Abstract
Treatment of articular cartilage injuries to the knee remains a considerable challenge today. Current procedures succeed in providing relief of symptoms, however damaged articular tissue is not replaced with new tissue of the same biomechanical properties and long-term durability as normal hyaline cartilage. Despite many arthroscopic procedures that often manage to achieve these goals, results are far from perfect and there is no agreement on which of these procedures are appropriate, particularly when full-thickness chondral defects are considered.Therefore, the search for biological solution in long-term functional healing and increasing the quality of wounded cartilage has been continuing. For achieving this goal and apply in wide defects, scaffolds are developed.The rationale of using a scaffold is to create an environment with biodegradable polymers for the in vitro growth of living cells and their subsequent implantation into the lesion area. Previously a few numbers of surgical treatment algorithm was described in reports, however none of them contained one-step or two –steps scaffolds. The ultimate aim of this article was to review various arthroscopic treatment options for different stage lesions and develop a new treatment algorithm which included the scaffolds.
Keywords: Chondral lesion, Microfracture, Osteochondral transplantation, Autologous chondrocyte implantation, Scaffolds
Core tip: This paper discusses the current arthroscopic treatment options of cartilage injuries. Over 1 cm2 full thickness chondral lesions are seen in 4%-5% of patients under 40 years undergone arthroscopy. Conventional arthroscopic treatment may not have successful results although chondral defects are observed with such a high incidence. Addition of novel scaffolds to conventional methods will provide beneficial effects on healing of articular cartilage lesions with hyaline. We now formulate a new treatment algorithm with scaffolds under the light of existing literature. In future, we expect the widespread use of arthroscopic surgery in chondral defects.
INTRODUCTION
Articular injuries that are related to trauma or overuse have plagued those afflicted for more than 200 years and are still problematic to treat. In 1743, Hueter[1] stated, “From Hippocrates down to the present age, we shall find, that an ulcerated cartilage is universally allowed to be a very troublesome disease; that it admits of a cure with more difficulty than carious bone; and that, when destroyed, it is not recovered’’.
Articular cartilage is vulnerable to both irreversible traumatic injury and degenerative disease[2]. The ability of damaged articular cartilage to recover with normal hyaline cartilage is limited because of two main factors: the absence of a vascular response and the relative absence of an undifferentiated cell population to respond to injury[3]. If patients with previous cartilage deformation (chondral defects) have not been treated properly, the osteoarthritis symptoms can be seen radiographically after 10 years and primary gonarthrosis related with osteoarthritis develops 10 years early[4,5]. Therefore, it can be said that cartilage deformation leads to osteoarthritis and chondral defects in weight-bearing regions that are at risk of developing osteoarthritis[6].
It is questionable whether every chondral defect results in osteoarthritis. A defect size under 10 mm does not increase the pressure in peripheral healthy cartilage. However, 64% more pressure is exerted on cartilage with a defect size greater than 10 mm[5]. After a 14-year follow-up of 10 mm sized defects, it has been reported that the joint gap was observed to be 50% narrower[7]. In animal studies where the treatment of cartilage defects has been evaluated, some artificial defects regenerated spontaneously. The defect sizes which do not regenerate without treatment are called “critical sized defects”. The sizes determined for every animal model can not be estimated for humans. In a clinical study, critical defect size was suggested to be 2 cm2[8]. Therefore, defect size is not the only factor for defect resolution. Three major factors must be taken into consideration when making the treatment decision. The first factor to be considered involves defect-specific factors such as size, depth, location, and degree of containment. The second includes patient-specific factors such as patient age, current and desired level of activity and patient expectations. The third area is joint-related factors, including alignment, stability and the status of the meniscus.
Some form of cartilage healing has been proven given certain conditions, although the terms “healing and repair” are rather non-specific. Most often, the repaired articular cartilage is unsuccessful in replicating the structure, composition and function of healthy articular cartilage. Today, there are various surgical procedures to treat articular injuries.
