Abstract
Behçet’s disease (BD) is a chronic inflammatory condition with multisystem involvement. Approximately 10%-15% of patients present with gastrointestinal involvement. Involved sites and the endoscopic view usually resemble Crohn’s disease (CD). In addition to intestinal involvement, oral mucosa, the eyes, skin, and joints are commonly affected. No pathognomonic laboratory test is available for the diagnosis of either disease. Management approaches are also similar in various aspects. Differentiating BD from CD is highly challenging. In this article, the similarities and differences between BD and CD in terms of epidemiology, etiopathogenesis, clinical and imaging findings, and histopathological and therapeutic approaches are reviewed.
Keywords: Behçet’s disease, Crohn’s disease
Core tip: Behçet’s disease and Crohn’s disease are chronic inflammatory conditions caused by lesions similar to those seen in the bowels. There are similar and different clinical findings, however both diseases show intestinal inflammation. The differential diagnosis may be difficult when the symptoms of the two disease processes are very similar. This review focuses on the similar and different characteristics of Behçet’s disease and Crohn’s disease.
INTRODUCTION
Behçet’s disease (BD), which was first defined by Hulusi Behçet, a Turkish dermatologist, in 1937, is a chronic inflammatory disease with multisystem involvement[1]. It presents with remission and exacerbation of mucocutaneous, ocular, articular, vascular, or gastrointestinal lesions. Crohn’s disease (CD), on the other hand, is a chronic relapsing inflammatory disorder of the gastrointestinal tract, presenting with BD-like extra-intestinal manifestations[2]. Both of these chronic immune-mediated inflammatory disorders are likely to affect patients at a younger age accompanied by fluctuating courses. It is possible that a patient with CD meets the criteria for BD. The differential diagnosis in some cases is quite difficult, particularly in the presence of gastrointestinal involvement. Differentiation is usually based on the involvement of different organs. This review aims to investigate the similar and different characteristics of BD and CD.
EPIDEMIOLOGY
The prevalence of BD varies geographically and the disease is more prevalent in certain groups. It is most common in populations clustered along the ancient Silk Road. Turkey has the highest prevalence (80-370 cases/100000), followed by Asia and the Middle Eastern countries, including Israel, Saudi Arabia and Iran. BD can be seen in all countries worldwide due to immigration[3-6]. The age at onset of the disease is usually between 20.8 and 40 years, as it is more common in young individuals[3]. Patients aged 16 years with initial symptoms, considered as childhood-onset BD, have also been reported. The male-to-female ratio varies regionally. The disease is more common among men in Russia, Saudi Arabia, Iraq, Lebanon, Jordan, Kuwait, Greece, Italy, Turkey, and Iran, while it is more frequent among women in Japan, South Korea, and Israel[3,7].
The incidence of CD may also vary regionally. The incidence of the disease is highest in the United Kingdom, North America, and the northern part of Europe. The prevalence of CD was found to be 133/100000 in the state of Minnesota, United States in 1991. In recent years, several studies showing an increasing incidence ratio have been reported. The incidence is highest in young individuals aged 15 to 29 years. The prevalence of the disease is similar in men and women (the male/female ratio is 2.9-0.76/1)[8-14].
GENETIC FACTORS
There are familial BD cases in the literature, suggesting that genetic factors play a role in the pathogenesis of the disease. The ratio of familial cases is between 0 and 18.2%. The genetic association between the HLA-B51 gene and BD was first reported in 1982 by Ohno[15]. This association has been confirmed in many different ethnic groups. The HLA-B5 gene, particularly the HLA-B5101 allele gene, may be a strong candidate locus responsible for the development of BD and HLA-B51 itself may be the major disease susceptibility gene for BD[16]. It is more likely that the HLA-B51 gene is directly involved in the hyperactivity of neutrophils. Increased neutrophil function has also been reported in HLA-B51-positive BD patients[17,18].
Familial aggregations and a high degree of disease concordance in twins with CD have been recognized for quite some time. The concordance rate has been reported to be 3% in dizygotic twins and up to 35% in monozygotic twins[2]. Recent studies have provided an insight into genetic disorders responsible for susceptibility of the disease. Furthermore, these studies have strengthened the evidence that major cytokines, cytokine receptors and cell types are involved in the underlying pathogenesis of the disease. Nucleotide oligomerization domain 2 (NOD2) is the major susceptibility gene for CD. Genome-wide association studies have demonstrated a number of susceptibility genes where NOD2 is encoded. The nucleotide oligomerization domain 2 gene is located at the CD susceptibility locus on chromosome 16q12[19,20].
PATHOGENESIS
Immunosuppressive agents, which are used in the management of autoimmune disorders, are highly effective in BD, and the role of autoimmunity has been widely discussed in the pathogenesis of the disease[21,22]. However, anti-nuclear antibody (ANA) positivity, anti-Ro, and anti-La antibodies, which are usually found in autoimmune disorders, have not been found in BD. Several studies have demonstrated the presence of anti-endothelial antibodies, anti-lymphocytic antibodies, and heat-shock protein 60 (HSP60) in BD; however, these antibodies have not been strongly associated with the disease[23]. Additionally, major histocompatibility complex (MHC) Class II molecules have been associated with autoimmunity. However, BD is strongly associated with HLA-B5, a MHC Class I antigen.
BD is likely to be an autoinflammatory disease, as it presents with mucocutaneous lesions and episodic arthritis without deformity with a very strong acute phase response during these episodes. In BD, neutrophils are implicated in the inflammatory process of natural immune system-mediated disease (caspase pathway, IL-1, IL-18) similar to autoinflammatory diseases[21]. Mediterranean fever (MEFV) gene mutations, which are the main causes of familial Mediterranean fever (FMF), an autoinflammatory disease, are frequently found in BD[24,25]. However, the presence of clinical manifestations including uveitis, vasculitis, and thrombosis, which are not seen in autoinflammatory diseases, and the absence of serositis, a very common pathology in autoinflammatory diseases, does not suggest its autoinflammatory nature. Thus, currently, BD is considered to be neither an autoinflammatory nor an autoimmune disorder[21].
Furthermore, large and small vessel vasculitides may be present in BD. Thrombotic occlusions of the venous branches and aneurysm formations in the arterial vessels may develop. Arterial involvement may lead to bleeding and organ failure, and ultimately death. Immunosuppressive therapies can be effective in the resolution of vasculitis[26]. Vasculitis-related alterations have been observed in biopsy specimens of oral aphthae, genital ulcers, and skin lesions[27]. As vasculitis is considered to be a major component involved in the pathogenesis of BD, it is recommended that the disease should be evaluated under systemic vasculitides[28].
