To the editor:
Human herpes virus 6 (HHV-6) infection occurs in >95% of the population before the age of 2 years and remains latent in adults.1 Although HHV-6 reactivation commonly occurs following cord-blood transplantation, the majority of cases are self-limiting. A minority who reactivate HHV-6 develop disease, particularly encephalitis, which can lead to cognitive impairment and death. Some centers use empiric foscarnet therapy in patients who reactivate HHV-6 with high copy numbers, because the risk for encephalitis is increased in this setting.2 Recently, it was discovered that 1% to 2% of individuals have germ-line integration of HHV-6, leading to vertical transmission of the virus.3,4 Such individuals have HHV-6 DNA integrated into every somatic cell, and high copy numbers of the virus are persistently detected by polymerase chain reaction (PCR) in the absence of viral reactivation and/or replication.5,6
Here, we present a case where a recipient of a combined cord/haploidentical transplant had exceedingly high copy numbers of HHV-6 detected during neutrophil recovery as a consequence of engraftment with cells from the haploidentical donor, who had genomic integration of HHV-6, rather than from viral reactivation. A 26-year-old man with treatment-refractory severe aplastic anemia underwent conditioning with cyclophosphamide/fludarabine and equine-ATG followed by transplantation of a 4/6 HLA-matched cord unit combined with granulocyte colony-stimulating factor (G-CSF)–mobilized CD34-selected hematopoietic progenitor cells from his haploidentical brother. Neutrophil recovery occurred on day +11 (absolute neutrophil count >500 cells/µL), with chimerism studies showing engrafting myeloid cells to be haplo-donor in origin and T cells to be cord, haplo-donor, and recipient in origin. Routine PCR on whole blood for HHV-6 before engraftment was negative but became highly positive (995 000 copies/mL) by day +14. The detection of high viral copy numbers of HHV-6 concomitant with neutrophil recovery and absence of symptoms led to the suspicion of germ-line integration of the virus in the haploidentical donor. Subsequent studies showed HHV-6 levels were high in whole-blood specimens (38 950 copies/mL) but virtually undetectable in the plasma (<250 copies/mL). A droplet digital PCR assay on mononuclear cells collected from the haplo-donor revealed a HHV-6 virus/cell ratio of 1.03 consistent with viral integration.7 Lineage-specific chimerism studies showed that early myeloid engraftment was 95% haplo-donor in origin when HHV-6 copy numbers were at their highest. Remarkably, HHV-6 copy numbers declined precipitously and proportionally to an observed switch from haplo to cord myeloid chimerism, as engrafting cord T cells eradicated hematopoiesis from the haplo-donor (Figure 1). Droplet digital PCR performed on mononuclear cells collected on day +42 revealed the HHV-6/cell ratio had declined precipitously to 0.02, consistent with the switch from haplo to cord myeloid chimerism.
Figure 1.
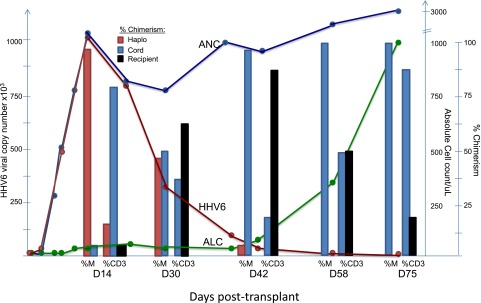
Correlation between HHV6 copy numbers, neutrophil recovery, and myeloid chimerism from the haploidentical donor. ANC, absolute neutrophil count; ALC, absolute lymphocyte count; %CD3, % T-cell chimerism; %M, % myeloid chimerism.
In most cases, the detection of HHV-6 in blood following stem-cell transplantation represents reactivation of latent virus in the recipient.8 As highlighted by this case, one should consider the possibility that genomic integration of HHV-6 in the stem-cell donor may account for HHV-6 detection, particularly when high copy numbers are detected early posttransplant, concomitant with neutrophil recovery. This phenomenon is dependent on the kinetics of donor engraftment; therefore, the time integrated HHV-6 becomes detectable posttransplant would likely be later and the slope in the rise of viral copy numbers lower following a typical single or double cord transplant compared with the case presented here where early robust engraftment of the haplo-donor occurred. In this case, HHV-6 copy numbers served as a marker for engraftment of the haploidentical donor rather than HHV-6 reactivation, highlighting a scenario where potentially toxic empiric antiviral therapy is unnecessary and should be avoided.
Authorship
Acknowledgments: This work was supported by the Intramural Research Program of the National Institutes of Health: National Heart, Lung, and Blood Institute. The content of this publication does not necessarily represent the views or policies of the National Institutes of Health.
Contribution: E.P., T.W., R.L.D., and R.W.C. were involved in patient care; G.A.F. and L.C. analyzed the HHV-6 copy numbers and chromosomal integration; D.M.Z. and K.R.J. contributed expertize; and E.P. and R.W.C. collected data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard W. Childs, National Heart Lung and Blood Institute, National Institutes of Health, 10 Center Dr, Rm 3E-5330, Bethesda, MD 20814; e-mail: childsr@nih.gov.
References
- 1.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogata M, Satou T, Kadota J, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;57:671–681. doi: 10.1093/cid/cit358. doi:10.1093/cid/cit358. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Caserta MT, Schnabel K, et al. Chromosomal integration of human herpesvirus 6 is the major mode of congenital human herpesvirus 6 infection. Pediatrics. 2008;122(3):513–520. doi: 10.1542/peds.2007-2838. [DOI] [PubMed] [Google Scholar]
- 4.Zerr DM. Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Curr Opin Infect Dis. 2012;25(4):438–444. doi: 10.1097/QCO.0b013e3283553362. [DOI] [PubMed] [Google Scholar]
- 5.Ward KN, Leong HN, Nacheva EP, et al. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol. 2006;44(4):1571–1574. doi: 10.1128/JCM.44.4.1571-1574.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamble RT, Clark DA, Leong HN, Heslop HE, Brenner MK, Carrum G. Transmission of integrated human herpesvirus-6 in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40(6):563–566. doi: 10.1038/sj.bmt.1705780. [DOI] [PubMed] [Google Scholar]
- 7.Sedlak RH, Cook L, Huang ML, et al. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Clin Chem. 2014;60(5):765–772. doi: 10.1373/clinchem.2013.217240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Pagter A PJ, Schuurman R, Meijer E, van Baarle D, Sanders EAM, Jan Boelens J. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol 2008;43:361-366. doi:10.1016/j.jcv.2008.08.008. [DOI] [PubMed]