GOALS, INDICATIONS AND CONTRAINDICATIONS FOR SURGICAL TREATMENT
Candidates for surgical treatment are patients who have documented articular damage and those with an associated ligamentous or meniscal injury that requires surgery. Today, the main purpose of surgical treatment of articular cartilage pathology is to lessen the pathology-related symptoms, stop the progression of articular damage, restore the articular surface anatomy and start a healing or repair process in order to transform damaged tissue into healthier new tissue. Currently, most surgical procedures lead to the formation of a fibrocartilage tissue replacement, which has an inferior biomechanical composition to normal hyaline cartilage. Surgical treatment of these articular lesions ultimately aims to replace the damaged tissue with normal hyaline cartilage that has an equivalent composition to that of the preexisting tissue. For this aim, indications are ranged according to chondral treatment options, however generally, distal femoral condyles lesions, symptomatic cartilage lesions, and asymptomatic lesion in patient who has an additional injury undergoing to surgical treatment . Contraindications of surgical treatment for articular cartilage lesions are wide-spread degenerative arthritis (including 3 compartment), systemic inflammatory or collagen vascular diseases, active infection in the related joint, body mass index > 30, opposed (kissing) full-thickness cartilage injury, untreated malignment and instability[9].
CLASSIFICATION OF CARTILAGE DEFECTS
It is important and necessary to throughly document and grade chondral lesions when treating patients with articular cartilage defects. In 1961, Outerbridge[10] described the simplest scale by directly observing damaged patellas during arthrotomy. The Outerbridge grading system is widely accepted, although it has size, depth and lesion locale descriptive limitations. Many other classification systems have been established to indicate the severity and type of articular cartilage. The international cartilage research society (ICRS) grading system observes the importance of subchondral osseous involvement and is used to describe the defect (area, depth, location)[11]. Table 1 shows the classification systems (Outerbridge, modified outerbridge grading system and ICRS) of articular lesions by severity[12,13].
Table 1.
Classification of articular lesions by severity
| Grade | Outerbridge | Modified outerbridge | ICRS |
| 0 | Normal cartilage | Intact cartilage | Intact cartilage |
| I | Softening and swelling | Chondral softening or blistering with intact surface | Superficial (soft indentation or superficial fissures and cracks) |
| II | Fragmentation and fissures in area less than 0.5 inch in diameter | Superficial ulceration, fibrillation, or fissuring less than 50% of depth of cartilage | Lesion less than half the thickness of articular cartilage |
| III | Fragmentation and fissures in area larger than 0.5 inch in diameter | Deep ulceration, fibrillation, fissuring or chondral flap more than 50% of cartilage without exposed bone | Lesion more than half the thickness of articular cartilage |
| IV | Exposed subchondral bone | Full-thickness wear with exposed subchondral bone | Lesion extending to subchondral bone |
ICRS: International Cartilage Repair Society.
SURGICAL TREATMENT OPTIONS AND RESULTS
During surgery, chondral defects in the knee joint are often observed. Those lesions do not always trigger symptoms. However, full thickness chondral lesions greater than 1 cm2 have been reported at 4%-5% in arthroscopy performed on patients aged under 40 years[14,15]. In chondral defects, while cartilage is treated, the problem causing the chondral defect should also be detected and resolved[16].The detection and treatment of the chondral problem influences the success of the treatment of the lesion[17]. During arthroscopic surgery, the defect is generally seen to be greater than as observed on magnetic resonance imaging (MRI)[18].
In a retrospective review of over 31000 knee arthroscopies, in all age groups, chondral lesions were found in 63% of patients, with an average of 2.7 lesions per knee. 19% of those were focal (not widespread) chondral or osteochondral lesion, 5.2% were grade III or grade IV and only focal cartilage lesions required treatment[14]. Most chondral defects (58%-80%) are seen in the medial femoral condyle, followed by the patella and tibial plateau. Defects in the lateral condyle, trochlea and medial tibial plateau are observed at lower incidence rates[15].
The main goal of arthroscopic surgical management of symptomatic chondral defects is to lessen symptoms, improve joint congruence and prevent additional cartilage deterioration. Options can be characterized as palliative, reparative or restorative for those lesions. For lesions discovered incidentally or symptomatic lesions in low-demand patients with a preponderance of mechanical symptoms or signs of meniscal pathology, palliative procedures such as debridement and lavage are used. In the area of the defect, reparative procedures promote a fibrocartilage healing response. Restorative techniques replace the damaged cartilage with new articular cartilage; these include autologous chondrocyte implantation, osteochondral autografting and fresh osteochondral allografting (Table 2).
Table 2.