Several microorganisms of the oral microbial flora have been indicated in the pathogenesis of BD[29,30]. Atypical streptococcal colonization is increased in the oral mucosa. A hyperimmune activity against Streptococci has been shown in various studies. Streptococcus sanguinis causes increased interleukin-6 (IL-6) and interferon gamma (IFN-γ) secretions in the peripheral blood T-cells[31]. Escherichia coli and Staphylococcaceae species have been reported to increase inflammatory cytokines in BD patients. There are also studies showing regression of BD lesions with antibiotherapy in the literature[32].
Microorganisms that are involved in normal colon microflora with a mutual relationship with the immune system are also considered to play a role in the underlying pathogenesis of CD. Several products that are produced by these microorganisms such as butyrate and propionate contribute to intestinal inflammation, by affecting immune system cells and cytokines in patients with genetic susceptibility to CD. Reduced mucin production in epithelial cells of the intestinal mucosa is another possible culprit. Genome-wide association studies have revealed a relationship between gene mutations in mucin expression (MUC1, MUC19 and PTGER4) and CD[2,19].
Innate immune system cells are mostly implicated in the immunopathogenesis of CD[2]. Pattern recognition receptors such as Toll-like receptors (TLR) and nucleotide binding domain (NOD) like receptors (NLR) have a critical role in the recognition of the molecular patterns of innate immune system cell pathogens. There is a strong association between NOD2/CARD15 polymorphisms and CD. NOD2/CARD15 encodes an intracellular receptor that is expressed predominantly in monocytes and Paneth cells. These pattern receptors are substantially expressed by dendritic cells lying beneath the intestinal epithelium. Dendritic cells may have reduced regulatory T-cell stimulation, which leads to immune tolerance in CD. These cells are responsible for organization of the relationship between microbial products and immune system cells, and identify immunity or tolerance development. The inflammasome complex of the lamina propria, which is implicated in mononuclear cells, is crucial for the immune response. The stimulation of NLPR3, caspase-1, and pro-interleukin-1 causes a significant increase in pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-alpha) and interleukin (IL)-6. IL-17, IL-23, and IL-27 are crucial players in inflammatory alterations during the disease process[19,33]. Some authorities have adopted CD as an autoinflammatory disease due to its potent inflammation pathways[34]. Unlike patients with BD, the incidence of MEFV gene mutations remains unchanged in patients with CD[35]. The ANA and anti-neutrophil cytoplasmic antibodies (ANCA) positivity is higher in the patient population than healthy individuals. Nearly 40% to 70% of patients also have positive anti-Saccharomyces cerevisiae antibodies (ASCA), which are associated with disease severity. The ASCA positivity is higher in patients with CD than BD[36-38].
CLINICAL MANIFESTATIONS
Extra-intestinal manifestations
BD is a multisystem condition usually presenting with oral mucosa, ocular, articular, and vascular involvement. Gastrointestinal, neurological, and cardiac involvement are relatively infrequent. Nearly all patients suffer from recurrent oral ulcers. These ulcers are classified as large, small, or herpetiform, based on their size. They are extremely painful and may involve the buccal mucosa, labial mucosa, tongue, the soft and hard palate, and the pharynx. The incidence of genital ulcers with scar formation is relatively low compared with oral ulcers. These painful ulcers are quite similar to oral ulcers in appearance. They may be found in the scrotum and penis in men, and the vulva, vagina, and cervix in women. Additionally, nearly two-thirds of patients with BD have skin changes including acne-like papules, pustules, pseudofolliculitis, and erythema nodosum-like lesions. Due to superficial thrombophlebitis, shifting, and painful subcutaneous nodules can be palpable[7,39-43].
The pathergy phenomenon is a hyper-reactive response to minor trauma. The test is based on the principle of using a 21-25-gauge needle inserted into the skin. Positive test results show papulopustular lesions in the skin or erythematous reactions of the surrounding tissue within 24-48 h. The positive predictive value of the pathergy test varies regionally as the rate of positive pathergy test differs in different countries, and is highest in countries along the ancient Silk Road (30%-70%). The diagnostic value of diagnostic criteria is reduced when pathergy positivity is excluded. In addition, the pathergy test, which involves intradermal monosodium urate crystals, is more sensitive[7,43].
Ocular involvement in BD includes anterior or posterior uveitis, vitritis, retinal vasculitis, retinal vein thrombosis, corneal ulcers, and retrobulbar neuritis. Ocular disease may be the initial manifestation of the disease. BD-associated uveitis is defined as chronic and recurrent non-granulomatous panuveitis and retinal vasculitis with a bilateral course. The disease usually presents with acute inflammatory episodes that resolve within days or weeks. Recurrent episodes may result in permanent vision loss. Furthermore, as uveitis is rarely accompanied by conjunctivitis, scleritis, episcleritis, or sicca syndrome, other conditions should be suspected in patients with ocular involvement[40-44].
Musculoskeletal disorders are also common in patients with BD. Palindromic asymmetric arthritic exacerbations involving the knee, wrist, and ankle may develop. Chronic erosive arthritis is relatively rare. The incidence of sacroiliitis has been reported to increase in patients with BD. Due to peripheral arthritis characteristics and sacroiliac joint involvement, BD is evaluated in the spectrum of seronegative spondyloarthropathy. An overlap of relapsing polychondritis and BD, known as mouth and genital ulcers with inflamed cartilage (MAGIC) syndrome, may also develop in patients with cartilaginous inflammation[42-45].
BD-related vasculopathy differs from other vasculitides, due to its pattern of arterial and venous involvement. Venous thrombus may develop. It may present with superficial thrombophlebitis or involve deep veins, as well as the inferior/superior vena cava, the right atrium, or intracranial large sinuses. The major hepatobiliary disease is Budd-Chiari syndrome, which is one of the leading causes of mortality[46]. Unlike other thrombotic events, embolism is not anticipated. Primary thrombosis, which is often accompanied by right atrial thrombi, may occur in the pulmonary artery and its thin branches. In addition, arterial aneurysms are common. Pulmonary artery aneurysms may lead to massive bleeding and a fatal outcome[40-42].
Moreover, central nervous system (CNS)-related symptoms may develop secondary to vascular events, such as sinus thrombus and intracranial aneurysms. Primary parenchymal involvement including meningitis and encephalitis, mostly in the pons and mesencephalon, is also seen in patients with BD. It is also known as Neuro-BD, accounting for 10% of patients. In addition, longitudinal extensive transverse myelitis (LETM), characterized by spinal cord lesions, may occur. Histopathological examination of neurological lesions typically shows an inflammatory cellular infiltration of the surrounding vessels[40,41].
