting and corrosion damage in metal-on-metal total hip arthroplasty devices?: a retrieval study. J Arthroplasty. 2013;28:2–6. doi: 10.1016/j.arth.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh G, Meyer H, Ruetschi M, Chamaon K, Feuerstein B, Lohmann CH. Large-diameter metal-on-metal total hip arthroplasties: a page in orthopedic history? J Biomed Mater Res A. 2013;101:3320–3326. doi: 10.1002/jbm.a.34619. [DOI] [PubMed] [Google Scholar]
- 18.Meyer H, Mueller T, Goldau G, Chamaon K, Ruetschi M, Lohmann CH. Corrosion at the cone/taper interface leads to failure of large-diameter metal-on-metal total hip arthroplasties. Clin Orthop Relat Res. 2012;470:3101–3108. doi: 10.1007/s11999-012-2502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill IP, Webb J, Sloan K, Beaver RJ. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012;94:895–900. doi: 10.1302/0301-620X.94B7.29122. [DOI] [PubMed] [Google Scholar]
- 20.Bishop N, Witt F, Pourzal R, Fischer A, Rütschi M, Michel M, Morlock M. Wear patterns of taper connections in retrieved large diameter metal-on-metal bearings. J Orthop Res. 2013;31:1116–1122. doi: 10.1002/jor.22326. [DOI] [PubMed] [Google Scholar]
- 21.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655–1661. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook RB, Bolland BJ, Wharton JA, Tilley S, Latham JM, Wood RJ. Pseudotumour formation due to tribocorrosion at the taper interface of large diameter metal on polymer modular total hip replacements. J Arthroplasty. 2013;28:1430–1436. doi: 10.1016/j.arth.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz SM, Kocagöz SB, Hanzlik JA, Underwood RJ, Gilbert JL, MacDonald DW, Lee GC, Mont MA, Kraay MJ, Klein GR, et al. Do ceramic femoral heads reduce taper fretting corrosion in hip arthroplasty? A retrieval study. Clin Orthop Relat Res. 2013;471:3270–3282. doi: 10.1007/s11999-013-3096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobb AG, Schmalzreid TP. The clinical significance of metal ion release from cobalt-chromium metal-on-metal hip joint arthroplasty. Proc Inst Mech Eng H. 2006;220:385–398. doi: 10.1243/09544119JEIM78. [DOI] [PubMed] [Google Scholar]
- 25.Billi F, Campbell P. Nanotoxicology of metal wear particles in total joint arthroplasty: a review of current concepts. J Appl Biomater Biomech. 2010;8:1–6. [PubMed] [Google Scholar]
- 26.Bowsher JG, Hussain A, Williams PA, Shelton JC. Metal-on-metal hip simulator study of increased wear particle surface area due to ‘severe’ patient activity. Proc Inst Mech Eng H. 2006;220:279–287. doi: 10.1243/09544119JEIM93. [DOI] [PubMed] [Google Scholar]
- 27.Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11:157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- 28.Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure. A discussion of causes of prosthetic loosening. Clin Orthop Relat Res. 1998;(352):75–80. [PubMed] [Google Scholar]
- 29.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 30.Harris WH. Osteolysis and particle disease in hip replacement. A review. Acta Orthop Scand. 1994;65:113–123. doi: 10.3109/17453679408993734. [DOI] [PubMed] [Google Scholar]
- 31.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–224. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozic KJ, Browne J, Dangles CJ, Manner PA, Yates AJ, Weber KL, Boyer KM, Zemaitis P, Woznica A, Turkelson CM, et al. Modern metal-on-metal hip implants. J Am Acad Orthop Surg. 2012;20:402–406. doi: 10.5435/JAAOS-20-06-402. [DOI] [PubMed] [Google Scholar]
- 33.Morrey BF, Berry DJ, An K, Kitaoka HB, Pagnano MW. Joint Replacement Arthroplasty: Basic Science, Hip, Knee and Ankle. Fourth Centennial Edition, Volume II. Amsterdam: Wolters Klumer Health/Lipincott Williams & Wilkins,; 2011. p. 510–514. [Google Scholar]
- 34.Delaunay C, Petit I, Learmonth ID, Oger P, Vendittoli PA. Metal-on-metal bearings total hip arthroplasty: the cobalt and chromium ions release concern. Orthop Traumatol Surg Res. 2010;96:894–904. doi: 10.1016/j.otsr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Browne JA, Bechtold CD, Berry DJ, Hanssen AD, Lewallen DG. Failed metal-on-metal hip arthroplasties: a spectrum of clinical presentations and operative findings. Clin Orthop Relat Res. 2010;468:2313–2320. doi: 10.1007/s11999-010-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann BS, Whittingham-Jones PM, Shaerf DA, Nawaz ZS, Harvie P, Hart AJ, Skinner JA. Metal-on-metal bearings, inflammatory pseudotumours and their neurological manifestations. Hip Int. 2012;22:129–136. doi: 10.5301/HIP.2012.9185. [DOI] [PubMed] [Google Scholar]
- 37.Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol AV, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br. 2011;93:572–579. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- 38.Shetty VD, Villar RN. Development and problems of metal-on-metal hip arthroplasty. Proc Inst Mech Eng H. 2006;220:371–377. doi: 10.1243/095441105X63264. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald SJ. Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop Relat Res. 2004:86–93. doi: 10.1097/01.blo.0000150309.48474.8b. [DOI] [PubMed] [Google Scholar]
- 40.Amstutz HC, Grigoris P. Metal on metal bearings in hip arthroplasty. Clin Orthop Relat Res. 1996;(429):S11–S34. doi: 10.1097/00003086-199608001-00003. [DOI] [PubMed] [Google Scholar]