Treatment options for articular cartilage lesions
| Procedure | Indications | Outcome |
| Arthroscopic debridement and lavage | Minimal symptoms | Palliative |
| Marrow stimulation | Smaller lesions, low-demand patient | Reparative |
| Osteochondral autograft | Smaller lesions, low-or high-demand patients | Restorative |
| Osteochondral allograft | Larger lesions with bone loss, low-or high-demand patients | Restorative |
| Autologous chondrocyte implantation | Small and large lesions with and without bone loss, high-demand patients | Restorative |
| Genetic engineering | Investigational | Restorative |
From Garrick JG, editor: Orthopaedic knowledge update: sports medicine, 3rd ed, Rosemont, IL, 2004, American Academy of Orthopaedic Surgeons.
Debridement and lavage
Over 60 years ago, Magnusson[19] described the benefits of knee joint debridement to relieve arthritic symptoms. Jackson et al[20] became a proponent of arthroscopic pallative procedures such as debridement and lavage for the treatment of a symptomatic arthritic knee with the arrival of arthroscopy. The purpose of this technique is to debride the loose chondral tissues. Removal of loose intra-articular tissue debris and inflammatory mediators generated by the synovial lining leads to acceptable short-term results for both acute and degenerative chondral lesions. Lavage most often provides short-term symptomatic relief. This procedure is appropriate for older sedentary patients, but when an active population is considered, the results are generally insufficient[21].
Evidence based analyses indicate that lavage and debridement is accepted as an effective technique in the short-term (up to 12 mo) in terms of pain management in patients with early osteoarthritis and those with mechanical symptoms. However, in patients with moderate to advanced osteoarthritis the results are contradictory[22].
Marrow stimulating techniques
The concept of penetrating the subchondral bone to allow for the release of blood, growth factors and mesenchymal cells into the chondral defect was popularized by the Pirdiean[23] open technique in 1959 and was then modified for arthroscopic use by Johnson[24]. Marrow-stimulating techniques such as abrasion arthroplasty, subchondral drilling and microfracture are the three described techniques used to penetrate the subchondral bone. All these techniques are used to stimulate fibrocartilage in growth into the chondral defect.
Abrasion arthroplasty: This technique involves debridement of the articular defect circumferentially using a motorized burr to remove 1-3 mm of subchondral bone[23]. However, excessive trauma to the underlying bone and thermal necrosis can be potentially more destructive than helpful. Therefore this technique is not used in current practice[11].
Subchondral drilling: This procedure is an extension of the abrasion arthroplasty technique in which the subchondral bone is drilled with multiple drill holes penetrating into the bone marrow to stimulate a vascular response. Considering the efficacy of this procedure, questions still remain due to poor access, thermal necrosis and long-term results[25]. On the other hand, a recent study indicated that drilling does not cause thermal injury and the drill holes actually allow more consistent channels for cell migration compared to microfracture holes that may be partially blocked with bony debris. Therefore, although less commonly used than microfracture, drilling is another alternative technique within the scope of marrow stimulating techniques[26].
Microfracture: In 1994, Steadman et al[27] developed the microfracture technique, which is now the currently preferred marrow-stimulation method. It includes arthroscopic debridement of the cartilage defect down to the subchondral bone but not through it. Damage to the subchondral bone should be avoided in over-aggressive shaving of the soft articular cartilage. When the subchondral bone is identified, an arthroscopic tapered awl is carefully used to make multiple drill holes approximately 3 to 4 mm apart and 4 mm in depth across the exposed surface of the lesion. The use of arthroscopic awls as opposed to subchondral drilling is thought to produce less thermal necrosis in creating the holes.
In spite of progression in chondral defect treatment, the current most widely-used therapy option is the chondral repair technique[28]. This technique is performed extensively as the first treatment choice due to its minimally invasive properties, technique simplicity, lower surgical morbidity and cost- effectiveness in focal chondral defects (< 2.5 cm2 ) in patients under 45 years old with a low level of activity[27,29,30]. After less than 2 years follow-up of small, full thickness chondral defects treated with the microfracture technique, 75% of patients reported a decrease in pain, increased function and good-excellent clinical results[27,29]. Other arthroscopic treatment options of autologous osteochondral transplantation (AOT) and autologous chondrocyte implantation (ACI) have been compared with microfracture and after 5 years follow-up, there were no differences in functional scores and postoperative MRI grades between the groups. Microfracture is the first therapy choice because of the simplicity and cost-effectiveness compared to AOT and ACI[31].