CD is a complex disorder, which primarily involves the small intestine and the colon. However, various extra-intestinal manifestations of the disease including oral and genital ulcers, erythema nodosum, uveitis, and arthritis may also be observed[2]. Skin changes may be seen in 5%-10% of patients. Erythema nodosum (5.6%-13.5%), pyoderma gangrenosum (0.75%-0.15%), and acute neutrophilic dermatoses, also termed Sweet’s disease, are among the main skin lesions. Other skin conditions include oral aphthous lesions, perianal lesions, large ulcers, fissures, fistulas, and aseptic abscesses[47,48]. Pathergy positivity is extremely low in patients with CD, compared to those with BD[49].
The most common ocular conditions are uveitis, episcleritis, conjunctivitis, and blepharitis. Non-granulomatous anterior uveitis may develop and recurrent episodes may result in permanent vision loss. Ocular complications are not associated with disease severity. Additionally, retinal vasculitis, which is extremely rare, has been reported in the literature as case studies[47-51].
The clinical association between spondyloarthropathy and CD has been well-established. Nearly 10%-15% of CD patients are complicated by spondyloarthropathy. Both peripheral and axial arthropathies may be seen in CD. Peripheral arthropathies often present as asymmetric pauciarticular involvement. It is usually acute and self-limited, and the severity of the disease is reduced in parallel with decreased disease activity without sequelae. Persistent erosive monoarthritis has been described. Axial involvement resembles ankylosing spondylitis. Bilateral sacroiliitis, as well as spondylitis of the lumbar vertebrae and syndesmophytes may be seen. Chronic low back pain is the main symptom. It is frequent in asymptomatic sacroiliitis. Half of patients with CD have sacroiliac joint abnormalities, as evidenced by X-ray images[52,53].
There are several studies showing a 1.5- to 3.5-fold increase in the risk of venous thromboembolism in CD. Some authors have suggested that it can be attributed to increased hospitalization and surgical interventions. On the other hand, the risk of arterial aneurysm and thromboembolism remain unchanged. However, mesenteric ischemia may occur[54,55]. The incidence of Takayasu’s arteritis has been reported to increase in patients with CD[56].
Primary sclerosing cholangitis is common in patients with CD, which has been reported in up to 10% of cases[48]. Although neurological signs of CD are not evident, neuroradiological imaging studies have demonstrated alterations in brain morphology[57].
Intestinal manifestations
Gastrointestinal manifestations are quite common in patients with BD. The most frequently observed signs include abdominal pain, diarrhea, nausea, anorexia, and abdominal distension. Despite the diffuse nature of the symptoms, ulcerations known as intestinal BD are relatively few. Gastrointestinal involvement varies regionally and according to the diagnostic method used. The incidence ranges from 15% to 50% based on symptoms alone and from 0.7% to 30% based on imaging or endoscopic findings[58]. Gastrointestinal involvement is higher in patients with childhood-onset BD[59]. BD-associated gastrointestinal involvement may affect all areas from the mouth to the anus. The terminal ileum and cecum are the main sites of ulcers, while few ulcers are seen in the esophagus and gastric duodenum. The most common site of involvement is in the segmental colon. Less than 15% of patients have diffuse intestinal involvement. The differentiation of intestinal BD from inflammatory bowel disease is sometimes quite challenging. The disease can be misdiagnosed as CD or ulcerative colitis during endoscopic examination. Fistulas, hemorrhage, and perforations mimicking CD may also be present. The shape of ulcers varies endoscopically from irregular to round and oval with a punched-out appearance, they are large (> 1 cm) and typically located in the deep layers. Longitudinal ulcers are rarely seen. The presence of less than six round and focal ulcers strongly indicate intestinal BD. Colonic ulcers include volcano-type and aphthous type lesions. Rectal and anal lesions are extremely rare[36,60-62].
Abdominal pain, diarrhea with or without bleeding, fatigue, weight loss, and fever are common manifestations of CD. Odynophagia, dysphagia, and dyspeptic symptoms are also seen in the case of esophageal and gastroduodenal involvement. Diarrhea is a common presentation, but often fluctuates over a long period of time. Fibrotic strictures may lead to repeated episodes of small bowel, or less commonly colonic, obstruction. Transmural bowel inflammation is associated with the development of sinus tracts, which may give rise to a fistula or abscess formation. Perianal disease, such as anal fissures, perirectal abscesses, and anorectal fistulas, occur in more than one-third of patients with CD[63]. CD may affect all areas from lips to the anus. Lesions were located in the terminal ileum in 40%-83%, colon in 32%, perianal region in 10%-15%, and the upper gastrointestinal tract in 4%[2,58,63]. Endoscopic findings of proximal CD include mucosal edema, focal and diffuse erythema, nodular lesions, erosion, and ulcers[64]. A diagnosis of CD should be considered in any patient who presents with isolated terminal ileum involvement and ileoscopy should be performed in all patients. The earliest lesions in CD consist of tiny punched-out ulcers. Deeper ulcers can occur throughout the entire wall of the colon. Cobblestoning–Serpiginous and linear ulcers are seen along the longitudinal axis of the entire colon. CD lesions are discontinuous and can be adjacent to normal tissue. Rectal involvement is suggestive of ulcerative colitis rather than CD. In addition, perianal lesions are frequently seen in CD with fistula formation[36,65].
PATHOLOGY
In BD, neutrophilic infiltration, lymphocyte aggregation of the surrounding vessels, and vascular proliferation have been observed in biopsy specimens of oral apthae and genital ulcers. Neutrophil-predominating infiltration, abscess formation and vasculitis-related changes may be present in skin lesions. Aggregation of lymphocytes, neutrophils, and eosinophils as well as edema and leukocytoclasia occur in the pathergy test site within the first 12 h. In the presence of large vessel involvement such as aortic involvement, medial elastic fiber ruptures or loss may be seen, while proliferation of the vaso vasorum and lymphocytic infiltration of the surrounding tissue may develop. Lymphocytic and necrotizing vasculitides are other conditions involving pulmonary arteries, veins, and septal capillaries. In addition, transmural necrosis and aneurysms of great vessels and pulmonary arteries may arise. Despite the non-specific nature, perivascular lymphocyte/plasma cell infiltration and myelin loss of parenchymal CNS lesions may develop[26,27,66-68].