- 41.Fabi D, Levine B, Paprosky W, Della Valle C, Sporer S, Klein G, Levine H, Hartzband M. Metal-on-metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics. 2012;35:e1009–e1016. doi: 10.3928/01477447-20120621-12. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez MH, Carr R, Walton S, Mihalko WM. The evolution and modern use of metal-on-metal bearings in total hip arthroplasty. Instr Course Lect. 2011;60:247–255. [PubMed] [Google Scholar]
- 43.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis. J Bone Joint Surg Br. 2011;93:608–615. doi: 10.1302/0301-620X.93B5.26309. [DOI] [PubMed] [Google Scholar]
- 44.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 45.Barrett WP, Kindsfater KA, Lesko JP. Large-diameter modular metal-on-metal total hip arthroplasty: incidence of revision for adverse reaction to metallic debris. J Arthroplasty. 2012;27:976–83.e1. doi: 10.1016/j.arth.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Cobalt and chromium ion release after large-diameter metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27:990–996. doi: 10.1016/j.arth.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Contzen H, Straumann F, Paschke E, Geissendörfer R: Grundlagen der Alloplastikmitmetallen und Kunststoffen ThiemeVerlag, Stuttgart, 1967, p50 [Google Scholar]
- 48.Daniel J, Holland J, Quigley L, Sprague S, Bhandari M. Pseudotumors associated with total hip arthroplasty. J Bone Joint Surg Am. 2012;94:86–93. doi: 10.2106/JBJS.J.01612. [DOI] [PubMed] [Google Scholar]
- 49.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallab NJ, Anderson S, Stafford T, Glant T, Jacobs JJ. Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. 2005;23:384–391. doi: 10.1016/j.orthres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Aroukatos P, Repanti M, Repantis T, Bravou V, Korovessis P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2135–2142. doi: 10.1007/s11999-009-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohmann CH, Nuechtern JV, Willert HG, Junk-Jantsch S, Ruether W, Pflueger G. Hypersensitivity reactions in total hip arthroplasty. Orthopedics. 2007;30:760–761. doi: 10.3928/01477447-20070901-12. [DOI] [PubMed] [Google Scholar]
- 53.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 54.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, Morlock MM, Pflüger G. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013;95:1561–1568. doi: 10.2106/JBJS.L.01273. [DOI] [PubMed] [Google Scholar]
- 55.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg Br. 2012;94:755–761. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 56.Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, Lord J, Nargol AV. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011;93:1011–1016. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- 57.Kawakita K, Shibanuma N, Tei K, Nishiyama T, Kuroda R, Kurosaka M. Leg edema due to a mass in the pelvis after a large-diameter metal-on-metal total hip arthroplasty. J Arthroplasty. 2013;28:197.e1–197.e4. doi: 10.1016/j.arth.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, Santambrogio L, Ross FP, Goldring SR, Purdue PE. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31:73–80. doi: 10.1002/jor.22190. [DOI] [PubMed] [Google Scholar]
- 59.Wang CT, Lin YT, Chiang BL, Lee SS, Hou SM. Over-expression of receptor activator of nuclear factor-kappaB ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials. 2010;31:77–82. doi: 10.1016/j.biomaterials.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 60.Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res A. 2010;93:1312–1321. doi: 10.1002/jbm.a.32627. [DOI] [PubMed] [Google Scholar]
- 61.Tuan RS, Lee FY, T Konttinen Y, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16 Suppl 1:S42–S48. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren W, Zhang R, Markel DC, Wu B, Peng X, Hawkins M, Wooley PH. Blockade of vascular endothelial growth factor activity suppresses wear debris-induced inflammatory osteolysis. J Rheumatol. 2007;34:27–35. [PubMed] [Google Scholar]
- 64.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 65.Lau YS, Danks L, Sun SG, Fox S, Sabokbar A, Harris A, Athanasou NA. RANKL-dependent and RANKL-independent mechanisms of macrophage-osteoclast differentiation in breast cancer. Breast Cancer Res Treat. 2007;105:7–16. doi: 10.1007/s10549-006-9438-y. [DOI] [PubMed] [Google Scholar]
- 66.Shen Z, Crotti TN, McHugh KP, Matsuzaki K, Gravallese EM, Bierbaum BE, Goldring SR. The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis. Arthritis Res Ther. 2006;8:R70. doi: 10.1186/ar1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collier JP, Suprenant VA, Jensen RE, Mayor MB, Suprenant HP. Corrosion between the components of modular femoral hip prostheses. J Bone Joint Surg Br. 1992;74-B:511–517. doi: 10.1302/0301-620X.74B4.1624507. [DOI] [PubMed] [Google Scholar]
- 68.Collier JP, Surprenant VA, Jensen RE, Mayor MB. Corrosion at the interface of cobalt-alloy heads on titanium-alloy stems. Clin Orthop Relat Res. 1991;(271):305–312. [PubMed] [Google Scholar]
- 69.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002;(401):149–161. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]