However in a recent review of microfracture tecniques, Goyal et al[32] emphasised that in young patients and smaller lesions, better results were seen in the first five years, but after 5 years the results worsened and resulted in osteoarthritis regardless of the defect size. Thus, with the aim of improving the quality of repair tissue with the microfracture technique and the management of long-term functional healing, biological solutions are being investigated[33].
Recent meta analyses and systematic review studies have indicated that microfracture technique is effective in smaller lesions (up to 4 cm2) with short-term follow up. The major short comings have included poor hyaline repair, variable cartilage volume and long-term functional deterioration[34] (Figure 1).
Figure 1.
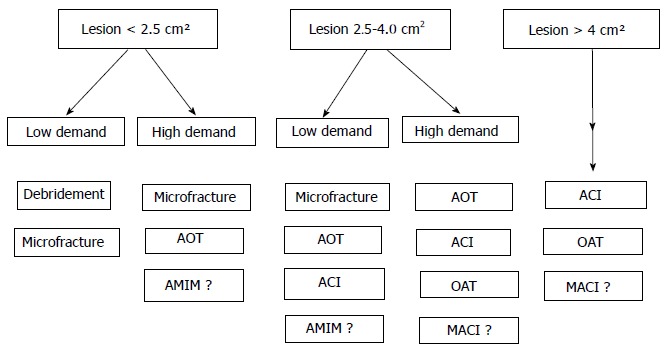
In small defects in high-demand patients, autologous osteochondral transplantation seems to be a reliable treatment alternative. AOT: Autologous osteochondral transplantation; ACI: Autologous chondrocyte implantation; MACI: Matrix-induced chondrocyte implantation; OAT: Osteochondral allograft transplantation; AMIM: Acellular matrix-induced microfracture.
Acellular matrix induced microfracture: Natural and artificial structure implants such as scaffolds have been developed for the improvement of the quality of repair tissue and treatment of wide defects with the microfracture technique[35]. Structure implants are implanted in 3 dimensional or liquid/gel-formed acellular materials to improve marrow inducement with the microfracture technique. The chondroconductive or osteoconductive properties of those implants do not contain vital cells.
In combination with microfracture this can stabilize the fibrin and provide an environment for mesenchymal root cells, keep them in place and support tissue differentiation. This type of microfracture has a scaffold to obtain hyaline chondral repair tissue.The advantages of the method are the placement of implants in single-stage surgery and no need for expensive cell production technology. Scaffolds are cost effective and non time-consuming devices. They are invaded by host tissue cells and resorb over time and are replaced with repair tissue. Chondrotissue®, Hyalofast®, AMIC®, CAIS®, Alginate Beads®, Trufit®, Maioregen® are examples of implanted one-step scaffold implants[36].
Siclari et al[37] reported that 52 patients, aged 25-65 years, treated with scaffold implants demonstrated an improvement in functional scores and the histological evaluation of 13 biopsy samples showed homogeneous hyaline-like chondral repair tissue. In a recent study, Gille et al[38] implanted scaffold membrane combined with microfracture in 27 patients with a mean defect size of 3.4 cm2 (range 1-12 cm2) and 87% of patients demonstrated significant clinical healing after a 37-mo follow-up period.
Current literature do not contain evidence-based researches or meta-analysis. Thus, to come to a decision with limited evidence, it could be speculated that one-step cell-free approaches have been developed to avoid the problems related to the ex vivo chondrocyte culture and expansion in a scaffold. Besides this, they reduce the costs and surgical time. Finally, osteochondral scaffolds have been proposed to treat lesions where the subchondral layer is also involved in the pathologic process and have shown promising preliminary results.
AOT
AOT is the cartilage restoration procedure which produces true hyaline articular cartilage. Osteochondral autografts can fill the articular defect with human articular cartilage tissue transplanted into damaged areas from areas of less weight-bearing on the femoral condyle as either a single large bone plug or multiple small plugs (mosaicplasty). The term mosaicplasty is reserved to describe the use of multiple, smaller diameter grafts. Autograft harvesting and transplantation techniques have the advantages of using the patient’s own tissue and immediate transplantation from the donor site to the recipient site without any additional cost to the patient.