Furthermore, inflamed intestinal BD may lead to mesenteric vasculitis with ischemia or necrosis of the intestines. Ulcer specimens often show non-specific patterns, including fibrinopurulent exudates and necrotic debris in active ulcers and transmural fibrosis in chronic ulcers. Inflammation from the lumen to the serosa is present in the perforated site with mural necrosis. Vasculitic changes secondary to the inflamed surrounding tissue and thrombus formation in the small vessels including both arteries and veins are other critical manifestations. Lymphoid follicles may be seen due to mucosal erosion in some cases. The differential diagnosis of these lesions, which are histopathologically suggestive of CD is highly challenging[26,68].
Histopathological characteristics of CD are discontinuous cryptic architectural abnormalities, mucin preservation at active sites, discontinuous inflammation, focal cryptitis, and epithelioid granulomas. Granulomas in histological sections are key features of CD, but are not necessary for diagnosis. In the submucosa, fibromuscular obliteration, nerve fiber hyperplasia and transmural lymphoid aggregates are found. Transmucosal increases in lamina propria cellularity and neutrophils are an indicator of disease activity[69].
Both BD and CD may present with transmural enteritis and colitis. Longitudinal ulcers, cobblestone appearance, and anorectal fistula are usual findings in Crohn’s colitis. The presence of granulomas in biopsy specimens indicates CD, while vasculitides are suggestive of BD[36].
DIAGNOSTIC CRITERIA
Although there is no specific diagnostic test for BD, diagnostic criteria sets described at different time points are available. The International Study Group (ISG) criteria[70], which were defined in 1990, are the most commonly used criteria for the diagnosis of BD (Table 1). These criteria are based on the most frequent clinical signs of BD. In addition, some cases of CD meet these criteria[71].
Table 1.
Diagnostic criteria for Behçet’s disease and Crohn’s disease
| International Study Group Diagnostic Criteria for Behçet’s disease[70] |
Proposed diagnostic criteria for Crohn’s disease |
|||
| Japan Criteria[72] | Lennard-Jones Criteria[73] | Copenhagen Criteria[8] | ||
| Major findings | Recurrent oral ulcerations | A: Longitudinal ulcer B: Cobblestone-like appearance C: Noncaseating epithelioid cell granuloma | Typical diarrhea history for at least 2 mo; 1 Radiological features of CD: segmental distribution, deep ulcerations or cobblestone pattern, thickened bowel wall, coarse mucosal relief, stenotic segments and fistulae; 2 Macroscopic diagnosis by endoscopy: patchy penetrating lesions, fissuring and strictures 3 Fistulas and/or abscesses with typical intestinal disease | 1 History of abdominal pain, weight loss and/or diarrhea for more than 3 mo 2 Characteristic endoscopic findings of ulceration (aphtous lesions, snail track ulceration) or cobble stoning or radiological features of stricture or cobble stoning 3 Histopathology consistent with Crohn’s disease (epitheloid granuloma of Langerhans type or transmural discontinuous focal or patchy inflammation) 4 Fistula and/or abscess in relation to affected bowel segments |
| Minor findings | Recurrent genital ulcerations Eye lesions Skin lesions Positive pathergy test | (1) Irregular-shaped and/or quasi-circular ulcers or aphthous ulcerations found extensively in the gastrointestinal tract (2) Characteristic perianal lesions (3) Characteristic gastric and/or duodenal lesions | ||
| Definite | Major finding plus two minor findings | 1 Major finding A or B 2 Major finding C, with minor finding (1) or (2) 3 All minor findings (1), (2), and (3) | Positive findings or one positive plus the finding of granuloma | At least two of the criteria present |
Several diagnostic and classification criteria for CD have been proposed[8,72-75] (Table 1). The location and appearance of lesions are important for the diagnosis of CD. According to the Vienna[74] and Montreal[75] classifications, the diagnosis of CD is established by three variables: (1) age at diagnosis; (2) disease location; and (3) behavior of the disease. The Lennard-Jones criteria are based on endoscopic, surgical/histopathological, radiological and clinical findings[73]. The Copenhagen criteria include histopathological confirmation of CD[8]. A diagnostic criteria set for CD based on alterations in gastrointestinal morphology was published in 2011[72]. However, no validated and widely adopted criteria set is currently available for the diagnosis of CD in clinical practice. The diagnosis usually relies on the patient history, physical examination, laboratory results, imaging studies, and endoscopic findings in combination with histopathological examination. Patients with BD, particularly with intestinal involvement, may be misdiagnosed and mismanaged as CD by clinicians with insufficient experience and knowledge on BD.
MANAGEMENT
As BD is a multisystem condition, effective management of the disease requires a multidisciplinary approach. Although the disease should be primarily managed by a rheumatologist, consultation is provided by a dermatologist, neurologist, gastroenterologist and cardiovascular surgeon, if necessary. The disease is inflammatory; therefore, immunosuppressive and immunomodulatory agents are first-line therapies. Due to the limited number of randomized-controlled clinical trials, management usually depends on the clinical experience of the treating physician. In 2008, the European League Against Rheumatism (EULAR) published a recommendation guideline for the management of BD[76].
The management of patients with BD is based on the presence of organ involvement and disease severity. Colchicine is a widely used treatment for BD. Corticosteroids and azathioprine can be prescribed if colchicine monotherapy is inadequate. Colchicine is used for the management of mucocutaneous and musculoskeletal findings. Corticosteroids and azathioprine can be combined in patients who are unresponsive to colchicine treatment and who have ocular, vascular, neurological, or intestinal involvement. Cyclosporine A and interferon-alpha are immunosuppressive agents used in the management of refractory uveitis and retinal vasculitis. A small number or patients with inadequate response may require mycophenolate mofetil and infliximab. Currently, these agents are used experimentally in the management of vascular involvement. In addition, cyclophosphamide is an effective immunosuppressive agent with increased side effects in patients with arterial, venous and neurological involvement who are refractory to other agents. Other agents that are preferred in unresponsive arthritis with a chronicity tendency include methotrexate and sulfasalazine. The latter is the most widely preferred agent in patients with intestinal BD, after corticosteroids and azathioprine. On the other hand, there are no randomized-controlled clinical trials in BD patients. Observational studies and case series have revealed that steroids, mesalazine, azathioprine, and sulfasalazine are likely to be used in the management of inflammatory bowel diseases. Recently, experience related to the use of anti-TNF agents have increased and some patients respond well to treatment. The efficacy of drugs in the treatment of CD and BD are compared in Table 2. In addition to immunosuppressive agents, antiaggregants, and anti-coagulants can be initiated in patients with venous and neurological involvement. However, no consensus on the use of antiaggregants and anti-coagulants has been reached yet, due to the low embolization tendency of BD-associated thrombosis and high bleeding risk secondary to arterial aneurysms. In clinical practice, these agents are prescribed in patients with low bleeding risk[7,41,76,77].