The upper age limit for mosaicplasty is under 50 years and it can be performed on patients with high physical expectations and 1-5 cm2 focal chondral defects. Hangody et al[39] stated that 1-4 cm2 sized defects are ideal for mosaicplasty. According to Ollat et al[40], defects of 2 cm2 or less and to Solheim et al[41], 3 cm2 or less, showed better results. Good prognostic factors are male gender, young age and small defects. Therefore, in small defects in high-demand patients, AOT seems to be a reliable treatment alternative (Figure 1).
Haklar et al[42] claimed that mosaicplasty is a reliable procedure in the treatment of full-thickness chondral lesions because it is minimally invasive, can be performed at a single session, and has a low complication rate and is cost effective.
Gudas et al[43] performed microfracture on 22 patients and AOT on 25 patients with a mean age of 24.3 years (range 15-40 years) and a follow-up period of 10 years. Patients treated with microfracture demonstrated good results immediately after surgery, which then worsened over time. The patients with AOT had better results compared with the microfracture group and a high rate of sportsmen in the AOT group were able to resume their previous sporting activities.
Osteochondral grafts in restorative techniques can be complicated by dislodgement of the graft from the transplant site, but this is rare with the press-fit technique. Additionally, graft collapse can occur through biomechanical overload or biological failure of the chondral or subchondral components.
Osteochondral allograft transplantation
The technique of osteochondral autograft plugs was first introduced by Yamashita[44] in 1985 and universalized by Hangody et al[45]. Fresh osteochondral allograft transplantation includes the implantation of a composite cadaveric graft that involves the subchondral bone and overlying hyaline cartilage in the site of the chondral defect with a single-stage procedure, and is not limited by its size. Osteochondral allograft transplants are used for medium to large articular lesions (up to 3 cm2) in relatively high-demand patients. These grafts are generally used on the femoral condyles but can also be used for the patella, trochlea, medial and lateral tibial plateau along with the donor meniscus. There is no donor site morbidity involved in the use of allografts. In addition, allografts may be taken from younger, healthier patients with better quality bone and cartilage. Allografts can also be used in large sized defects. In a study by Giorgini et al[46] 11 patients were treated and followed up for mean 26.5 mo between 2006-2011. The average defect size was 10.3 cm2 (range 3-20 cm2). The results of this study determined success in 10 patients who showed pain regression and functional recovery. It was emphasized that this technique had better results in lesions smaller than 8 cm2, although larger lesions also showed good results.
In another recent study, Chahal et al[47] conducted a systematic review of clinical outcomes after osteochondral allograft transplantation in the knee. There were 19 eligible studies with 644 knees in total. The mean age was 37 years and the mean follow-up period, 58 mo. The mean defect size across the studies was 6.3 cm2. The methods of procurement and storage time included fresh (61%), prolonged fresh (24%) and fresh frozen (15%). It was emphasized that osteochondral allograft transplantation for focal and diffuse (single compartment) chondral defects leads to predictably favorable outcomes and high satisfaction rates at intermediate follow-up. The major drawback of this technique is the use of fresh allogenic tissue, which has the potential for disease transmission. Cost and size mismatch are other issues, which should also be considered.
Autologous chondrocyte implantation
As an alternative for the treatment of articular cartilage injuries with a hyaline-like cartilage repair, Brittberg et al[48] were the first to report Autologous chondrocyte implantation (ACI) in 1994. Being a two-stage technique, the first stage in ACI involves an arthroscopic evaluation of the chondral lesion and biopsy by harvesting of chondrocytes. In this stage, cartilage is taken from lesser weight-bearing regions of the knee. The preferred locations are the lateral edge of the intercondylar notch or the superomedial trochlea. The total size of the biopsy should be between 200 and 300 mg. The cartilage specimens are sent to the laboratory for the chondroyctes to be isolated from the harvested cartilage. The cells are cultured for 2 wk to increase the number of cells as the implanted cartilage cells require a stable environment in which to heal. This procedure comes 6 wk after the biopsy. Following this, the second stage involves implantation through a mini-arthrotomy. Coverage is obtained by a periosteal patch sewn according to the defect size with 6-0 Vicryl sutures and sealed with fibrin glue. The aim is to achieve a more durable “hyaline-like” repair tissue that resembles hyaline articular cartilage.
ACI can be used as the primary treatment choice in over 4cm2, focal, ICRS grade III and IV lesions of the knee joint and in patients with chondral defects who have a high activity demand, excellent compliance and are aged 15-55 years. It can be indicated as a second treatment in patients with 2.5 cm2 unhealed lesions where microplasty or microfracture has been previously performed.
In a systematic review, Bekkers et al[49] concluded that for defects over 2.5 cm2 in young patients, ACI can be performed successfully. In another study by Harris et al[50] microfracture (n = 271), mosaicplasty (n = 42) and ACI (n = 604) were compared in 917 patients. The clinical results of mosaicplasty were similar to those of ACI in the short term but worsened over a period of two years. In defects over 4 cm2 in size, ACI was found to be superior to all the other treatments.
The ACI technique has better results in 70%-80% of patients not only in the short term but also in mid and long-term follow-up[51]. Moseley et al[52] showed that 69% of patients maintained the successful treatment results over 6-10 years. In a 5-year follow-up study, ACI was found to be better than microfracture in patients whose complaints had been ongoing for less than 3 years[53]. Mosaicplasty and ACI were compared in a 10-year follow-up study and the functional outcome of patients with a surviving graft was significantly better in patients who had undergone ACI compared with the mosaicplasty group[54].
Matrix induced chondrocyte implantation
In the ACI process, complications, such as graft hypertrophy seen while using periosteal patches, have led to increased interest in utilizing bioabsorbable covers as an alternative. One such technique is matrix-induced chondrocyte implantation (MACI). The MACI membrane involves a porcine-derived collagen bilayer, which is seeded with the patient’s harvested chondrocytes. MACI is a two-stage technique, which includes an arthroscopic evaluation of the chondral lesion and biopsied arthroscopically in the first stage and implanted generally through an arthrotomy in the second stage which is also applied by arthroscopic surgery[55]. During implantation, the graft is secured to the defect by fibrin glue alone, without sutures. Although there are several implants, Cares®, Hyalograft ®, NeoCart®, Novocart®, Bioseed C®, Chondron®, Cartipatch®, Atelocollagen® are examples of implants containing autologous chondrocytes, which have produced satisfactory clinical results[36].
Marlovits et al[56] reported 2 failures from 21 patients treated with ACI after 5 years and with MRI evaluation it was observed that in 80% of patients,defects had totally healed and integrated with peripheral chondral tissue. Eber et al[57] reported successful results in 20 patients treated with arthroscopic surgical technique and MACI after 2 years. The follow-up MRI showed 90% defect healing and 70% integration. Vijayan et al[58] observed good results in deep lesions > 8 mm with MACI, bone graft and double layer MACI. Several studies have compared MACI with current treatment options[59,60]. Basad et al[59] performed microfracture in 20 patients and MACI in 40 patients with a defect size over 4 cm2 and reported that MACI was superior to microfracture in the treatment of articular defects over 2 years. The MACI technique represents a significant advance in terms of reproducibility, safety, and reduced invasiveness. Zeifang et al[60] found no difference in clinical results between ACI and MACI in 21 patients after 2 years. Both treatments had similar results but MACI had significantly better scores in chondral healing when evaluated by MRI.
CONCLUSION
Appropriate patient selection for articular cartilage lesion treatment is paramount to reduce symptoms and successfully improve function. In smaller lesions of up to 2.5 cm2 in size, and in larger (up to 4 cm2) lesions in low-demand patients, debridement and microfracture are the most commonly used techniques. In lesions up to 4 cm2, and in high demand patients, AOT is a reliable method. Although indications of the use of microfracture plus scaffolds are not clear, this technique is commonly used in high demand patients with lesions of up to 4 cm2. However, in larger lesions more sophisticated cell based techniques such as ACI or MACI should be use.
In this article a treatment algorithm were formulated to help guide the decision.
Cartilage restoration techniques will most certainly evolve over the next several decades. With the addition of biological scaffolds and gene therapy techniques, the future holds much promise for patients for the natural healing of articular cartilage lesions. Alternative tissue techniques will be available to replace damaged articular cartilage or modifications of existing technology will lead to better results or fewer complications. Moreover, continued advances in arthroscopic techniques will allow procedures,which are commonly performed through an open arthrotomy, to be performed arthroscopically.