Table 2.
Treatment options for Behçet’s and Crohn’s disease
|
BD |
CD |
|||
| Extraintestinal BD | Intestinal BD | Extraintestinal CD | Intestinal CD | |
| Colchicine | S, M, A | - | - | - |
| Corticosteroids | All manifestations | + | All manifestations | + |
| Azathioprine | S, M, O, V, N | + | S | + |
| 6-mercaptopurine | - | ?? | - | + |
| Cyclosporine A | O | - | - | - |
| Interferon-alpha | O, N | - | - | - |
| Mycophenolate Mofetil | O | - | - | - |
| Cyclophosphamide | O, V, N | - | - | - |
| Methotrexate | A, N | - | A, S | - |
| Sulfasalazine | A | + | A | + |
| Mesalazine | - | + | - | + |
| Anti-TNF agents | A, O, N | + | A, S, O | + |
A: Arthritis; S: Skin; M: Mucosal; O: Ocular; V: Vascular; N: Neurogical Involvement; (+): Effective; (-): Non-Effective; BD: Behçet’s Disease; CD: Crohn’s disease.
Corticosteroids have been used in the management of CD for over five decades. Corticosteroids are the most effective therapeutic agents in relieving disease exacerbations. They exert remarkable effects in suppressing pro-inflammatory cytokines and active lymphocytes and inhibiting inflammatory processes of the intestinal lamina propria. Although corticosteroids are more effective in higher concentrations, treatment-related side effects are likely to increase. Prednisolone treatment is usually initiated at 40-60 mg/d and reduced on a gradual basis. Nearly 48%-58% of the patients achieve complete remission, while 26%-32% achieve partial remission following 30 d of treatment. Approximately 16%-20% of patients are unresponsive. Six-mercaptopurine and its pro-drug azathioprine are the most commonly used agents in patients unresponsive to corticosteroids and maintenance therapy. Methotrexate is an alternative agent in patients who are intolerant or unresponsive to these agents. On the other hand, controversial data are available on the efficacy of 5-aminosalicylic acid (5-ASA) preparations. In several meta-analyses, mesalazine 4 g/d significantly reduced disease activity in patients with mild to moderate activity. All these agents are frequently prescribed due to their low side-effect potential[78,79]. Anti-TNF agents including infliximab, adalimumab, and certolizumab pegol can be used in refractory patients with relapsing disease. Meta-analyses have demonstrated that anti-TNF agents are effective as both induction and maintenance therapy in CD patients with fistulizing disease[80]. Surgery is indicated in patients with perianal involvement, fistulas, fissures, and intra-abdominal abscesses.
Medical and surgical management approaches for CD and intestinal BD are similar. Recently, a retrospective case series with long-term outcomes for both diseases was reported[81]. Ten year-follow-up data after diagnosis showed no significant difference in the need for surgery between the study groups with CD and intestinal BD. However, CD patients required a higher dose of corticosteroids and immunosuppressive agents. The doses of biological agents were also higher in CD patients compared to patients with intestinal BD (14.2% vs 1.4%). Based on these results, long-term prognosis appears to be similar in patients with CD and intestinal BD.
CONCLUSION
CD primarily involves the gastrointestinal system and can present with various extra-intestinal signs and symptoms. However, BD is a condition or syndrome that presents with multisystem involvement. The gastrointestinal tract is also one of the main sites of involvement in these patients. Both diseases have a true overlap, affecting the gastrointestinal tract. Furthermore, both conditions share similar characteristics with respect to age of onset, gender, and inflammation biomarkers such as erythrocyte sedimentation rate and C-reactive protein (increased levels). Despite these similarities, the immunopathogenesis, genetic factors, and regional distribution are quite different. Although both diseases involve similar systems, they have distinct histopathological characteristics. For instance, uveitis is more common in BD, and CD patients are more likely to suffer from episcleritis or conjunctivitis. Figure 1 shows the similarities and differences in BD and CD. Table 3 summarizes the incidence of similarities, the distribution of gastrointestinal involvement, and endoscopic and histopathological differences.
Figure 1.
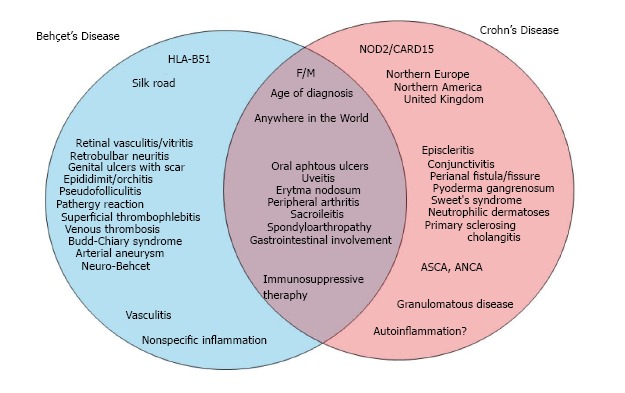
Similar and different characteristics of Behçet’s disease and Crohn’s disease. F: Female; M: Male; ASCA: Anti-Saccharomyces cerevisiae antibodies; ANCA: Anti-neutrophil cytoplasmic antibodies.
Table 3.
Distribution of similarities and differences in the differential diagnosis of Behçet’s disease and Crohn’s disease[2,3,6,8,9,14,58,60,62,68,81]
| Behçet’s Disease | Crohn’s Disease | |
| Gender (M/F) | 4.9-0.57 | 2.9-0.76 |
| Symptoms onset age (yr) | 20.8-40 | 15-29 |
| Average age at diagnosis (yr) | 24.7-35.7 | 29.5-31 |
| Oral aphtous ulcers (%) | Approximately 100 | < 10 |
| Uveitis (%) | 57-69 | < 10 |
| Skin lesions (%) | 61-87 | < 10 |
| Arthritis (%) | 30-57 | 2-24.7 |
| Gastrointestinal involvement (%) | ||
| Ileocecal area | 50-94 | 40-83 |
| Colon | 10-15 | 32-50 |
| Upper GI | 1-3 | 4 |
| Perianal | 1-2 | 10-15 |
| Intestinal complications (%) | ||
| Perforation | 12.7 | 8.7 |
| Fistula | 7.6 | 24.7 |
| Stricture | 7.2 | 38.3 |
| Abscess | 3.3 | 19.6 |
| Endoscopic Morphology | Round-oval shape, | Longitudinal ulcers with a cobblestone appearance |
| Focal, solitary | (segmental and diffuse distribution) | |
| Volcano-shaped | ||
| Deep ulcers | ||
| Mucosal Biopsy | Vasculitis | Granuloma |
| Neutrophilic infiltration | Focal cryptitis | |
| Fibrinopurulent exudates | Nerve fiberhyperplasia | |
| Necrotic debris | Lymphoid aggregates |
As mentioned above, BD is more common in Asian and Mediterranean populations, while CD is more frequently seen in north European and American individuals. However, given the fact that we live in a globalizing world, the number of patients in whom the differential diagnosis of both conditions is of the utmost importance has increased. Therefore, rheumatologists and gastroenterologists who are mainly involved in the diagnosis and management of BD and CD should be well aware of the typical characteristics of both diseases.