Footnotes
P- Reviewer: Zhou S S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
References
- 1.Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res. 1995;(317):3–6. [PubMed] [Google Scholar]
- 2.Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952–962. doi: 10.1007/s11999-007-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, Goodnight JL, McIlwraith CW. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;(407):215–227. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Prakash D, Learmonth D. Natural progression of osteo-chondral defect in the femoral condyle. Knee. 2002;9:7–10. doi: 10.1016/s0968-0160(01)00133-8. [DOI] [PubMed] [Google Scholar]
- 5.Kock NB, Smolders JM, van Susante JL, Buma P, van Kampen A, Verdonschot N. A cadaveric analysis of contact stress restoration after osteochondral transplantation of a cylindrical cartilage defect. Knee Surg Sports Traumatol Arthrosc. 2008;16:461–468. doi: 10.1007/s00167-008-0494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand. 1996;67:165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 7.Coons DA, Barber FA. Arthroscopic osteochondral autografting. Orthop Clin North Am. 2005;36:447–458. doi: 10.1016/j.ocl.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Nehrer S, Chiari C, Domayer S, Barkay H, Yayon A. Results of chondrocyte implantation with a fibrin-hyaluronan matrix: a preliminary study. Clin Orthop Relat Res. 2008;466:1849–1855. doi: 10.1007/s11999-008-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18:9–32. doi: 10.1053/jars.2002.31783. [DOI] [PubMed] [Google Scholar]
- 10.Outerbridge RE. The etiology of chondromalacia patellae. 1961. Clin Orthop Relat Res. 2001;(389):5–8. doi: 10.1097/00003086-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 12.Mandelbaum BR, Browne JE, Fu F, Micheli L, Mosely JB, Erggelet C, Minas T, Peterson L. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853–861. doi: 10.1177/03635465980260062201. [DOI] [PubMed] [Google Scholar]
- 13.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 14.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 15.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 16.Hangody L, Sükösd L, Szabó Z. [Repair of cartilage defects. Technical aspects] Rev Chir Orthop Reparatrice Appar Mot. 1999;85:846–857. [PubMed] [Google Scholar]
- 17.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cole BJ, Lee SJ. Complex knee reconstruction: articular cartilage treatment options. Arthroscopy. 2003;19 Suppl 1:1–10. doi: 10.1016/j.arthro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Magnuson PB. Technic of debridement of the knee joint for arthritis. Surg Clin North Am. 1946:249–266. [PubMed] [Google Scholar]
- 20.Jackson RW. Arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:1717–179; author reply 1717-1719. doi: 10.1056/NEJM200211213472117. [DOI] [PubMed] [Google Scholar]
- 21.Krüger T, Wohlrab D, Birke A, Hein W. Results of arthroscopic joint debridement in different stages of chondromalacia of the knee joint. Arch Orthop Trauma Surg. 2000;120:338–342. doi: 10.1007/s004020050478. [DOI] [PubMed] [Google Scholar]
- 22.Health Quality Ontario. Arthroscopic lavage and debridement for osteoarthritis of the knee: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5:1–37. [PMC free article] [PubMed] [Google Scholar]
- 23.Insall J. The Pridie debridement operation for osteoarthritis of the knee. Clin Orthop Relat Res. 1974;(101):61–67. [PubMed] [Google Scholar]
- 24.Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2:54–69. doi: 10.1016/s0749-8063(86)80012-3. [DOI] [PubMed] [Google Scholar]
- 25.Shah MR, Kaplan KM, Meislin RJ, Bosco JA. Articular cartilage restoration of the knee. Bull NYU Hosp Jt Dis. 2007;65:51–60. [PubMed] [Google Scholar]
- 26.Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, McKee MD, Shive MS, Buschmann MD. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;27:1432–1438. doi: 10.1002/jor.20905. [DOI] [PubMed] [Google Scholar]
- 27.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 28.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 29.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 30.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Südkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180–1186. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470:2261–2267. doi: 10.1007/s11999-012-2304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579–1588. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Wakitani S, Kawaguchi A, Tokuhara Y, Takaoka K. Present status of and future direction for articular cartilage repair. J Bone Miner Metab. 2008;26:115–122. doi: 10.1007/s00774-007-0802-8. [DOI] [PubMed] [Google Scholar]
- 34.Negrin L, Kutscha-Lissberg F, Gartlehner G, Vecsei V. Clinical outcome after microfracture of the knee: a meta-analysis of before/after-data of controlled studies. Int Orthop. 2012;36:43–50. doi: 10.1007/s00264-011-1364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Wang F, Yang L. [Research progress of articular cartilage scaffold for tissue engineering] Zhongguo Xiufu ChongJian Waike Zazhi. 2012;26:1247–1250. [PubMed] [Google Scholar]
- 36.Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 2013;29:174–186. doi: 10.1016/j.arthro.2012.05.891. [DOI] [PubMed] [Google Scholar]
- 37.Siclari A, Mascaro G, Gentili C, Cancedda R, Boux E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Relat Res. 2012;470:910–919. doi: 10.1007/s11999-011-2107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, Anders S. Outcome of Autologous Matrix Induced Chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133:87–93. doi: 10.1007/s00402-012-1621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65–72. [PubMed] [Google Scholar]
- 40.Ollat D, Lebel B, Thaunat M, Jones D, Mainard L, Dubrana F, Versier G. Mosaic osteochondral transplantations in the knee joint, midterm results of the SFA multicenter study. Orthop Traumatol Surg Res. 2011;97:S160–S166. doi: 10.1016/j.otsr.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Solheim E, Hegna J, Øyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20:287–290. doi: 10.1016/j.knee.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Haklar U, Tüzüner T, Kocaoğlu B, Güven O. [Mosaicplasty technique in the treatment of osteochondral lesions of the knee] Acta Orthop Traumatol Turc. 2008;42:344–349. doi: 10.3944/aott.2008.344. [DOI] [PubMed] [Google Scholar]
- 43.Gudas R, Gudaite A, Pocius A, Gudiene A, Cekanauskas E, Monastyreckiene E, Basevicius A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita F, Sakakida K, Suzu F, Takai S. The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1985;(201):43–50. [PubMed] [Google Scholar]
- 45.Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262–267. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 46.Giorgini A, Donati D, Cevolani L, Frisoni T, Zambianchi F, Catani F. Fresh osteochondral allograft is a suitable alternative for wide cartilage defect in the knee. Injury. 2013;44 Suppl 1:S16–S20. doi: 10.1016/S0020-1383(13)70005-6. [DOI] [PubMed] [Google Scholar]
- 47.Chahal J, Gross AE, Gross C, Mall N, Dwyer T, Chahal A, Whelan DB, Cole BJ. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29:575–588. doi: 10.1016/j.arthro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 49.Bekkers JE, Inklaar M, Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37 Suppl 1:148S–155S. doi: 10.1177/0363546509351143. [DOI] [PubMed] [Google Scholar]
- 50.Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220–2233. doi: 10.2106/JBJS.J.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhosale AM, Kuiper JH, Johnson WE, Harrison PE, Richardson JB. Midterm to long-term longitudinal outcome of autologous chondrocyte implantation in the knee joint: a multilevel analysis. Am J Sports Med. 2009;37 Suppl 1:131S–138S. doi: 10.1177/0363546509350555. [DOI] [PubMed] [Google Scholar]
- 52.Moseley JB, Anderson AF, Browne JE, Mandelbaum BR, Micheli LJ, Fu F, Erggelet C. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in US patients. Am J Sports Med. 2010;38:238–246. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- 53.Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566–2574. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 54.Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504–509. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- 55.Abelow SP, Guillen P, Ramos T. Arthroscopic technique for matrix-induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Oper Tech Orthop. 2006;16:257–261. [Google Scholar]
- 56.Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, Trattnig S. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am J Sports Med. 2012;40:2273–2280. doi: 10.1177/0363546512457008. [DOI] [PubMed] [Google Scholar]
- 57.Ebert JR, Fallon M, Ackland TR, Wood DJ, Janes GC. Arthroscopic matrix-induced autologous chondrocyte implantation: 2-year outcomes. Arthroscopy. 2012;28:952–964.e1-2. doi: 10.1016/j.arthro.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Vijayan S, Bartlett W, Bentley G, Carrington RW, Skinner JA, Pollock RC, Alorjani M, Briggs TW. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: a two- to eight-year follow-up study. J Bone Joint Surg Br. 2012;94:488–492. doi: 10.1302/0301-620X.94B4.27117. [DOI] [PubMed] [Google Scholar]
- 59.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 60.Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]