Footnotes
P- Reviewer: Ahluwalia NK, Castro FJ, Garip Y, Lakatos PL S- Editor: Ji FF L- Editor: Webster JR E- Editor: Lu YJ
References
- 1.Behçet H. Uber rezidivierende, aphthose, durch ein Virus verursachte Geschwure am Mund, am Auge und an den Genitalien. Dermatol Wochenschr. 1937;36:1152–1157. [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn‘s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Nadji A, Akhlaghi M, Faezi T, Ghodsi Z, Faridar A, Ashofteh F, et al. Behcet‘s disease: from East to West. Clin Rheumatol. 2010;29:823–833. doi: 10.1007/s10067-010-1430-6. [DOI] [PubMed] [Google Scholar]
- 4.Mahr A, Belarbi L, Wechsler B, Jeanneret D, Dhote R, Fain O, Lhote F, Ramanoelina J, Coste J, Guillevin L. Population-based prevalence study of Behçet‘s disease: differences by ethnic origin and low variation by age at immigration. Arthritis Rheum. 2008;58:3951–3959. doi: 10.1002/art.24149. [DOI] [PubMed] [Google Scholar]
- 5.Yurdakul S, Günaydin I, Tüzün Y, Tankurt N, Pazarli H, Ozyazgan Y, Yazici H. The prevalence of Behçet‘s syndrome in a rural area in northern Turkey. J Rheumatol. 1988;15:820–822. [PubMed] [Google Scholar]
- 6.Yurdakul S, Yazıcı Y. Epidemiology of Behçet’s Syndrome and Regional Differences in Disease Expression. 1st ed. In: Yazici Y, Yazici H, editors. Behçet’s Syndrome (NY): Springer; 2010. pp. 35–53. [Google Scholar]
- 7.Ambrose NL, Haskard DO. Differential diagnosis and management of Behçet syndrome. Nat Rev Rheumatol. 2013;9:79–89. doi: 10.1038/nrrheum.2012.156. [DOI] [PubMed] [Google Scholar]
- 8.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–1282. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 9.Thia KT, Loftus EV, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–3182. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 10.Yapp TR, Stenson R, Thomas GA, Lawrie BW, Williams GT, Hawthorne AB. Crohn’s disease incidence in Cardiff from 1930: an update for 1991-1995. Eur J Gastroenterol Hepatol. 2000;12:907–911. doi: 10.1097/00042737-200012080-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hovde Ø, Moum BA. Epidemiology and clinical course of Crohn’s disease: results from observational studies. World J Gastroenterol. 2012;18:1723–1731. doi: 10.3748/wjg.v18.i15.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonager K, Sørensen HT, Olsen J. Change in incidence of Crohn’s disease and ulcerative colitis in Denmark. A study based on the National Registry of Patients, 1981-1992. Int J Epidemiol. 1997;26:1003–1008. doi: 10.1093/ije/26.5.1003. [DOI] [PubMed] [Google Scholar]
- 13.Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein CN, Wajda A, Svenson LW, MacKenzie A, Koehoorn M, Jackson M, Fedorak R, Israel D, Blanchard JF. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohno S, Ohguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M. Close association of HLA-Bw51 with Behçet’s disease. Arch Ophthalmol. 1982;100:1455–1458. doi: 10.1001/archopht.1982.01030040433013. [DOI] [PubMed] [Google Scholar]
- 16.Durrani K, Papaliodis GN. The genetics of Adamantiades-Behcet’s disease. Semin Ophthalmol. 2008;23:73–79. doi: 10.1080/08820530701745264. [DOI] [PubMed] [Google Scholar]
- 17.Takeno M, Kariyone A, Yamashita N, Takiguchi M, Mizushima Y, Kaneoka H, Sakane T. Excessive function of peripheral blood neutrophils from patients with Behçet’s disease and from HLA-B51 transgenic mice. Arthritis Rheum. 1995;38:426–433. doi: 10.1002/art.1780380321. [DOI] [PubMed] [Google Scholar]
- 18.Kaneoka H, Furukawa H, Takeno M, Mizushima Y, Sakane T. HLA-B51 involved in the hyperfunction of peripheral blood neutrophils from patients Behcet’s disease. In: Wechsler B, Godeau P, editors. Behcet’s Disease Amsterdam: Excerpta Medica; 1993. pp. 29–32. [Google Scholar]
- 19.Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkes M. The genetics universe of Crohn’s disease and ulcerative colitis. Dig Dis. 2012;30 Suppl 1:78–81. doi: 10.1159/000341130. [DOI] [PubMed] [Google Scholar]
- 21.Direskeneli H. Autoimmunity vs autoinflammation in Behcet’s disease: do we oversimplify a complex disorder? Rheumatology (Oxford) 2006;45:1461–1465. doi: 10.1093/rheumatology/kel329. [DOI] [PubMed] [Google Scholar]
- 22.Yazici H. The place of Behçet’s syndrome among the autoimmune diseases. Int Rev Immunol. 1997;14:1–10. doi: 10.3109/08830189709116840. [DOI] [PubMed] [Google Scholar]
- 23.Kibaroglu A, Eksioglu-Demiralp E, Akoglu T, Direskeneli H. T and NK cell subset changes with microbial extracts and human HSP60-derived peptides in Behçet’s disease. Clin Exp Rheumatol. 2004;22:S59–S63. [PubMed] [Google Scholar]
- 24.Atagunduz P, Ergun T, Direskeneli H. MEFV mutations are increased in Behçet’s disease (BD) and are associated with vascular involvement. Clin Exp Rheumatol. 2003;21:S35–S37. [PubMed] [Google Scholar]
- 25.Tasliyurt T, Yigit S, Rustemoglu A, Gul U, Ates O. Common MEFV gene mutations in Turkish patients with Behcet’s disease. Gene. 2013;530:100–103. doi: 10.1016/j.gene.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Hamuryudan V, Melikoğlu M. Vascular Disease in Behçet’s Syndrome. 1st ed. In: Yazici Y, Yazici H, editors. Behçet’s Syndrome (NY): Springer; 2010. pp. 115–135. [Google Scholar]
- 27.Chun SI, Su WP, Lee S. Histopathologic study of cutaneous lesions in Behçet’s syndrome. J Dermatol. 1990;17:333–341. doi: 10.1111/j.1346-8138.1990.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 28.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 29.Mumcu G, Ergun T, Inanc N, Fresko I, Atalay T, Hayran O, Direskeneli H. Oral health is impaired in Behçet’s disease and is associated with disease severity. Rheumatology (Oxford) 2004;43:1028–1033. doi: 10.1093/rheumatology/keh236. [DOI] [PubMed] [Google Scholar]
- 30.Direskeneli H. Behçet’s disease: infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Dis. 2001;60:996–1002. doi: 10.1136/ard.60.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirohata S, Oka H, Mizushima Y. Streptococcal-related antigens stimulate production of IL6 and interferon-gamma by T cells from patients with Behcet’s disease. Cell Immunol. 1992;140:410–419. doi: 10.1016/0008-8749(92)90207-6. [DOI] [PubMed] [Google Scholar]
- 32.Calgüneri M, Kiraz S, Ertenli I, Benekli M, Karaarslan Y, Celik I. The effect of prophylactic penicillin treatment on the course of arthritis episodes in patients with Behçet’s disease. A randomized clinical trial. Arthritis Rheum. 1996;39:2062–2065. doi: 10.1002/art.1780391216. [DOI] [PubMed] [Google Scholar]
- 33.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidder H, Chowers Y, Ackerman Z, Pollak RD, Crusius JB, Livneh A, Bar-Meir S, Avidan B, Shinhar Y. The familial Mediterranean fever (MEVF) gene as a modifier of Crohn’s disease. Am J Gastroenterol. 2005;100:338–343. doi: 10.1111/j.1572-0241.2005.40810.x. [DOI] [PubMed] [Google Scholar]
- 36.Grigg EL, Kane S, Katz S. Mimicry and deception in inflammatory bowel disease and intestinal behçet disease. Gastroenterol Hepatol (N Y) 2012;8:103–112. [PMC free article] [PubMed] [Google Scholar]
- 37.Filik L, Biyikoglu I. Differentiation of Behcet’s disease from inflammatory bowel diseases: anti-Saccharomyces cerevisiae antibody and anti-neutrophilic cytoplasmic antibody. World J Gastroenterol. 2008;14:7271. doi: 10.3748/wjg.14.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi CH, Kim TI, Kim BC, Shin SJ, Lee SK, Kim WH, Kim HS. Anti-Saccharomyces cerevisiae antibody in intestinal Behçet’s disease patients: relation to clinical course. Dis Colon Rectum. 2006;49:1849–1859. doi: 10.1007/s10350-006-0706-z. [DOI] [PubMed] [Google Scholar]
- 39.Shang Y, Han S, Li J, Ren Q, Song F, Chen H. The clinical feature of Behçet’s disease in Northeastern China. Yonsei Med J. 2009;50:630–636. doi: 10.3349/ymj.2009.50.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet’s disease. Int J Dermatol. 2003;42:346–351. doi: 10.1046/j.1365-4362.2003.01741.x. [DOI] [PubMed] [Google Scholar]
- 41.Yilmaz S, Karadag O, Yazisiz V, Altun B, Gezer M, Karaman M, Cinar M, Erdem H, Pay S, Dinc A. Systemic involvements and preferred treatments in a large cohort of Behçet’s disease. Rheumatol Int. 2013;33:3025–3030. doi: 10.1007/s00296-013-2830-0. [DOI] [PubMed] [Google Scholar]
- 42.Alpsoy E, Donmez L, Onder M, Gunasti S, Usta A, Karincaoglu Y, Kandi B, Buyukkara S, Keseroglu O, Uzun S, et al. Clinical features and natural course of Behçet’s disease in 661 cases: a multicentre study. Br J Dermatol. 2007;157:901–906. doi: 10.1111/j.1365-2133.2007.08116.x. [DOI] [PubMed] [Google Scholar]
- 43.Mat MC, Bang D, Melikoğlu M. The Mucocutaneous Manifestations and Pathergy Reaction in Behcet’s Disease. 1st ed. In: Yazici Y, Yazici H, editors. Behçet’s Syndrome (NY): Springer; 2010. pp. 53–73. [Google Scholar]
- 44.Tugal-Tutkun I, Gupta V, Cunningham ET. Differential diagnosis of behçet uveitis. Ocul Immunol Inflamm. 2013;21:337–350. doi: 10.3109/09273948.2013.795228. [DOI] [PubMed] [Google Scholar]
- 45.Benamour S, Zeroual B, Alaoui FZ. Joint manifestations in Behçet’s disease. A review of 340 cases. Rev Rhum Engl Ed. 1998;65:299–307. [PubMed] [Google Scholar]
- 46.Ben Ghorbel I, Ennaifer R, Lamloum M, Khanfir M, Miled M, Houman MH. Budd-Chiari syndrome associated with Behçet’s disease. Gastroenterol Clin Biol. 2008;32:316–320. doi: 10.1016/j.gcb.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 48.Ardizzone S, Puttini PS, Cassinotti A, Porro GB. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40 Suppl 2:S253–S259. doi: 10.1016/S1590-8658(08)60534-4. [DOI] [PubMed] [Google Scholar]
- 49.Hatemi I, Hatemi G, Celik AF, Melikoglu M, Arzuhal N, Mat C, Ozyazgan Y, Yazici H. Frequency of pathergy phenomenon and other features of Behçet’s syndrome among patients with inflammatory bowel disease. Clin Exp Rheumatol. 2008;26:S91–S95. [PubMed] [Google Scholar]
- 50.Lanna CC, Ferrari Mde L, Rocha SL, Nascimento E, de Carvalho MA, da Cunha AS. A cross-sectional study of 130 Brazilian patients with Crohn’s disease and ulcerative colitis: analysis of articular and ophthalmologic manifestations. Clin Rheumatol. 2008;27:503–509. doi: 10.1007/s10067-007-0797-5. [DOI] [PubMed] [Google Scholar]
- 51.Yilmaz S, Aydemir E, Maden A, Unsal B. The prevalence of ocular involvement in patients with inflammatory bowel disease. Int J Colorectal Dis. 2007;22:1027–1030. doi: 10.1007/s00384-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 52.Colombo E, Latiano A, Palmieri O, Bossa F, Andriulli A, Annese V. Enteropathic spondyloarthropathy: a common genetic background with inflammatory bowel disease? World J Gastroenterol. 2009;15:2456–2462. doi: 10.3748/wjg.15.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gravallese EM, Kantrowitz FG. Arthritic manifestations of inflammatory bowel disease. Am J Gastroenterol. 1988;83:703–709. [PubMed] [Google Scholar]
- 54.Miehsler W, Reinisch W, Valic E, Osterode W, Tillinger W, Feichtenschlager T, Grisar J, Machold K, Scholz S, Vogelsang H, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J Crohns Colitis. 2014;8:469–479. doi: 10.1016/j.crohns.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Reny JL, Paul JF, Lefèbvre C, Champion K, Emmerich J, Blétry O, Piette JC, Fiessinger JN. Association of Takayasu’s arteritis and Crohn’s disease. Results of a study on 44 Takayasu patients and review of the literature. Ann Med Interne (Paris) 2003;154:85–90. [PubMed] [Google Scholar]
- 57.Agostini A, Benuzzi F, Filippini N, Bertani A, Scarcelli A, Farinelli V, Marchetta C, Calabrese C, Rizzello F, Gionchetti P, et al. New insights into the brain involvement in patients with Crohn’s disease: a voxel-based morphometry study. Neurogastroenterol Motil. 2013;25:147–e82. doi: 10.1111/nmo.12017. [DOI] [PubMed] [Google Scholar]
- 58.Cheon JH, Çelik AF, Kim WH. Behçet’s Disease: Gastrointestinal Involvement. 1st ed. In: Yazici Y, Yazici H, editors. Behçet’s Syndrome (NY): Springer; 2010. pp. 165–189. [Google Scholar]
- 59.Hung CH, Lee JH, Chen ST, Yang YH, Lin YT, Wang LC, Yu HH, Chiang BL. Young children with Behçet disease have more intestinal involvement. J Pediatr Gastroenterol Nutr. 2013;57:225–229. doi: 10.1097/MPG.0b013e3182936ec4. [DOI] [PubMed] [Google Scholar]
- 60.Wu QJ, Zhang FC, Zhang X. Adamantiades-Behcet’s disease-complicated gastroenteropathy. World J Gastroenterol. 2012;18:609–615. doi: 10.3748/wjg.v18.i7.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ, Kim YN, Jang HW, Jeon HH, Jung ES, Park SJ, Hong SP, Kim TI, Kim WH, Nam CM, et al. Correlations between endoscopic and clinical disease activity indices in intestinal Behcet’s disease. World J Gastroenterol. 2012;18:5771–5778. doi: 10.3748/wjg.v18.i40.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SK, Kim BK, Kim TI, Kim WH. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy. 2009;41:9–16. doi: 10.1055/s-0028-1103481. [DOI] [PubMed] [Google Scholar]
- 63.Mekhjian HS, Switz DM, Melnyk CS, Rankin GB, Brooks RK. Clinical features and natural history of Crohn’s disease. Gastroenterology. 1979;77:898–906. [PubMed] [Google Scholar]
- 64.Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618–1623. doi: 10.1007/s10620-012-2072-0. [DOI] [PubMed] [Google Scholar]
- 65.Pera A, Bellando P, Caldera D, Ponti V, Astegiano M, Barletti C, David E, Arrigoni A, Rocca G, Verme G. Colonoscopy in inflammatory bowel disease. Diagnostic accuracy and proposal of an endoscopic score. Gastroenterology. 1987;92:181–185. [PubMed] [Google Scholar]
- 66.Matsumoto T, Uekusa T, Fukuda Y. Vasculo-Behçet’s disease: a pathologic study of eight cases. Hum Pathol. 1991;22:45–51. doi: 10.1016/0046-8177(91)90060-3. [DOI] [PubMed] [Google Scholar]
- 67.Hamuryudan V, Oz B, Tüzün H, Yazici H. The menacing pulmonary artery aneurysms of Behçet’s syndrome. Clin Exp Rheumatol. 2004;22:S1–S3. [PubMed] [Google Scholar]
- 68.Demirkesen C, Oz B, Göksal S. Behçet’s Disease: Pathology. 1st ed. In: Yazici Y, Yazici H, editors. Behçet’s Syndrome (NY): Springer; 2010. pp. 215–243. [Google Scholar]
- 69.Geboes K. What histologic features best differentiate Crohn’s disease from ulcerative colitis? Inflamm Bowel Dis. 2008;14 Suppl 2:S168–S169. doi: 10.1002/ibd.20598. [DOI] [PubMed] [Google Scholar]
- 70.Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 71.Tunç R, Uluhan A, Melikoğlu M, Ozyazgan Y, Ozdoğan H, Yazici H. A reassessment of the International Study Group criteria for the diagnosis (classification) of Behçet’s syndrome. Clin Exp Rheumatol. 2001;19:S45–S47. [PubMed] [Google Scholar]
- 72.Matsui T, Hirai F, Hisabe T. Proposed diagnostic criteria for Crohn’s disease. Annual reports of the research group of intractable inflammatory bowel disease subsidized by the Ministry of Health, Labour, and Welfare of Japan, Tokyo, Japan. Behçet’s Syndrome (NY): Springer; 2011. pp. 52–54. [Google Scholar]
- 73.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16-19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 74.Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 76.Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, Houman MH, Kötter I, Olivieri I, Salvarani C, et al. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis. 2008;67:1656–1662. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 77.Arida A, Fragiadaki K, Giavri E, Sfikakis PP. Anti-TNF agents for Behçet’s disease: analysis of published data on 369 patients. Semin Arthritis Rheum. 2011;41:61–70. doi: 10.1016/j.semarthrit.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Girardin M, Manz M, Manser C, Biedermann L, Wanner R, Frei P, Safroneeva E, Mottet C, Rogler G, Schoepfer AM. First-line therapies in inflammatory bowel disease. Digestion. 2012;86 Suppl 1:6–10. doi: 10.1159/000341951. [DOI] [PubMed] [Google Scholar]
- 79.Ueno F, Matsui T, Matsumoto T, Matsuoka K, Watanabe M, Hibi T. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31–72. doi: 10.1007/s00535-012-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawalec P, Mikrut A, Wiśniewska N, Pilc A. Tumor necrosis factor-α antibodies (infliximab, adalimumab and certolizumab) in Crohn’s disease: systematic review and meta-analysis. Arch Med Sci. 2013;9:765–779. doi: 10.5114/aoms.2013.38670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Long-term clinical outcomes of Crohn’s disease and intestinal Behcet’s disease. Inflamm Bowel Dis. 2013;19:99–105. doi: 10.1002/ibd.22991. [DOI] [PubMed] [Google Scholar]


